Abstract
Bothrops asper (Squamata: Viperidae) is the most important venomous snake in Central America, being responsible for the majority of snakebite accidents. Four basic PLA2s (pMTX-I to -IV) were purified from crude venom by a single-step chromatography using a CM-Sepharose ion-exchange column (1.5 × 15 cm). Analysis of the N-terminal sequence demonstrated that pMTX-I and III belong to the catalytically active Asp49 phospholipase A2 subclass, whereas pMTX-II and IV belong to the enzymatically inactive Lys49 PLA2s-like subclass. The PLA2s isolated from Panama Bothrops asper venom (pMTX-I, II, III, and IV) are able to induce myotoxic activity, inflammatory reaction mainly leukocyte migration to the muscle, and induce J774A.1 macrophages activation to start phagocytic activity and superoxide production.
1. Introduction
Envenomation resulted from snakebites is the cause of considerable morbidity and mortality in many tropical and subtropical countries, being an important public health problem [1, 2]. According to Kasturiratne et al. [2], the estimative number of deaths per year due to snakebites in 2007 for Central America ranged from 193 to 1,461. Since snakebites affect, in most cases, poor people living in rural parts of tropical countries [3], the World Health Organization (WHO), incorporated snakebite envenoming in its list of neglected diseases (http://www.who.int/neglected_diseases/diseases/en/).
Lance-headed pit vipers belonging the Viperidae family, especially the Bothrops asper snake (Figure 1) are responsible for the most severe cases of snakebite envenoming, being the main cause of the largest numbers of bites and fatalities in Central America [1]. Among the American countries, Panama has thehighest incidence of snakebite cases, showing an average number of 40 to 65 cases per 100,000 population per year and a estimated total number of 1,300 to 1,800 cases per year [4, 5], which B. asper is responsible for 90% of all snakebites cases of major clinical importance [4, 6]. B. asper is able to inoculate a relative large amount of venom and is considered extreme aggressive, being able to cause severe accidents [7].
Figure 1.
Bothrops asper resting in limestone cave, Central Panama.
According to Gutiérrez et al. [5], the envenomation produced by B. asper induces prominent local tissue damage, characterized by swelling, blistering, prominent oedema, haemorrhage, dermonecrosis, and myonecrosis with clinical manifestations that include bleeding, effects on platelet aggregation, coagulopathy, hypotension, hemodynamic alterations, pulmonary oedema, and acute renal failure. Other less common effects include intravascular haemolysis, acute myocardial damage multiple organ failure, and death. The clinical features of the envenomation are affected by the venom components, which vary according to snake species, geographic region, age, sex, and environment [5, 8, 9].
Snake venoms are characterized as a complex mixture of bioactive molecules, which proteins compose more than 90% of the venom dry weight [10–12]. Many of these proteins are enzymes, in which the most abundant are phospholipases A2 (PLA2s; E.C.3.1.1.4) [10]. PLA2s are members of a protein superfamily that comprise several groups of enzymes with different catalytic mechanisms, as well as different functional and structural features, that cleavage the sn-2 acyl ester bond of glycerolphospholipids producing free fatty acids and lysophospholipids [13, 14]. Snake venom PLA2s (svPLA2s) have been grouped into four classifications according to minor structural differences as group I and II, both sub-classified as type A or B. The group II is found in venoms from Viperidae family, while the group I is found in Elapidae and Hydrophiidae venoms [14]. svPLA2s from Viperidae family are placed into group IIB and are mainly subdivided in two types: Asp49 PLA2s, which have an Asp residue at position 49, and Lys49 PLA2s, showing a Lys residue at position 49. Different from Asp49 PLA2s, Lys49 PLA2s have low or any catalytic activity upon artificial substrates [13, 15, 16].
This present paper describes the biochemical and toxicological characterization of crude B. asper venom from Panama, and the isolation, purification, and biochemical characterization of four basic cytotoxic PLA2 from this venom and its effects on gastrocnemius muscle and inflammation.
2. Materials and Methods
2.1. Materials
Bothrops asper snake venom was collected from adult specimens, captured in Caldera and Gomez (Provence of Chiriquí, Panama) and in Arraiján (Provence of Panama, Panama). The snakes were maintained in a serpentarium at the Gamboa Rainforest Resort, Panamá, where the crude venom was obtained by inducing the snake to bite a parafilm-wrapped jar. Venoms were centrifuged at 1,000 xg for 15 min, and supernatants were lyophilized and stored at −20°C in Microbiology Department at the Medicine Faculty of Panama University until used. Male albino Swiss mice, weighing 18–22 g, were used for the assays.
