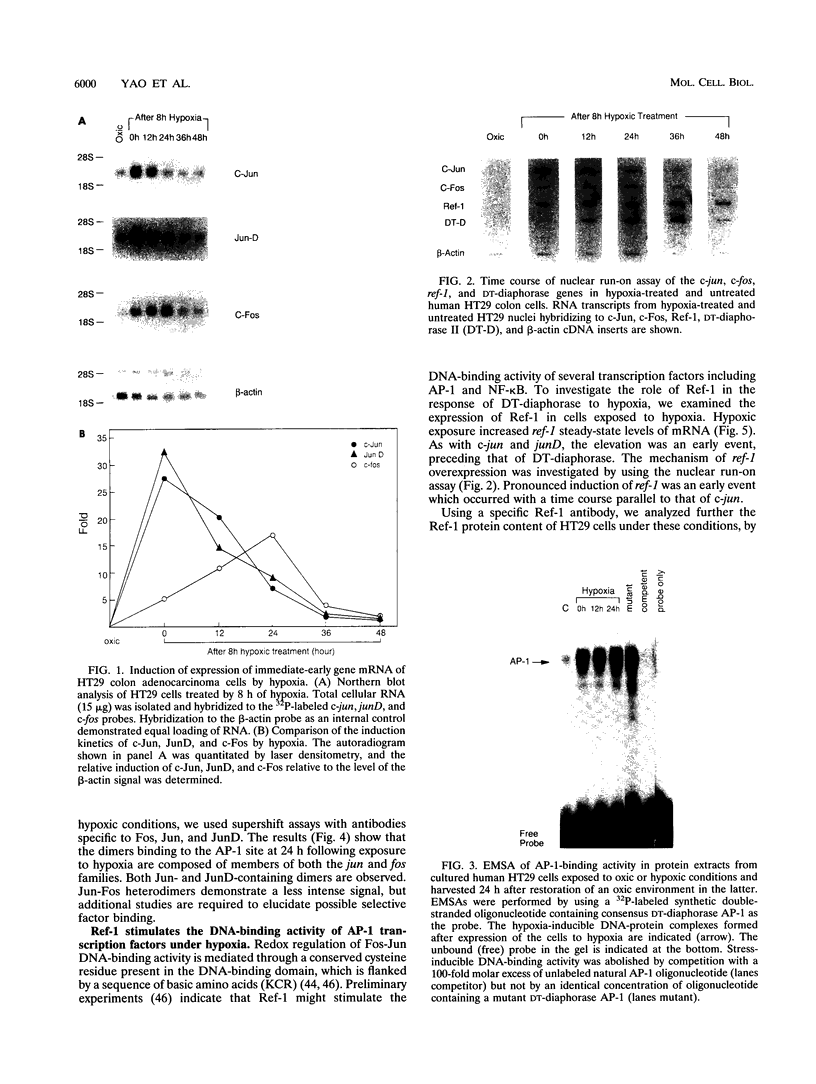
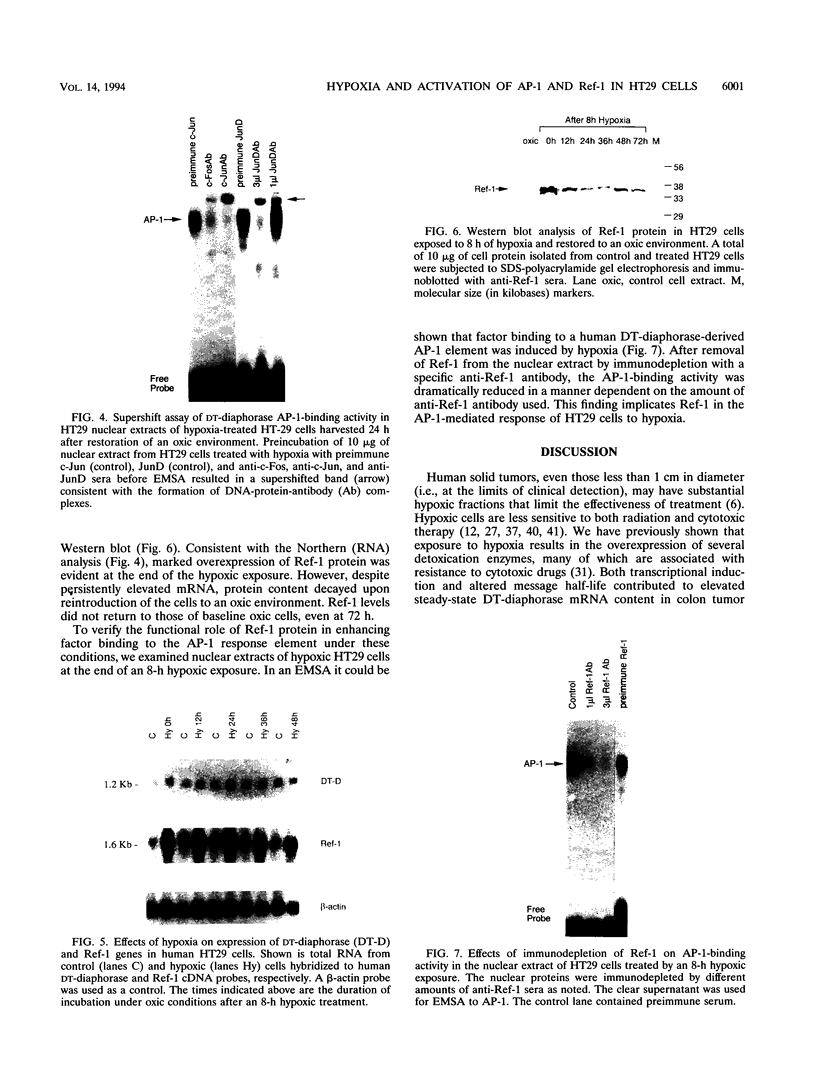
Abstract
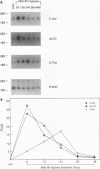
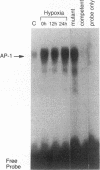
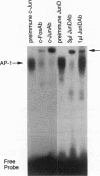
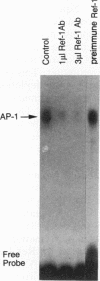
Many solid tumors contain substantial fractions of hypoxic cells which are relatively resistant to both radiation therapy and certain cytotoxic drugs. We have previously shown that exposure of human HT29 cells to hypoxic conditions results in the overexpression of certain enzymes involved in the detoxication of xenobiotics, including NAD(P)H:(quinone acceptor) oxidoreductase (DT)-diaphorase, and gamma-glutamylcysteine synthetase, the rate-limiting enzyme in glutathione synthesis. This hypoxic effect on DT-diaphorase was shown to involve both transcriptional induction and altered message stability. We have investigated the effects of hypoxia on elements in the promoter region of DT-diaphorase. Electrophoretic mobility shift assays demonstrate the induction of a binding activity to the AP-1 response element of DT-diaphorase. Supershift assays suggest that this binding is due to AP-1 nuclear factors and that members of the jun family are induced to a greater degree than fos by hypoxia. Analysis of the kinetics of transcription factor expression indicates that the expression of c-jun and junD is induced during hypoxic exposure; mRNA levels fall during reoxygenation. Induction of fos on the other hand is not as florid during hypoxia (5-fold) and is most pronounced (17-fold) 24 h after the restoration of an oxic environment. Thus, the hypoxic response of DT-diaphorase expression is mediated in part through AP-1, initially by a jun-related mechanism and then by the involvement of fos. The affinity of transcription factors for the AP-1 binding site depends on the redox state of a cysteine residue located close to the DNA-binding region of both Fos and Jun. A nuclear protein, Ref-1, maintains the reduced state of Fos and Jun and promotes binding to AP-1. Nuclear extracts of HT29 cells exposed to hypoxia show markedly increased Ref-1 protein content. Elevation of ref-1 steady-state mRNA levels occurs as an early event following induction of hypoxia and persists when cells are restored to a normally oxygenated environment. Nuclear run-on analysis demonstrates that induction of transcription is the mechanism of ref-1 mRNA elevation. Electrophoretic mobility shift assays and