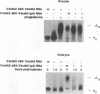
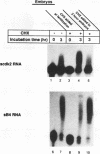
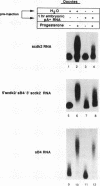
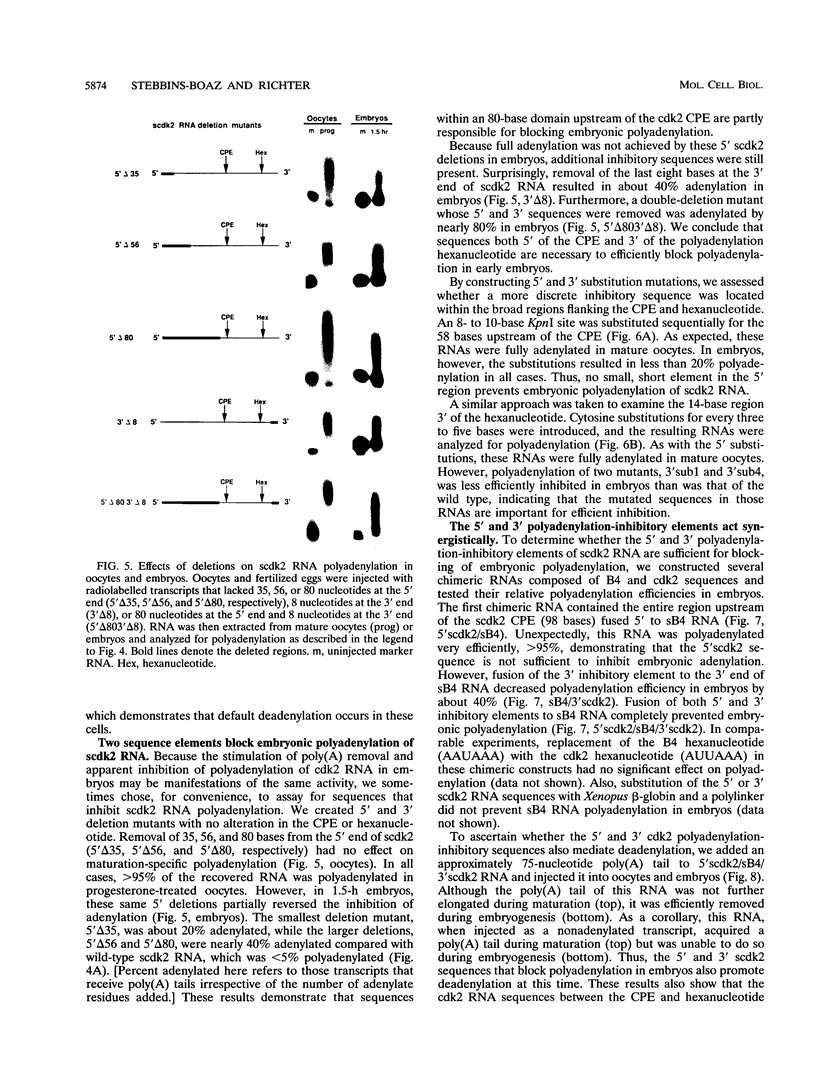
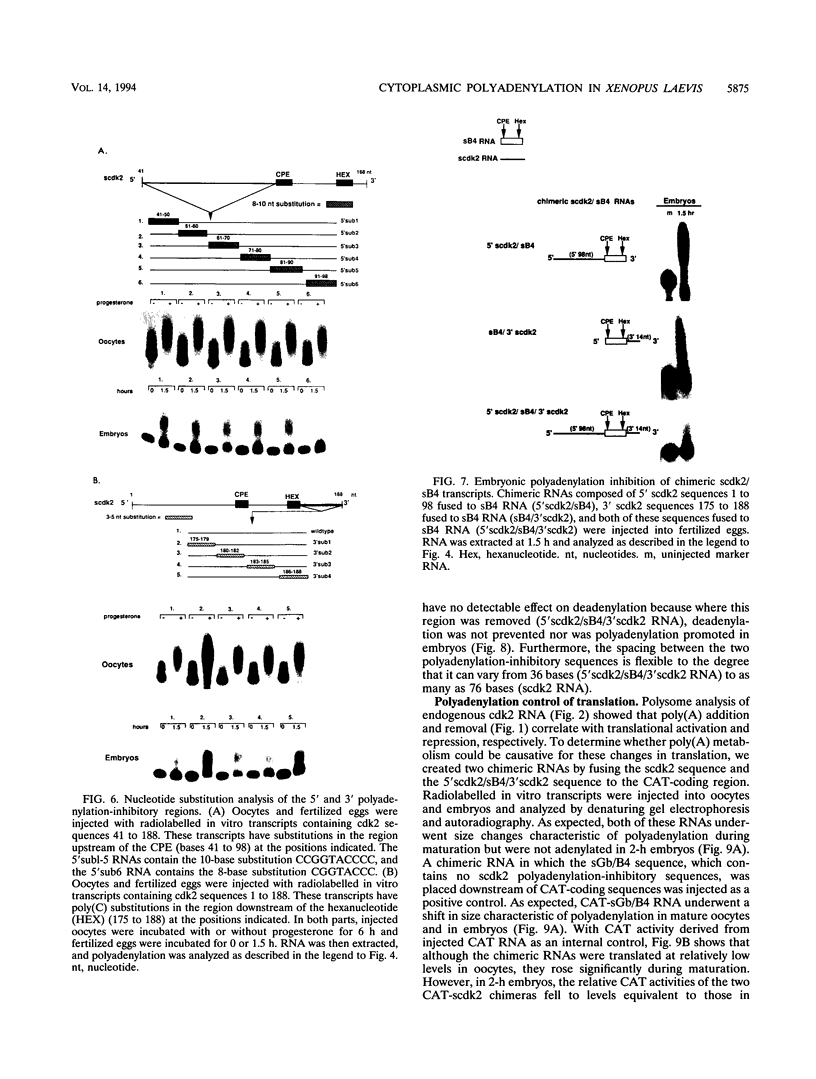
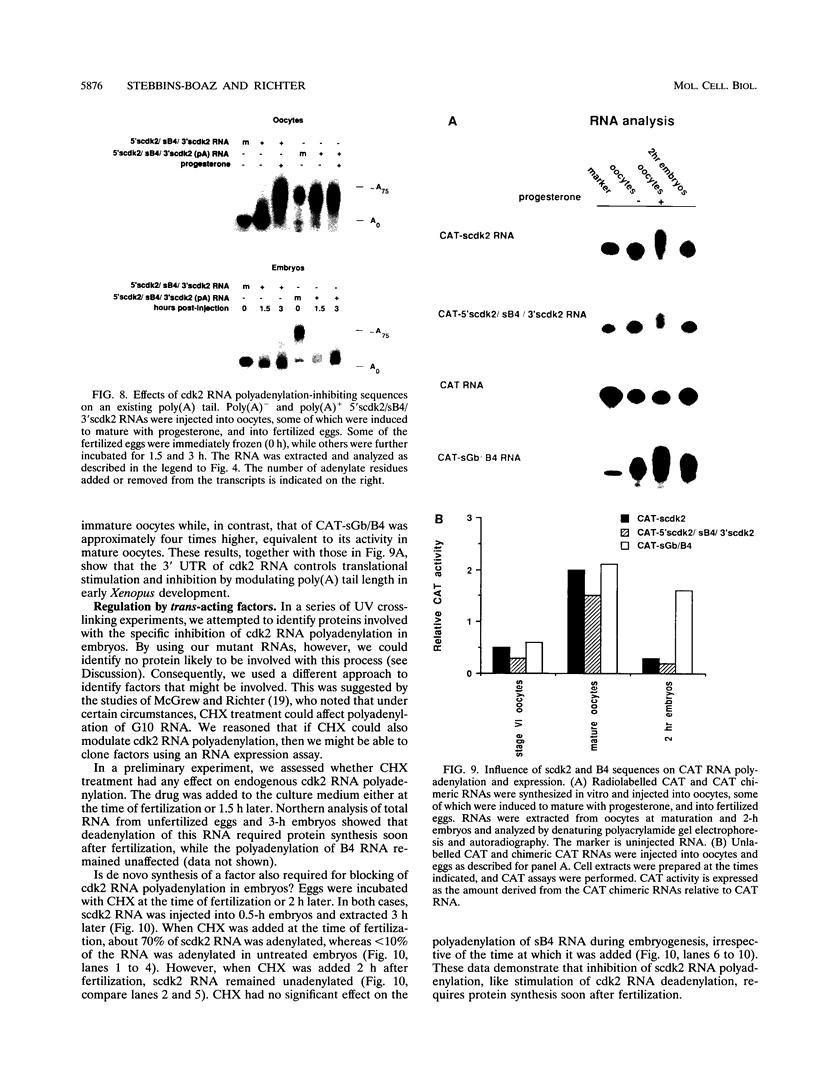
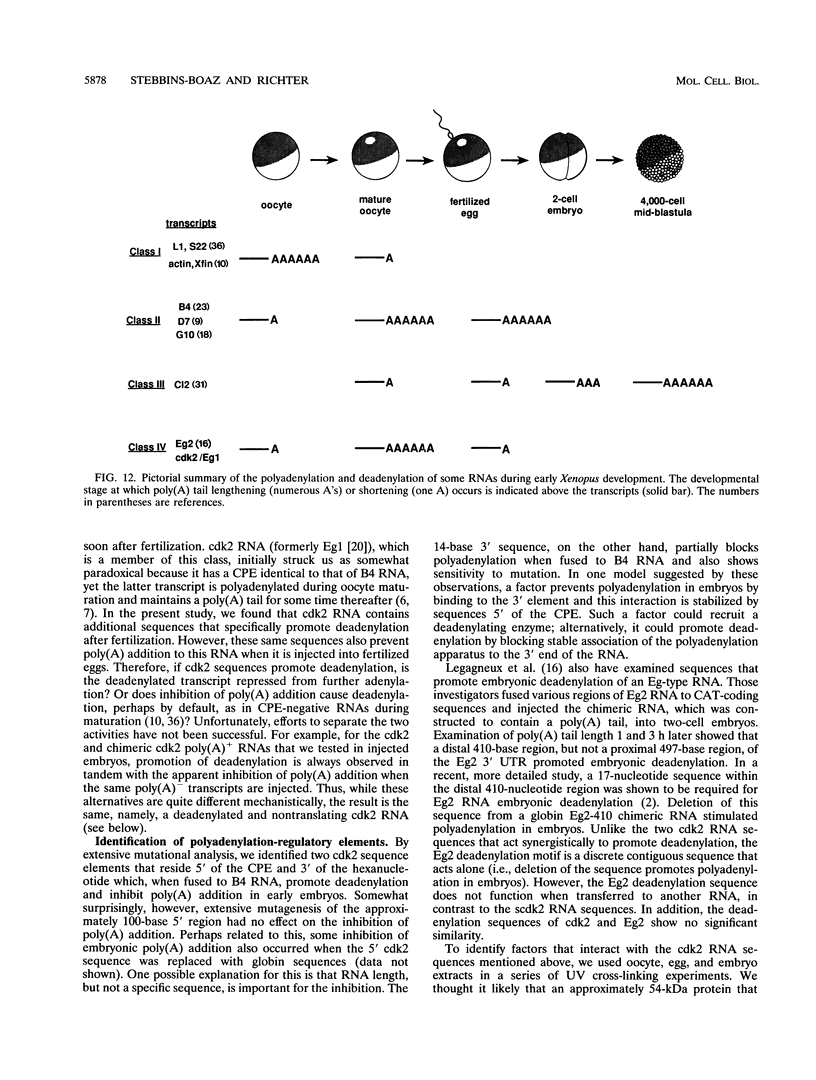
Abstract
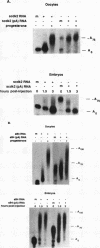
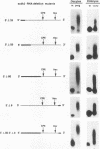
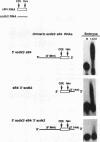
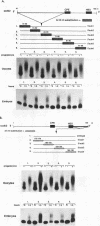
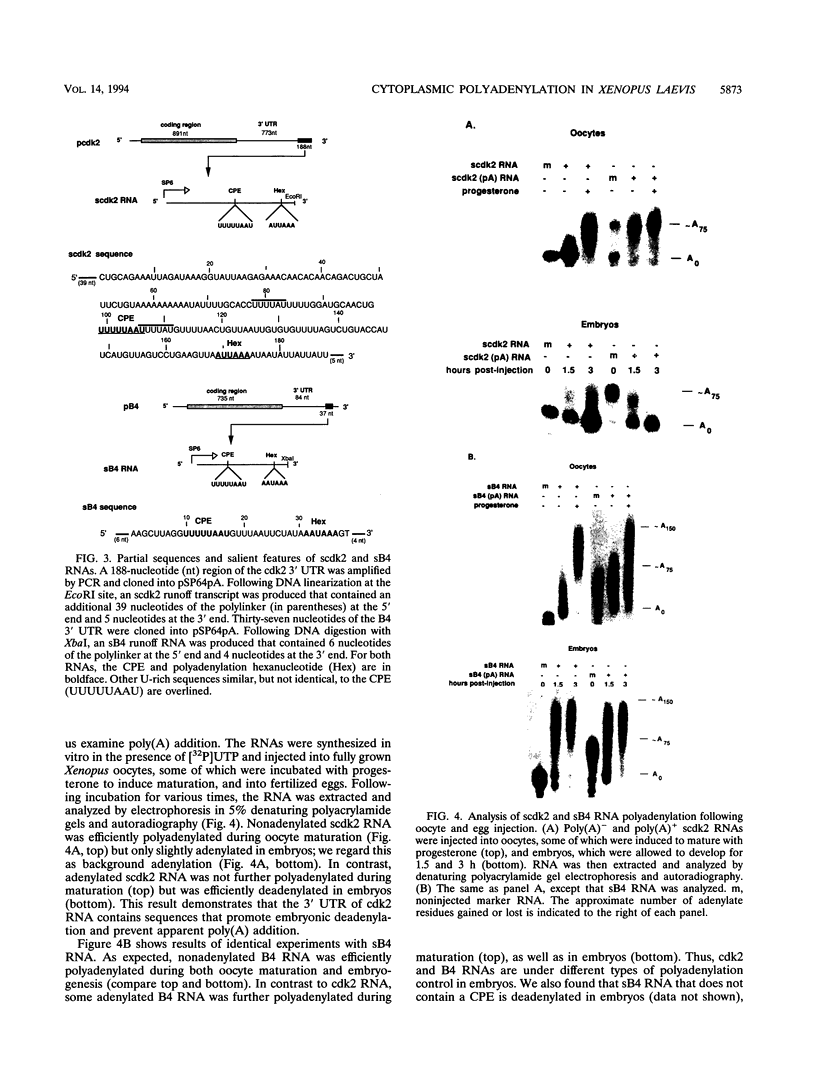
Cytoplasmic poly(A) elongation is one mechanism that regulates translational recruitment of maternal mRNA in early development. In Xenopus laevis, poly(A) elongation is controlled by two cis elements in the 3' untranslated regions of responsive mRNAs: the hexanucleotide AAUAAA and a U-rich structure with the general sequence UUUUUAAU, which is referred to as the cytoplasmic polyadenylation element (CPE). B4 RNA, which contains these sequences, is polyadenylated during oocyte maturation and maintains a poly(A) tail in early embryos. However, cdk2 RNA, which also contains these sequences, is polyadenylated during maturation but deadenylated after fertilization. This suggests that cis-acting elements in cdk2 RNA signal the removal of the poly(A) tail at this time. By using poly(A) RNA-injected eggs, we showed that two elements which reside 5' of the CPE and 3' of the hexanucleotide act synergistically to promote embryonic deadenylation of this RNA. When an identical RNA lacking a