The murine macrophage cell lines (J774A.1) were obtained from Rio de Janeiro Cell Bank Collection (Brazil). RPMI-1640, penicillin, streptomycin, and L-glutamine were purchased from Sigma-Aldrich (MO, USA); fetal bovine serum (FBS) was from Cultilab (Brazil). All reagents were low endotoxin or endotoxin-free grades. Animal care was in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA) and was approved by the Committee for Ethics in Animals Utilization of Universidade de São Paulo (CEUA no. 06.1.291.53.3). CM-Sepharose and Phenyl-Sepharose resins were purchased from Amersham Biosciences, Uppsala, Sweden. The Kit CK-UV- Kinetic was purchased from Bioclin, Brazil. The following reagents: ethylenediaminetetraacetic acid (EDTA), molecular weight protein standards, and acrylamide were obtained from Sigma Chemical Co. All other chemicals reagents were of analytical grade from Merck, Aldrich or Pharmacia Biotech.
2.2. Purification and Biochemical Characterization of PLA2s
B. asper crude venom (300 mg) was dissolved in 1.5 mL of 0.05 M ammonium bicarbonate buffer, pH 8.1 and applied on a CM-Sepharose column (1.5 × 15 cm) according to Soares et al. [17]. All fractions were analyzed by SDS-PAGE, and PLA2 activity was evaluated in vitro by indirect erythrocyte lysis in agar containing human erythrocytes and egg yolk, as previously described [18], being the fraction with PLA2 activity selected. Polyacrylamide gel electrophoresis was performed in the presence of sodium dodecyl sulfate (SDS-PAGE) [19]. Isoelectric focusing was performed according to previously described. Buffalyte, pH range 3.0–9.0 (Pierce, IL), was used to generate the pH gradient. To determinate the protein concentration, the microbiuret method was used. The mol. wt of PLA2s was estimated by mass spectrometry (Quattro II, Micromass). A Procise-491 (Applied Biosystems) automatic sequencer was used for the N-terminal sequencing [17]. The phenylthiohydantoin (PTH) amino acids were identified by comparing their retention times with the 20 PTH-amino acid standard mixture. The peptides obtained were compared with the sequences of other related proteins in the SWISS-PROT/TrEMBL databases using the FASTA and BLAST tools.
2.3. Biological and Pharmacological Characterization
2.3.1. Lethality
Groups of four mice (18–22 g) were injected by IP route with various amounts of crude venom (in a volume of 0.1 mL) eluted with PBS, (phosphate buffered saline, 0.12 M NaCl, 0.04 M Na2HPO4, and pH 7.2). Deaths were recorded at 1, 3, 6, 12, 24, and 48 hours. LD50 was calculated using the Spearman-Karber method [20].
2.3.2. Edema-Inducing Activity
Groups of four male Swiss mice (18–22 g) were injected in the subplantar region with various amounts of crude venom (in a volume of 50 μL) prepared with PBS, pH 7.2. Then, the paw increase was measured at different time intervals (30, 60, 120, and 180 min), subtracting the initial paw measure (time 0 h). The paw edema was measured with the aid of a low-pressure pachymeter (Mitutoyo, Japan).
2.3.3. Hemorrhage
Groups of four male Swiss mice (18–22 g) were injected by ID route in the dorsal region with various amounts of crude venom (in a volume of 50 μL) prepared in PBS, pH 7.2. The hemorrhagic activity of the venom was determined as follows. Different doses of venom were injected intradermally, in a volume of 0.1 mL, into groups of four mice (18–22 g); 2 h later, they were sacrificed with CO2, their skin removed, and the area of the hemorrhagic spot was measured. Diameters were calculated, and the minimum hemorrhagic dose was defined as the dose of venom, which induced a lesion of 10 mm diameter [21].
2.3.4. Coagulant Effect
Platelet-poor plasma was obtained from rabbit citrated blood by centrifuging the plasma twice at 2,500 xg for 15 min at 4°C. Aliquots of 0.5 mL of platelet-poor plasma were incubated with various amounts of crude venom (dissolved in 100 μL of PBS, pH 7.2). Incubation was carried out for 5 min at 37°C. Then, 0.1 mL of 0.25 M CaCL2 was added to each tube, and they were checked for the formation of a clot every 30 seconds for a total period of 2 h. All experiments were carried out in triplicate.
2.3.5. Fibrinolytic Activity
The fibrinolytic activity was measured using 0.6% bovine plasminogen-free fibrin plates [22]. For this purpose, 30 μL of sample was placed on a fibrin plate, and the lysis area was measured after incubation at 37°C for 18 h. PBS was used as negative control. The specific activity was calculated from a standard curve for the lysis area obtained with plasmin on the plasminogen-free fibrin plates. All experiments were carried out in triplicate.
