Abstract
Ovarian cancer is the most deadly gynecological cancer, with previous studies implicating lysophosphatidic acid (LPA) in the progression of approximately 90% of all ovarian cancers. LPA potently stimulates the tyrosine phosphorylation of p130Cas, a scaffolding protein, which, upon phosphorylation, recruits an array of signaling molecules to promote tumor cell migration. Our work presented here identifies Gαi2 as the major G protein involved in tyrosine phosphorylation of p130Cas in a panel of ovarian cancer cells consisting of HeyA8, SKOV3, and OVCA429. Our results also indicate that the G12 family of G proteins that are also involved in LPA-mediated migration inhibits tyrosine phosphorylation of p130Cas. Using p130Cas siRNA, we demonstrate that p130Cas is a necessary downstream component of LPA Gαi2–induced migration and collagen-1 invasion of ovarian cancer cells. Considering the fact that LPA stimulates invasive migration through the coordination of multiple downstream signaling pathways, our current study identifies a separate unique signaling node involving p130Cas and Gαi2 in mediating LPA-mediated invasive migration of ovarian cancer cells.
Keywords: LPA, Gαi2, p130Cas, G protein, ovarian cancer, migration
Introduction
Ovarian cancer is currently the most fatal gynecological cancer with a 5-year survival rate of only 45%.1 This is primarily due to the lack of a clear understanding of the causative factors involved in the genesis and progression of the disease as well as the absence of any early diagnostic biomarkers. In this context, the identification of lysophosphatidic acid (LPA) as a novel “ovarian cancer–activating factor” that is present at high concentrations in ascitic samples of ovarian cancer patients is highly significant in that it identifies the LPA-mediated signaling pathway as a potential therapeutic or diagnostic target for the control of ovarian cancer.2,3 To this end, LPA has been convincingly linked to ovarian cancer progression and metastasis.4-16 It has been shown that signaling by LPA involves the activation of different LPA receptors that are rather permissively coupled to several heterotrimeric G proteins. Of these G proteins, the α-subunits of Gi, Gq, and G12 families have been shown to be involved in LPA-stimulated cancer cell survival, proliferation, and invasive migration.17-20 Previous studies from several laboratories including ours have shown that LPA stimulates both proliferation and invasive migration in ovarian cancer cells. While our previous studies have established a crucial role for Gα12 in LPA-stimulated proliferation of ovarian cancer cells,18 studies from several laboratories including ours have also established a role for multiple α-subunits, including those of Gi, G12, and G13, in LPA-stimulated invasive migration of ovarian cancer cells. It is of interest to note here that the downstream signaling pathways regulated by the Gi and G12 families in response to LPA are quite different. Considering the complex array of spatiotemporal signaling networks involved in cell migration, it can be reasoned that the different α-subunits regulated by LPA-LPAR signaling are involved in the regulation of specific signaling nodes in the vast array of interconnected networks involved in cell migration. Based on this rationale, the present study is focused on investigating whether a distinct signaling node can be ascribed to a specific G protein in LPA-stimulated cell migration.
Of the different signaling nodes involved in cell migration, the role of Gα13 in the regulation of Rho-dependent cytoskeletal changes and the role of Gαi/Gα13 in the regulation of Rac-dependent cytoskeletal changes have been well documented.9,21 However, the identity of the G α-subunit that mediates LPA-stimulated tyrosine kinase signaling is not fully resolved. Focusing on defining the α-subunit involved in this process, we first sought to identify LPA-responsive tyrosine kinase signaling pathways using an antibody array. Results presented here indicate that LPA stimulates the potent phosphorylation of p130Cas, an adaptor protein that acts as a signaling hub from which signals are sorted out to different kinases, structural proteins, and GTPases. Furthermore, we demonstrate that LPA stimulation of ovarian cancer cells activates Src and directly leads to the phosphorylation of Tyr-410, allowing p130Cas to recruit a host of downstream target proteins involved in invasive migration. Our studies also indicate that LPA stimulates the phosphorylation of p130Cas via VPC32183-sensitive LPA1 and/or LPA3 receptors. Silencing of p130Cas drastically attenuates LPA-mediated migration of a panel of ovarian cancer cells. More interestingly, our studies using Gαi2-silenced ovarian cancer cells clearly provide unequivocal evidence that LPA-stimulated activation of p130Cas is mediated by Gαi2 and not by Gαq, Gα12, or Gα13 in the ovarian cancer cell lines tested. Thus, our results presented here demonstrate for the first time a nonoverlapping, unique role for Gαi2 in LPA-stimulated invasive migration of ovarian cancer cells.
Results
LPA stimulates the tyrosine phosphorylation of p130Cas in ovarian cancer cells
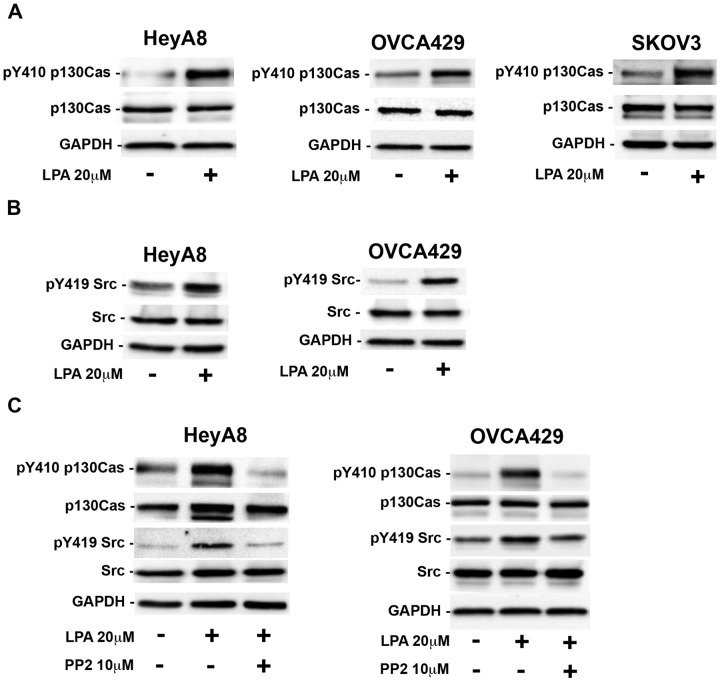
To identify proteins that were tyrosine phosphorylated in response to LPA stimulation, we carried out tyrosine phosphorylation profiling of HeyA8 ovarian cancer cells that were stimulated with 20 µM of LPA for 20 minutes using an antibody array containing antibodies targeting 60 different signaling proteins. Results from such tyrosine phosphorylation profiling indicated positive signals with varying strengths of tyrosine phosphorylation for 4 proteins (Fig. 1A, lower panel). These spots in descending order of signal strengths are p130Cas (D4), FAK (E2), integrin β3 (H3), and CDC25A (F1), respectively. In contrast, reprobing the antibody array with the lysates from untreated control HeyA8 cells failed to show a strong signal in any of the spots (Fig. 1A, upper panel), indicating that LPA stimulated the tyrosine phosphorylation of these proteins, including p130Cas, in a highly specific manner. Due to the fact that the p130Cas spot (D4) showed a relatively stronger signal and that the increased expression and/or phosphorylation of p130Cas has been associated with an increased aggressiveness of different cancers,22-29 we focused on verifying whether LPA stimulates the tyrosine phosphorylation of p130Cas in several other ovarian cancer cell lines and then characterized the upstream signaling components of LPA-mediated phosphorylation of p130Cas, specifically focusing on the G protein(s) that lead to p130Cas phosphorylation. To verify the antibody array data, we performed immunoprecipitation of p130Cas coupled with immunoblotting with an antibody that recognizes phosphorylated tyrosine residues in a panel of ovarian cancer cells consisting of HeyA8, OVCA429, and SKOV3 that were stimulated with 20 µM of LPA for 20 minutes. As shown in Figure 1B, our results indicated that LPA potently stimulates the phosphorylation of p130Cas in HeyA8, SKOV3, and OVCA429 cells, thereby validating the data obtained from the antibody array (Fig. 1A, spot D4).