poly(A) tail was injected, these sequences also prevented poly(A) addition. When fused to CAT RNA, the cdk2 3' untranslated region, which contains these elements, as well as the CPE and the hexanucleotide, promoted poly(A) addition and enhanced chloramphenicol acetyltransferase activity during maturation, as well as repression of these events after fertilization. Incubation of fertilized eggs with cycloheximide prevented the embryonic inhibition of cdk2 RNA polyadenylation but did not affect the robust polyadenylation of B4 RNA. This suggests that a maternal mRNA, whose translation occurs only after fertilization, is necessary for the cdk2 deadenylation or inhibition of RNA polyadenylation. This was further suggested when poly(A)+ RNA isolated from two-cell embryos was injected into oocytes that were then allowed to mature. Such oocytes became deficient for cdk2 RNA polyadenylation but remained proficient for B4 RNA polyadenylation. These data show that CPE function is developmentally regulated by multiple sequences and factors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum E. Z., Hyman L. E., Wormington W. M. Post-translational control of ribosomal protein L1 accumulation in Xenopus oocytes. Dev Biol. 1988 Mar;126(1):141–149. doi: 10.1016/0012-1606(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Omilli F., Arlot-Bonnemains Y., Legagneux V., Roghi C., Bassez T., Osborne H. B. The deadenylation conferred by the 3' untranslated region of a developmentally controlled mRNA in Xenopus embryos is switched to polyadenylation by deletion of a short sequence element. Mol Cell Biol. 1994 Mar;14(3):1893–1900. doi: 10.1128/mcb.14.3.