2.3.6. PLA2 Activity
It was determined by incubating 0.5 mL of crude venom solution (at various amounts) with 50 μL of egg yolk diluted 1 : 5 with 0.1 M Tris, 10 mM CaCl2, and pH 8.5 buffer containing 1% Triton X-100. Incubations were carried out for 10 min at 37°C. The liberated free fatty acids were extracted and titrated according to the method of Dole [23]. Crude venom from Bothrops asper (10 μg) and PBS were used as positive and negative controls, respectively. All experiments were carried out in triplicate.
2.3.7. Myotoxic Activity
Groups of four male Swiss mice (18–22 g) were injected in the right gastrocnemius muscle with crude venom (50 μg/50 μL of PBS), MTXs (50 μg/50 μL of PBS), or PBS alone (50 μL). After 3 h, blood was collected from the tail in heparinized capillary tubes and centrifuged for plasma separation. Activity of creatine kinase (CK) was then determined using 4 μL of plasma, which was incubated for 3 min at 37°C with 1.0 mL of the reagent according to the kinetic CK-UV protocol from Bioclin, Brazil. The activity was expressed in U/L, where one unit corresponds to the production of 1 mmol of NADH per minute.
2.3.8. Histological Examination of Myonecrosis
Myotoxic activity was assayed on the basis of the morphologic alterations induced by IM injections of crude venom or MTXs (50 μg) and negative control PBS (50 μL) in the right gastrocnemius muscle of Swiss mice (18–22 g, n = 4). After 24 h, the animals were euthanized with CO2, and a small section of the central region of the muscle was excised and soaked in fixing solution (10% formaldehyde in PBS, v/v). The material was then dehydrated by increasing concentrations of ethanol and processed for inclusion in paraffin. The resulting blocks were sliced in 2.5 μm thick sections, stained with 0.25% (w/v) hematoxylin-eosin and examined under a light microscope [17].
2.3.9. Cytotoxic Assay
Cell viability was measured by Trypan blue exclusion. In brief, monolayers of J774A.1 cells grown in RPMI-1640 medium with 100 μg/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine were withdrawn and after counting 2 × 105 cells/80 μL were added to plastic vials and incubated with 20 μL of different concentrations of pMTX-I, II, and III (1.5, 3 and 6 μg/mL) diluted in RPMI (control), for 1 h at 37°C in a humidified atmosphere (5% CO2). Then, 20 μL 0.1% Trypan blue was added to 100 μL of J774A.1 macrophage suspension. Viable cell index was determined in a Neubauer's chamber by counting a total number of 100 cells. Results were expressed as percentage of viable cells.
2.3.10. Colorimetric NBT Assay
The colorimetric NBT assay was conducted in J744A.1 cells. In this assay, the generation of superoxide was estimated by reducing nitroblue tetrazolium (NBT), a yellow liposoluble compound that becomes insoluble and blue in its reduced form [24]. For this test, the cells J774A.1 had their concentration adjusted to 2 × 105/100 μL and were incubated with 100 μL of RPMI containing NBT 0.1% (control) or 100 μL of 2 × 106 zymosan particles suspension diluted in RPMI containing NBT 0.1% (positive control) or 100 μL of different concentrations of pMTX-I, II, and III (1.5, 3 and 6 μg/mL) diluted in RPMI containing NBT 0.1%, and incubated for 1 h at 37°C in humidified atmosphere (5% CO2). At the end of the incubation period, the vials were centrifugated for 30 seconds at 4,500 xg, and the cells were washed twice with warm PBS. The NBT reduced deposited inside the cells were then dissolved, first by adding 120 μL of 2 M KOH to solubilize cell membranes and then by adding 140 μL of DMSO to dissolve blue formazan with gentle shaking for 10 min at room temperature. The dissolved NBT solution was then transferred to a 96-well plate and absorbance was read on a microplate reader at 620 nm. Data were expressed as absorbance.
2.3.11. Phagocytic Activity of J774A.1 Cells of Nonopsonized Zymosan
J774A.1 cells were plated on 13 mm diameter glass coverslips (Glass Tecnica, Brazil) in 24-well plates at a density of 2 × 105 cells per coverslip and allowed to attach for 2 h at 37°C under a 5% CO2 atmosphere. Nonadherent cells were removed by washing with PBS. Cell monolayers were cultured for 1 h with RPMI supplemented with 100 μg/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine at 37°C and 5% CO2, and then challenged with RPMI (control) or 6 μg/mL of pMTX-I, II, and III diluted in RPMI. After washing in cold PBS, the monolayers were incubated for 1 h at 37°C and 5% CO2 with nonopsonized zymosan, prepared as described below, and unbound particles were removed by washing with PBS. Cells were fixed with 2.5% glutaraldehyde for 15 min at room temperature, and the coverslips were mounted in microscope slides. The extent of phagocytosis was quantified by contrast phase microscopic observation. At least 200 macrophages were counted in each determination, and those containing three or more internalized particles were considered positive for phagocytosis [25, 26]. Results were presented as the percentage of cells positive for phagocytosis.
