Abstract
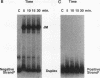
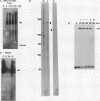
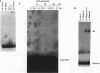
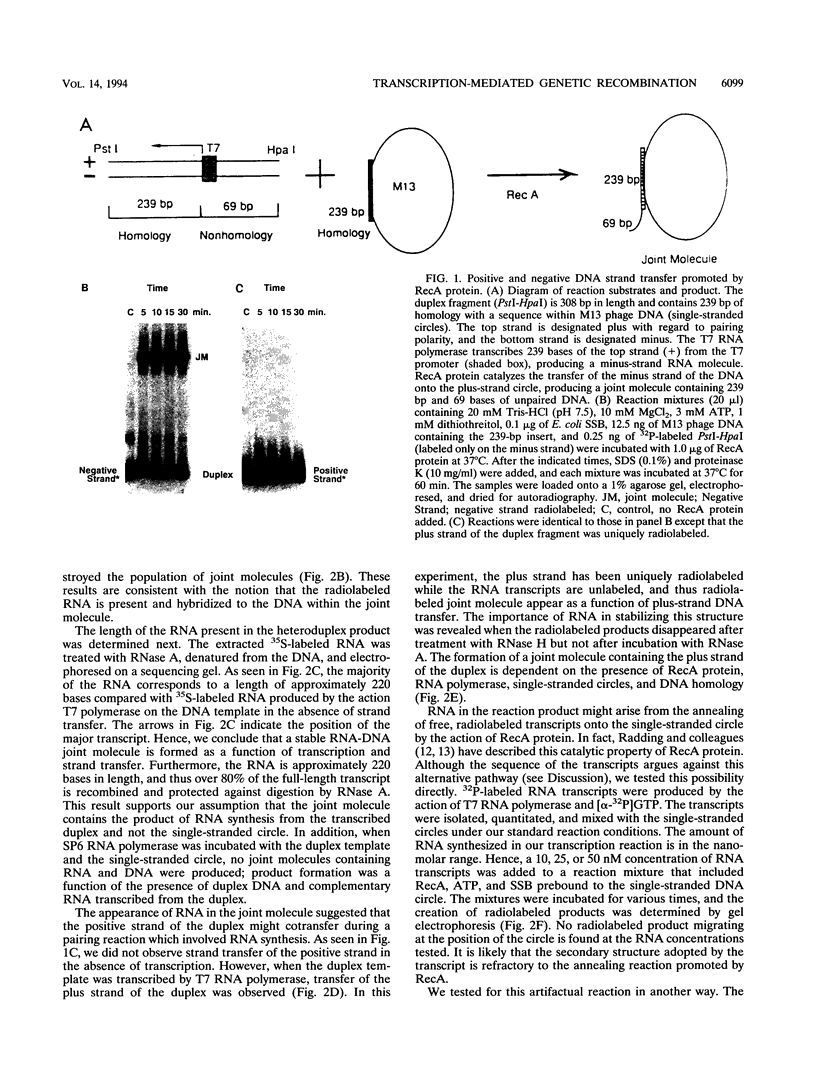
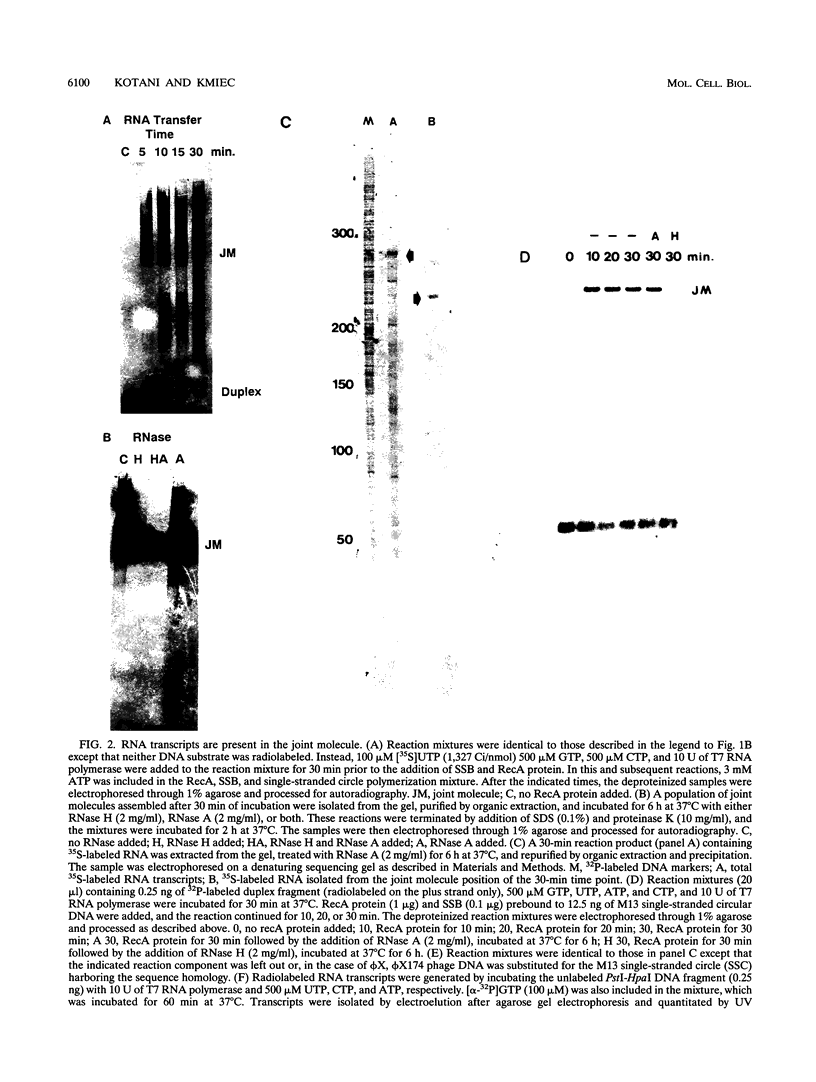
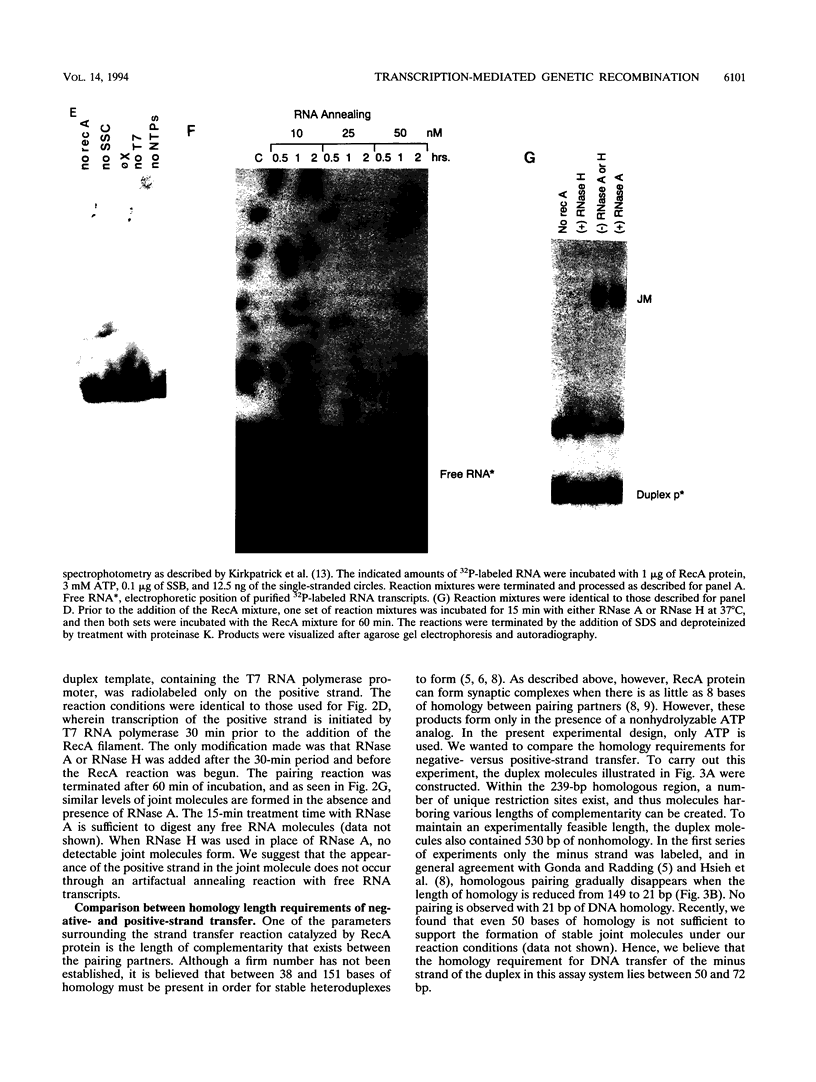
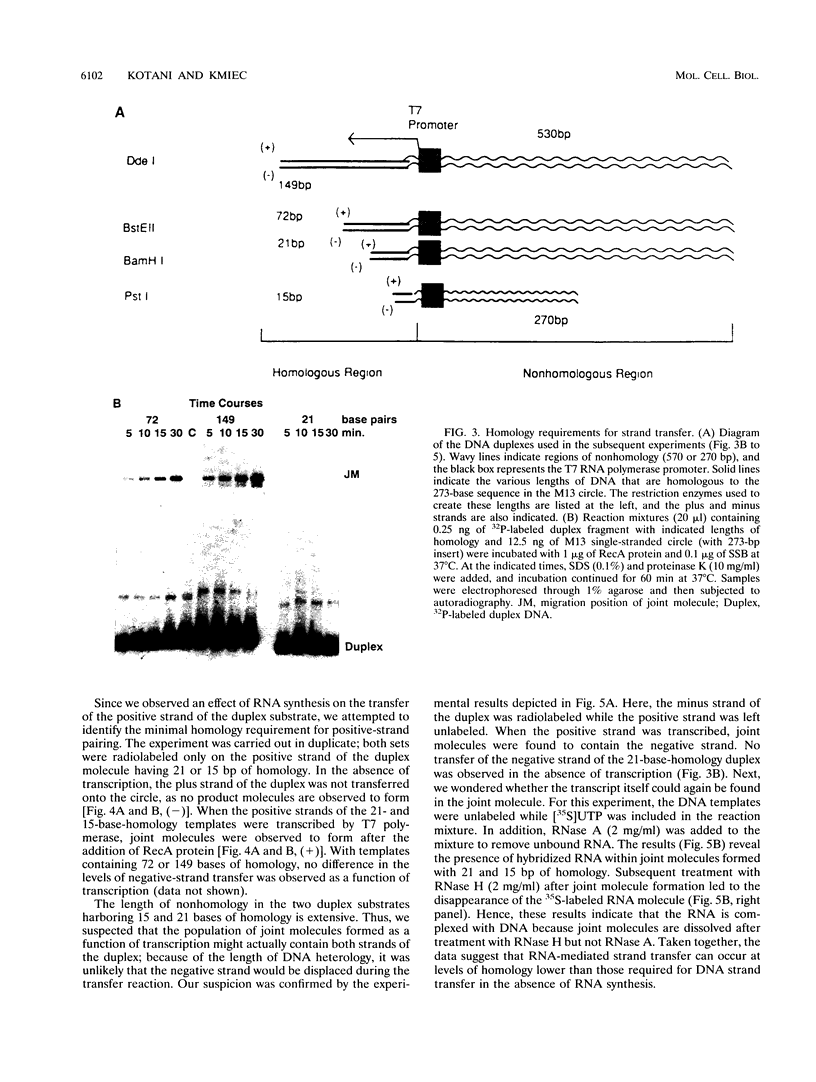
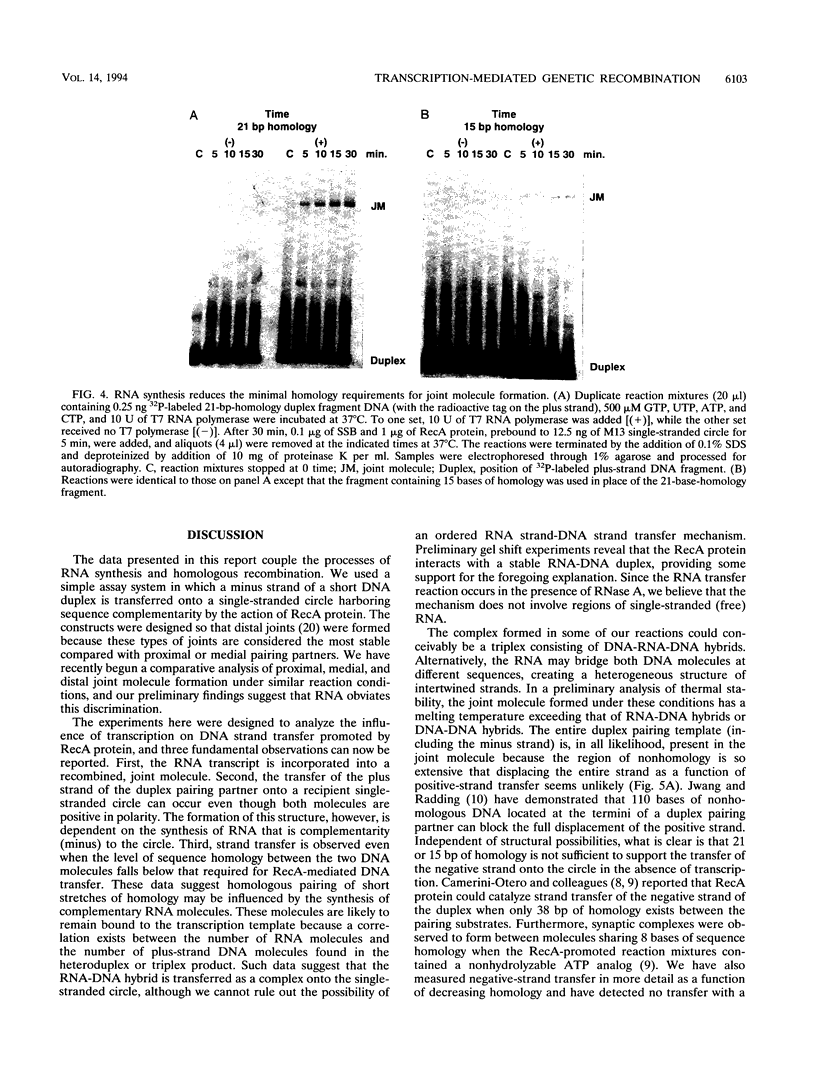
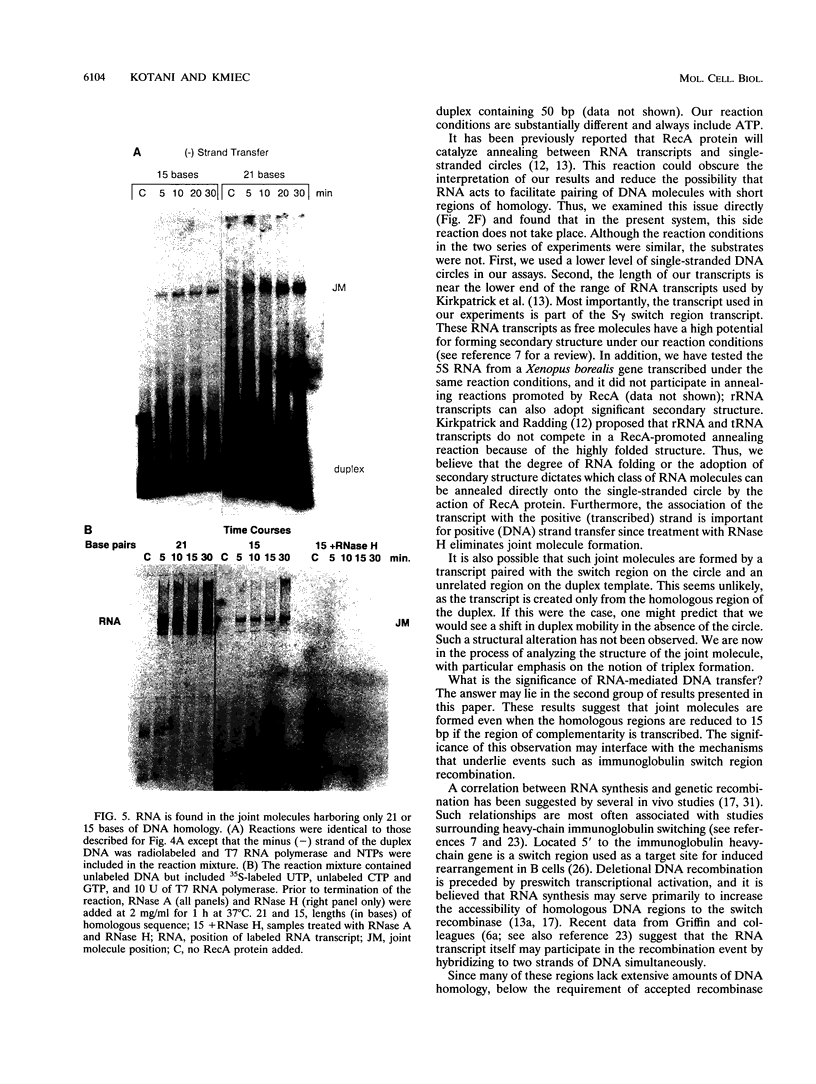
The relationship between RNA synthesis and homologous pairing in vitro, catalyzed by RecA protein, was examined by using an established strand transfer assay system. When a short DNA duplex is mixed with single-stranded circles, RecA protein promotes the transfer of the minus strand of the duplex onto the complementary region of the plus-strand circle, with the displacement of the plus strand of the duplex. However, if minus-strand RNA is synthesized from the duplex pairing partner, joint molecules containing the RNA transcript, the plus strand of the DNA duplex, and the plus-strand circle are also observed to form. This reaction, which is dependent on RNA polymerase, sequence homology, and RecA protein, produces a joint