Abstract
BrTX-I, a PLA2, was purified from Bothrops roedingeri venom after only one chromatographic step using reverse-phase HPLC on μ-Bondapak C-18 column. A molecular mass of 14358.69 Da was determined by MALDI-TOF mass spectrometry. Amino acid analysis showed a high content of hydrophobic and basic amino acids as well as 14 half-cysteine residues. The total amino acid sequence was obtained using SwissProt database and showed high amino acid sequence identity with other PLA2 from snake venom. The amino acid composition showed that BrTX-I has a high content of Lys, Tyr, Gly, Pro, and 14 half-Cys residues, typical of a basic PLA2. BrTX-I presented PLA2 activity and showed a minimum sigmoidal behavior, reaching its maximal activity at pH 8.0, 35–45°C, and required Ca2+. In vitro, the whole venom and BrTX-I caused a neuromuscular blockade in biventer cervicis preparations in a similar way to other Bothrops species. BrTX-I induced myonecrosis and oedema-forming activity analyzed through injection of the purified BrTX-I in mice. Since BrTX-I exerts a strong proinflammatory effect, the enzymatic phospholipid hydrolysis might be relevant for these phenomena; incrementing levels of IL-1, IL-6, and TNFα were observed at 15 min, 30 min, one, two, and six hours postinjection, respectively.
1. Introduction
PLA2s (phosphatide 2-acylhydrolase, EC 3.1.14) represent a superfamily of lipolytic enzymes which specifically catalyze the hydrolysis of the ester bond at the sn-2 position of glycerophospholipids resulting in the generation of fatty acid (arachidonate) and lysophospholipids. The PLA2 superfamily consists of about 15 groups which are further subdivided into several subgroups, all of which display differences in terms of their structural and functional specificities. However, the four main types or classes of PLA2s are the secreted, the cytosolic, the Ca2+-independent and the lipoprotein-associated PLA2 [1], PLA2 structure/function, mechanism, and signaling [2].
Snake venom PLA2s displays a variety of activities, such as neurotoxicity, myotoxicity, cardiotoxicity, and hemolysis that may be modulated by specific receptors located on target cells [3–6]. Indeed, PLA2 receptors classified as kinds M and N [7] have been identified in various kinds of cells, including vascular smooth muscle cells, platelets, neutrophils, chondrocytes, fibroblasts, hepatocytes, and mesangial cells, as well as in brain, lung, and skeletal muscle [8, 9]. Snake venom PLA2 can bind to M receptors, which are the most common kind found in human macrophages and muscle cells, and these may mediate some of the deleterious actions of venom PLA2s, although that was not conclusively demonstrated [5, 6].
Peru has a rich and diverse herpetofauna that includes venomous snake species of the families Elapidae (16 species of Micrurus and the pelagic sea snake Pelamis platurus) and Viperidae (15 species) [10]. Snakebite envenomations represent a public health problem in this country. The vast majority of snakebites in Peru are inflicted by species of the genus Bothrops (familyViperidae) [11]. Bothrops atrox, Bothrops brazili, and Bothrops bilineatus are distributed in the tropical rainforests located in the eastern part of the country, whereas Bothrops barnetti and Bothrops roendingeri are found in the western dry coastal regions [10–12].
This variety of pharmacological roles derives from an accelerated microevolutionary process through which a high rate of amino acid substitutions have occurred in molecular regions located mainly at the surface of these molecules [13–15]. The purpose of this paper is to isolate, biochemically and pharmacologically characterize a basic PLA2 from Bothrops roedingeri venom, BrTX-I.
2. Materials and Methods
2.1. Venom and Reagents
The venom was obtained from the adult specimens of Bothrops roedingeri captured in the vicinity of Arequipa-Perú. Swiss mice (18–20 g) were supplied by the Animal Services Unit of the State University of Campinas (UNICAMP). All experiments were conduced in accordance with guidelines of the Committee for Ethics in Animal Research, UNICAMP No. 2006-1 (Campinas-Brazil). The reagents used in this work were of analytical or sequencing grade.
2.2. PLA2 Activity
PLA2 activity was measured using the assay described in [16, 17], modified for 96-well plates [18]. The standard assay mixture contained 200 μL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 μL of substrate (4-nitro-3-octanoyloxy-benzoic acid), 20 μL of water, and 20 μL of PLA2 in a final volume of 260 μL. After the addition of PLA2 (20 μg), the mixture was incubated for up to 40 min at 37°C, with the absorbance being read at 10 min intervals. The enzyme activity, expressed as the initial velocity of the reaction (Vo), was calculated based on the increase in absorbance after 20 min.
All assays were done three times and the absorbances at 425 nm were measured using a VersaMax 190 multiwell plate reader (Molecular Devices, Sunnyvale, CA, USA).
2.3. Reversed-Phase HPLC (RP-HPLC)
Five milligrams of the venom was dissolved in 200 μL solvent A (TFA 0.1%, pH 3.5). The resulting solution was clarified by centrifugation and the supernatant was applied to a μ-Bondapak C18 column (0.78 × 30 cm; Waters 991-PDA system). Fractions were eluted using a linear gradient (0–100%, v/v) of acetonitrile (solvent B) at a constant flow rate of 1.0 mL/min over 40 min. The elution profile was monitored at 280 nm, and the collected fractions were lyophilized and conserved at −20°C.
2.4. Electrophoresis SDS-PAGE
The relative molecular mass of the protein was determined by SDS-PAGE [19]. The molecular mass markers were (in kDa): phospholipase B—94, albumin—67, ovalbumin—43, carbonic anhydrase—30, soybean trypsin inhibitor—20, and lysozyme—14.
2.5. Amino Acid Analysis
Amino acid analysis was done on a Pico-Tag amino acid analyzer (Waters Corporation, Massachusetts, USA) as described by [20]. The purified protein (30 μg) was hydrolyzed at 105°C for 24 h in 6 M HCl acid (Pierce sequencing grade) containing 1% phenol (w/v). The hydrolyzates were reacted with 20 μL of derivatization solution (ethanol : triethylamine : water : phenylisothiocyanate, 7 : 1 : 1 : 1, v/v) for 1 h at room temperature after the phenylthiohydantoin (PTC)-amino acids were identified and quantified by HPLC by the comparison of their retention times and peak areas with those of a standard amino acid mixture.
2.6. Reduction and Alkylation
Purified lyophilized protein from RP-HPLC was resuspended in 8 M urea containing 10 mM DTT at pH 8.0 and the disulfide bridges were then reduced by incubation at 37°C for 2 h. Since the number of cysteine residues in the protein was initially unknown, the optimum concentration of iodoacetamide for alkylating the free thiols was derived empirically, based on results obtained from incubations using various concentrations of iodoacetamide and different amounts of protein, with each mixture being analyzed by mass spectrometry [21]. Based on these preliminary experiments, a 30% molar excess of iodoacetamide relative to the total number of thiols was eventually chosen and the mixture was incubated for 1.5 h at 37°C in the dark. The reaction was ceased by injecting the mixture onto a RP-HPLC column followed by lyophilization of the collected peak.
2.7. Enzymatic Hydrolysis
The purified proteins were hydrolyzed with sequencing grade bovine pancreatic trypsin in 0.4% ammonium bicarbonate, pH 8.5, for 4 h at 37°C, at an enzyme : substrate ratio of 1 : 100 (w/w). The reaction was ceased by lyophilization.