The zymosan particles, obtained from yeast cell walls, were suspended in PBS providing a concentration of 3 mg/mL. After that, the zymosan suspension was sonicated for 15 min, and total zymosan particles were determined in a Neubauer's chamber. The ratio of zymosan per macrophage was 1 : 10.
2.4. Statistical Analysis
Results are presented as mean ± S.D. obtained with the indicated number of tests. The statistical significance of differences between groups was evaluated using t student test. A 0.1 < P < 0.05 value was considered to indicate significance.
3. Results and Discussion
Panamanian B. asper snake venom induced hemorrhage, edema, myonecrosis, coagulation, and fibrinolytic activities in vitro, and lethality, also presenting PLA2 activity evidenced by titrimetric and indirect hemolytic assays (Table 1). The toxicological profile was qualitatively similar to that previously described for B. asper from Costa Rica [12] and Guatemala [27]. The nature and biological properties of snake venom components are peculiar to each species [8], whereas the presence and concentration of several venom components could vary intraspecifically as a function of geographic distribution, age, sex, feeding, size, season, and the time elapsed between venom extraction [9, 11, 28]. These intraspecific variations have evident clinical and therapeutic implications and can affect the capacity of antivenoms to neutralize venoms from snakes of geographically separated populations [5, 11, 13, 29, 30].
Table 1.
Toxic activities induced by B. asper snake venom from Panama.
| Effecta | Activity |
|---|---|
| Lethal (LD50; μg/mouse) | 55 |
| Edema-inducing (DEM; μg)ab | 1 ± 0.1 |
| Hemorrhagic (MHD, μg)ab | 1.2 ± 0.2 |
| Coagulant (MCD; μg)ab | 3.5 ± 0.05 |
| Fibrinolytic (MFD; μg) | 0.5 |
| Indirect hemolitic (MHeD; μg)ab | 7.2 ± 0.01 |
| Phospholipase A2 (mEq/mg·min)ab | 27.2 ± 0.45 |
| Myotoxic (MMD, μg)ab | 10 ± 1 |
aResults are presented as mean ± SD. Except for lethality and fibrinolytic activity.
bAll experiments were carried out in triplicate.
Four basic svPLA2s were highly purified in a single step purification using an ion-exchange chromatography performed on a CM-Sepharose column. The elution of absorbed proteins with a linear gradient of concentrated buffer resulted in seven fractions (Figure 2(a)), which fractions Ba-4 to Ba-7 were related to PLA2s. Ba-4 and Ba-5 fraction were related to PLA2 enzymatically active, whereas Ba-6 and Ba-7 were related to enzymatically inactive PLA2. The purity degree of the isolated proteins was further demonstrated by SDS-PAGE, mass spectrometry, and N-terminal sequence (Figures 2 and 3) and named as pMTX-I, pMTX-III, pMTX-IV, and pMTX-II, respectively. Different from our study, others research groups have exhaustively purified PLA2s from Bothrops venoms using different combinations of chromatographic methods: gel filtration, ion exchange, RP-HPLC, and affinity with antibodies and heparin [13, 31].
Figure 2.
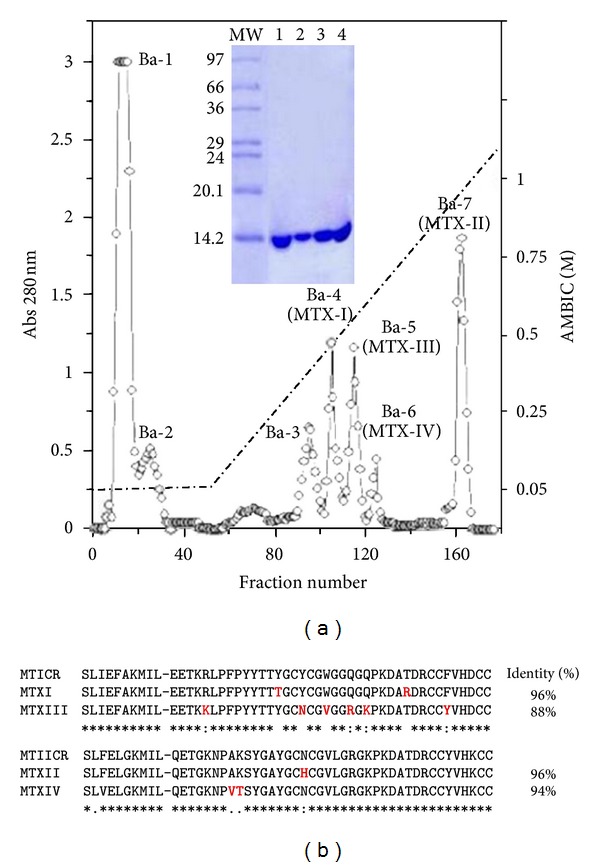
(a) Purification of myotoxins: ion exchange column of Panama B. asper venom (300 mg) on a CM-Sepharose column equilibrated with AMBIC 0.05 M pH 8.0 and eluted with a concentration gradient of AMBIC up to 1 M at a flow rate of 1.5 mL/minute. Inserted: SDS-PAGE 12%. Samples: MW (molecular weight markers); (1) pMTX-I (20 μg); (2) pMTX-II (20 μg); (3) pMTX-III (20 μg); (4) pMTX-IV (20 μg). (b) Comparison of the N-terminal amino acid sequence of phospholipases A2 isolated from Panama B. asper venom: pMTX-I and III belong to the subgroup Asp49, whereas pMTX-II and IV belong to the subgroup Lys49 when compared with myotoxin III (P20474-PA21 BOTAS) Asp49 and myotoxin II (P24605-PA2H2 BOTAS) Lys49 from Costa Rica B. asper venom.