molecule that can be dissolved by treatment with RNase H but not RNase A. Under these reaction conditions, product molecules form even when the length of shared homology between duplex and circle is reduced to 15 bp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Bianchi M. E., Radding C. M. Insertions, deletions and mismatches in heteroduplex DNA made by recA protein. Cell. 1983 Dec;35(2 Pt 1):511–520. doi: 10.1016/0092-8674(83)90185-x. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., DasGupta C., Shibata T., Radding C. M. Homologous pairing in genetic recombination: recA protein makes joint molecules of gapped circular DNA and closed circular DNA. Cell. 1980 May;20(1):223–235. doi: 10.1016/0092-8674(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Gonda D. K., Radding C. M. By searching processively RecA protein pairs DNA molecules that share a limited stretch of homology. Cell. 1983 Sep;34(2):647–654. doi: 10.1016/0092-8674(83)90397-5. [DOI] [PubMed] [Google Scholar]
- Gonda D. K., Radding C. M. The mechanism of the search for homology promoted by recA protein. Facilitated diffusion within nucleoprotein networks. J Biol Chem. 1986 Oct 5;261(28):13087–13096. [PubMed] [Google Scholar]
- Gritzmacher C. A. Molecular aspects of heavy-chain class switching. Crit Rev Immunol. 1989;9(3):173–200. [PubMed] [Google Scholar]
- Hsieh P., Camerini-Otero C. S., Camerini-Otero R. D. Pairing of homologous DNA sequences by proteins: evidence for three-stranded DNA. Genes Dev. 1990 Nov;4(11):1951–1963. doi: 10.1101/gad.4.11.1951. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Camerini-Otero C. S., Camerini-Otero R. D. The synapsis event in the homologous pairing of DNAs: RecA recognizes and pairs less than one helical repeat of DNA. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6492–6496. doi: 10.1073/pnas.89.14.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwang B., Radding C. M. Torsional stress generated by RecA protein during DNA strand exchange separates strands of a heterologous insert. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7596–7600. doi: 10.1073/pnas.89.16.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil R. L., Roeder G. S. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell. 1984 Dec;39(2 Pt 1):377–386. doi: 10.1016/0092-8674(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D. P., Radding C. M. RecA protein promotes rapid RNA-DNA hybridization in heterogeneous RNA mixtures. Nucleic Acids Res. 1992 Aug 25;20(16):4347–4353. doi: 10.1093/nar/20.16.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D. P., Rao B. J., Radding C. M. RNA-DNA hybridization promoted by E. coli RecA protein. Nucleic Acids Res. 1992 Aug 25;20(16):4339–4346. doi: 10.1093/nar/20.16.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Kmiec E. B. Transcription activates RecA-promoted homologous pairing of nucleosomal DNA. Mol Cell Biol. 1994 Mar;14(3):1949–1955. doi: 10.1128/mcb.14.3.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa R., Brown D. D. A bacteriophage RNA polymerase transcribes in vitro through a nucleosome core without displacing it. Cell. 1987 Aug 28;50(5):801–808. doi: 10.1016/0092-8674(87)90338-2. [DOI] [PubMed] [Google Scholar]
- Lutzker S., Alt F. W. Structure and expression of germ line immunoglobulin gamma 2b transcripts. Mol Cell Biol. 1988 Apr;8(4):1849–1852. doi: 10.1128/mcb.8.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- McCarthy J. G., Sander M., Lowenhaupt K., Rich A. Sensitive homologous recombination strand-transfer assay: partial purification of a Drosophila melanogaster enzyme and detection of sequence effects on the strand-transfer activity of RecA protein. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5854–5858. doi: 10.1073/pnas.85.16.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont G., Degroote F., Picard G. Illegitimate recombination in the histone multigenic family generates circular DNAs in Drosophila embryos. Nucleic Acids Res. 1988 Sep 26;16(18):8817–8833. doi: 10.1093/nar/16.18.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. J., Dutreix M., Radding C. M. Stable three-stranded DNA made by RecA protein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2984–2988. doi: 10.1073/pnas.88.8.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaban M. E., Griffin J. A. Induction of RNA-stabilized DNA conformers by transcription of an immunoglobulin switch region. Nature. 1990 Nov 22;348(6299):342–344. doi: 10.1038/348342a0. [DOI] [PubMed] [Google Scholar]
- Schofield M. A., Agbunag R., Miller J. H. DNA inversions between short inverted repeats in Escherichia coli. Genetics. 1992 Oct;132(2):295–302. doi: 10.1093/genetics/132.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986 Mar;112(3):441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Whoriskey S. K., Nghiem V. H., Leong P. M., Masson J. M., Miller J. H. Genetic rearrangements and gene amplification in Escherichia coli: DNA sequences at the junctures of amplified gene fusions. Genes Dev. 1987 May;1(3):227–237. doi: 10.1101/gad.1.3.227. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Drew H. R. Initiation of transcription on nucleosomal templates. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9817–9821. doi: 10.1073/pnas.86.24.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., DePinho R. A., Zimmerman K. A., Lutzker S. G., Rosenberg N., Alt F. W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986 Dec 1;5(12):3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]