Figure 1.

LPA induces the tyrosine phosphorylation of p130Cas. (A) Antibody array analysis indicated that p130Cas is tyrosine phosphorylated in an LPA-dependent manner. The dark spots, in decreasing order of magnitude, at D4, E2, F1, and H3 represent the tyrosine phosphorylation of p130Cas, FAK, CDC25A, and integrin β3, respectively. HeyA8 cells were plated at 5 × 105 per plate, allowed to adhere and grow overnight, serum starved for 16 hours and either left untreated or treated with 20 µM of LPA for 20 minutes, and then lysed with RIPA buffer. The lysates were incubated with an antibody array for 2 hours at room temperature, washed, and then incubated with an HRP-conjugated pan-tyrosine at 4°C overnight. After 16 hours, the antibody array was washed and developed. (B) Immunoprecipitation of p130Cas confirmed the antibody array data that p130Cas is tyrosine phosphorylated in an LPA-dependent manner in ovarian cancer cells. Lysates from HeyA8, OVCA429, or SKOV3 cells were subjected to immunoprecipitation using an antibody specific to p130Cas. Immunoprecipitates containing p130Cas were resolved by 10% SDS-PAGE, and immunoblot analysis was carried out using the same antibody specific to phosphotyrosine as used in the antibody array. The blot was then stripped and analyzed for total p130Cas levels.
LPA-stimulated phosphorylation of p130Cas involves the Src family of kinases
p130Cas is a scaffold protein, which has been shown to recruit a multitude of signaling molecules when tyrosine phosphorylated. It has previously been shown that p130Cas is necessary for v-Src–mediated transformation of cells.30 It has been well established that Src directly phosphorylates p130Cas on YXXP motifs in the substrate-binding domain of p130Cas, and Tyr-410 of p130Cas is one of the Src-targeted YXXP sites in the substrate-binding domain of p130Cas. Reasoning that the phosphorylation at Tyr-410 of p130Cas should represent a good proxy for total tyrosine phosphorylation of p130Cas in addition to being indicative of the involvement of Src in this pathway,31 we first investigated whether Tyr-410 is phosphorylated in response to LPA using an antibody specific to Tyr-410 of p130Cas. Our results in Figure 2A indicate that Tyr-410 of p130Cas is phosphorylated when the cells are stimulated with LPA, demonstrating a potential role of Src in LPA-stimulated phosphorylation of p130Cas. If Src indeed is involved in the phosphorylation of p130Cas, it can be reasoned that LPA is involved in the activation of Src as well. To test this, we monitored the phosphorylation of Src on Tyr-410 as an index of activation in response to LPA. Our results showed that LPA stimulated the activation of Src (Fig. 2B). In order to confirm that Src mediates LPA-induced phosphorylation of p130Cas, we tested whether LPA-stimulated phosphorylation of p130Cas on Tyr-410 was attenuated by PP2, an inhibitor of the Src family of kinases. As shown in Figure 2C, treatment of HeyA8 and OVCA429 cells with PP2 inhibited the phosphorylation of Tyr-410 on p130Cas, suggesting that Src or a member of the Src family of kinases is mediating the phosphorylation of p130Cas on Tyr-410.
Figure 2.
The Src family of kinases stimulates the phosphorylation of p130Cas. (A) LPA stimulation of ovarian cancer cells leads to the tyrosine phosphorylation of tyrosine 410 on p130Cas. HeyA8 ovarian cancer cell lines were stimulated with 20 µM of LPA for 20 minutes and then immunoblotted for phosphorylation of p130Cas on Tyr-410. The blot was stripped and reprobed for total p130Cas, and GAPDH was a loading control. (B) Stimulation of HeyA8 and OVCA429 cells with LPA leads to the activation of Src. HeyA8 and OVCA429 cells were plated (5 × 105 and 7 × 105, respectively), allowed to adhere and grow overnight, serum deprived for 16 hours, and stimulated with 20 µM of LPA for 20 minutes or left untreated as a control. Lysates from these cells were resolved by 10% SDS-PAGE, and immunoblot analysis was carried out using antibodies specific to phospho–Tyr-419 of Src and GAPDH. The blots were stripped and reprobed for total Src. (C) The Src family inhibitor PP2 inhibited the tyrosine phosphorylation of p130Cas. HeyA8 and OVCA429 cells were plated (5 × 105 and 7 × 105, respectively), allowed to adhere overnight, serum starved for 16 hours, and treated with 10 µM of the Src family–specific inhibitor PP2 for 1 hour or with DMSO as a control. PP2-treated as well as DMSO-treated cells were stimulated with 20 µM of LPA for 20 minutes. Lysates from these cells were subjected to immunoblot analysis by probing the blots with antibodies to Tyr-410–phosphorylated p130Cas, Tyr-419–phosphorylated Src, and GAPDH and were stripped and reprobed for total p130Cas and total Src. These experiments were repeated 3 times, and the results depicted are from a representative experiment.
LPA1 and/or LPA3 receptors mediate LPA-stimulated phosphorylation of p130Cas
The involvement of the Edg family of LPA receptors in ovarian cancer has been well characterized.32 Since LPA can stimulate at least 6 distinct receptors, we sought to identify the LPA receptor(s) that mediates the phosphorylation of p130Cas. First, we determined the expression levels of the 3 LPA receptors commonly expressed in the panel of ovarian cancer cells utilized in this study using quantitative RT-PCR. As shown in Figure 3A, the levels of each LPA receptor varied in these 3 cell lines. HeyA8 cells expressed high levels of LPAR3 with low expression of LPAR1 and LPAR2, while OVCA429 cells expressed high levels of LPAR1 and LPAR2 with low levels of LPAR3, whereas SKOV3 cells showed approximately equal levels of expression of all the 3 LPA receptors. Next, we investigated which LPA receptor(s) lead to the phosphorylation of p130Cas using 2 known inhibitors of LPA receptors, KI16425 and VPC32183. Previous studies have shown that KI16425 inhibited LPA-stimulated responses by inhibiting LPA1, LPA2, and LPA3 receptors in a concentration-dependent manner in the following order: LPAR1 (Ki: 0.34 µM) > LPAR2 (0.93 µM) > LPAR3 (6.5 µM).33 Therefore, we carried out a dose escalation study with the LPA receptor inhibitor KI16425 to assess which endogenous LPA receptor(s) were contributing to p130Cas phosphorylation. As shown in Figure 3B, inhibition of p130Cas was seen at a dose of 0.5 µM, and maximal inhibition, although not a complete return to basal levels, was seen at a dosage of 1 µM. These results indicated that LPAR1 and/or LPAR3 are the LPA receptors inducing p130Cas phosphorylation in these cell lines. To confirm this further, we used the LPA receptor inhibitor VPC32183 that has been shown to inhibit only LPAR1 and LPAR3 and not LPAR2.34-37 As shown in Figure 3C, treatment of HeyA8, OVCA429, and SKOV3 with 10 µM of VPC32183 reduced p130Cas to nearly basal levels and led to the inactivation of Src, suggesting that LPAR1 and LPAR3 are the major endogenous LPA receptors that lead to p130Cas phosphorylation.