Figure 3.

Analysis of mass spectra of PLA2s isolated: pMTX-I, Mr = 14,156; pMTX-II, Mr = 14,249; pMTX-III, Mr = 14,253.
The purified proteins were characterized as single polypeptide chains, with an isoelectric point ranging from 8.1 to 8.3. The average molecular mass estimated by mass spectrometry was 14,156.5 for pMTX-I, 14,249.5 for pMTX-II, and 14,253.0 for pMTX-III (Figure 3). The N-terminal sequence alignment of pMTX-I and pMTX-III with MTICR myotoxic III PLA2 (Uniprot accession no.: P20474) from Costa Rican B. asper showed, respectively, 96% and 88% of identity, and the sequence alignment of pMTX-II and pMTX-IV showed, respectively, 96% and 94% of identity with B. asper MTIICR myotoxic IV PLA2 (Uniprot accession no.: P24605). Additionally, multiple sequence alignment of pMTX-I, II, III, and IV with Costa Rica Bothrops PLA2s showed highly conserved amino acids, such as cysteine residues involved in disulfide bond formation. Several other conserved residues important to PLA2 catalytic activity, such as the catalytic site (D42XCCXXHD49) and the calcium-binding site (X27CGXGG32) [13, 31] were shown. The N-terminal sequences (Figure 2(b)) of the isolated MTXs demonstrated that pMTX-I and III are basic PLA2s with an aspartate residue at position 49 (Asp49), therefore catalytically active (Figure 4(a)), whereas MTX-II and IV are basic PLA2s displaying a lysine residue at the same position (Lys49) (Figure 4(a)), therefore, catalytically inactive.
Figure 4.

(a) Phospholipase activity (indirect hemolysis) of the isolated enzymes from Panama B. asper venom. Samples: pMTX-I (5 μg); pMTX-II (10 μg); pMTX-III (5 μg); pMTX-IV (10 μg). Negative control (PBS) and positive control (VBasp, B. asper venom, 10 μg); (b) myotoxic activity of the PLA2s isolated from Panama B. asper venom. Swiss mice were injected with 50 μL of the different samples in the right gastrocnemius muscle and, after 3 h, the blood from the tail was collected in capillaries with heparine. Negative (PBS), positive control (BaspV, B. asper venom, 25 μg), and isolated myotoxins (50 μg) were used. Values represent the mean ± S.D. from 3 independent experiments.
Panamanian B. asper crude venom, pMTX-I, II, III, and IV showed a high myotoxic activity (Figure 4(b)). Histopathological analysis revealed a drastic myonecrosis, displaying contracted and clumped fibers in different stages of degeneration and leukocyte infiltrate induced by myotoxins. Our results agree with Gutiérrez and Lomonte [32] and suggest that Lys49 myotoxins pMTX-II and pMTX-IV can affect the cell membrane of skeletal muscle fibers by a phospholipid hydrolysis independent mechanism. These results suggest that, moreover the catalytic site, this toxin may possess another molecular region that can bind and disorganize skeletal muscle plasma membrane [31, 32]. Some studies have suggested that myotoxic PLA2s may induce muscle cell damage by affecting the integrity of plasmatic membranes, thereby leading to hyper contraction and other intracellular effects [13, 31, 32].
