Abstract
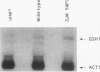
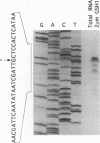
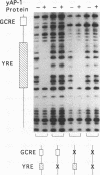
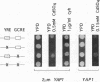
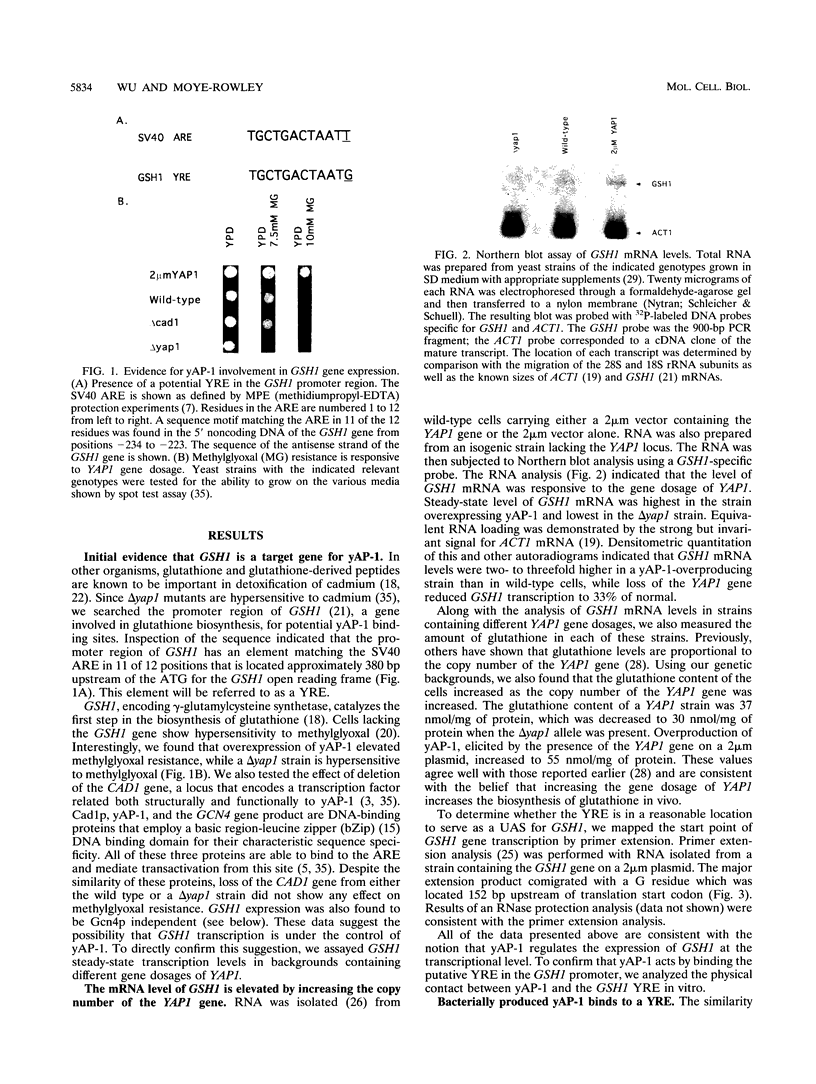
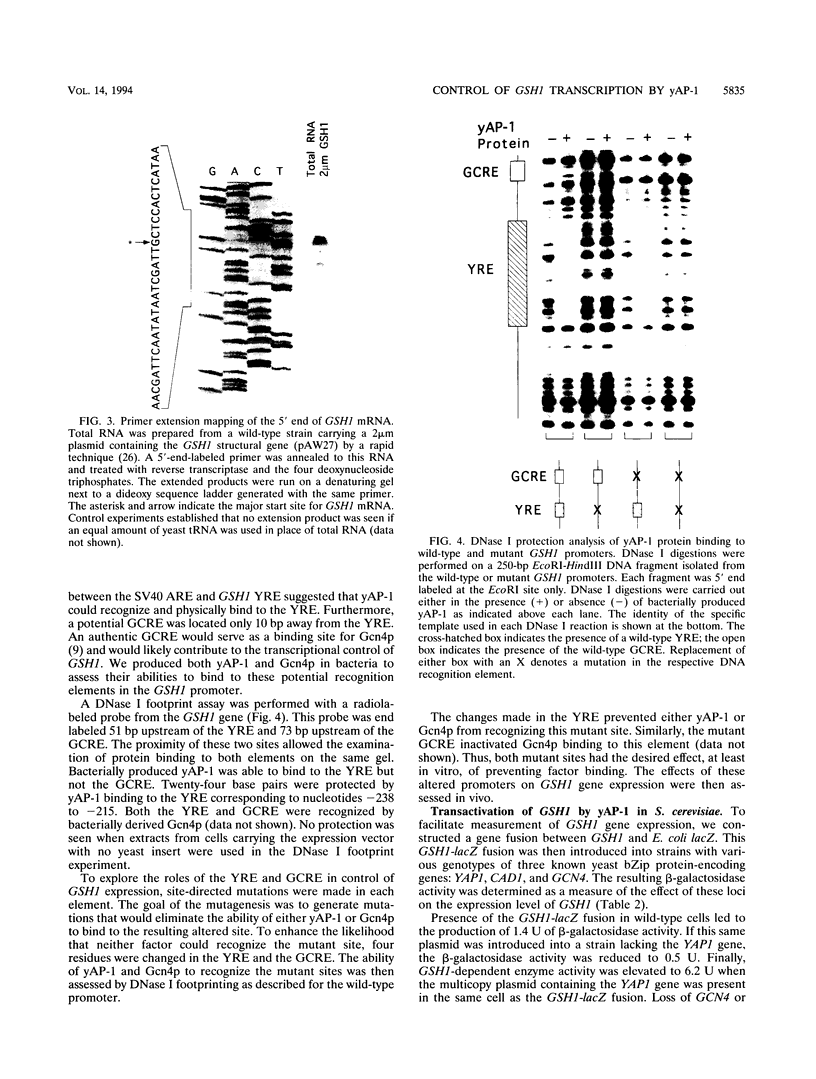
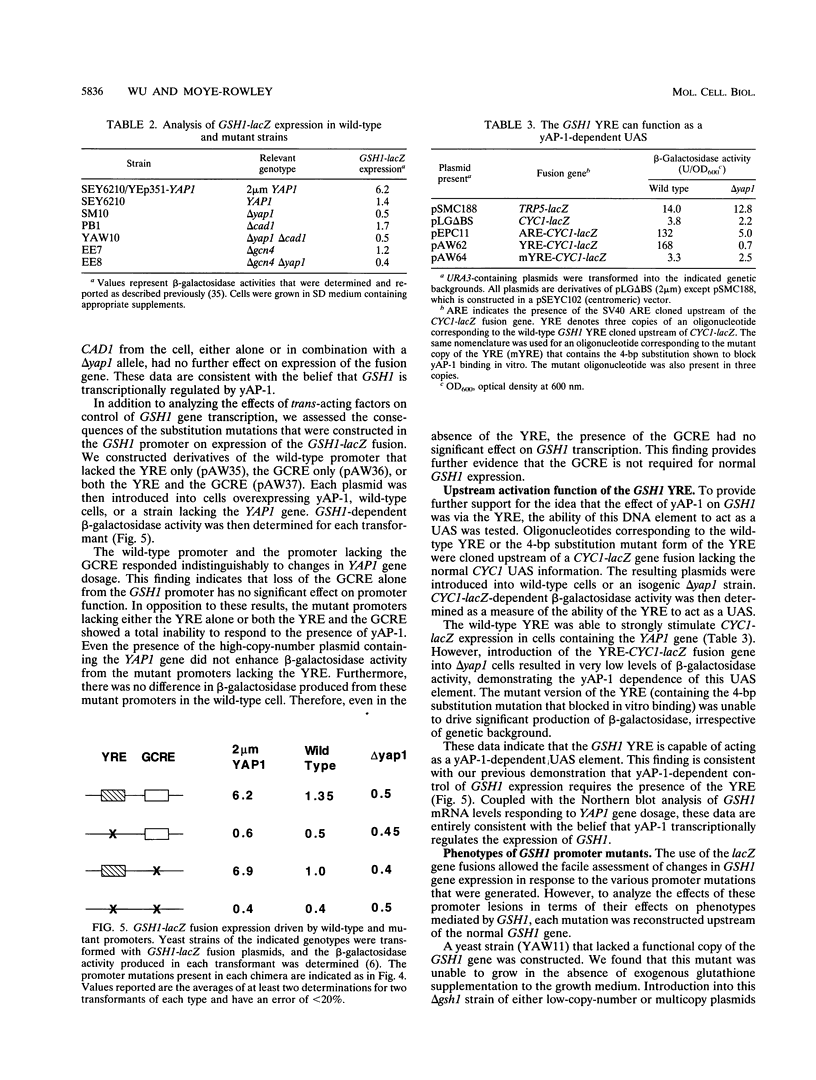
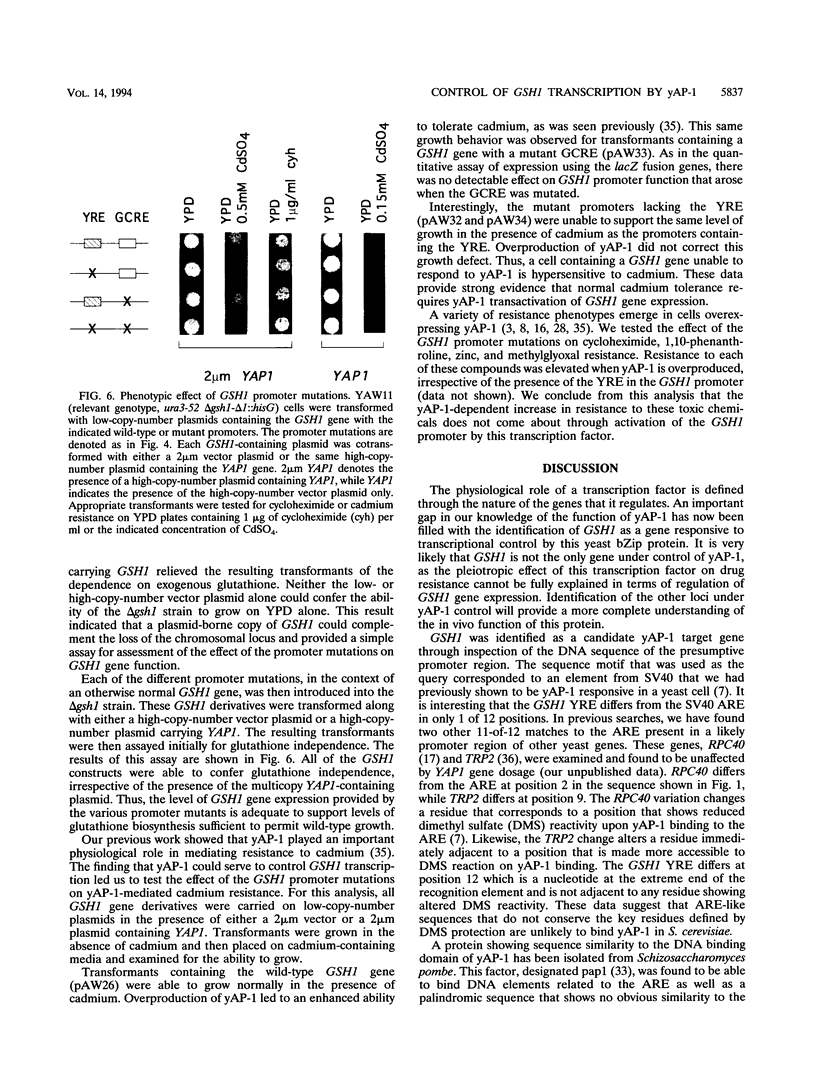
Changes in gene dosage of the YAP1 gene, encoding the yAP-1 transcriptional regulatory protein, cause profound alterations in cellular drug and metal resistance. Previous studies on yAP-1 action in yeast cells have used the AP-1 response element (ARE) from simian virus 40 as an artificial site for yAP-1-mediated transcriptional activation. No authentic yeast target sites for control of gene expression by yAP-1 are known. Here we show that the GSH1 gene, encoding gamma-glutamylcysteine synthetase, is transcriptionally responsive to the yAP-1 protein. GSH1 encodes the rate-limiting step in yeast glutathione biosynthesis and contains within its promoter region a DNA element that matches the ARE in 11 of 12 positions. The GSH1 yAP-1 response element (YRE) was recognized by yAP-1 protein in vitro. Northern (RNA) blot analysis showed that GSH1 mRNA levels were responsive to YAP1 gene dosage. A site-directed mutation in the YRE that blocked yAP-1 binding in vitro prevented the mutant GSH1 promoter from responding to elevation in YAP1 gene dosage. A delta gsh1 mutant strain was constructed and unable to grow in