Figure 3.
LPA stimulates p130Cas phosphorylation through LPA receptors 1 and/or 3. (A) Quantification of the mRNA levels of LPA receptors via quantitative RT-PCR in HeyA8, OVCA429, and SKOV3 cells. (B) Effect of LPAR inhibitor KI16425. OVCA429 cells were plated and allowed to grow overnight. The following day, the cells were serum starved for 16 hours and then pretreated with the indicated dosages of KI16425 for 15 minutes. Following KI16425 pretreatment, the cells were then stimulated with 20 µM of LPA for 20 minutes and then lysed. Immunoblot analysis was carried out with the lysates using antibodies for Tyr-410 p130Cas. The blot was stripped and reprobed for total p130Cas levels. GAPDH was checked to ensure equal loading of each lane. (C) Effect of the LPAR1/3 inhibitor VPC32183. HeyA8, OVCA429, and SKOV3 cells were plated and allowed to adhere overnight. The following day, the cells were serum starved for 16 hours and then pretreated with the indicated dosages of VPC32183 for 15 minutes. After 15-minute pretreatment with VPC32183, the cells were treated with 20 µM of LPA for 20 minutes and subsequently lysed. The lysates were subjected to immunoblot analyses using antibodies to Tyr-410 p130Cas or Tyr-419 Src and then stripped and reprobed for total p130Cas and Src. The level of GAPDH was checked to ensure equal loading of each lane.
Gαi2 mediates LPA-stimulated phosphorylation of p130Cas
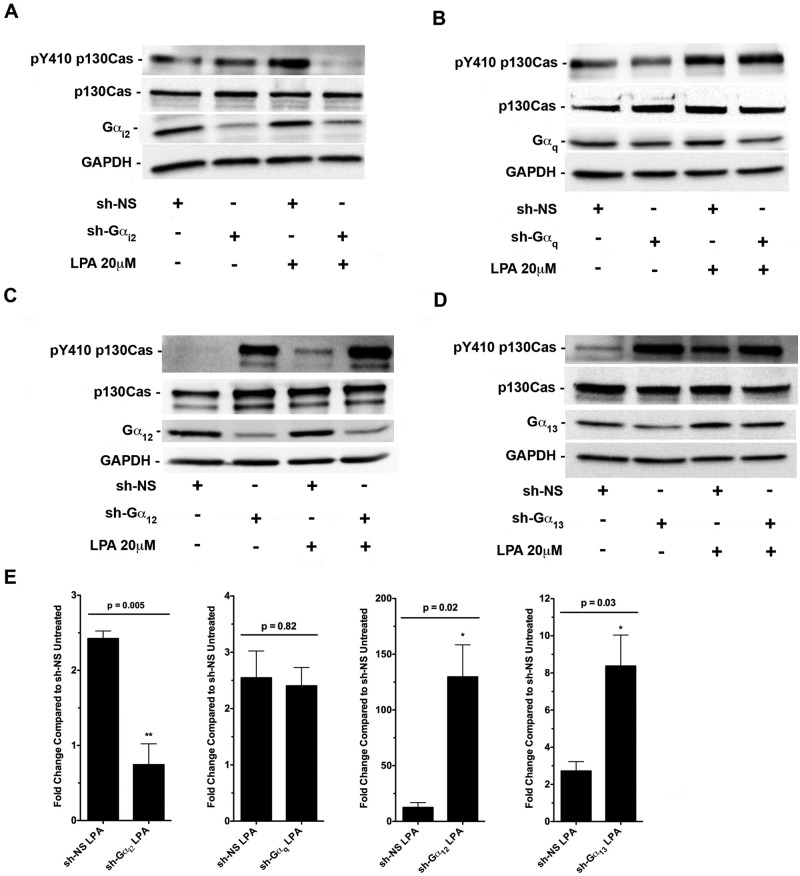
Several past studies, including ours, have shown that oncogenic signaling by LPA involves the activation of LPA receptors, which in turn activates several of the α-subunits of heterotrimeric G protein families Gi, Gq, and/or G12/13, and these activated G proteins can lead to the activation of multiple downstream signaling cascades in the cell.17-19,38,39 To determine the identity of the G α-subunit(s) involved in transmitting the signals from LPA receptors to induce p130Cas phosphorylation, we monitored Tyr-410 phosphorylation of p130Cas in response to 20 µM of LPA for 20 minutes in HeyA8 cells in which the expression of Gαi2, Gαq, Gα12, or Gα13 was silenced by shRNAs specific to each of these α-subunits (Fig. 4A-D). The results from 3 replicates were then quantified in Figure 4E. Our data indicated that only silencing of Gαi2 abrogated LPA-stimulated Tyr-410 phosphorylation of p130Cas (Fig. 4A and 4E). In addition, our results (Fig. 4C-E) demonstrated that knockdown of Gα12 and Gα13 significantly enhanced the phosphorylation of p130Cas in the context of LPA-mediated signaling, suggesting that the G12/13 family plays an inhibitory role in p130Cas phosphorylation.
Figure 4.
LPA-stimulated Tyr-410 phosphorylation of p130Cas is mediated by Gαi2. HeyA8 cells were transiently transfected with either nonsense shRNA or shRNA that targeted (A) Gαi2, (B) Gαq, (C) Gα12, or (D) Gα13. After 24 hours, the transfected cells were serum starved for 16 hours and treated with 20 µM of LPA for 20 minutes, while each control group was left untreated. Lysates were subjected to immunoblot analysis using antibodies specific to Tyr-410–phosphorylated p130Cas and then stripped and probed for total p130Cas. Silencing of Gαi2, Gαq, Gα12, or Gα13 was confirmed by probing the respective blots with antibodies specific to each α-subunit. The blots were stripped and reprobed with antibodies to GAPDH to monitor equal loading of proteins. (E) Quantification and statistical analysis of the experiments represented in A to D (n = 3).
To further confirm that Gαi2 is directly involved in p130Cas phosphorylation, we transiently transfected HeyA8 ovarian cancer cells with a vector encoding a constitutively active mutant of Gαi2 (Gαi2Q205L), Gαq (GαqQ209L), Gα13 (Gα13Q226L), or Gα12 (Gα12Q229L) and monitored Tyr-410 phosphorylation of p130Cas (Fig. 5A-E; results of 3 replicates quantified in Fig. 5E). Results from these analyses indicated that only expression of Gαi2Q205L resulted in the phosphorylation of Tyr-410 of p130Cas, whereas expression of GαqQ209L, Gα12Q229L, or Gα13Q226L failed to have such a stimulatory effect on Tyr-410 phosphorylation of p130Cas. Interestingly, overexpression of constitutively active Gα13 and Gα12 resulted in a statistically significant reduction (Fig. 5E) of basal p130Cas phosphorylation (Fig. 5C and 5D). Taken together with the results obtained with the silencing of individual G α-subunits (Fig. 4), these findings clearly establish a specific role for Gαi2 in LPA-stimulated phosphorylation of p130Cas.