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Changes in RNA titers and polyadenylation during oogenesis and oocyte maturation in Xenopus laevis. Dev Biol. 1985 Dec;112(2):451–457. doi: 10.1016/0012-1606(85)90417-8. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Shrutkowski A., Dworkin-Rastl E. Mobilization of specific maternal RNA species into polysomes after fertilization in Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7636–7640. doi: 10.1073/pnas.82.22.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wahle E., Wickens M. Polyadenylation of maternal mRNA during oocyte maturation: poly(A) addition in vitro requires a regulated RNA binding activity and a poly(A) polymerase. EMBO J. 1992 Dec;11(13):5021–5032. doi: 10.1002/j.1460-2075.1992.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wickens M. P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989 Dec;3(12B):2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Wickens M. Poly(A) removal during oocyte maturation: a default reaction selectively prevented by specific sequences in the 3' UTR of certain maternal mRNAs. Genes Dev. 1990 Dec;4(12B):2287–2298. doi: 10.1101/gad.4.12b.2287. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli A., Strickland S., Vassalli J. D. Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev. 1987 Dec;1(10):1201–1211. doi: 10.1101/gad.1.10.1201. [DOI] [PubMed] [Google Scholar]
- Huarte J., Stutz A., O'Connell M. L., Gubler P., Belin D., Darrow A. L., Strickland S., Vassalli J. D. Transient translational silencing by reversible mRNA deadenylation. Cell. 1992 Jun 12;69(6):1021–1030. doi: 10.1016/0092-8674(92)90620-r. [DOI] [PubMed] [Google Scholar]
- Hyman L. E., Wormington W. M. Translational inactivation of ribosomal protein mRNAs during Xenopus oocyte maturation. Genes Dev. 1988 May;2(5):598–605. doi: 10.1101/gad.2.5.598. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Legagneux V., Bouvet P., Omilli F., Chevalier S., Osborne H. B. Identification of RNA-binding proteins specific to Xenopus Eg maternal mRNAs: association with the portion of Eg2 mRNA that promotes deadenylation in embryos. Development. 1992 Dec;116(4):1193–1202. doi: 10.1242/dev.116.4.1193. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Osborne H. B., Couturier A., Le Guellec R., Philippe M. Changes in the polyadenylation of specific stable RNA during the early development of Xenopus laevis. Gene. 1988 Dec 10;72(1-2):169–176. doi: 10.1016/0378-1119(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Paris J., Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990 Jul;140(1):221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Paris J., Richter J. D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990 Nov;10(11):5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Swenson