Abstract
Background
We evaluated the usefulness of follow-up BRAFV600E mutation analysis using ultrasonography-guided fine-needle aspiration (US-FNA) in diagnosis of thyroid nodules showing negative BRAFV600E mutation on prior analysis.
Methodology/Principal Finding
A total of 49 patients (men: 6, women: 43, mean age: 50.4 years) with 49 thyroid nodules were included. Patients had undergone initial and follow-up US-FNA and subsequent BRAFV600E mutation analysis from US-FNA aspirates. All patients had negative results on initial BRAFV600E mutation analysis. Clinicopathologic findings, US assessment, and BRAFV600E mutation results were analyzed according to the final pathology. Of the 49 nodules, 12 (24.5%) were malignant and 37 (75.5%) were benign. Seven (58.3%) of the 12 malignant nodules were positive for BRAFV600E mutation on follow-up, all showing suspicious US features. Initial US-FNA cytology of the 7 nodules were non-diagnostic (n = 2), benign (n = 2), or atypia (n = 3), while follow-up were benign (n = 1), indeterminate (n = 1), suspicious for malignancy (n = 4), and malignancy (n = 1).
Conclusions/Significance
Follow-up BRAFV600E mutation analysis may be helpful in the diagnosis of selected thyroid nodules negative for BRAFV600E mutation on initial analysis, which are assessed as suspicious malignant on US, diagnosed as non-diagnostic, benign or atypia on follow-up US-FNA.
Introduction
Recent reports worldwide show rapid increase in the incidence of papillary thyroid carcinoma (PTC), the most common form of thyroid cancer [1]–[4]. Molecular genetics have evolved remarkably in the recent decade, and at the present time BRAF mutation is the most commonly known genetic alteration in PTC [5]–[6]. BRAFV600E mutations found in PTC are a thymine to adenine transversion at nucleotide 1799 (T1799A), leading to a substitution of valine to glutamic acid at residue 600 of the protein (V600E)[5], [7]–[8]. This point mutation leads stimulation of MAPK pathway, which is results in oncogenic activation in the thyroid. BRAFV600E mutation is common and highly specific for conventional PTC, and can be used for diagnosis, optimal sugical planning, and postoperative patient management [9].
Up to the present date, ultrasonography-guided fine-needle aspiration (US-FNA) has showed excellent performances and considered the standard diagnostic method in the diagnosis of various thyroid nodules detected on ultrasonography (US) [10]–[14]. Major limitations of US-FNA are the indeterminate cytology results such as ‘inadequate or non-diagnostic’, ‘follicular cells with atypia’, ‘follicular neoplasm’, or ‘suspicious for malignancy’, which consist of 10–30% of all cytology results [15]. BRAFV600E mutation analysis has proven to be both useful and accurate as an adjunctive diagnostic tool to US-FNA in providing additional information in the differential diagnosis of PTC showing non-diagnostic or indeterminate cytology results [7], [16]–[18], especially in a BRAFV600E mutation prevalent area such as Korea. Mostly, BRAFV600E mutation analysis accompanies the initial US-FNA of a thyroid nodule based on clinical or radiological suspicion for malignancy, and has been proven more effective when performed at the time of initial cytologic analysis [17]. To our knowledge, no other studies have reported results on the diagnostic significances of performing consecutive follow-up BRAFV600E mutation analysis on the differential diagnosis of thyroid nodules, especially in nodules with negative results on prior BRAFV600E mutation analysis which had ambiguous cytology such as non-diagnostic and atypia, or discordant cytology to US features, and whether if this additional analysis of BRAFV600E mutation is useful or not has not been established. Therefore, we evaluated the usefulness of follow-up BRAFV600E mutation analysis using US-FNA in the differential diagnosis of thyroid nodules which were initially negative for BRAFV600E mutation on prior analysis, and in which circumstances this additional analysis can be effectively used.
Materials and Methods
This study has been approved by the institutional review board (IRB) of Severance Hospital, Yonsei University (Seoul, Korea) and informed consent has been waived by the IRB committee since the study design was in a retrospective form. Informed consent for US-FNA and BRAFV600E mutation analysis was obtained from all patients included in this study, prior to all procedures.