Figure 5.
Tyr-410 phosphorylation of p130Cas is specific to Gαi2. HeyA8 (2 × 106) cells were transiently transfected with either a vector control or (A) Gai2Q205L, (B) GαqQ209L, (C) Gα13Q226L, or (D) Gα12Q229L and incubated in 6-well plates in normal growth media. After approximately 48 hours, the cells were lysed and subjected to immunoblot analysis using antibodies specific to Tyr-410–phosphorylated p130Cas and then stripped and probed for total p130Cas. Overexpression of each constitutively active G protein was confirmed with antibodies to Gai2, Gαq, Gα12, or Gα13 on their respective blots. The blots were stripped and reprobed with antibodies to GAPDH to monitor equal loading of proteins. (E) Quantification and statistical analysis of the experiments represented in A to D (n = 3).
p130Cas is a critical signaling node in LPA-mediated migration
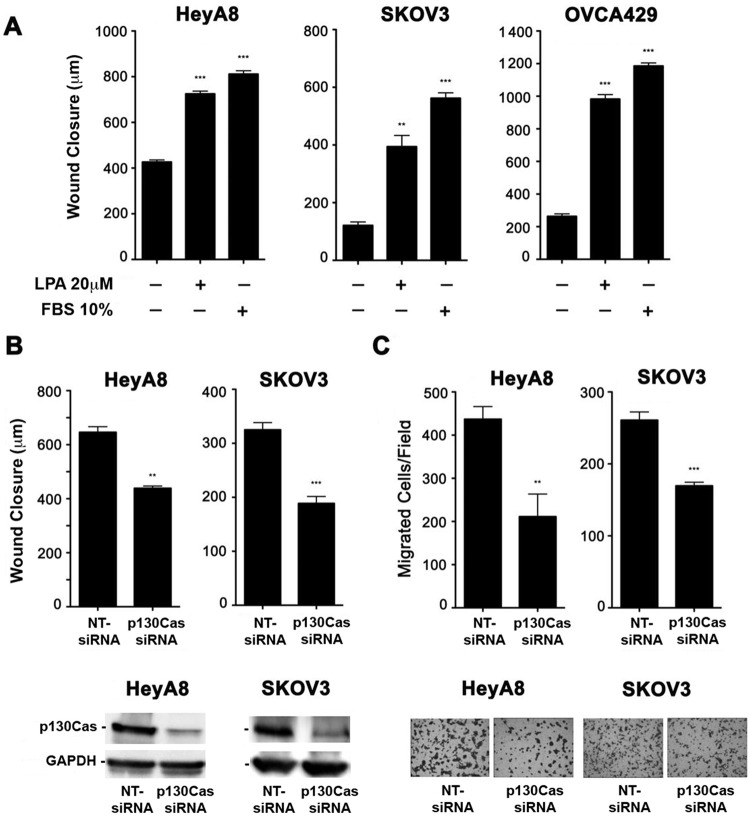
Phosphorylation of p130Cas on Tyr-410 has been shown to be necessary for the recruitment of Crk to p130Cas, which leads to the migration and invasion of cells.27,40 Therefore, we hypothesized that LPA stimulates the phosphorylation of p130Cas to promote the migration and invasion of ovarian cancer cells. To test this hypothesis, we first sought to confirm previous studies18,38,39 that pathological levels of LPA could lead to the migration of ovarian cancer cells. As shown in Figure 6A, 24-hour stimulation with 20 µM of LPA led to the migration of 3 separate ovarian cancer cell lines, confirming that LPA induces the migration of ovarian cancer cells. Next, we examined whether knocking down p130Cas, via p130Cas-specific siRNA, attenuated LPA-mediated migration of ovarian cancer cells. Using a standard wound healing assay to quantify migration (Fig. 6B), we found that knockdown of p130Cas significantly reduces LPA-mediated migration of ovarian cancer cells. In addition, to test whether p130Cas is involved in LPA-mediated invasive migration into collagen-1, we monitored the migration of p130Cas-silenced ovarian cancer cells in response to 20 µM of LPA using a transwell assay in which the wells were precoated with collagen-1. Similar to the results obtained from the wound healing assay, LPA-mediated invasive migration of ovarian cancer cells was significantly attenuated by silencing p130Cas (Fig. 6C). Together, these data clearly establish that p130Cas is a necessary component for initiating LPA-mediated invasive migration of ovarian cancer cells.
Figure 6.
Silencing p130Cas inhibits LPA-mediated invasive migration of ovarian cancer cells. (A) LPA stimulation induced the migration of ovarian cancer cells. HeyA8 (5 × 105), SKOV3 (7 × 105), and OVCA429 (7 × 105) cells were plated in 6-well plates and allowed to grow to confluence. The cells were then serum starved overnight and treated with mitomycin C (10 µg/mL) to inhibit proliferation, and then 3 separate wounds were made per well. Pictures were taken at 3 random fields per wound and marked, and the cells were then stimulated with 20 µM of LPA for 24 hours. After 24-hour stimulation, pictures were taken of the same location on each wound, and wound closure was measured with the CellSens program. Ten percent FBS was used as a positive control. (B) Silencing p130Cas inhibits LPA-mediated migration of ovarian cancer cells. There was 100 nM of siRNA that specifically targeted p130Cas or nontargeting siRNA that was transiently transfected into HeyA8 and SKOV3 cells. At 48 hours after transfection, the cells were then serum starved overnight, and the wound healing assay was performed as described in A. Each group was done in triplicate. (C) p130Cas mediates LPA-induced invasive migration. HeyA8 and SKOV3 cells were transfected with p130Cas-specific siRNA or nontargeting siRNA. Collagen-1–coated cell culture inserts containing 2.5 × 104 (HeyA8) or 1 × 105 (SKOV3) cells suspended in 200 mL serum-free media were placed in their respective well of a 24-well plate. Each well contained 500 mL serum-free media that was treated with 20 µM of LPA. The cells were incubated at 37°C and allowed to migrate for 20 hours. Nonmigrating cells on the proximal side of the inserts were removed with a cotton swab, and the migrated cells on the distal side of the insert were fixed and stained with Hemacolor. Images were obtained at 200× in 5 random fields for each experimental group. The experiments were repeated at least 3 times, and the results presented are from a typical experiment. **P < 0.01. ***P < 0.001.