2.8. Mass Spectrometry
All mass spectra were acquired using a quadrupole-time of flight (Q-TOF) hybrid mass spectrometer Q-TOF Ultima from Micromass (Manchester, UK) equipped with a nano Zspray source operating in a positive ion mode. The ionization conditions of usage included a capillary voltage of 2.3 kV, a cone voltage and RF1 lens of 30 V and 100 V, respectively, and a collision energy of 10 V. The source temperature was 70°C and the cone gas was N2 at a flow of 80 L/h; nebulizing gas was not used to obtain the sprays. Argon was used for collisional cooling and for fragmentation of ions in the collision cell. External calibration with sodium iodide was made over a mass range from 50 to 3000 m/z. All spectra were acquired with the TOF analyzer in “Vmode” (TOF kV = 9.1) and the MCP voltage set at 2150 V.
2.9. Analysis of Native and Alkylated Protein
Lyophilised RP-HPLC fractions of intact native and alkylated protein were dissolved in 10% acetonitrile in 0.1% TFA and was introduced into the mass spectrometer source with a syringe pump at a flow rate of 500 nL/min. Mass spectra were acquired over the mass range of 1000–2800 m/z for the native protein and over the range of 800–2000 m/z for the alkylated protein, both at a scan speed of 1 s/scan. The masses were analyzed by the MassLynx-MaxEnt 1 deconvolution algorithm. The data obtained were processed using the Mascot MS/MS Ion Search software http://www.matrixscience.com/.
2.10. De Novo Sequencing of Tryptic Peptides
Alkylated tryptic peptides fractionated by RP-HPLC were lyophilized and re-suspended in 20% acetonitrile in 0.1% TFA prior to injection into the mass spectrometer source at a flow rate of 500 nL/min. Before performing a tandem mass spectrum, an ESI/MS mass spectrum (TOF MS mode) was acquired for each HPLC fraction over the mass range of 400–2000 m/z, in order to select the ion of interest, subsequently, these ions were fragmented in the collision cell (TOF MS/MS mode). Different collision energies were used, depending on the mass and charge state of the ions. The resulting ion spectra was acquired in the TOF analyser and deconvoluted using the MassLynx-MaxEnt 3 algorithm. Singly charged spectra were processed manually using the PepSeq application included in MassLynx.
2.11. Pharmacological Activity
2.11.1. Young Chicken Biventer Cervicis Preparation
Male chicks (4–8-days-old) were killed with isoflurane and the biventer cervicis muscle was removed [22]. The biventer cervicis muscles were mounted under a tension of 0.5 g, in a 5 mL organ bath (Automatic organ multiple-bath LE01 Letica Scientific Instruments. Barcelona, Spain) at 37°C containing aerated (95% O2 - 5% CO2) Krebs solution (pH 7.5) of the following composition (mM): NaCl 118.7, KCl 4.7, CaCl2 1.88, KH2PO4 1.17, MgSO4 1.17, NaHCO3 25.0 and glucose 11.65. Contracture to exogenously applied acetylcholine (ACh; 55 and 110 μM for 60 s) and KCl (20.1 mM for 130 s) was obtained in the absence of field stimulation, prior to the addition of a single dose of BrTX-I (50 μg/mL). A bipolar platinum ring electrode was placed around the tendon, which runs the nerve trunk supplying the muscle. Indirect stimulation was performed with a (MAIN BOX LE 12404 Panlab s.l. Powerlab AD Instruments Barcelona, Spain) stimulator (0.1 Hz, 0.2 ms, 3-4 V). Muscle contractions and contractures were isometrically recorded by force-displacement transducers (Model MLT0201 Force transducer 5 mg–25 g Panlab s.l. AD Instruments Pty Ltd. Spain) connected to a PowerLab/4SP (OUAD Bridge AD Instruments, Barcelona, Spain).
2.11.2. Myotoxic Activity
Groups of four Swiss mice (18–20 g) received an intramuscular (i.m.) or an intravenous (i.v.) injection of variable amounts of BrTX-I, in 100 μL of PBS, in the gastrocnemius. A control group received 100 μL of PBS. At different intervals of time (2, 4, 6, 9, and 24 h) blood was collected from the tail into heparinized capillary tubes, and the plasma creatine kinase (CK; EC 2.7.3.2) activity was determined by a kinetic assay (Sigma 47-UV). Activity was expressed in U/L, one unit defined as the phosphorylation of 1 μmol of creatine/min at 25°C.
2.11.3. Edema-Forming Activity
The ability of BrTX-I to induce edema was studied in groups of five Swiss mice (18–20 g) according Ponce-Soto et al. [6, 23, 24]. Twenty microliters of phosphate-buffered saline (PBS; 0.12 M NaCl, 0.04 M sodium phosphate, pH 7.2) with BrTX-I (1, 5, 10 and 20 μg/paw) were injected in the subplantar region of the right footpad. The control group received an equal volume of PBS alone. The swelling of the paw was measured at 0.5; 1; 3; 6, and 24 h after administration. Edema was expressed as the percentage increased in the volume of the treated group to that of the control group at each time interval.
2.11.4. Cytokines
The percentage of cytotoxicity was of IL-1, IL-6, and TNF-α in the plasma were collected and measured at 30, 60, 180, and 360 min after i.p. injection of the BrTX-I PLA2 (1.0 mg/kg) (20 μg/100 μL) or sterile saline. After centrifugation, the supernatants were used for determination of IL-1 and IL-6 levels by a specific EIA. The levels of cytokines IL-1, IL-6, and TNF-α in the serum from BALB/c mice were assayed by a two-site sandwich enzyme-like immunosorbent assay (ELISA). In brief, ELISA plates were coated with 100 μL (1 μg/mL) of the monoclonal antibodies anti-IL-1, in 0.1 M sodium carbonate buffer (pH 8.2) and incubated for 6 hours at room temperature. The wells were then washed with 0.1% phosphate-buffered saline (PBS/Tween-20) and blocked with 100 μL of 10% fetal calf serum (FCS) in PBS for 2 hours at room temperature. After washing, duplicate sera samples of 50 μL were added to each well. After 18 hours of incubation at 4°C, the wells were washed and incubated with 100 μL (2 μg/mL) of the biotinylated monoclonal antibodies anti-IL-1, anti-IL-6,as second antibodies for 45 minutes at room temperature. After a final wash, the reaction was developed by the addition of orthophenyldiamine (OPD) to each well. Optical densities were measured at 405 nm in a microplate reader were measured using a VersaMax 190 multiwell plate reader (Molecular Devices, Sunnyvale, CA, USA).
The cytokine content of each sample was read from a standard curve established with the appropriate recombinant cytokines (expressed in picograms per millilitre). The minimum levels of each cytokine detectable in the conditions of the assays were 10 pg/mL for IL-1, IL-6.
2.12. Statistical Analysis
The results are reported as the means ± SEM. The significance of differences among the means was assessed by ANOVA followed by Dunnett's test when various experimental groups were compared to the control group. A value of P < 0.05 indicated significance.
3. Results
The elution profile of Bothrops roendigeri venom following RP-HPLC performed on a C18 column showed fifteen fractions (1–15) (Figure 1). The fifteen eluted peaks were screened for PLA2 activity. Only the fraction labeled in figure peak 8 presented PLA2 activity, which was eluted with 58% of buffer B.
Figure 1.