the absence of exogenous glutathione. A mutant GSH1 gene lacking the YRE was unable to confer normal cadmium tolerance, although other yAP-1-mediated phenotypes remained normal. Thus, GSH1 is one of several genes that are transcriptionally controlled by yAP-1 and influence drug resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bossier P., Fernandes L., Rocha D., Rodrigues-Pousada C. Overexpression of YAP2, coding for a new yAP protein, and YAP1 in Saccharomyces cerevisiae alleviates growth inhibition caused by 1,10-phenanthroline. J Biol Chem. 1993 Nov 5;268(31):23640–23645. [PubMed] [Google Scholar]
- Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb;102(2):523–533. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor E. J., Yen T. S., Rutter W. J. A mammalian viral enhancer confers transcriptional regulation in yeast. J Biol Chem. 1988 Jul 15;263(20):9569–9572. [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Harshman K. D., Moye-Rowley W. S., Parker C. S. Transcriptional activation by the SV40 AP-1 recognition element in yeast is mediated by a factor similar to AP-1 that is distinct from GCN4. Cell. 1988 Apr 22;53(2):321–330. doi: 10.1016/0092-8674(88)90393-5. [DOI] [PubMed] [Google Scholar]
- Hertle K., Haase E., Brendel M. The SNQ3 gene of Saccharomyces cerevisiae confers hyper-resistance to several functionally unrelated chemicals. Curr Genet. 