The LPAR1/3-Gαi2-p130Cas signaling node plays a critical role in LPA-mediated migration of ovarian cancer cells
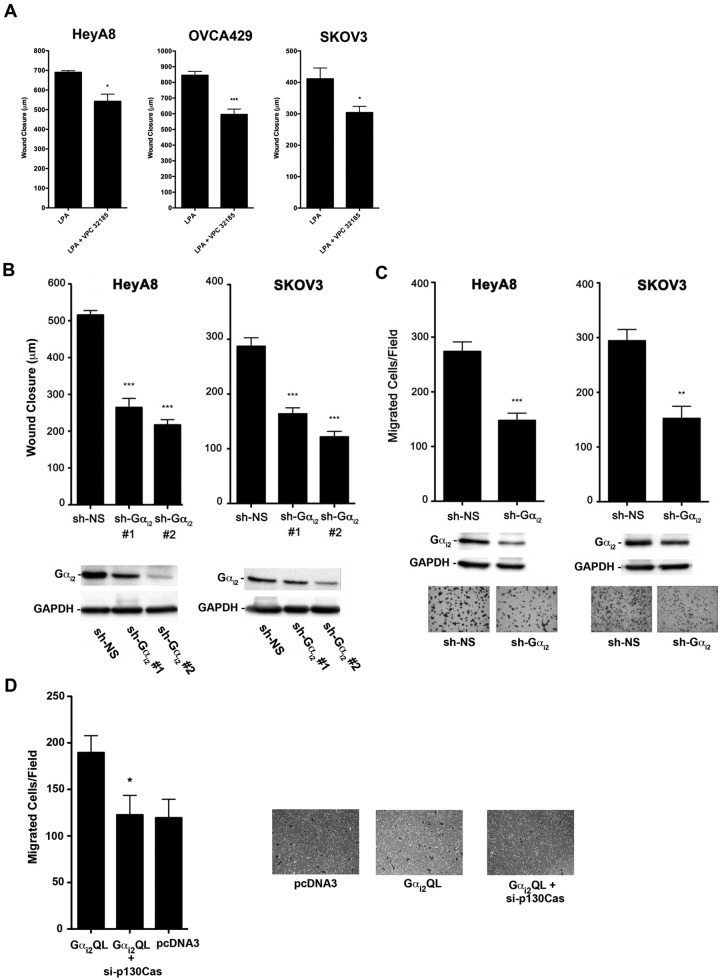
It has been previously shown that LPA-mediated cell migration involves the Gi family in many different cell types, including ovarian cancer cells.39 From our results that demonstrate that silencing p130Cas abrogates LPA-induced migration of ovarian cancer cells and that LPAR1/3 as well as Gαi2 are involved in the phosphorylation of p130Cas, it can be deduced that LPA-mediated migration of ovarian cancer involves the LPAR1/3-Gαi2-p130Cas signaling nexus. To establish such a role, first we investigated whether the inhibition of LPAR1/3 led to an attenuation of LPA-mediated migration in ovarian cancer cells (Fig. 7A). HeyA8, OVCA429, and SKOV3 cells were treated with VPC32183, and the migratory potential of these cells in response to 20 µM of LPA was analyzed using a wound healing assay. As shown in Figure 7A, treatment of these ovarian cancer cells with VPC32183 significantly attenuated their migration. Following this, we verified the role of Gαi2 in LPA-mediated migration by silencing the expression of Gαi2 and performing a wound healing assay (Fig. 7B). As shown in Figure 7B, knockdown of Gαi2 led to a significant decrease in LPA-mediated migration in HeyA8 and SKOV3 cells. We also found that knockdown of Gαi2 significantly reduced LPA-mediated collagen-1 invasion (Fig. 7C). The results presented in Figure 7B and 7C clearly demonstrate that LPA induces invasive migration of ovarian cancer cells in response to LPA and that knockdown of Gαi2 significantly attenuates this invasive migration. To confirm this further, in addition to establishing a role for p130Cas in this process, we investigated whether the transient expression of the activated mutant of Gαi2 could stimulate invasive migration of ovarian cancer cells and, if so, whether the siRNA-mediated silencing of p130Cas would abrogate such an anticipated stimulatory effect of constitutively activated Gαi2 (Fig. 7D). To test this, HeyA8 cells were transfected with Gαi2Q205L, and the migration of these cells was monitored using a collagen-1–coated transwell assay. As shown in Figure 7D, expression of constitutively active Gαi2 led to a statistically significant increase in invasive migration of these cells compared to the control. More interestingly, when p130Cas was silenced via p130Cas-specific siRNA in the cells expressing constitutively active Gαi2, the observed increase in cell migration seen with Gαi2 was completely inhibited, suggesting that p130Cas plays a vital role in Gαi2-mediated invasive migration of ovarian cancer cells. Together with the results obtained from the use of LPA receptor subtype–specific inhibitor VPC32183, our results establish a critical role for the LPAR1/3-Gαi2-p130Cas signaling nexus in LPA-responsive invasive migration of ovarian cancer cells.
Figure 7.
Inhibition of the LPAR1/3-Gαi2-p130Cas signaling axis attenuates LPA-stimulated migration of ovarian cancer cells. (A) Role of LPAR1/3 in LPA-stimulated cell migration. HeyA8 (5 × 105), SKOV3 (7 × 105), and OVCA429 (7 × 105) cells were plated in 6-well plates and allowed to grow to confluence. The cells were then serum starved overnight and treated with mitomycin C (10 µg/mL) to inhibit proliferation, and then 3 separate wounds were made per well. Pictures were taken at 3 random fields per wound and marked, and the cells were then stimulated with 20 µM of LPA or 20 µM of LPA plus 10 µM VPC32183 for 24 hours. After 24-hour stimulation, pictures were taken of the same location, and cell movement was measured with the CellSens program. *P < 0.05. ***P < 0.001 (n = 3). (B) Silencing Gαi2 attenuates LPA-stimulated migration of ovarian cancer cells. Two different shRNAs that target different regions of Gαi2 or nonsense shRNA were transiently transfected into HeyA8 and SKOV3 (2 × 106) cells. The wound closure assay was carried out at 48 hours following transfection as described in Figure 6A. Silencing of Gαi2 in these cells was verified by immunoblot analysis (lower panel). (C) Silencing Gαi2 attenuates LPA-stimulated invasive migration of ovarian cancer cells. HeyA8 and SKOV3 (2 × 106) cells were transfected with Gαi2-specific shRNA or nonsense shRNA. Silencing of Gαi2 in these cells was verified by immunoblot analysis (lower panel). The transwell assay was carried out as described in Figure 6C. Representative images from each experimental group are shown. (D) Gαi2-stimulated invasive migration is dependent on p130Cas. HeyA8 cells were transiently transfected with Gαi2Q205L, Gαi2Q205L + si-p130Cas, or with vector alone (pcDNA3). The cells were allowed to grow in 60-mm plates for approximately 48 hours. There were 2.5 × 104 cells counted and suspended in 200 mL of serum-free media and subsequently plated on collagen-1–coated transwell inserts in 24-well plates with 500 mL of serum-free media. The cells were incubated at 37°C and allowed to migrate for 20 hours. The transwells were then processed as described in Figure 6C. The experiments were repeated at least 3 times, and the results presented are from a typical experiment. *P < 0.05. **P < 0.01. ***P < 0.001 (n = 3).