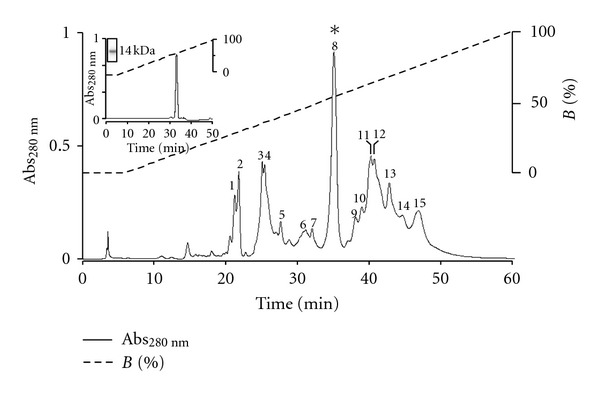
Elution profile of Bothrops roedingeri venom by RP-HPLC on an m-Bondapack C18 column. Fraction 4 (BrTX-I) contained PLA2 activity. Insert Re-chromatography on RP-HPLC chromatography of the BrTX-I and electrophoretic profile of BrTX-I with molecular mass ~14 kDa).
To confirm the level of purity, peak 8 was re-purified in a μ-Bondapack C 18 column (0.78 cm × 30 cm; Waters 991-PDA system) in HPLC of the reverse phase, showing a high level of molecular homogeneity (95%), for the presence of a single peak for the peak 8 (BrTX-I, with a very small retention time difference (37.19 ± 0.34 min) (Figure 1 insert). SDS-PAGE show of PLA2 BrTX-I only band with molecular masses of ~14 kDa (Figure 1 insert) confirmed by MALDI-TOF mass spectrometry in 14,358.69 Da (Figure 2).
Figure 2.
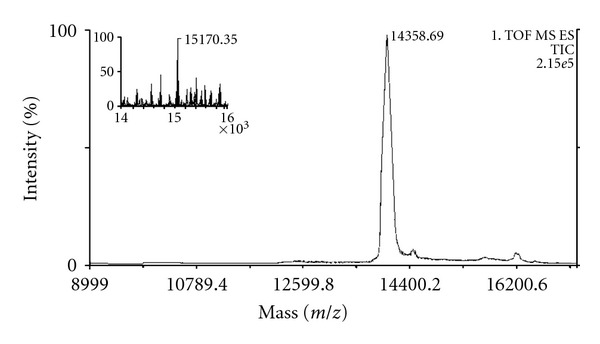
Mass determinations of BrTX-I by mass spectrometry, using a Q-Tof Ultima API ESI/MS (TOF MS mode). Insert mass spectrum, showing multiple alkylation channels of alkylated BrTX-I PLA2 isolated from Bothrops roedingeri.
The amino acid composition determined was: N, D/10; Q, E/7; S/6; G/6; H/3; R/9; T/6; A/5; P/7; Y/8; V/5; M/1; C/14; I/5; L/7; F/3; K/18; W/Not determined (Figure 5(f)).
Figure 5.
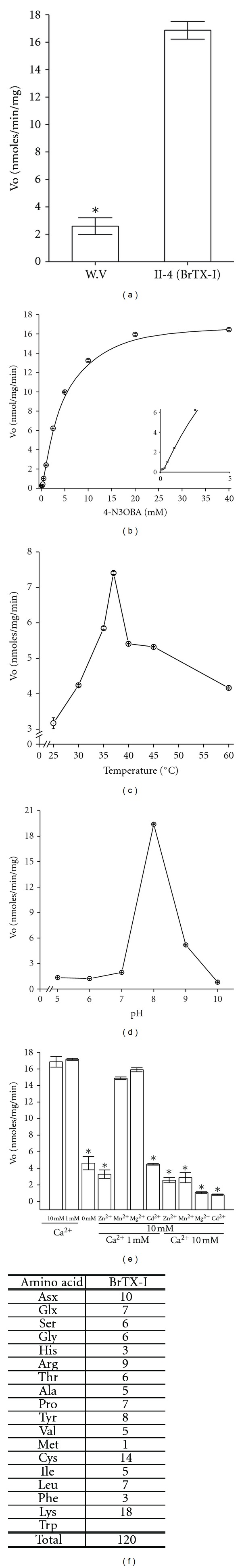
(a) PLA2 activity of Bothrops roedingeri venom and peak 4 (BrTX-I); (b) effect of substrate concentration on the kinetics of BrTX-I (PLA2) activity. (c) effect of temperature on the PLA2 activity of BrTX-I; (d) effect of pH on BrTX-I activity; (e) influence of ions (10 mM each) on PLA2 activity in the absence or presence of 1 mM Ca2+. The results of all experiments are the mean ± SE, of three determinations (P < 0.05) and (f) amino acid composition of BrTX-I from Bothrops roendingeri snake venom.
Samples of the native with mass 14,358.69 Da (Figure 2) and alkylated 15,170.35 Da (Figure 2 inserted) BrTX-I were digested with trypsin and the digests were analyzed by RP-HPLC. Table 1 shows the masses of the tryptic peptides obtained for from the BrTX-I. It is possible to see that these proteins presented five common peptides to the other Bothrops snake venoms. The data obtained were processed using the Mascot MS/MS Ion Search software (http://www.matrixscience.com/).
Table 1.
Measured molecular masses and deduced amino acid sequences obtained by ESI-MS/MS based on the alkylated tryptic peptides of BrTX-I. The peptides were separated by RP-HPLC and sequenced by mass spectrometry. C = alkylated cysteine, lysine residues shown in bold were deduced on the cleavage and missed cleavage by trypsin. All molecular masses are reported as monoisotopic.
| BrTX-I HPLC fraction | Measured mass (Da) | Amino acid sequence | Theoretical mass (Da) |
|---|---|---|---|
| 1 | 1360.65 | DL/IWQ/KWNK/QMI/LK/Q | 1360.61 |
| 2 | 1404.67 | DI/LTI/LVCGEDL/IPCK/Q | 1404.64 |
| 3 | 1791.07 | AAAVCFYENL/IGTYNK/QK/Q | 1791.03 |
| 4 | 1120.28 | YGCYCGWGGR | 1020.25 |
| 5 | 616.79 | L/ITGCPK/Q | 616.75 |
To obtain detailed structural information, the native protein was alkylated and then digested to be analyzed through ESI-MS/MS. The alkylated protein digest was fractionated by RP-HPLC and each chromatographic peak marked in the chromatogram was manually collected and lyophilized. De novo sequencing by ESI-MS/MS was carried out for each peptide peak. The sequences were deduced using ESI-MS/MS and 5 peptides were obtained from the alkylated BrTX-I (Table 1).
Ile and Leu residues were not discriminated in any of the sequences, since they were indistinguishable in low-energy collision-induced dissociation spectra. Due to the external calibration applied to all the spectra, it was also not possible to distinguish between Gln and Lys residues based on the 0.035 Da that separates these amino acids, except for Lys, marked in bold in Table 1, which was deduced by analysis of the cleavage and missed cleavage sites of the enzyme.
Each de novo sequenced peptide of the BrTX-I was submitted separately to the NCBI database, using the protein search program BLAST-p with the search being restricted to the sequenced proteins from the PLA2 from snake venom family. In order to determine the presence and number of cysteine residues, BrTX-I was reduced and alkylated as described in Section 2.6.
The protein mass registered in peak 1–4 after alkylation was 15170.35 Da; the mass increase of 812 Da indicated the presence of 14 Cys modified residues. The primary structure of the BrTX-I was determined by sequence tryptic digested and deduction of the SwissProt database http://br.expasy.org/. BrTX-I presented a sequence of 54 amino acid residues sequenced, being BrtX-I: DLWQWNKMIK - - - - - - - - - - - - -YGCYCGW GGR- - - - - - - - - - - - - - - - - - - - - - -LTGC P- - - - - - - - - -KDITIVCGE DLPC- - - - - - -KAAAVCFYE NLGTYNKK- - - - - - - - - - - - -
From BrTX-I, five peptides, with molecular masses of 1,360.65 Da (peak 1), 1,404.67 Da (peak 2), 1,791.07 Da (peak 3), 1,120.28 Da (peak 4), and 616.79 Da (Peak 5). After the determination of these molecular masses and with the utilization of iodoacetamide, the cysteines presented in the peptides were alkylated (Table 1).