Patients
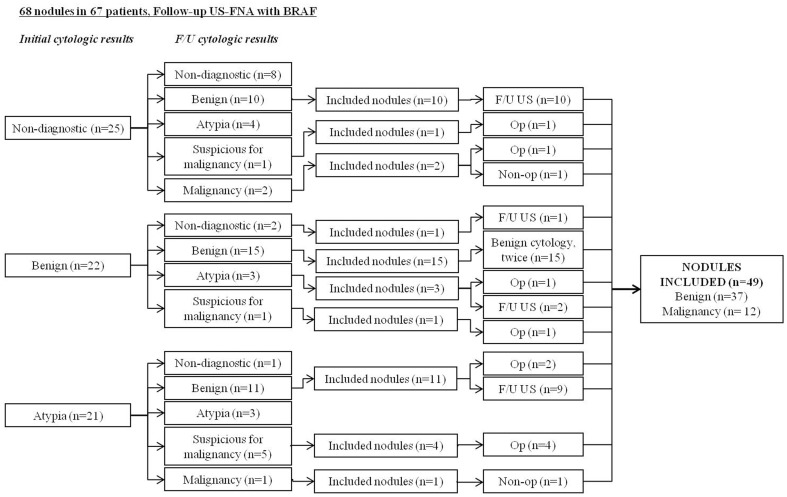
This study was conducted at our institution (a referral center) from June 2009 to April 2011. During this period, 2,365 patients has undergone US-FNA and subsequent BRAFV600E mutation analysis, of which 67 (2.8%) patients had undergone follow-up US-FNA and BRAFV600E mutation analysis from the aspiration specimen in 68 thyroid nodules. Among them, nodules showing repeated non-diagnostic cytology on initial and follow-up US-FNA (n = 9), benign (n = 2), or atypia (n = 8) on initial US-FNA cytology without further follow-up surgical or imaging evaluation were excluded. Thyroid nodules with negative BRAFV600E mutation results on initial US-FNA which fulfilled the following criteria were included: 1) surgery after US-FNA (n = 10), 2) benign cytology results on both initial and follow-up US-FNA (n = 15), 3) no changes on the follow-up US for at least 12 months after a benign cytology result on initial or follow-up US-FNA (n = 22), or 4) follow-up US-FNA cytology resulting in malignancy but without surgery (n = 2) [17]. A total of 49 thyroid nodules in 49 patients were finally included in this study (Fig 1).
Figure 1. Inclusion of the study population.
US-FNA: ultrasonography-guided fine-needle aspiration biopsy, F/U: follow-up, BRAF: BRAFV600E mutation analysis, Op: operation.
At our institution, initial BRAFV600E mutation analysis was performed based on requests from the referring clinicians based on clinical features of the patient, the judegements of radiologists performing US-FNA due to presence of suspicious US features of the targeted nodule during US examinations. Follow-up BRAFV600E mutation analysis was recommended in patients with initial negative BRAFV600E mutation by the clinician, due to clinial features arising suspicion for thyroid malignancy such as family history of PTC (n = 1) or prior non-diagnostic (n = 13), atypia of undetermined significance (n = 16) on initial US-FNA cytology and benign cytology results in spite of the presence of suspicious US features (n = 14), and unknown causes (n = 5).
Imaging Analysis
US examinations and subsequent US-FNA procedures were performed using either 5– to 12– MHz linear probe (iU22, Philips Medical Systemts, Bothell, WA) or 6– to 13– MHz linear probe (EUB-7500, Hitachi Medical, Tokyo, Japan). Compoung imaging was performed when using the iU22 machine.
During the study period, US and US-FNA were performed by 1 of 14 board-certified radiologists with 1–14 years of experience in thyroid imaging. All radiologists had access to the medical record of the patients such as clinical symptoms, past history, and prior cytology and BRAFV600E mutation analysis results when performing US and US-FNA. US features of the thyroid nodules were analyzed by the radiologist performing real-time US examinations prospectively, according to the following categories; internal composition, echogenicity, margin, presence of calcifications, shape and final assessment [19]. Malignant US features were defined as markedly hypoechogenicity (echogenicity lower than the adjacent strap muscle), non-circumscribed margins (microlobulated or irregular margins), microcalcifications (tiny, punctate, echogenic foci measuring less than 1 mm) [20] or mixed calcifications (mixed macrocalcifications, including eggshell calcifications, and microcalcifications), and non-parallel ‘taller-than-wide’ shape, based on a previously published criteria [19]. Final assessments were given as ‘probably benign’, when none of the suspicious US features mentioned above were present, and ‘suspicious malignant’, when 1 or more suspicious US features were present in the thyroid nodule. Final reporting and assessments of complicated cases was done after consulting senior faculty members.
US-guided Fine-Needle Aspiration (US-FNA)
US-FNA was performed by the same radiologist who obtained real-time US images of the thyroid nodule. US-FNAs were performed on either thyroid nodules showing suspicious US features or the largest nodule with probably benign assessment on US, when none of the multiple thyroid nodules showed any suspicious US features.
US-FNA was performed at least twice from each targeted thyroid nodule using 23-gauge needles attatched to a 2 mL disposable plastic syringe, without an aspirator. Aspirated material was expelled on glass slides, which were smeared and immediately placed into 95% alcohol, for Papanicolaou staining. The remaining material in the syringe and needle hub was rinsed in normal saline for cell block processing. Cytopathologists were not present during US-FNA procedures, and additional staining of cytology slides were performed, if needed, on a case-by-case basis according to the request of the interpreting cytopathologist.