immunodepletion assays were used to further define the interaction of Ref-1 with specific AP-1-binding proteins under hypoxic conditions. These data demonstrate that the induction of detoxicating enzyme expression in HT29 cells exposed to hypoxia results from the induction of both transactivating factors that bind to the AP-1 element and of redox proteins that enhance their affinity for this element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T., HOWARD-FLANDERS P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature. 1956 Nov 3;178(4540):978–979. doi: 10.1038/178978a0. [DOI] [PubMed] [Google Scholar]
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Benjamin I. J., Kröger B., Williams R. S. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6263–6267. doi: 10.1073/pnas.87.16.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M. Sensitizers and protectors in radiotherapy. Cancer. 1985 May 1;55(9 Suppl):2222–2228. doi: 10.1002/1097-0142(19850501)55:9+<2222::aid-cncr2820551426>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- Celano P., Baylin S. B., Casero R. A., Jr Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem. 1989 May 25;264(15):8922–8927. [PubMed] [Google Scholar]
- Celano P., Berchtold C., Casero R. A., Jr A simplification of the nuclear run-off transcription assay. Biotechniques. 1989 Oct;7(9):942–944. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C. N., Bump E. A., Kramer R. A. Chemical modifiers of cancer treatment. J Clin Oncol. 1988 Apr;6(4):709–733. doi: 10.1200/JCO.1988.6.4.709. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau L. V., Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Characterization of a DNA-protein interaction at the antioxidant responsive element and induction by 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1993 Sep 15;268(26):19875–19881. [PubMed] [Google Scholar]
- Favreau L. V., Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991 Mar 5;266(7):4556–4561. [PubMed] [Google Scholar]
- Fornace A. J., Jr Mammalian genes induced by radiation; activation of genes associated with growth control. Annu Rev Genet. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Groshar D., McEwan A. J., Parliament M. B., Urtasun R. C., Golberg L. E., Hoskinson M., Mercer J. R., Mannan R. H., Wiebe L. I., Chapman J. D. Imaging tumor hypoxia and tumor perfusion. J Nucl Med. 1993 Jun;34(6):885–888. [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991 Nov 5;30(44):10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A., Teicher B. A., Rockwell S., Sartorelli A. C. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980 Jan 1;29(1):1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- Li Y., Jaiswal A. K. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992 Jul 25;267(21):15097–15104. [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Okuno H., Akahori A., Sato H., Xanthoudakis S., Curran T., Iba H. Escape from redox regulation enhances the transforming activity of Fos. Oncogene. 1993 Mar;8(3):695–701. [PubMed] [Google Scholar]
- Robson C. N., Milne A. M., Pappin D. J., Hickson I. D. Isolation of cDNA clones encoding an enzyme from bovine cells that repairs oxidative DNA damage in vitro: homology with bacterial repair enzymes. Nucleic Acids Res. 1991 Mar 11;19(5):1087–1092. doi: 10.1093/nar/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Weinstein I. B. Inducible cellular responses to ultraviolet light irradiation and other mediators of DNA damage in mammalian cells. Cell Biol Toxicol. 1990 Jan;6(1):105–126. doi: 10.1007/BF00135030. [DOI] [PubMed] [Google Scholar]
- Rozek D., Pfeifer G. P. In vivo protein-DNA interactions at the c-jun promoter: preformed complexes mediate the UV response. Mol Cell Biol. 1993 Sep;13(9):5490–5499. doi: 10.1128/mcb.13.9.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K., Kwok T. T., Murphy B. J., Laderoute K. R., Gordon G. R., Sutherland R. M. Hypoxia-induced drug resistance: comparison to P-glycoprotein-associated drug resistance. Br J Cancer. 1991 Nov;64(5):809–814. doi: 10.1038/bjc.1991.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler D. L., Anderson G. R., Russo C. A., Spina A. M., Beerman T. A. Anoxia-inducible endonuclease activity as a potential basis of the genomic instability of cancer cells. Cancer Res. 1992 Aug 15;52(16):4372–4378. [PubMed] [Google Scholar]
- Tannock I., Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981 Feb;43(2):245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., al-Achi A., Herman T. S. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990 Jun 1;50(11):3339–3344. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. J., Robson C. N., Black E., Gillespie D., Hickson I. D. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993 Sep;13(9):5370–5376. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G. G., Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]