The peptide eluted in fraction 4 of BrTX-I, having the sequence Y G C Y C G W G G R (tandem MS spectra shown in Figure 3) and the sequence of the BrTX-I protein was deduced and returns high homology with the others PLA2s from snake from Bothrops snake genus present of the venoms snake registered in the date base Blast-p and showed high sequence homology with other PLA2 in the region associated with the catalytic site (Figure 4).
Figure 3.
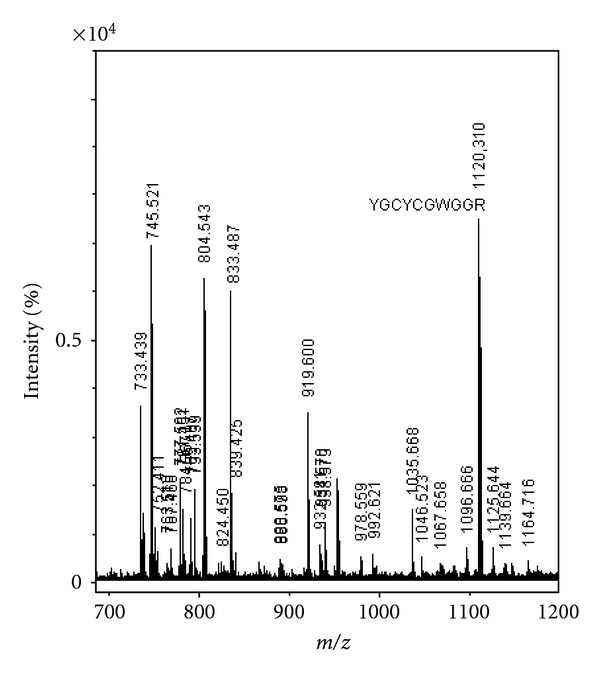
MS/MS spectrum of the peptide tryptic ion of m/z 1120.310. Ion of the major sequence-specific peptide of the complementing ions YGCYCGWGGR, from which the sequence of BrTX-I tag was deduced.
Figure 4.
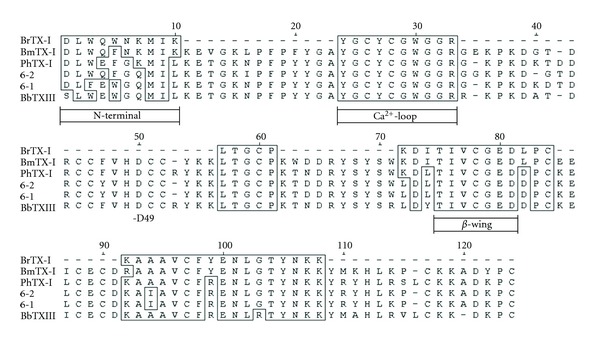
Alignment of the deduced amino acid sequence of the new PLA2 BrTX-I with PLA2 present in venom of PLA2 (BmTX-I) from Bothrops moojeni [26], PLA2 PhTX-I from Porthidium hyoprora [27], PLA2 isoforms (6-1 and 6-2) of the fraction BthTX-II from Bothrops jararacuçu [15], and BbTX-III from Bothrops brazili [27].
The PLA2 activity was examined in the Bothrops roedingeri venom and in BrTX-I using the synthetic substrate 4-nitro-3(octanoyloxy) benzoic acid [25]. The PLA2 activity was higher in BrTX-I c (Figure 5(a)). Under the conditions used, BrTX-I showed a discrete sigmoidal behavior (Figure 5(b) insert), mainly at low substrate concentrations. Maximum enzyme activity occurred at 35–40°C (Figure 5(c)) and the pH optimum was 8.0 (Figure 5(d)). PLA2s require Ca+2 for full activity, being only 1 mM of Ca+2 needed for BrTX-I to present phospholipase A2 activity. The addition of Zn2+, Mg2+, Mn2+, and Cd2+ (10 mM) in the presence of low Ca2+ concentration (1 mM) decreases the enzyme activity. The substitution of Ca2+ by Mg2+, Cd2+and Mn2+ also reduced the activity to levels similar to those in the absence of Ca2+ (Figure 5(e)).
In the neuromuscular activity in chick nerve-muscle preparation, the whole venom concentrations of the 50 μg/mL were tested as well as the concentrations of 5, 20, 50, and 100 μg/mL of BrTX-I. The tested concentration, in both venom and BrTX-I, caused an irreversible dose-dependent blockade of the neuromuscular transmission (P < 0.05). The time required for the venom to achieve 50% twitch tension blockade, through an indirect stimulation, was: 22.60 ± 0.61 min (50 μg/mL) (Figure 6). The time required for BrTX-I to achieve 50% twitch tension blockade, also through indirect stimulation only doses of 50 (31.51 ± 0.52 min) and 100 μg/mL (25.29 ± 0.28 min) (Figure 6(a)). The twitch tension records of the control preparation remain stable at 98% to the venom and 97% to the BrTX-I (5 μg) along the 120 min of incubation with Krebs solution.
Figure 6.
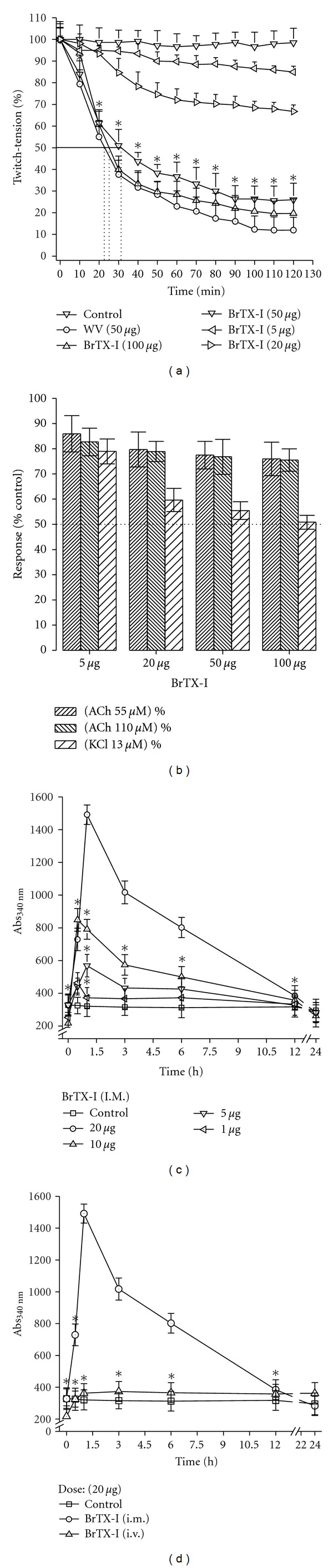
(a) Neuromuscular blockade in chick biventer cervicis muscle preparation (BCP), after addition of B. roedingeri whole venom (50 μg/mL), or fraction BII-4 (BrTX-I; 5, 20, 50, and 100 μg/mL). (b) Inhibition of the response to ACh and KCl, after a 120 min incubation with PLA2 BrTX-I of Bothrops roedingeri (5, 20, 50, and 100 μg/mL) in chick biventer cervicis muscle preparation. Each point represents the average from five experiments ± SEM. P < 0.05 compared with control. In (c), a group of five Swiss mice (18–20 g) received an intramuscular (i.m.) injection of BrTX-I (1 to 20 μg in 50 μL of PBS), in the gastrocnemius muscle of mice. (d) CK serum levels after control (□) or PLA2 BrTX-I injection by the i.m. route (○) and i.v route (Δ). At different times, blood was collected, and serum CK levels were measure. Values are means ± SEM of five mice at each point.