One of five cytopathologists specializing in thyroid pathology interpreted the slides obtained from US-FNA. During the study period of June 2009 to November 2009, cytology reports were divided into the following categories: 1) non-diagnostic, indicating specimen showing less than the required minimum of six groupings of well-preserved thyroid follicular cells, each consisting of at least 10 cells per group [21], 2) benign, 3) indeterminate cytology, which includes follicular neoplasm and Hürthle cell neoplasm (22–23). 4) suspicious for malignancy, and 5) malignancy. From December 2009 to the present, the Bethesda System for reporting thyroid cytopathology was used in the classification of cytology reports [21].
BRAFV600E Mutation Analysis
After US-FNA for cytology examinations, aspiration was performed once more for BRAFV600E mutation analysis. Aspirated material obtained was rinsed in 1 mL of normal saline, which was sent for BRAFV600E mutation analysis.
Direct DNA sequencing was used in mutation analysis. Exon 15, which contains the BRAFV600E mutation, was amplified by PCR with the foward primer AGGAAAGCATCTCACCTCATC and the reverse primer GATCACACCTGCCTTAAATTGC. The PCR parameters were as follows: 94°C for 5 minutes, 35 cycles at 94°C for 0.5 minutes, 60°C for 0.5 minutes, and 72°C for 10 minutes. The amplified products were purified with a QIAGEN PCR purification kit and sequenced using the foward primer described previously with Big Dye Terminator (ABI Systems, Applied Biosystems, Foster City, CA), and an ABI PRISM 3100 Avant Genetic Analyzer (Perkin-Elmer).
Data and Statistical Analysis
Histopathology results from surgery were considered the standard reference. Cytology results were used as standard reference for nodules which had not undergone surgery. Thyroid nodules showing benign cytology results on either initial or follow-up US-FNA cytology which did not show change on follow-up US for at least 1 year were considered benign. Nodules diagnosed as malignancy on US-FNA cytology which had not undergone surgery were considered malignant.
Statistical comparison was performed using t-test for parametric variables. Χ 2-test or Fisher's exact test was used in comparison of non-parametric variables. Generalized estimated equation (GEE) methods were used in comparing diagnostic performances. P values of less than 0.05 were considered to have statistical significance. Statistical analyses were performed using SAS software (SAS system for Windows, version 9.1.3; SAS Institute, Cary, NC).
Results
Of the 49 thyroid nodules included in this study, 12 (24.5%) were malignancy and 37 (75.5%) were benign. Of the 49 patients, 6 (12.2%) were men and 43 (87.8%) were women. Mean age of the patients were 50.4 years (range, 29 to 67 years), and mean size of the thyroid nodules were 12.0 mm (range, 3 to 41 mm). Mean interval between the initial and follow-up US-FNA and BRAFV600E mutation analysis was 6.3 months (range, 3 to 21 months).
Clinical, radiological, and cytopathologic features of the patients and thyroid nodules according to final pathology are summarized in Table 1. Mean size of the thyroid nodules were significantly smaller in malignancy (7.18±2.93 mm) compared to benign (13.43±8.76 mm, P = 0.023). No significant differences were seen in mean age and gender between the benign and malignant nodules.
Table 1. Clinical, radiological, and cytopathologic characteristics of the 49 patients and thyroid nodules according to final pathology.
| Benign | Malignancy | P | |
| Number of nodules | 37 (75.5%) | 12 (24.5%) | - |
| Mean age (years±SD) | 50.73±9.40 | 49.50±10.39 | 0.431 |
| Mean size of nodules (mm±SD) | 13.43±8.76 | 7.18±2.93 | 0.022 |
| Patient gender | 0.634 | ||
| Men (%) | 5 (13.5) | 1 (8.3) | |
| Women (%) | 32 (86.5) | 11 (91.7) | |
| US Assessment | 0.004 | ||
| Probably benign (%) | 17 (45.9) | 0 (0.0) | |
| Suspicious malignant (%) | 20 (54.1) | 12 (100.0) | |
| 2nd BRAFV600E mutation | <0.001 | ||
| Negative (%) | 37 (100.0) | 5 (41.7) | |
| Positive (%) | 0 (0.0) | 7 (58.3) |
SD: standard deviation
SD: standard deviation
On US examinations, all 12 (100.0%) malignant nodules showed 1 or more suspicious US features, while 20 (54.1%) benign ones were assessed as suspicious malignant on US final assessment. Malignant nodules showed significantly higher rates of suspicious US features compared to benign ones (P = 0.004). Seven (14.3%) of the 49 thyroid nodules was positive for the second BRAFV600E mutation analysis, among which all were proven as malignancy (Table 2). Initial US-FNA cytology of the 7 nodules were non-diagnostic (n = 2), benign (n = 2), or atypia of undetermined significance (n = 3), while follow-up US-FNA cytology were benign (n = 1), indeterminate (n = 1), suspicious for malignancy (n = 4), and malignancy (n = 1).