1991 Jun;19(6):429–433. doi: 10.1007/BF00312733. [DOI] [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Moreno S., Nurse P., Jones N. C. Expression of the SV40 promoter in fission yeast: identification and characterization of an AP-1-like factor. Cell. 1988 May 20;53(4):659–667. doi: 10.1016/0092-8674(88)90581-8. [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Leppert G., McDevitt R., Falco S. C., Van Dyk T. K., Ficke M. B., Golin J. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics. 1990 May;125(1):13–20. doi: 10.1093/genetics/125.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C., Buhler J. M., Treich I., Sentenac A. RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell. 1987 Feb 27;48(4):627–637. doi: 10.1016/0092-8674(87)90241-8. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Ng R., Domdey H., Larson G., Rossi J. J., Abelson J. A test for intron function in the yeast actin gene. Nature. 1985 Mar 14;314(6007):183–184. doi: 10.1038/314183a0. [DOI] [PubMed] [Google Scholar]
- Ohtake Y., Yabuuchi S. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast. 1991 Dec;7(9):953–961. doi: 10.1002/yea.320070907. [DOI] [PubMed] [Google Scholar]
- Reese R. N., Mehra R. K., Tarbet E. B., Winge D. R. Studies on the gamma-glutamyl Cu-binding peptide from Schizosaccharomyces pombe. J Biol Chem. 1988 Mar 25;263(9):4186–4192. [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D. Identification and characterization of a Saccharomyces cerevisiae gene (PAR1) conferring resistance to iron chelators. Eur J Biochem. 1991 Sep 1;200(2):487–493. doi: 10.1111/j.1432-1033.1991.tb16209.x. [DOI] [PubMed] [Google Scholar]