Regarding the venom, the concentration of 50 μg/mL altered significantly the ACh (110 μM) and KCl (20 mM) induced contractures when compared to the control values. In the concentration of the 50 μg/mL, the complete blockade was not accompanied by significantly inhibition of the response to ACh and KCl (Figure 6(b)). In the control preparations, the contracture to ACh and KCl was kept stable after a 120 min indirect stimulation.
In vivo, BrTX-I induced a conspicuous local myotoxic effect when injected by the i.m. route, only doses 10 and 20 μg (Figure 6(c)), but no increase in plasma CK levels occurred after their i.v. injection even in the same dose of 20 μg. Time-course analysis showed a maximum increase in plasma CK 1 h after i.m. injection, returning to normal by 24 h (Figure 6(d)).
Compared to PBS-injected animals, those which received subplantar injections of the BrTX-I (1, 5, 10 and 20 μg/paw) presented marked paw edema all doses (Figure 7(a)). Maximal activity was attained 2 h to BrTX-I after injection and receded to normal levels after 24 h. The level of edema induction by 20 μg of BrTX-I PLA2 was similar to the other doses tested.
Figure 7.
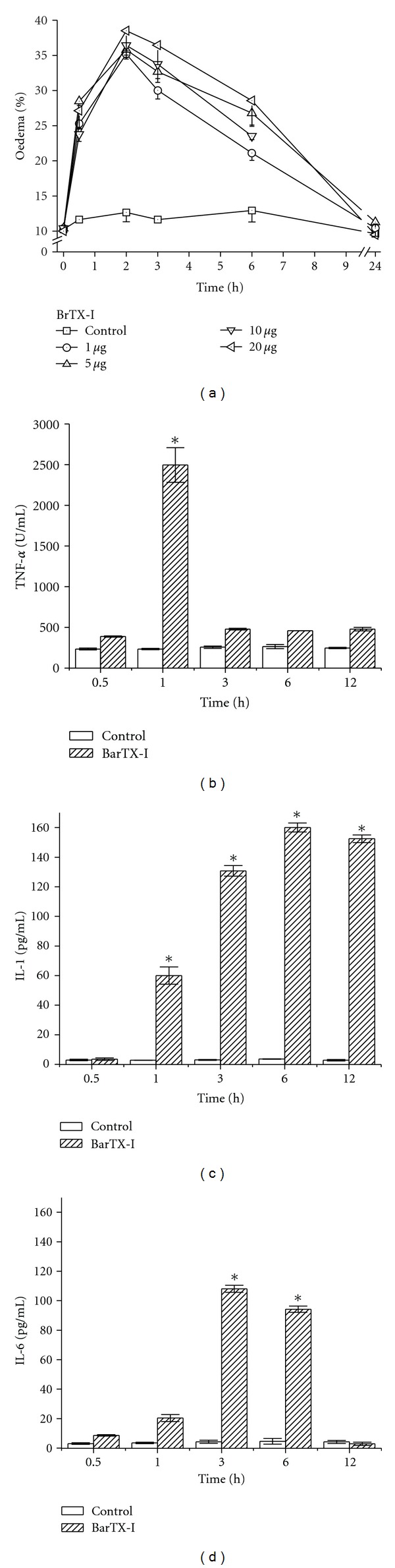
In (a), time-course of the mice paw oedema induced by selected doses of BrTX-I (1–20 μg). The oedema, which was expressed as the percentage increased in the volume of the treated group to that of the control group at each time interval, was maximal around 2 h and decreased thereafter. Levels of TNF-α, IL-1 and IL-6 ((b), (c), and (d), resp.) in the serum after injection of BrTX-I. Animals were injected i.m. with BrTX-I (1.0 mg/kg) or sterile saline alone (control) in a final volume of 1 mL. TNF-α, IL-1 and IL-6 ((b), (c), and (d), resp.) were quantified by specific ELISA, in serum collected at the indicated time intervals after BrTX-I or saline injection as described in Section 2. Each bar represents mean GSEM of 5 animals. *P < 0.05 when compared with the corresponding control.
To further analyze and compare the mechanisms of the inflammatory events induced by BrTX-I PLA2, the concentrations of the IL-1, IL-6, and TNF-α in the serum were measured. BrTX-I caused a marked increase in the TNF-α concentrations only at 1 h (Figure 7(b)). In both the case of IL-1, the maximum peak was recorded at 6 h, on the other hand for IL-6 level the peak was at 3 h (Figures 7(c) and 7(d)).
4. Discussion
The purification procedure for basic PLA2s developed by Ponce-Soto et al. [6, 15, 23, 24] showed to be also efficient for the obtainment of the Bothropsroedingeritoxin I PLA2 (BrTX-I) from Bothrops roedingeri snake venom. Fractionation protocol of this crude venom using a single pass chromatographic in a column μ-Bondapack C-18 coupled to a system of reverse phase HPLC (0.78 cm–30 cm; Waters 991-PDA system) gave rise to 15 fractions at 280 nm, the eight last being the basic PLA2 named BrTX-I (Figure 1).
SDS-PAGE showed (Figure 1 insert) the isolated toxin, BrTX-I have Mr of ~14 kDa similarly to basic PLA2 isolated from other myotoxins from Bothrops snake venoms.
The molecular masses obtained by MALDI TOF mass spectrometry showed to be similar to that of other snake venom PLA2s (14358.69 Da) (Figure 2). Sequence homology studies had showed that there are extremely conserved positions in the PLA2s. In positions 1 and 2, there is a predominance of the amino acids sequence (DL), in position 4 (Q). One of the highly conserved regions in the amino acid sequences of PLA2 is the Ca2+-binding loop, segment from…YGCYCGXGG… and HD(49)CC (Figure 3). Residues forming the Ca2+-binding loop and the catalytic network of BrTX-I PLA2 show a high conservation grade, reflecting the nondecreased catalytic activity.
The primary structure of BrTX-I determined by deduced sequencing (SwissProt database http://br.expasy.org/) method is aligned with the sequences of some other homologous snake venom PLA2 from snake of the crotalidae family. It was very similar to that of other PLA2 (Figure 4).
The PLA2 activity showed to be higher in BrTX-I (16.87 ± 0.643 nmoles/min/mg) when compared with the whole venom (2.59 ± 0.617 nmoles/min/mg) (Figure 5(a)). The PLA2 from Crotalus durissus terrificus venom is a typical PLA2, since it hydrolyzes synthetic substrates at position 2 and preferentially attacks substrates in their micellar state [28]. They can hydrolyze phospholipids in monomeric, micellar or lipid bilayer phases. PLA2 enzymes exhibit a large and abrupt increase (up to 10,000 times) in their catalytic activity when monomeric phospholipids aggregate forms micelles at their critical micellar concentration [29]. This is due to the higher efficiency of interfacial catalysis, which depends on the absorption of the enzyme onto the lipid-water interface, strongly promoted by the presence of anionic amphipatic molecules within the membrane [30]. With synthetic substrate, BrTX-I behaved allosterically, especially at low substrate concentrations, which is in agreement with the results obtained by Beghini et al. [31], Bonfim et al. [32, 33], Ponce-Soto et al. [18], Calgarotto et al. [26], Huancahuire-Vega et al. [27] and for other PLA2 using the same nonmicellar substrate also observed that the dependence of activity on substrate concentration was markedly sigmoidal (Figure 5(b)).