Table 2. Demographics of the 7 patients with positive BRAFV600E mutation on follow-up analysis.
| No. | Age | Gender | Size (mm) | Inital US-FNA | Follow-up US-FNA | Final diagnosis |
| 1 | 46 | F | 8 | non-diagnostic | suspicious for malignancy | cPTC based on surgery |
| 2 | 57 | F | 10 | non-diagnostic | malignancy | Follow-up evaluation based on cytology |
| 3 | 44 | F | 4 | benign | indeterminate | cPTC based on surgery |
| 4 | 45 | F | 7 | benign | suspicious for malignancy | cPTC based on surgery |
| 5 | 38 | M | 8 | atypia | benign | cPTC based on surgery |
| 6 | 54 | F | 5 | atypia | suspicious for malignancy | cPTC based on surgery |
| 7 | 58 | F | 6 | atypia | suspicious for malignancy | FVPTC based on surgery |
F: women, M: men
cPTC: conventional papillary thyroid carcinoma, FVPTC: follicular variant papillary thyroid carcinoma
Of the 17 nodules with probably benign US final assessment, initial US-FNA cytology results are as follows; 4 of non-diagnostic, 6 of benign, and 7 of atypia of undetermined significance. All 17 nodules were diagnosed as benign on follow-up cytology, showed negative results on follow-up BRAFV600E mutation analysis, and did not show significant changes on the serial follow-up US examinations.
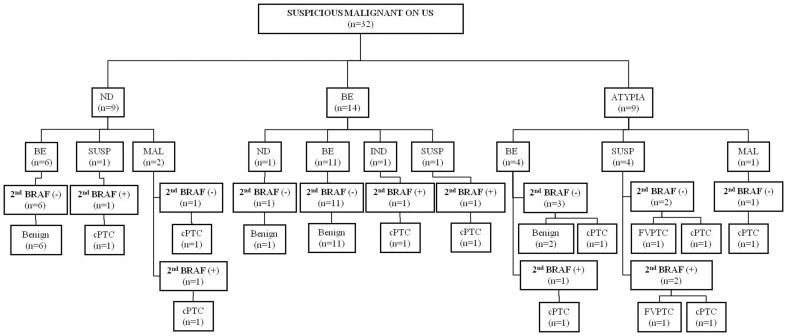
Final outcome of the 32 nodules with suspicious malignant US assessment are summarized in Figure 2. Of the 32 nodules with suspicious malignant US assessment, 12 (37.5%) were malignancy and 20 (62.5%) were benign on final pathology. Of the 9 nodules with initial non-diagnostic cytology, 3 nodules were diagnosed as suspicious for malignancy (n = 1) or malignancy (n = 2) on follow-up US-FNA. All 3 nodules were confirmed as PTC with surgery, among which 2 showed positive results on follow-up BRAFV600E mutation analysis (Fig 3). Of the 14 nodules with initial benign cytology, 2 were diagnosed as indeterminate (n = 1) or suspicious for malignancy (n = 1) on follow-up US-FNA, both with positive results on follow-up BRAFV600E mutation analysis, and confirmed as PTC on surgery. Eleven of the 14 nodules were diagnosed was once again diagnosed as benign on follow-up US-FNA, showed negative results on follow-up BRAFV600E mutation analysis. All 11 nodules were confirmed as benign. Of the 9 nodules with atypia on initial cytology, 4 were diagnosed as benign on follow-up US-FNA, of which 1 nodule was positive for BRAFV600E mutation on follow-up analysis, and was confirmed as PTC on surgery (Fig 4).
Figure 2. Final outcome of the 32 thyroid nodules with suspicious malignant US assessment.
ND: non-diagnostic, BE: benign, IND: indeterminate, SUSP: suspicious for malignancy, MAL: malignancy, cPTC: conventional papillary thyroid carcinoma, FVPTC: follicular variant of papillary thyroid carcinoma
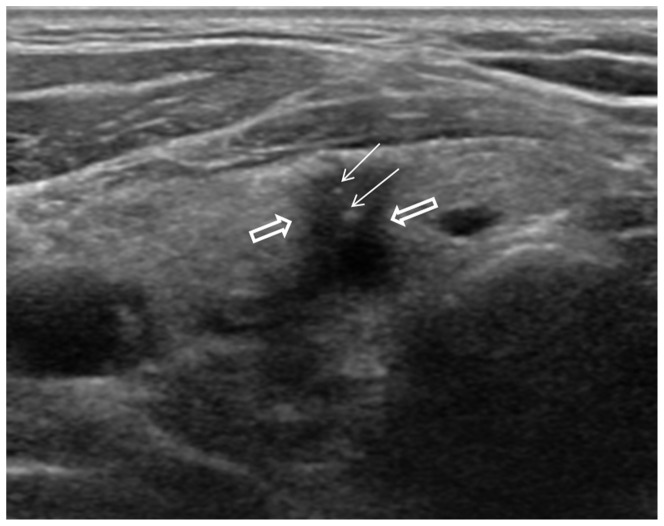
Figure 3. 46-year-old woman with multiple thyroid nodules.