K., Piwnica-Worms H., Richter J. D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991 Sep;5(9):1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- Richter J. D. Information relay from gene to protein: the mRNP connection. Trends Biochem Sci. 1988 Dec;13(12):483–486. doi: 10.1016/0968-0004(88)90236-8. [DOI] [PubMed] [Google Scholar]
- Richter J. D. Translational control during early development. Bioessays. 1991 Apr;13(4):179–183. doi: 10.1002/bies.950130406. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Tansey T. R., Ruderman J. V. Sequence-specific adenylations and deadenylations accompany changes in the translation of maternal messenger RNA after fertilization of Spisula oocytes. J Mol Biol. 1983 May 25;166(3):309–327. doi: 10.1016/s0022-2836(83)80087-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenborn E. T., Mierendorf R. C., Jr A novel transcription property of SP6 and T7 RNA polymerases: dependence on template structure. Nucleic Acids Res. 1985 Sep 11;13(17):6223–6236. doi: 10.1093/nar/13.17.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. D., Fox C. A., Hunt T., Vande Woude G., Wickens M. The 3'-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994 Apr 15;8(8):926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Simon R., Tassan J. P., Richter J. D. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev. 1992 Dec;6(12B):2580–2591. doi: 10.1101/gad.6.12b.2580. [DOI] [PubMed] [Google Scholar]