The PLA2s from snake are highly stable and resistant to heat, acid, and urea, but catalytic activity is inactivated at high pH. When micellar substrates are used, maximum catalytic activity occurs at pH 7-8 and 30–55°C [17, 28, 34–36] (Figure 5(c)). BrTX-I showed maximum enzyme activity at 35–45°C and greatest activity at around pH 8.0 (Figure 5(d)).
A strict requirement for Ca2+ is characteristic of some PLA2 [18, 31, 35, 37]. BrTX-I showed typical Ca2+-dependent PLA2 activity similar to other PLA2 and this activity was lower in the presence of other divalent cations. Beghini et al. [31] observed the same for PLA2 from Crotalus durissus cascavella venom and Ponce-Soto et al. [18] for PLA2 from Crotalus durissus collilineatus (Figure 5(e)).
The amino acid composition of the BrTX-I PLA2 toxin revealed a high content of basic and hydrophobic residues, with 14 half-Cys, in agreement with the reported compositions and primary structures of PLA2 toxins isolated from Bothrops venoms (Figure 5(f)), [6, 15, 38, 39]. The pharmacological activities investigated for BrTX-I PLA2 includes neurotoxicity ex vivo in preparation BCP, in vivo inducing rapid damaging action to skeletal muscle tissue, paw oedema and increase of IL-1, IL-6 and TNF-α in the mice serum.
Some authors [3, 4, 6, 15, 40–44] have proposed several models to explain PLA2 catalytic and pharmacological activities. In these models PLA2 has two separated places; one is responsible for catalitic activity and other for biological activity expression. In according to them, the pharmacological place would be located in the surface of PLA2 molecules.
The BrTX-I caused an irreversible concentration-dependent blockade of the indirectly elicited twitch responses of the chick biventer cervicis muscle preparation (BCP). Only doses 20, 50, and 100 μg/mL caused an irreversible dose-dependent blockade of the neuromuscular transmission (Figure 6(a)). The complete blockade of the muscle contraction all of the doses, was not accompanied by any significant inhibition of the responses to ACh. Inhibition response to KCl was progressive in terms of increasing the dose, suggesting a myotoxic effects due to destabilization of the membrane (Figure 6(b)).
Thus, the neuromuscular blockade produced by BrTX-I may be attributed to presynaptic activity, either by blocking axonal conduction or by affecting transmitter release at the motor nerve-terminal. The fact that the BrtX-I from Bothrops moojeni did not significantly affect the response to ACh and KCl, except when high doses were used, suggests that the venom presents a primordial presynaptic nature. Such neuromuscular blockade characteristics have been attributed to presynaptic-acting PLA2 from snake [45, 46] as those of Crotalus durissus terrificus [47], Micrurus species [48, 49], and other Bothrops, Bothrops insularis [50], Bothrops pauloensis [47, 51], and Bothriopsis bilineata smargadina [52], which did not show any detectable effect on the nicotinic receptor and, in some cases, showed only a mild muscle alteration.
In according to the model proposed by [42], the anticoagulant place would be located in a region between the 53 and 76 residues, considering this region charged positively in the PLA2 with high anti-coagulant activity. In PLA2 with moderate or low anti-coagulant activity, there is a predominancy of negative chargings. This region is placed in a distinct local and separated of foreseen regions by neurotoxicity and myotoxicity.
Local and systemic skeletal muscle degeneration is a common consequence of envenomations due to snakebites and mass bee attacks. PLA2 is an important myotoxic component in these venoms, inducing a similar pattern of degenerative events in muscle cells. The bothropics PLA2 myotoxins generally present low systemic toxicity, in contrast to myotoxic PLA2 that are also strongly neurotoxic [5, 53].
Our studies on local and systemic myotoxicity in vivo reveal the BrTX-I is nonsystemic myotoxin with local action due to decrease of the plasmatic CK levels (Figures 6(a) and 6(b)). This fact reinforces the hypothesis of differentiated action of local and systemic myotoxicity proposed by Gutiérrez and Ownby [5] and also the unspecificity and specificity proposed by Kini [3], Ponce-Soto et al. [6] and Gutiérrez et al. [54].
PLA2s from snake venoms exert a large number of pharmacological activities [35, 54] due to a process of accelerated micro-evolution through which a high mutational rate in the coding regions of their genes has allowed the development of new functions, mainly associated with the exposed regions of the molecules [13]. The integral analysis of the inflammation elicited by BrTX-I from Bothrops roedingeri venom in the mouse serum performed in the present study allowed a parallel evaluation of the increase in microvascular permeability, by paw oedema and the production of various inflammatory mediators.
The PLA2s from snake induced an increase in vascular permeability in peritoneal cavity of mice. This is in agreement with previous observations on the edema forming activity of similar molecules in the rodent footpad model [55, 56]. The increase of vascular permeability was detected after BrTX-I injection and developed rapidly, indicating that the observed plasma extravasation is primarily due to formation of endothelial gaps in vessels of microcirculation (Figure 7(a)). Previous studies have documented polymorphonuclear and mononuclear cellular infiltrate after injection of myotoxic PLA2s from the venoms of Bothrops asper [57], Bothrops nummifer [58], and Bothrops jararacussu [59] in mouse skeletal muscle, and after intrapleural administration of similar myotoxins from Bothrops jararacussu and Bothrops pirajai venoms [60]. The mediators involved in this effect of BrTX-I was not addressed in this study. However, the immediate plasma extravasation in response to BrTX-I, strongly suggests the involvement of vasoactive mediators derived from mast cell granules. Previously, the ability of venom PLA2 to degranulate mast cells has been shown [55].
TNF-α is also likely to be involved in inflammation induced by BrTX-I, since the PLA2 caused a significant increase of TNF-α levels in the serum. TNF-α is also likely to be involved in leukocyte infiltration induced by BrTX-I, since the PLA2 caused a significant increase of TNF-α levels in the serum. TNF-α induces the expression of E-selectin, CD11b/CD18 and ICAM-1 and triggers the release of several cytokines such as IL-1 and IL-6 (Figure 7(b)). Thus, our results suggest that TNF-α may have a role in the expression of CD18 and the release of other cytokines following BrTX-I injection, thereby being relevant for neutrophil influx and for increase of vascular permeability on the paw edema.
Cytokines, such as IL-1, IL-6, and TNF-α, are also relevant mediators for leukocyte migration and participate in several inflammatory conditions. Our results showed that BrTX-I induce increase in IL-1 and IL-6 in the serum, exerting a stronger effect (Figures 7(c) and 7(d)). IL-1 induced the expression of adhesion molecules by endothelial cells and stimulates the release of both IL-6 and TNF-α [61]. Thus, our results suggest that IL-1 may contribute for the leukocyte migration.
All these biological effects induced by the BrTX-I occur in the presence of a measurable PLA2 activity. Although the catalytic activity of PLA2 contributes to pharmacological effects, it is not a prerequisite [55, 56, 62–64]. However, further studies are necessary to identify the structural determinants involved in these pharmacological activities.
Acknowledgments
The authors thank Daniel Martins-de-Souza from Max Planck Institute of Psychiatry, Munich, Germany, Salomón Huancahuire-Vega, and Frey F. Romero-Vargas for general technical help. This work was supported by CAPES and is part of a Ph.D. thesis by Maurício Aurélio Gomes Heleno.