US (a: transverse, b: longitudinal) revealed a 8-mm sized hypoechoic nodule (arrows) with peripheral calcifications in the mid pole of left thyroid. US assessment was suspicious malignant. Initial US-FNA showed non-diagnostic cytology and negative for BRAFV600E mutation. This nodule was diagnosed as suspicious for malignancy on follow-up US-FNA performed 11 months later, and positive for BRAFV600E mutation. Surgery confirmed this lesion as PTC.
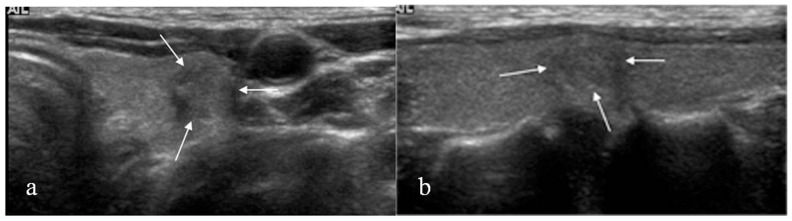
Figure 4. 38-year-old man with multiple thyroid nodules.
A 8-mm sized hypoechoic nodule (empty arrows) with suspicious calcifications (arrows), and spiculated margins was seen in right lower isthmus on US. US assessment was suspicious malignant. Initial US-FNA was diagnosed as atypia of undetermined significance, and negative for BRAFV600E mutation. Follow-up US-FNA and BRAFV600E mutation analysis was performed 6 months later, and follow-up cytology was diagnosed as benign, but positive results on BRAFV600E mutation analysis. Surgery was performed, and this nodule was confirmed as PTC.
Diagnostic performances of the follow-up US-FNA, follow-up BRAFV600E mutation analysis alone, and follow-up US-FNA combined with BRAFV600E mutation analysis are summarized in Table 3. Sensitivity of US-FNA combined to BRAFV600E mutation analysis was significantly higher than US-FNA alone (P = 0.02), while none of the other values showed significant differences.
Table 3. Diagnostic performances of follow-up US-FNA, BRAFV600E mutation analysis, and follow-up US-FNA combined to BRAFV600E mutation.
| All nodules (n = 49) | Follow-up US-FNA | BRAFV600E | Follow-up US-FNA+BRAFV600E | P* | Nodules with suspicious US feature (n = 32) | Follow-up US-FNA | BRAFV600E | Follow-up US-FNA+BRAFV600E | P* |
| Sensitivity | 83.3 (10/12) | 58.3 (7/12) | 91.7 (11/12) | 0.021 | Sensitivity | 83.3 (10/12) | 58.3 (7/12) | 91.7 (11/12) | 0.021 |
| Specificity | 100.0 (37/37) | 100.0 (37/37) | 100.0 (37/37) | - | Specificity | 100.0 (20/20) | 100.0 (20/20) | 100.0 (20/20) | - |
| PPV | 100.0 (10/10) | 100.0 (7/7) | 100.0 (11/11) | - | PPV | 100.0 (10/10) | 100.0 (7/7) | 100.0 (11/11) | - |
| NPV | 94.9 (37/39) | 88.1 (37/42) | 97.4 (37/38) | 0.167 | NPV | 90.0 (20/22) | 80.0 (20/25) | 95.2 (20/21) | 0.289 |
| Accuracy | 95.9 (47/49) | 89.8 (44/49) | 98.0 (48/49) | 0.23 | Accuracy | 93.8 (30/32) | 84.4 (27/32) | 96.9 (31/32) | 0.241 |
Note: Raw data are in parentheses
PPV: positive predictive value, NPV: negative predictive value, *: P values when comparing US-FNA and US-FNA+BRAFV60
Discussion
There is no doubt in that US-FNA is a safe, easy, and reliable diagnostic method in the differential diagnosis of variable thyroid nodules, but US-FNA has its limitations in that up to 10–30% of the cytology derived from it can show inconclusive results, such as insufficient material or indeterminate morphologic criteria of the cells [15], [21]. Molecular analysis of cytology specimen has developed and contributed in providing additional information in the differential diagnosis of thyroid nodules, with BRAF, RAS mutations, and RET/PTC rearrangements most popularly used. BRAFV600E mutation, in particular, is present in 29–70% of papillary thyroid carcinoma [7]–[8], [18], reaching up to 84% in Korea, a BRAFV600E mutation-prevalent area [4], [22]. Many studies have looked into the factors related to BRAFV600E mutation, mostly focusing on clinical [9], [23]–[25], imaging or cytology features [1], [26]–[31]. However, to our knowledge there are no studies in published literature that have investigated the usefulness of follow-up BRAFV600E mutation analysis among nodules negative for BRAFV600E mutation on initial analysis.