In order to evaluate the activation of leukocytes, the toxicity of B. asper myotoxins on macrophage J774A.1 cell line were studied. The cells were incubated with different concentrations of pMTX-II, III, and IV during 1 hour. These myotoxins did not affect the macrophage viability, which are in agreement with Zuliani et al. [25], showing their low toxicity on this cell type. Additionally, the effect of the same myotoxins on J774A.1 phagocytosis ability was evaluated via β-glucan receptor, by the uptake of nonopsonized zymosan particles incubated with noncytotoxic concentrations of pMTX-II, III, and IV was investigated. Our data showed that J774A.1 macrophages incubated with RPMI showed an average of phagocytosis of 16.5 ± 0.5%. Incubation of macrophages with pMTX-II, III, and IV, at 6 μg/mL, resulted in phagocytic indexes of 30.6 ± 0.6%, 29.3 ± 5.5%, and 36.5 ± 2.5%, respectively. These results showed that the myotoxins studied were able to stimulate phagocytosis of non-opsonized zymosan particles by J774A.1 macrophages (Figure 5(a)), which are in agreement with Zuliani et al. [25]. Moreover, these results suggest that phospholipid hydrolysis catalytic activity is not essential for the activity observed and argue with the hypothesis that other molecular regions distinct from the active site may be involved in this effect.
Figure 5.
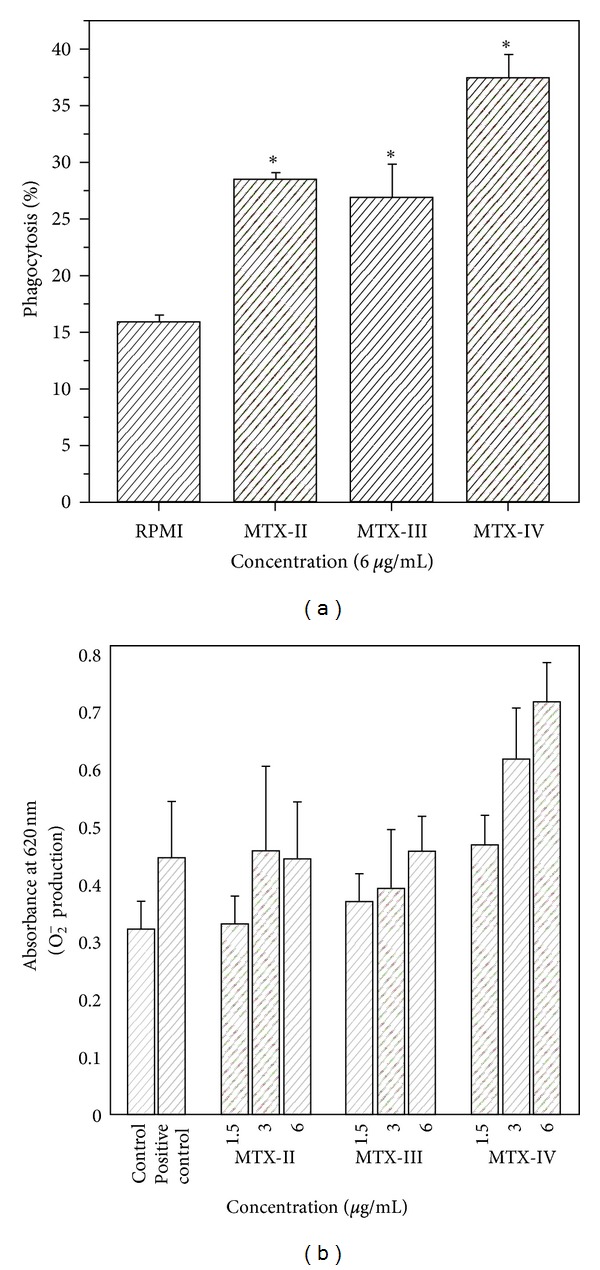
Effect of pMTX-II, pMTX-III, and pMTX-IV on phagocytosis (a) and O2 − production (b) by J774A.1 macrophages. The phagocytosis of nonopsonized zymosan particles was determined by phase-contrast microscopy. J774A.1 macrophages were incubated with 6 μg/mL of pMTX-II, pMTX-III,pMTX-IV, and RPMI (control) during 60 minutes before addition of non-opsonized zymosan particles. Values represent the mean ± S.D.M. from 3–5 independent experiments. *P < 0.05 compared with control # P < 0.05 compared with positive control (ANOVA).