References
- 1.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovascular Drugs and Therapy. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. Journal of Lipid Research. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42(8):827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Majunatha Kini R, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2 . Toxicon. 1989;27(6):613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42(8):915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Ponce-Soto LA, Martins D, Novello JC, Marangoni S. Structural and biological characterization of two crotamine isoforms IV-2 and IV-3 isolated from the Crotalus durissus cumanensis venom. Protein Journal. 2007;26(8):533–540. doi: 10.1007/s10930-007-9094-z. [DOI] [PubMed] [Google Scholar]
- 7.Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2 . Trends in Pharmacological Sciences. 1999;20(4):162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 8.Bonventre JV, Sapirstein A. Group IV cytosolic phospholipase A2 (PLA2) function: insights from the knockout mouse. Advances in Experimental Medicine and Biology. 2002;507:25–31. doi: 10.1007/978-1-4615-0193-0_5. [DOI] [PubMed] [Google Scholar]
- 9.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins and Other Lipid Mediators. 2002;68-69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 10.Campbell J, Lamar WW. The Venomous Reptiles of Latin America. New York, NY, USA: Cornell Univ. Press; 2004. [Google Scholar]
- 11.Zavaleta A, Salas M. Ofidismo: envenenamiento por mordedura de serpientes. In: Martínez-Villaverde JR, León-Barúa R, Vidal-Neira L, Losno-García R, editors. Lima, Perú: Emergencias en Medicina Interna; 1996. pp. 241–260. [Google Scholar]
- 12.Fan HW, Cardoso JLC. Clinical toxicology of snake bites in South America. In: Meier J, White J, editors. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Boca Raton, Fla, USA: CRC Press; [Google Scholar]
- 13.Kini RM, Chan YM. Accelerated evolution and molecular surface of venom phospholipase A2 enzymes. Journal of Molecular Evolution. 1999;48:125–132. doi: 10.1007/pl00006450. [DOI] [PubMed] [Google Scholar]
- 14.Kordiš D, Gubenšek F. Bov-B long interspersed repeated DNA (LINE) sequences are present in Vipera ammodytes phospholipase A2 genes and in genomes of Viperidae snakes. European Journal of Biochemistry. 1997;246(3):772–779. doi: 10.1111/j.1432-1033.1997.00772.x. [DOI] [PubMed] [Google Scholar]
- 15.Ponce-Soto LA, Bonfim VL, Rodrigues-Simioni L, Novello JC, Marangoni S. Determination of primary structure of two isoforms 6-1 and 6-2 PLA 2 D49 from Bothrops jararacussu snake venom and neurotoxic characterization using in vitro neuromuscular preparation. Protein Journal. 2006;25(2):147–155. doi: 10.1007/s10930-006-0006-4. [DOI] [PubMed] [Google Scholar]
- 16.Cho W, Kezdy FJ. Chromogenic substrates and assay of phospholipases A2 . Methods in Enzymology. 1991;197:75–79. doi: 10.1016/0076-6879(91)97134-k. [DOI] [PubMed] [Google Scholar]
- 17.Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2 . Toxicon. 1996;34(10):1149–1155. doi: 10.1016/0041-0101(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 18.Ponce-Soto LA, Toyama MH, Hyslop S, Novello JC, Marangoni S. Isolation and preliminary enzymatic characterization of a novel PLA2 from Crotalus durissus collilineatus venom. Journal of Protein Chemistry. 2002;21(3):131–136. doi: 10.1023/a:1015332314389. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Heinrikson RL, Meredith SC. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Analytical Biochemistry. 1984;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- 21.Ponce-Soto LA, Martins-De-souza D, Marangoni S. Neurotoxic, myotoxic and cytolytic activities of the new basic PLA 2 isoforms BmjeTX-I and BmjeTX-II isolated from the Bothrops marajoensis (marajó lancehead) snake venom. Protein Journal. 2010;29(2):103–113. doi: 10.1007/s10930-010-9229-5. [DOI] [PubMed] [Google Scholar]
- 22.Ginsborg BL, Warriner J. The isolated chick Biventer cervicis nerve-muscle preparation. British Journal of Pharmacology and Chemotherapy. 1960;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponce-Soto LA, Lomonte B, Rodrigues-Simioni L, Novello JC, Marangoni S. Biological and structural characterization of crotoxin and new isoform of crotoxin B PLA2 (F6a) from Crotalus durissus collilineatus snake venom. Protein Journal. 2007;26(4):221–230. doi: 10.1007/s10930-006-9063-y. [DOI] [PubMed] [Google Scholar]
- 24.Ponce-Soto LA, Baldasso PA, Romero-Vargas FF, Winck FV, Novello JC, Marangoni S. Biochemical, pharmacological and structural characterization of two PLA2 isoforms Cdr-12 and Cdr-13 from Crotalus durissus ruruima snake venom. Protein Journal. 2007;26(1):39–49. doi: 10.1007/s10930-006-9042-3. [DOI] [PubMed] [Google Scholar]
- 25.Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2 . Toxicon. 1996;34(10):1149–1155. doi: 10.1016/0041-0101(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 26.Calgarotto AK, Damico DCS, Ponce-Soto LA, et al. Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon. 2008;51(8):1509–1519. doi: 10.1016/j.toxicon.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Huancahuire-Vega S, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Structural and functional characterization of brazilitoxins II and III (BbTX-II and -III), two myotoxins from the venom of Bothrops brazili snake. Toxicon. 2009;54(6):818–827. doi: 10.1016/j.toxicon.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Breithaupt H. Enzymatic characteristics of crotalus phospholipase A2 and the crotoxin complex. Toxicon. 1976;14(3):221–233. doi: 10.1016/0041-0101(76)90010-6. [DOI] [PubMed] [Google Scholar]
- 29.Verheij HM, Slotboom AJ, de Haas GH. Structure and function of phospholipase A2 . Reviews of Physiology, Biochemistry & Pharmacology. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 30.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological Reviews. 2000;80(2):717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 31.Beghini DG, Toyama MH, Hyslop S, Sodek LC, Novello, Marangoni S. Enzymatic characterization of a novel phospholipase A2 from Crotalus durissus cascavella rattlesnake (maracambóia) venom. Protein Journal. 2000;19(8):679–684. doi: 10.1023/a:1007152303179. [DOI] [PubMed] [Google Scholar]
- 32.Bonfim VL, Toyama MH, Novello JC, et al. Isolation and enzymatic characterization of a basic phospholipase A 2 from Bothrops jararacussu snake venom. Protein Journal. 2001;20(3):239–245. doi: 10.1023/a:1010956126585. [DOI] [PubMed] [Google Scholar]
- 33.Bonfim VL, Ponce-Soto LA, Novello JC, Marangoni S. Structural and functional properties of Cr 5, a new Lys49 phospholipase A2 homologue isolated from the venom of the snake Calloselasma rhodostoma . Protein Journal. 2006;25(7-8):492–502. doi: 10.1007/s10930-006-9033-4. [DOI] [PubMed] [Google Scholar]
- 34.Habermann E, Breithaupt H. The crotoxin complex—an example of biochemical and pharmacological protein complementation. Toxicon. 1978;16(1):19–30. doi: 10.1016/0041-0101(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 35.Kini RM. Phospholipase A2: a complex multifuncional protein puzzle. In: Kini RM, editor. Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Chichester, UK: Wiley; 1997. pp. 1–28. [Google Scholar]