BRAFV600E mutation rate of the malignant nodules included in this study was 58.3% (7 of 12 nodules), which is lower than the reported 70–80% prevalence among Korean popluation [4], [16]. This may be explained by the inclusion of thyroid nodules which had initial negative BRAFV600E mutation results. Our study included 49 thyroid nodule which had undergone two consecutive rounds of US-FNA and BRAFV600E mutation analysis, all showing negative BRAFV600E mutation on initial analysis. When putting US assessements of the 49 thyroid nodules in consideration, all 17 nodules with probably benign features had negative results on follow-up BRAFV600E mutation analysis and finally confirmed as benign. Many recent reports show low malignancy rates among thyroid nodules diagnosed as non-diagnostic, benign or atypia on initial cytology showing no suspicious US features [21], [32]–[33], which may be further supported by the fact that this study included nodules with negative results on initial BRAFV600E mutation analysis. In contrast, 7 of the 32 nodules with suspicious malignant US assessment were positive for BRAFV600E mutation on follow-up analysis. Follow-up cytology results of the 7 nodules were benign (n = 1), indeterminate (n = 1), suspicious for malignancy (n = 4), and malignancy (n = 1). The Bethesda System for Reporting Thyroid Cytopathology recommends repeating US-FNA on thyroid nodules with non-diagnostic or atypia, and diagnostic lobectomy on those with indeterminate or suspicious for malignancy [21]. Similarly, the American Thyroid Association management guidelines recommend follow-up US-FNA on nodules diagnosed as non-diagnostic or atypia on cytology, and either lobectomy or total thyroidectomy on those diagnosed as indeterminate and suspicious for malignancy [15]. Follow-up US-FNA is also recommended on thyroid nodules showing suspicious US features which are diagnosed as benign on cytology [34]–[36]. When considering these recommendations, follow-up BRAFV600E mutation analysis confidently lead surgeons towards deciding upon therapeutic surgery in 5 nodules with indeterminate and suspicious for malignancy on follow-up cytology. Also, false-negative results can occur in US-FNA [11], [37]. and follow-up BRAFV600E mutation contributed in detecting malignancy in 1 nodule with false-negative cytology.
Diagnostic performances analysed in this study shows that when combining the follow-up BRAFV600E mutation analysis results to follow-up US-FNA alone, sensitivity was significantly improved, 83.3% to 91.7%, with specificity 100% in all combinations. Among the 32 thyroid nodules with suspicious US features, 11 benign nodules had benign cytology on initial and follow-up US-FNA, along with negative results on the two consecutive BRAFV600E mutation analyses. When considering the high specificity of BRAFV600E mutation analysis, two consecutive negative results of BRAFV600E mutation analysis along with negative cytology results may reassure clinicians in deciding upon follow-up rather than diagnostic lobectomy, reducing unnecessary diagnostic surgical procedures. But, among the 7 nodules showing positive results on follow-up BRAFV600E mutation analysis, 6 were had indeterminate, suspicious for malignancy or malignant cytology results on follow-up US-FNA, presenting suspicious US features. These results alone without the addition of follow-up BRAFV600E mutation analysis may be an indication for therapeutic surgery. But, US features in which surgeons rely upon in deciding surgery involves a significant amount of subjectivity produced by the operator, therefore, BRAFV600E mutation analysis has its role in that it provides more objective information regarding the targeted nodule. Although follow-up BRAFV600E mutation analysis may not be useful in nodules diagnosed as malignancy on follow-up US-FNA, it still has its strong points in choosing upon therapeutic methods among nodules with indeterminate or suspicious for malignancy results on follow-up US-FNA.
Differences in BRAFV600E mutation results between US-FNA cytology and pathology specimen obtained with surgery have been reported in several studies [7], [38]. As in a study on detection of BRAFV600E mutation in indeterminate thyroid nodules [38], the tumor cell contents of cytology specimen can limit the detection of BRAFV600E mutation; even if the cancer tissues contain mutant cells, if the cytology specimen does not include enough mutant cells BRAFV600E mutation analysis may show negative results. Recently, highly sensitive analysis methods compared to direct DNA sequencing such as pyrosequencing and dual priming oligonucleotide (DPO)- based multiplex polymerase chain reaction (PCR) have been introduced, showing abilities to detect less than 2–10% of mutant cells among the aspirated specimen [22], [39], but still, it is clear that obtaining enough mutant cells during US-FNA procedures is important in getting accurate results. The gap between the two consecutive BRAFV600E mutation analysis of our study may be explained by the differences between the initial and follow-up cytology results of the 7 nodules; while initial cytology results were non-diagnostic, benign or atypia, follow-up cytology consists of suspicious for malignancy or malignancy in 5 of the 7 nodules. In contrast to the previously mentioned studies, although obtaining enough cells during aspiration is critical, BRAFV600E mutation analysis has shown high sensitivity regardless of the analysis method, even in cytology specimen somewhat insufficient for accurate diagnosis [22], [40]–[41]. Even though BRAFV600E mutation has its limitations, it has improved the selection of thyroid nodules for surgical treatment in our study; BRAFV600E mutation was detected in the 2 nodules with benign and indeterminate follow-up cytology results in our study, further confirming its efficacy in the diagnosis of PTC.