- Schnell N., Krems B., Entian K. D. The PAR1 (YAP1/SNQ3) gene of Saccharomyces cerevisiae, a c-jun homologue, is involved in oxygen metabolism. Curr Genet. 1992 Apr;21(4-5):269–273. doi: 10.1007/BF00351681. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Saka Y., Yamano H., Adachi Y., Shirakawa M., Kyogoku Y., Yanagida M. Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol Cell Biol. 1992 Dec;12(12):5474–5484. doi: 10.1128/mcb.12.12.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991 Jan;5(1):60–73. doi: 10.1101/gad.5.1.60. [DOI] [PubMed] [Google Scholar]
- Wemmie J. A., Wu A. L., Harshman K. D., Parker C. S., Moye-Rowley W. S. Transcriptional activation mediated by the yeast AP-1 protein is required for normal cadmium tolerance. J Biol Chem. 1994 May 20;269(20):14690–14697. [PubMed] [Google Scholar]
- Wu A., Wemmie J. A., Edgington N. P., Goebl M., Guevara J. L., Moye-Rowley W. S. Yeast bZip proteins mediate pleiotropic drug and metal resistance. J Biol Chem. 1993 Sep 5;268(25):18850–18858. [PubMed] [Google Scholar]
- Zalkin H., Paluh J. L., van Cleemput M., Moye W. S., Yanofsky C. Nucleotide sequence of Saccharomyces cerevisiae genes TRP2 and TRP3 encoding bifunctional anthranilate synthase: indole-3-glycerol phosphate synthase. J Biol Chem. 1984 Mar 25;259(6):3985–3992. [PubMed] [Google Scholar]