- 36.Pieterson WA, Vidal JC, Volwerk JJ, De Haas GH. Zymogen-catalyzed hydrolysis of monomeric substrates and the presence of a recognition site for lipid-water interfaces in phospholipase A2 . Biochemistry. 1974;13(7):1455–1460. doi: 10.1021/bi00704a021. [DOI] [PubMed] [Google Scholar]
- 37.Dennis EA. Diversity of groups types, regulation and function of phospholipase A2 . The Journal of Biological Chemistry. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 38.Gutiérrez J, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. In: Kini RM, editor. Venom Phospholipase A2 Enzymes, Structure, Function and Mechanism. New York, NY, USA: John Wiley; 1997. pp. 321–352. [Google Scholar]
- 40.Kini RM, Iwanaga S. Structure-function relationships of phospholipases I: prediction of presynaptic neurotoxicity. Toxicon. 1986;24(6):527–541. doi: 10.1016/0041-0101(86)90173-x. [DOI] [PubMed] [Google Scholar]
- 41.Kini RM, Iwanaga S. Structure-function relationships of phospholipases II: charge density distribution and the myotoxicity of presynaptically neurotoxic phospholipases. Toxicon. 1986;24(9):895–905. doi: 10.1016/0041-0101(86)90090-5. [DOI] [PubMed] [Google Scholar]
- 42.Kini RM, Evans HJ. Structure-function relationships of phospholipases. The anticoagulant region of phospholipases A2 . Journal of Biological Chemistry. 1987;262(30):14402–14407. [PubMed] [Google Scholar]
- 43.Kini RM, Evans HJ. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. International Journal of Peptide and Protein Research. 1989;34(4):277–286. doi: 10.1111/j.1399-3011.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 44.Kini RM, Evans HJ. Role of cationic residues in cytolytic activity: modification of lysine residues in the cardiotoxin from Naja nigricollis venom and correlation between cytolytic and antiplatelet activity. Biochemistry. 1989;28(23):9209–9215. doi: 10.1021/bi00449a037. [DOI] [PubMed] [Google Scholar]
- 45.Harvey AL, Barfaraz A, Thomson E, Faiz A, Preston S, Harris JB. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon. 1994;32(3):257–265. doi: 10.1016/0041-0101(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 46.Lewis RL, Gutmann L. Snake venoms and the neuromuscular junction. Seminars in Neurology. 2004;24(2):175–179. doi: 10.1055/s-2004-830904. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues-Simioni L, Zamunér SR, Cogo JC, et al. Pharmacological evidence for a presynaptic action of venoms from Bothrops insularis (Jararaca ilhoa) and Bothrops neuwiedi (Jararaca pintada) Toxicon. 2004;43(6):633–638. doi: 10.1016/j.toxicon.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Dal Belo CA, Leite GB, Toyama MH, et al. Pharmacological and structural characterization of a novel phospholipase A2 from Micrurus dumerilii carinicauda venom. Toxicon. 2005;46(7):736–750. doi: 10.1016/j.toxicon.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Vital Brazil O, Fontana MD. Ações pré-juncionais e pós-juncionais da peçonha da cobra coral Micrurus corallinus na junção neuromuscular. Memórias do Instituto Butantan. 1984;47-48:13–26. [Google Scholar]
- 50.Cogo JC, Prado-Franceschi J, Cruz-Hofling MA, Corrado AP, Rodrigues-Simioni L. Effect of Bothrops insularis venom on the mouse and chick nerve-muscle preparation. Toxicon. 1993;31(10):1237–1247. doi: 10.1016/0041-0101(93)90397-2. [DOI] [PubMed] [Google Scholar]
- 51.Borja-Oliveira CR, Kassab BH, Soares AM, et al. Purification and N-terminal sequencing of two presynaptic neurotoxic PLA2, neuwieditoxin-I and neuwieditoxin-II, from Bothrops neuwiedi pauloensis (Jararaca pintada) venom. Journal of Venomous Animals and Toxins Including Tropical Diseases. 2007;13(1):103–121. [Google Scholar]
- 52.Rodrigues-Simioni L, Floriano RS, Rostelato-Ferreira S, et al. Presynaptic action of Bothriopsis bilineata smargadina (forest viper) venom in vitro . Toxicon. 2011;58(1):140–145. doi: 10.1016/j.toxicon.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cellular and Molecular Life Sciences. 2008;65(18):2897–2912. doi: 10.1007/s00018-008-8113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutiérrez JM, Alberto Ponce-Soto L, Marangoni S, Lomonte B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon. 2008;51(1):80–92. doi: 10.1016/j.toxicon.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Landucci ECT, Castro RC, Pereira MF, et al. Mast cell degranulation induced by two phospholipase A2 homologues: dissociation between enzymatic and biological activities. European Journal of Pharmacology. 1998;343(2-3):257–263. doi: 10.1016/s0014-2999(97)01546-x. [DOI] [PubMed] [Google Scholar]
- 56.Chaves F, León G, Alvarado VH, Gutiérrez JM. Pharmacological modulation of edema induced by Lys-49 and Asp-49 myotoxic phospholipases A2 isolated from the venom of the snake Bothrops asper (terciopelo) Toxicon. 1998;36(12):1861–1869. doi: 10.1016/s0041-0101(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 57.Lomonte B, Gutiérrez JM, Ramírez M, Díaz C. Neutralization of myotoxic phospholipases A2 from the venom of the snake Bothrops asper by monoclonal antibodies. Toxicon. 1992;30:239–245. doi: 10.1016/0041-0101(92)90866-4. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez JM, Chaves F, Gene JA, Lomonte B, Camacho Z, Schosinsky K. Myonecrosis induced in mice by a basic myotoxin isolated from the venom of the snake Bothrops nummifer (jumping viper) from Costa Rica. Toxicon. 1989;27(7):735–745. doi: 10.1016/0041-0101(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 59.Gutiérrez JM, Nuñez J, Díaz C, Cintra AC, Homsi-Brandeburgo MI, Giglio JR. Skeletal muscle degeneration and regeneration after injection of bothropstoxin-II, a phospholipase A2 isolated from the venom of the snake Bothrops jararacussu. Experimental and Molecular Pathology. 1991;55:217–229. doi: 10.1016/0014-4800(91)90002-f. [DOI] [PubMed] [Google Scholar]
- 60.De Castro RC, Landucci ECT, Toyama MH, et al. Leucocyte recruitment induced by type II phospholipases A2 into the rat pleural cavity. Toxicon. 2000;38(12):1773–1785. doi: 10.1016/s0041-0101(00)00107-0. [DOI] [PubMed] [Google Scholar]
- 61.Stylianou E, Saklatvala J. Interleukin-1. International Journal of Biochemistry & Cell Biology. 1998;30:1075–1079. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- 62.Landucci ECT, De Castro RC, Toyama M, et al. Inflammatory oedema induced by the Lys-49 phospholipase A2 homologue piratoxin-I in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromide. Biochemical Pharmacology. 2000;59(10):1289–1294. doi: 10.1016/s0006-2952(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 63.Andrião-Escarso SH, Soares AM, Rodrigues VM, et al. Myotoxic phospholipases A2 in Bothrops snake venoms: effect of chemical modifications on the enzymatic and pharmacological properties of Bothrops toxins from Bothrops jararacussu . Biochimie. 2000;82(8):755–763. doi: 10.1016/s0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- 64.Kanashiro MM, De Escocard RCM, Petretski JH, et al. Biochemical and biological properties of phospholipases A2 from Bothrops atrox snake venom. Biochemical Pharmacology. 2002;64(7):1179–1186. doi: 10.1016/s0006-2952(02)01288-1. [DOI] [PubMed] [Google Scholar]