There are several limitations to our study. First, a limited number of patients and thyroid nodules with initial and follow-up BRAFV600E mutation were included in this retrospective study. As the indication or the cost-effectiveness of follow-up BRAFV600E mutation analysis has not yet been proven, few patients had undergone follow-up analysis, and selection bias may have existed. Further studies in a large series is anticipated. Second, 14 radiologists with various experiences in thyroid imaging were involved in US assessment and US-FNA procedures. Observer variability among radiologists may have affected US assessments and cytology results of thyroid nodules. Similarly, 5 cytopathologists were involved in cytology slide interpretation, and interobserver variability among cytopathologists may have affected the US-FNA cytology results. Last, 2 of the 13 malignant nodules were diagnosed based on cytology only, and the 17 benign nodules were considered benign based on cytology results and US follow-up examinations. False-positive and false-negative results can occur in US-FNA, reported false-positive rates are 0.2–5.7% [42]–[43], false-negative rates 1.9–5.8% [34], [44], which may have had effect on our results.
In conclusion, follow-up BRAFV600E mutation analysis may be helpful in the diagnosis of selected thyroid nodules negative for BRAFV600E mutation on initial analysis, which are assessed as suspicious malignant on US, diagnosed as non-diagnostic, benign or atypia on follow-up US-FNA.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0007711). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nam SY, Han BK, Ko EY, Kang SS, Hahn SY, et al. (2010) BRAF V600E mutation analysis of thyroid nodules needle aspirates in relation to their ultrasongraphic classification: a potential guide for selection of samples for molecular analysis. Thyroid 20: 273–279. [DOI] [PubMed] [Google Scholar]
- 2. Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295: 2164–2167. [DOI] [PubMed] [Google Scholar]
- 3. Leenhardt L, Grosclaude P, Cherie-Challine L (2004) Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14: 1056–1060. [DOI] [PubMed] [Google Scholar]
- 4. Kim KH, Kang DW, Kim SH, Seong IO, Kang DY (2004) Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J 45: 818–821. [DOI] [PubMed] [Google Scholar]
- 5. Nikiforov YE (2011) Molecular analysis of thyroid tumors. Mod Pathol 24 Suppl 2S34–43. [DOI] [PubMed] [Google Scholar]
- 6. Trovisco V, Vieira de Castro I, Soares P, Maximo V, Silva P, et al. (2004) BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol 202: 247–251. [DOI] [PubMed] [Google Scholar]
- 7. Cohen Y, Rosenbaum E, Clark DP, Zeiger MA, Umbricht CB, et al. (2004) Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res 10: 2761–2765. [DOI] [PubMed] [Google Scholar]
- 8. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, et al. (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63: 1454–1457. [PubMed] [Google Scholar]
- 9. Xing M (2007) BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28: 742–762. [DOI] [PubMed] [Google Scholar]
- 10. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A (1998) Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 8: 15–21. [DOI] [PubMed] [Google Scholar]
- 11. Gharib H, Goellner JR (1993) Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med 118: 282–289. [DOI] [PubMed] [Google Scholar]
- 12. Lee MJ, Hong SW, Chung WY, Kwak JY, Kim MJ, et al. (2011) Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: emphasis on correlation with sonographic findings. Yonsei Med J 52: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, et al. (2011) Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosen IB, Azadian A, Walfish PG, Salem S, Lansdown E, et al. (1993) Ultrasound-guided fine-needle aspiration biopsy in the management of thyroid disease. Am J Surg 166: 346–349. [DOI] [PubMed] [Google Scholar]
- 15. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19: 1167–1214. [DOI] [PubMed] [Google Scholar]
- 16. Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, et al. (2010) BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab 95: 3693–3700. [DOI] [PubMed] [Google Scholar]
- 17. Moon HJ, Kim EK, Chung WY, Choi JR, Yoon JH, et al. (2011) Diagnostic value of BRAF(V600E) mutation analysis of thyroid nodules according to ultrasonographic features and the time of aspiration. Ann Surg Oncol 18: 792–799. [DOI] [PubMed] [Google Scholar]
- 18. Xing M, Tufano RP, Tufaro AP, Basaria S, Ewertz M, et al. (2004) Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab 89: 2867–2872. [DOI] [PubMed] [Google Scholar]
- 19. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, et al. (2002) New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 178: 687–691. [DOI] [PubMed] [Google Scholar]