One of the most immediate responses of macrophages during phagocytosis is the production of the potent oxygen free radical, superoxide anion. The enzyme complex primarily responsible for the production of this highly reactive oxygen species is the NADPH oxidase complex [33]. This reaction parallels the release of a variety of inflammatory mediators that play crucial roles in the host defense by microbial killing, but may also cause injury to surrounding tissues [33–35]. In order to investigate the ability of pMTX-II, III, and IV to induce the production of superoxide by a macrophage cell line J774A.1, the cells were incubated with non-cytotoxic concentrations of myotoxins. As shown in Figure 5(b), J774A.1 macrophages incubated with RPMI (negative control) showed a superoxide production average of 0.316 ± 0.05 D.O., and J774A.1 incubated with RPMI plus non-opsonized zymosan (positive control) showed a superoxide production average of 0.455 ± 0.1 D.O. Incubation of macrophages with pMTX-II, III, and IV, at 3 and 6 μg/mL, respectively, induced a significant production of O2 − in J774A.1 macrophages, showing that myotoxins are able to induce superoxide production by J774A.1 macrophages, indicating the ability of these toxins to activate these cells. Again, these results suggest that the PLA2 catalytic activity is not important in macrophage activation. Thus, in accordance with our results, increments in hydrogen peroxide (H2O2), another reactive oxygen specie generated by a multicomponent enzyme system, NADPH-oxidase, have been described in thioglycollate-elicited macrophages incubated with MTX-II and III from Costa Rica B. asper venom [25].
It is important to note that phagocytosis mediated by β-glucan receptors and also by mannose and Fcγ receptors are coupled to the production of both proinflammatory and microbicidal molecules, such ROS [36, 37]. Release of ROS by phagocytic cells has been implicated in microbial killing [38] as well as in the damage to host surrounding tissue [39]. Considering that MT-II and MT-III isolated from B. asper venom from Costa Rica display a broad cytolytic activity and affect a variety of cell types in culture [40, 41], our findings suggest the role of O2 − in the cytotoxicity induced by these myotoxins, a hypothesis that can be addressed with the use of antioxidant agents.
In conclusion, the Panamanian B. asper venom has qualitatively a similar toxicological profile to those previously described for B. asper from Costa Rica and Guatemala despite the observation of quantitative variations in these activities. The PLA2s isolated from Panama Bothrops asper venom (pMTX-I, pMTX-II, pMTX-III, and pMTX-IV) induced myotoxic activity, inflammatory reaction mainly leukocyte migration to the muscle, activation of macrophages to exert phagocytic activity, and production of superoxide.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
The authors thank the financial support of Matzumae International Foundation (MIF), Japan, Organization of American States (Grantee 20050150, OAS), Instituto para la Formación y Aprovechamiento de los Recursos Humanos-Secretaria Nacional de Ciencia y Tecnología, (IFARHU-SENACYT) Panamá, Caja de Seguro Social (CSS), Panamá, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Financiadora de Estudos e Projetos-FINEP, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, projeto Nanobiotec Brasil 10 e Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
References
- 1.Gutiérrez JM, Theakston RDG, Warrell DA. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Medicine. 2006;3(6):0727–0731. doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasturiratne A, Wickremasinghe AR, De Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Medicine. 2008;5(11):1591–1604. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindhauser M. Communicable Diseases, 2002: Global Defense Against the Infectious Disease Threat (WHO/CDS/2003.15) Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 4.Quintero A. El envenenamiento ofIdico en Panamá: epidemiología, fisiopatología y evaluación por el Laboratorio Clínico. Tecnomédica. 2000;1:24–27. [Google Scholar]
- 5.Gutiérrez JM, Fan HW, Silvera CLM, Angulo Y. Stability, distribution and use of antivenoms for snakebite envenomation in Latin America: report of a workshop. Toxicon. 2009;53(6):625–630. doi: 10.1016/j.toxicon.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Jutzy DA, Biber SH, Elton NW, Lowry EC. A clinical and pathological analysis of snake bites on the Panama Canal Zone. The American Journal of Tropical Medicine and Hygiene. 1953;2(1):129–141. doi: 10.4269/ajtmh.1953.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso JLC, França FOS, Wen FH, Málaque CMS, Haddad V. Animais Peçonhentos no Brasil: Biologia, Clínica e Terapêutica dos acidentesofídicos. São Paulo, Brazil: Ed. Sarvier; 2009. [Google Scholar]
- 8.Assakura MT, De Fatima Furtado M, Mandelbaum FR. Biochemical and biological differentiation of the venoms of the lancehead vipers (Bothrops atrox, Bothrops asper, Bothrops marajoensis and Bothrops moojeni) Comparative Biochemistry and Physiology B. 1992;102(4):727–732. [Google Scholar]
- 9.Ferreira ML, Moura-Da-Silva AM, Franca FOS, Cardoso JL, Mota I. Toxic activities of venoms from nine Bothrops species and their correlation with lethality and necrosis. Toxicon. 1992;30(12):1603–1608. doi: 10.1016/0041-0101(92)90032-z. [DOI] [PubMed] [Google Scholar]