Discussion
In this report, we have shown that the LPAR1/3-Gαi2-p130Cas signaling network is intimately involved in invasive migration of ovarian cancer cells. More importantly, we demonstrate that Gαi2 mediates the tyrosine phosphorylation of the scaffold protein p130Cas to induce invasive migration of ovarian cancer cells in response to LPA. Although the role of the Gi family in LPA-stimulated migration in multiple cell types, including ovarian cancer cells, has been demonstrated previously,39 the specific locus in which Gαi2 is involved in the regulation of invasive cell migration has thus far not been identified. Our finding here that LPA-Gαi2 signaling involves Src-mediated p130Cas phosphorylation is a novel and highly significant finding. This gains further significance in light of the observation that p130Cas is a scaffold protein, which has been shown to recruit a multitude of signaling molecules when tyrosine phosphorylated, and that p130Cas is “essential” for the neoplastic transformation of various cell types in response to many different oncogenes.8,23,30,41 Furthermore, it has been demonstrated that p130Cas is directly involved in the increased aggressiveness of several cancers, including a recent report showing its importance in ovarian cancer.24,27,29,42,43 Overall, the critical observations made in this report are that LPA stimulates the Tyr-410 phosphorylation of p130Cas in ovarian cancer cells and that the phosphorylation of p130Cas is dependent on Gαi2. What is more, we have conclusively shown that the LPA-LPAR-Gαi2-p130Cas signaling network is intimately involved in the migration of ovarian cancer cells. Therefore, this report is the first study to establish that LPAR1/3-LPAR Gαi2–mediated stimulation of p130Cas is involved in invasive migration of ovarian cancer.
Analyses of our results also unravel the possible mechanisms through which LPA activates multiple G proteins to regulate distinct signaling nodes to coordinate cell migration in general. Previously, it has been observed that LPA stimulates ovarian cancer cell migration through the activation of Gαi 39 as well as Gα12/13,18,38 suggesting the possibility that the migratory response of ovarian cancer cells to LPA involves a network of interconnected pathways. It has been well established that LPA stimulates Rho-dependent cytoskeletal changes via Gα12/13. Likewise, previous studies have linked the Gi family to the activation of Rac. One particular study of a ligand with very similar properties to LPA, S1P, was shown to induce Rac1 activation and invasive migration via a Gi family–dependent pathway.44 An additional report demonstrated that Gi family activation via a PI3 kinase–dependent manner led to the activation of Tiam1, inducing the activation of Rac1.45 What is more, previous studies have shown that when p130Cas is phosphorylated by Src on Tyr-410, it leads to the recruitment of Crk, which then leads to the activation of Dock180 and subsequent activation of Rac1.27 Indeed, Sharma and Mayer46 recently demonstrated that phosphorylation of p130Cas by Src initiates Rac activation and subsequent membrane ruffling and lamellipodium formation. Likewise, it has also been established that LPA can stimulate Rac and subsequent Rac-dependent cytoskeletal changes in some cell types. Considering the fact that the stimulation of a p130Cas-mediated hub can result in the activation of Rac, it is rather tempting to speculate that LPA through specific receptors recruits Gα13 to stimulate Rho-dependent events and Gαi2 to stimulate Rac-dependent events. Nevertheless, it has been observed that Gα13 can stimulate Rac activation in several different cell types.4-7 Similarly, Gαi2 has been shown to be involved in the activation of Rho as well.47,48 To this end, an interesting possibility relates to the recent observation that Gα13 can play a determinant role in the inhibition of Rac signaling in dorsal ruffles while mediating its activation in the leading edge.49,50 While the role of Gα13 in the dissolution of dorsal ruffles through inactivating Rac has been established, the identity of the G protein involved in the activation of Rac to stimulate dorsal ruffle formation is hitherto unknown. Our observation here that Gαi2 phosphorylates Tyr-410 of p130Cas, which is well characterized in leading to the activation of Rac and the observed inhibition of this pathway by Gα13, may be indicative that Gαi2 is the α-subunit involved in such locus- specific activation of Rac essential for cell migration. Thus, it is quite likely that LPA stimulates, presumably through distinct LPA receptors, Rac in dorsal ruffles via Gαi2 while utilizing Gα13 for Rac inhibition in dorsal ruffles as well as Rac activation in other cellular loci. Based on this reasoning, it can be envisioned that the dynamic spatiotemporally regulated balance between Gαi2 and Gα13 signaling, both stimulated by LPA, is required for cell migration, and the overexpression or silencing of one versus another unravels a seemingly antagonistic activity of the respective G protein. This is consistent with the observation that the silencing of Gαi2 converts the stimulatory effect of Gα13 into an inhibitory effect on cytoskeletal changes involved in cell migration.21
Finally, recent findings by Nick et al. 28 have demonstrated that when p130Cas is overexpressed in ovarian cancer patients, there is a very significant correlation with a decreased survival of these patients. When Nick et al.’s28 findings are paired with the knowledge that over 90% of ovarian cancer patients have high levels of LPA found in the surrounding ascitic fluid, it is tempting to postulate that p130Cas overexpression paired with LPA signaling via a Gαi2 pathway may be contributing to the decrease in the survival of these patients; yet, this remains to be experientially demonstrated. Therefore, in addition to providing evidence for such a context-specific role for these α-subunits, our findings with the LPAR1/3-Gαi2-p130Cas signaling nexus gain greater significance in ovarian cancer in which p130Cas signaling appears to play a critical role. Thus, the identification of this critical signaling node in ovarian cancer pathophysiology as presented here (Fig. 8) could potentially lead to the development of novel targeted therapeutics for ovarian cancer, especially those patients who have an increased expression of p130Cas.
Figure 8.
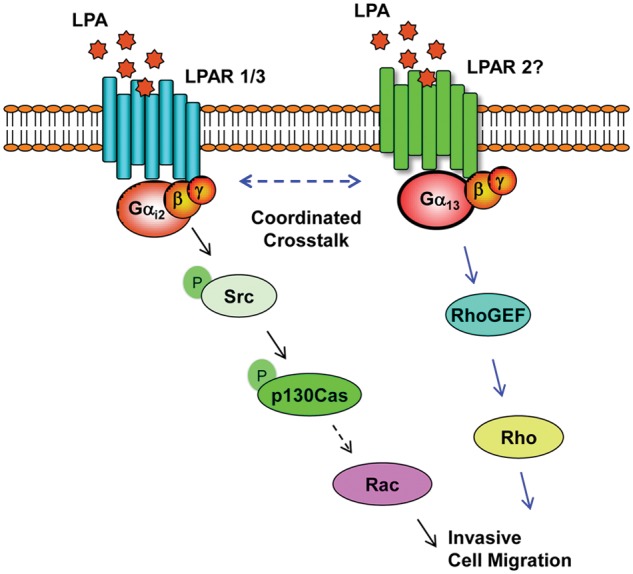
Schematic representation of LPA-LPAR1/3 Gαi2–mediated activation of p130Cas in cell migration. LPA stimulates the phosphorylation at Tyr-410 of p130Cas via Gαi2 in a Src-dependent pathway, and p130Cas is critically required for LPA-mediated invasive migration of ovarian cancer cells. The blue arrows indicate the results derived from previous studies (see text), whereas the black arrows indicate the results obtained from the present study. Taken together with the previous findings, it can be envisioned that LPA, through distinct LPA receptors, regulates the coordinated signaling of spatially and/or temporally compartmentalized Gαi2-p130Cas–mediated stimulation of Rac as well as Gα13-Rho-GEF–mediated activation of Rho to promote invasive migration of ovarian cancer cells.