- Standart N., Dale M., Stewart E., Hunt T. Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3'-untranslated region. Genes Dev. 1990 Dec;4(12A):2157–2168. doi: 10.1101/gad.4.12a.2157. [DOI] [PubMed] [Google Scholar]
- Sturgess E. A., Ballantine J. E., Woodland H. R., Mohun P. R., Lane C. D., Dimitriadis G. J. Actin synthesis during the early development of Xenopus laevis. J Embryol Exp Morphol. 1980 Aug;58:303–320. [PubMed] [Google Scholar]
- Varnum S. M., Hurney C. A., Wormington W. M. Maturation-specific deadenylation in Xenopus oocytes requires nuclear and cytoplasmic factors. Dev Biol. 1992 Oct;153(2):283–290. doi: 10.1016/0012-1606(92)90113-u. [DOI] [PubMed] [Google Scholar]
- Varnum S. M., Wormington W. M. Deadenylation of maternal mRNAs during Xenopus oocyte maturation does not require specific cis-sequences: a default mechanism for translational control. Genes Dev. 1990 Dec;4(12B):2278–2286. doi: 10.1101/gad.4.12b.2278. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Belin D., Gubler P., Vassalli A., O'Connell M. L., Parton L. A., Rickles R. J., Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989 Dec;3(12B):2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]
- Wormington M. Poly(A) and translation: development control. Curr Opin Cell Biol. 1993 Dec;5(6):950–954. doi: 10.1016/0955-0674(93)90075-2. [DOI] [PubMed] [Google Scholar]
- Wormington M. Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol. 1991;36:167–183. doi: 10.1016/s0091-679x(08)60277-0. [DOI] [PubMed] [Google Scholar]