- 20. Yoon JH, Kim EK, Son EJ, Moon HJ, Kwak JY (2011) Diffuse Microcalcifications Only of the Thyroid Gland Seen on Ultrasound: Clinical Implication and Diagnostic Approach. Ann Surg Oncol [DOI] [PubMed] [Google Scholar]
- 21. Cibas ES, Ali SZ (2009) The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 22. Kwak JY, Kim EK, Kim JK, Han JH, Hong SW, et al. (2010) Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck 32: 490–498. [DOI] [PubMed] [Google Scholar]
- 23. Colanta A, Lin O, Tafe L, Ghossein R, Nafa K, et al. (2011) BRAF Mutation Analysis of Fine-Needle Aspiration Biopsies of Papillary Thyroid Carcinoma: Impact on Diagnosis and Prognosis. Acta Cytol 55: 563–569. [DOI] [PubMed] [Google Scholar]
- 24.Ricarte-Filho J, Ganly I, Rivera M, Katabi N, Fu W, et al.. (2011) Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, number of nodal metastasis and extra-nodal extension. Thyroid. [DOI] [PMC free article] [PubMed]
- 25. Tang KT, Lee CH (2010) BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 73: 113–128. [DOI] [PubMed] [Google Scholar]
- 26. Moon HJ, Kwak JY, Kim EK, Choi JR, Hong SW, et al. (2009) The role of BRAFV600E mutation and ultrasonography for the surgical management of a thyroid nodule suspicious for papillary thyroid carcinoma on cytology. Ann Surg Oncol 16: 3125–3131. [DOI] [PubMed] [Google Scholar]
- 27. Chung KW, Yang SK, Lee GK, Kim EY, Kwon S, et al. (2006) Detection of BRAFV600E mutation on fine needle aspiration specimens of thyroid nodule refines cyto-pathology diagnosis, especially in BRAF600E mutation-prevalent area. Clin Endocrinol (Oxf) 65: 660–666. [DOI] [PubMed] [Google Scholar]
- 28. Hwang J, Shin JH, Han BK, Ko EY, Kang SS, et al. (2010) Papillary thyroid carcinoma with BRAFV600E mutation: sonographic prediction. AJR Am J Roentgenol 194: W425–430. [DOI] [PubMed] [Google Scholar]
- 29.Kabaker AS, Tublin ME, Nikiforov YE, Armstrong MJ, Hodak SP, et al.. (2012) Suspicious Ultrasound Characteristics Predict BRAF V600E-Positive Papillary Thyroid Carcinoma. Thyroid. [DOI] [PMC free article] [PubMed]
- 30. Lee EJ, Song KH, Kim DL, Jang YM, Hwang TS, et al. (2011) The BRAF(V600E) mutation is associated with malignant ultrasonographic features in thyroid nodules. Clin Endocrinol (Oxf) 75: 844–850. [DOI] [PubMed] [Google Scholar]
- 31. Mazzaferri E (2011) Fine-needle aspiration biopsy, ultrasound and BRAF(v600E) analysis - is this the best FNAB combination for most patients? Clin Endocrinol (Oxf) 75: 740–742. [DOI] [PubMed] [Google Scholar]
- 32. Yoon JH, Moon HJ, Kim EK, Kwak JY (2011) Inadequate cytology in thyroid nodules: should we repeat aspiration or follow-up? Ann Surg Oncol 18: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 33. Dincer N, Balci S, Yazgan A, Guney G, Ersoy R, et al. (2012) Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology [DOI] [PubMed] [Google Scholar]
- 34. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, et al. (2009) How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol [DOI] [PubMed] [Google Scholar]
- 35. Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, et al. (2009) Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology 253: 854–860. [DOI] [PubMed] [Google Scholar]
- 36. Mazzawi SJ, Rosen G, Luboshitzky R, Dharan M (2000) Management of benign thyroid nodules based on the findings of fine-needle aspiration. J Otolaryngol 29: 95–97. [PubMed] [Google Scholar]
- 37. Hamburger JI, Hamburger SW (1986) Fine needle biopsy of thyroid nodules: avoiding the pitfalls. N Y State J Med 86: 241–249. [PubMed] [Google Scholar]
- 38. Rowe LR, Bentz BG, Bentz JS (2006) Utility of BRAF V600E mutation detection in cytologically indeterminate thyroid nodules. Cytojournal 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim SK, Kim DL, Han HS, Kim WS, Kim SJ, et al. (2008) Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol 17: 118–125. [DOI] [PubMed] [Google Scholar]
- 40. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, et al. (2011) Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96: 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sapio MR, Posca D, Raggioli A, Guerra A, Marotta V, et al. (2007) Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol (Oxf) 66: 678–683. [DOI] [PubMed] [Google Scholar]
- 42. Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, et al. (2010) Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology 254: 292–300. [DOI] [PubMed] [Google Scholar]
- 43. Haberal AN, Toru S, Ozen O, Arat Z, Bilezikci B (2009) Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: correlation with histopathology in 260 cases. Cytopathology 20: 103–108. [DOI] [PubMed] [Google Scholar]
- 44. Ylagan LR, Farkas T, Dehner LP (2004) Fine needle aspiration of the thyroid: a cytohistologic correlation and study of discrepant cases. Thyroid 14: 35–41. [DOI] [PubMed] [Google Scholar]