Materials and Methods
Cell lines and regents
The ovarian cancer cell lines SKOV3 and HeyA8 were kindly provided by E. Premkumar Reddy (Mount Sinai School of Medicine, New York, NY). The ovarian cancer cell line OVCA429 was kindly provided by Susan K. Murphy (Duke University, Durham, NC). All of the cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Manassas, VA) containing 10% FBS (Gemini Bio-Products, West Sacramento, CA), 50 U/mL penicillin, and 50 mg/mL streptomycin (Mediatech) at 37°C in a 5% CO2 incubator. For serum starvation, the media used were DMEM with 0.1% BSA Fraction V (heat shock, fatty acid ultra free) (Roche, Indianapolis, IN), 50 U/mL penicillin, and 50 mg/mL streptomycin (Mediatech). All cells were lysed in RIPA buffer (PBS, pH 7.4, 1% Nonidet P-40 (Fisher Scientific, Pittsburgh, PA), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 0.2 mM sodium vanadate, 2 mg/mL leupeptin, 2 mg/mL pepstatin A, and 4 mg/mL aprotinin). LPA was obtained from Avanti Polar Lipids (Alabaster, AL) and dissolved into 10 mM stock solutions in PBS with 0.1% BSA and stored at −80°C until use. PP2 was purchased from EMD Chemicals (Gibbstown, NJ), dissolved in DMSO, stored at −20°C until use, and used at the indicated concentrations. si-p130Cas (5′-GGUCGACAGUGGUGUGUAUUU-3′) and nontargeting siRNA ON-TARGETplus nontargeting siRNA #1 were purchased from Dharmacon (Lafayette, CO). sh-RNA for Gαi2 #1 (5′-CCAGAGTAAGTTTGAGGACCT-3′) and Gαi2 #2 (5′-GCATGAGAGCATGAAGCTATT-3′) was used in all experiments that had a single sh-RNA for Gαi2 knockdown; Gαq (5′-CCATACAAGTATGAGCACAAT- 3′), Gα13 (5′-GTCTCCATAATTCTGTTCTTA-3′), Gα12 (5′- CGTCAACAACAAGCTCTTCTT-3′), and nonsense shRNA were purchased from Open Biosystems (Lafayette, CO). Peroxidase-conjugated anti-rabbit IgG was purchased from Promega (Madison, WI), and peroxidase-conjugated anti-mouse was purchased from GE Healthcare (Little Chalfont, UK). HRP-conjugated pan-tyrosine antibody (#5465), Tyr-410 p130Cas (#4011), Tyr-419 Src (#2101), Src (#2109), and GAPDH (#2118) were purchased from Cell Signaling Technology (Danvers, MA). p130Cas (sc-20029), Gαi2 (sc-13534), Gαq (sc-393), Gα12 (sc-20), and Gα13 (sc-410) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Antibody array
HeyA8 cells were plated at 5 × 105 per plate, allowed to adhere and grow overnight, serum starved for 16 hours and either left untreated or treated with 20 µM of LPA for 20 minutes, and then lysed with RIPA buffer. The lysates (912 µg total protein) were incubated with an antibody array (#HM-3000-100T, Hypromatrix, Worcester, MA) for 2 hours at room temperature, washed in TBST 3 times, and then incubated with an HRP-conjugated pan-tyrosine antibody at 4°C with rocking for 16 hours. After 16 hours, the antibody array was washed 3 times in TBST and developed by a standard chemiluminescence protocol using a Kodak Image Station 4000MM (Rochester, NY).
Immunoblotting and immunoprecipitation
Lysates (500 µg) from HeyA8, OVCA429, or SKOV3 cells were subjected to immunoprecipitation using an antibody specific for p130Cas. Immunoprecipitates containing p130Cas were resolved by 10% SDS-PAGE, and immunoblot analysis was carried out using the same phosphotyrosine antibody as used in the antibody array. The blot was then stripped and analyzed for total p130Cas. Immunoblot analysis with the indicated antibodies was carried out following previously published procedures51 and developed with a Kodak Image Station 4000MM. The blots were cut at particular molecular weights relevant to each experiment, so that several antibodies could be incubated and developed in parallel. Stripping of membranes was performed at 70°C for 10 minutes in stripping buffer (1 M β-mercaptoethanol, 0.0625 M Tris, pH 6.8, 0.2% SDS). Quantification of immunoblots was performed with Carestream Molecular Imaging Software version 5 (Rochester, NY), and the respective values were imported into GraphPad Prism (La Jolla, CA) for graphing and statistical analysis.
Wound healing assay
All cells were transfected using an Amaxa Nucleofector II apparatus (Cologne, Germany). For knockdown of p130Cas, 100 nM of siRNA that specifically targeted p130Cas or nontargeting siRNA was transiently transfected into 2 × 106 HeyA8 or SKOV3 cells and placed into a 6-well plate in triplicate. At 48 hours after transfection, the cells were then serum starved overnight and treated with mitomycin C (10 µg/mL) to inhibit proliferation, and then 3 separate wounds were made per well. Pictures were taken at 3 random fields per wound and marked with a pen, and the cells were then stimulated with 20 µM of LPA for 24 hours. After 24-hour stimulation, pictures were taken of the same location, and cell movement was measured with the CellSens program (Olympus America, Center Valley, PA). For monitoring the effect of Gαi2 knockdown, 2 different shRNAs that target different regions of Gαi2 or nonsense shRNA were transiently transfected into 2 × 106 HeyA8 or SKOV3 cells, and wound healing was performed as described above. To monitor the effect of LPA on 3 different ovarian cancer cell lines, HeyA8 (5 × 105), SKOV3 (7 × 105), and OVCA429 (7 × 105) cells were plated in 6-well plates and allowed to grow to confluence, and the same wound healing assay was performed as indicated above.
Collagen-1 invasion assay
Collagen type 1 was coated overnight onto 8-mm pore transwells at 4°C. The following day, the collagen-coated cell culture inserts containing 2.5 × 104 (HeyA8) or 1 × 105 (SKOV3) cells suspended in 200 mL serum-free media were placed in the well of a 24-well companion plate. Each well contained 500 mL media containing serum-free media control or serum-free media containing 20 µM of LPA. The cells were incubated for 20 hours. Nonmigrating cells on the proximal side of the inserts were removed with a cotton swab, and the migrated cells on the distal side of the inserts were fixed and stained with Hemacolor (EMD Chemicals). Images were obtained at 200× in 5 random fields for each group. The experiments were repeated 3 times. Each cell was transfected with the indicated plasmid or si-RNA and plated into 6-well plates for a total of 48 hours. Twenty-four hours after transfection, the cells were serum starved for an additional 24 hours, trypsinized, counted, and plated at the above-mentioned numbers for each cell line.
Statistical analysis
All statistical analysis was performed using GraphPad Prism by a 2-tailed Student t test with Welch correction. All depicted error bars represent the standard error of the mean.
Acknowledgments
Helpful discussions with Dr. Ji Hee Ha are gratefully acknowledged.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Institutes of Health (CA116984, CA123233).