- 10.Koh DCI, Armugam A, Jeyaseelan K. Snake venom components and their applications in biomedicine. Cellular and Molecular Life Sciences. 2006;63(24):3030–3041. doi: 10.1007/s00018-006-6315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chippaux JP. Snake Venoms and Envenomations. Florida, Fla, USA: Krieger Publishing Company; 2006. [Google Scholar]
- 12.Angulo Y, Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper . Toxicon. 2009;54(7):949–957. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Kini RM. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester, UK: John Wiley & Son; 1997. [Google Scholar]
- 14.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica et Biophysica Acta. 2006;1761(11):1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Lomonte B, Angulo Y, Calderón L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42(8):885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2 . Toxicon. 2003;42(8):947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Soares AM, Rodrigues VM, Homsi-Brandeburgo MI, et al. A rapid procedure for the isolation of the LYS-49 myotoxin II from Bothrops moojeni (caissaca) venom: biochemical characterization, crystallization, myotoxic and edematogenic activity. Toxicon. 1998;36(3):503–514. doi: 10.1016/s0041-0101(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez JM, Avila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411–413. doi: 10.1016/0041-0101(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.WHO. World Health Organization Progress in the Characterization of Venoms and Standardization of Antivenoms. Geneva, Switzerland: WHO; 1981. [PubMed] [Google Scholar]
- 21.Gutiérrez JM, Gené JA, Rojas G, Cerdas L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon. 1985;23(6):887–893. doi: 10.1016/0041-0101(85)90380-0. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues RS, Izidoro LFM, Teixeira SS, et al. Isolation and functional characterization of a new myotoxic acidic phospholipase A2 from Bothrops pauloensis snake venom. Toxicon. 2007;50(1):153–165. doi: 10.1016/j.toxicon.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. The Journal of clinical investigation. 1956;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhavi N, Das UN. Effect of n-6 and n-3 fatty acids on the survival of vincristine sensitive and resistant human cervical carcinoma cells in vitro . Cancer Letters. 1994;84(1):31–41. doi: 10.1016/0304-3835(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 25.Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, Teixeira CFP. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A 2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45(3):335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Zuliani JP, Gutiérrez JM, Casais E Silva LL, Sampaio SC, Lomonte B, Pereira Teixeira CDF. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2 . Toxicon. 2005;46(5):523–532. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Saravia P, Rojas E, Escalante T, et al. The venom of Bothrops asper from Guatemala: toxic activities and neutralization by antivenoms. Toxicon. 2000;39(2-3):401–405. doi: 10.1016/s0041-0101(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 28.Chippaux JP, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon. 1991;29(11):1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- 29.Solórzano A. Snakes of Costa Rica. San Jose, Costa Rica: Editorial INBio; 2004. [Google Scholar]
- 30.Segura A, Herrera M, Villalta M, et al. Venom of Bothrops asper from Mexico and Costa Rica: intraspecific variation and cross-neutralization by antivenoms. Toxicon. 2012;59(1):158–162. doi: 10.1016/j.toxicon.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Soares AM, Fontes MRM, Giglio JR. Phospholipase A2 myotoxins from Bothrops snake venoms: structure-function relationship. Current Organic Chemistry. 2004;8(17):1677–1690. [Google Scholar]
- 32.Gutiérrez J, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 33.Babior BM. NADPH oxidase. Current Opinion in Immunology. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Naito M. Macrophage heterogeneity in development and differentiation. Archives of Histology and Cytology. 1993;56(4):331–351. doi: 10.1679/aohc.56.331. [DOI] [PubMed] [Google Scholar]
- 35.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. American Journal of Respiratory and Critical Care Medicine. 2002;166(12):S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 36.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual Review of Immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 37.Park JB. Phagocytosis induces superoxide formation and apoptosis in macrophages. Experimental and Molecular Medicine. 2003;35(5):325–335. doi: 10.1038/emm.2003.44. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Wu TH, Rubin RL. Bridging of neutrophils to target cells by opsonized zymosan enhances the cytotoxicity of neutrophil-produced H2O2. Journal of Immunology. 1997;159(5):2468–2475. [PubMed] [Google Scholar]
- 39.Clark RA, Klebanoff SJ. Neutrophil mediated tumor cell cytotoxicity: role of the peroxidase system. Journal of Experimental Medicine. 1975;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bultrón E, Gutiérrez JM, Thelestam M. Effects of Bothrops asper (terciopelo) myotoxin III, a basic phospholipase A2, on liposomes and mouse gastrocnemius muscle. Toxicon. 1993;31(2):217–222. doi: 10.1016/0041-0101(93)90289-u. [DOI] [PubMed] [Google Scholar]
- 41.Lomonte B, Lundgren J, Johansson B, Bagge U. The dynamics of local tissue damage induced by Bothrops asper snake venom and myotoxin II on the mouse cremaster muscle: an intravital and electron microscopic study. Toxicon. 1994;32(1):41–55. doi: 10.1016/0041-0101(94)90020-5. [DOI] [PubMed] [Google Scholar]