References
- 1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-36 [DOI] [PubMed] [Google Scholar]
- 2. Mills GB, May C, McGill M, Roifman CM, Mellors A. A putative new growth factor in ascitic fluid from ovarian cancer patients: identification, characterization, and mechanism of action. Cancer Res. 1988;48(5):1066-71 [PubMed] [Google Scholar]
- 3. Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309(Pt 3):933-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang X, Gaudette D, Furui T, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann N Y Acad Sci. 2000;905:188-208 [DOI] [PubMed] [Google Scholar]
- 5. Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6(6):2482-91 [PubMed] [Google Scholar]
- 6. Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279(10):9653-61 [DOI] [PubMed] [Google Scholar]
- 7. Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61(7):3194-9 [PubMed] [Google Scholar]
- 8. Frankel A, Mills GB. Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clin Cancer Res. 1996;2(8):1307-13 [PubMed] [Google Scholar]
- 9. Fujita T, Miyamoto S, Onoyama I, Sonoda K, Mekada E, Nakano H. Expression of lysophosphatidic acid receptors and vascular endothelial growth factor mediating lysophosphatidic acid in the development of human ovarian cancer. Cancer Lett. 2003;192(2):161-9 [DOI] [PubMed] [Google Scholar]
- 10. Luquain C, Singh A, Wang L, Natarajan V, Morris AJ. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J Lipid Res. 2003;44(10):1963-75 [DOI] [PubMed] [Google Scholar]
- 11. Mukai M, Imamura F, Ayaki M, et al. Inhibition of tumor invasion and metastasis by a novel lysophosphatidic acid (cyclic LPA). Int J Cancer. 1999;81(6):918-22 [DOI] [PubMed] [Google Scholar]
- 12. Pustilnik TB, Estrella V, Wiener JR, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5(11):3704-10 [PubMed] [Google Scholar]
- 13. Schwartz BM, Hong G, Morrison BH, et al. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81(2):291-300 [DOI] [PubMed] [Google Scholar]
- 14. Symowicz J, Adley BP, Woo MM, Auersperg N, Hudson LG, Stack MS. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65(6):2234-42 [DOI] [PubMed] [Google Scholar]
- 15. Tanyi JL, Morris AJ, Wolf JK, et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003;63(5):1073-82 [PubMed] [Google Scholar]
- 16. Xie Y, Gibbs TC, Meier KE. Lysophosphatidic acid as an autocrine and paracrine mediator. Biochim Biophys Acta. 2002;1582(1-3):270-81 [DOI] [PubMed] [Google Scholar]
- 17. Radhika V, Hee Ha J, Jayaraman M, Tsim ST, Dhanasekaran N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Galpha12. Oncogene. 2005;24(28):4597-603 [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN. Lysophosphatidic acid stimulates the proliferation of ovarian cancer cells via the gep proto-oncogene Galpha(12). Genes Cancer. 2011;2(5):563-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870-81 [DOI] [PubMed] [Google Scholar]
- 20. Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26(22):3122-42 [DOI] [PubMed] [Google Scholar]
- 21. Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582-91 [DOI] [PubMed] [Google Scholar]
- 22. Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem. 2004;279(37):38331-7 [DOI] [PubMed] [Google Scholar]
- 23. Cabodi S, Tinnirello A, Di Stefano P, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66(9):4672-80 [DOI] [PubMed] [Google Scholar]
- 24. Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10(12):858-70 [DOI] [PubMed] [Google Scholar]
- 25. Fromont G, Vallancien G, Validire P, Levillain P, Cussenot O. BCAR1 expression in prostate cancer: association with 16q23 LOH status, tumor progression and EGFR/KAI1 staining. Prostate. 2007;67(3):268-73 [DOI] [PubMed] [Google Scholar]
- 26. Wendt MK, Smith JA, Schiemann WP. p130Cas is required for mammary tumor growth and transforming growth factor-beta-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem. 2009;284(49):34145-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67(7):1025-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nick AM, Stone RL, Armaiz-Pena G, et al. Silencing of p130cas in ovarian carcinoma: a novel mechanism for tumor cell death. J Natl Cancer Inst. 2011;103(21):1596-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fromont G, Cussenot O. The integrin signalling adaptor p130CAS is also a key player in prostate cancer. Nat Rev Cancer. 2011;11(3):227. [DOI] [PubMed] [Google Scholar]
- 30. Honda H, Oda H, Nakamoto T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19(4):361-5 [DOI] [PubMed] [Google Scholar]
- 31. Vultur A, Buettner R, Kowolik C, et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther. 2008;7(5):1185-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu S, Murph MM, Lu Y, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100(22):1630-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohta H, Sato K, Murata N, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64(4):994-1005 [DOI] [PubMed] [Google Scholar]
- 34. Heasley BH, Jarosz R, Lynch KR, Macdonald TL. Initial structure-activity relationships of lysophosphatidic acid receptor antagonists: discovery of a high-affinity LPA1/LPA3 receptor antagonist. Bioorg Med Chem Lett. 2004;14(11):2735-40 [DOI] [PubMed] [Google Scholar]
- 35. Heasley BH, Jarosz R, Carter KM, Van SJ, Lynch KR, Macdonald TL. A novel series of 2-pyridyl-containing compounds as lysophosphatidic acid receptor antagonists: development of a nonhydrolyzable LPA3 receptor-selective antagonist. Bioorg Med Chem Lett. 2004;14(15):4069-74 [DOI] [PubMed] [Google Scholar]
- 36. Lapierre DM, Tanabe N, Pereverzev A, et al. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J Biol Chem. 2010;285(33):25792-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu YJ, Tappia PS, Goyal RK, Dhalla NS. Mechanisms of the lysophosphatidic acid-induced increase in [Ca(2+)](i) in skeletal muscle cells. J Cell Mol Med. 2008;12(3):942-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bian D, Mahanivong C, Yu J, et al. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25(15):2234-44 [DOI] [PubMed] [Google Scholar]
- 39. Bian D, Su S, Mahanivong C, et al. Lysophosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK kinase 1 pathway. Cancer Res. 2004;64(12):4209-17 [DOI] [PubMed] [Google Scholar]
- 40. Lai YJ, Chen CS, Lin WC, Lin FT. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25(14):5859-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cabodi S, Tinnirello A, Bisaro B, et al. p130Cas is an essential transducer element in ErbB2 transformation. FASEB J. 2010;24(10): 3796-808 [DOI] [PubMed] [Google Scholar]
- 42. Schrecengost RS, Riggins RB, Thomas KS, Guerrero MS, Bouton AH. Breast cancer antiestrogen resistance-3 expression regulates breast cancer cell migration through promotion of p130Cas membrane localization and membrane ruffling. Cancer Res. 2007;67(13):6174-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fathers KE, Bell ES, Rajadurai CV, et al. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 2012;14(3):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Devine KM, Smicun Y, Hope JM, Fishman DA. S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecol Oncol. 2008;110(2):237-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleming IN, Gray A, Downes CP. Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J. 2000;351(Pt 1):173-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma A, Mayer BJ. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biol. 2008;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fleming IN, Elliott CM, Exton JH. Differential translocation of rho family GTPases by lysophosphatidic acid, endothelin-1, and platelet-derived growth factor. J Biol Chem. 1996;271(51):33067-73 [DOI] [PubMed] [Google Scholar]
- 48. Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459-89 [DOI] [PubMed] [Google Scholar]
- 49. Shan D, Chen L, Wang D, Tan YC, Gu JL, Huang XY. The G protein G alpha(13) is required for growth factor-induced cell migration. Dev Cell. 2006;10(6):707-18 [DOI] [PubMed] [Google Scholar]
- 50. Dhanasekaran DN. Transducing the signals: a G protein takes a new identity. Sci STKE. 2006;2006(347):pe31. [DOI] [PubMed] [Google Scholar]
- 51. Kumar RN, Ha JH, Radhakrishnan R, Dhanasekaran DN. Transactivation of platelet-derived growth factor receptor alpha by the GTPase-deficient activated mutant of Galpha12. Mol Cell Biol. 2006;26(1):50-62 [DOI] [PMC free article] [PubMed] [Google Scholar]