Abstract
A long list of chemotherapeutical drugs used in the treatment of the peripheral and the central nervous systems possess anti-microbial activity. Some of these neurotropic compounds are chiral, with the one stereo isomeric form exaggerating reduced neurotropism. This is the case for the levorotatory form of thioridazine. The phenothiazine thioridazine is an interesting compound, characterized by exhibiting a significant growth inhibiting activity on a wide array of micro-organisms. Thioridazine is characterized by another challenging feature, because the compound is concentrated in certain human tissue cells. The present study describes a comparative study of the two enantiomers as well as the racemic form of thioridazine. The study exploits the stereochemical aspect and the in vitro and in vivo potential of these compounds, with a focus on the effects on Gram negative organism Salmonella enterica serover Typhimurium. In summary, the results of this study yielded a significant antibacterial activity of all forms of thioridazine, indicating the levorotatory (–)- form to be superior in terms of both its in vitro and in vivo efficacies.
Introduction
An antibiotic may be defined as a product from a microorganism capable of inhibiting the growth of another microorganism at distinctly low levels. The chemotherapeutics, on the other hand, are primarily synthetic compounds that are able to act on microorganisms in a very similar manner, but at much higher concentrations. It is now known that both antibiotics and antibacterial chemotherapeutics have lost the battle to a large extent in the fight against multidrug resistant (MDR) bacterial pathogens. However, intensive studies by various groups of scientists throughout the world have revealed that there are medicinal compounds used for the therapy of non-infectious pathology possess distinct antimicrobial properties [1]–[13]. These compounds are termed as non-antibiotics [10]. Non-antibiotics exhibit properties that render them important for the therapy of different MDR infections. Phenothazines being one of the most important group of non-antibiotics have been studied extensively for their antimicrobial potentiality [1]–[13], [14]. These non-antibiotics possess most of the characteristics of antibiotics and their antibacterial action can be further potentiated by suitable combinations [15]–[18].
The phenothiazine thioridazine (Tz) is a unique non-antibiotic which is highly bactericidal for Gram positive bacteria and acts as a bacteriostatic agent against Gram negative organisms [19].
Thioridazine is chiral and previous studies have reported that the levorotatory form (−) thioridazine is concentrated in human tissue cells at higher levels than the dextrorotatory form (+) [20]. Furthermore the (–) form of Tz has been reported to have less challenging pharmaco-dynamic activity, e.g. reduced blocking activity on centrally located dopamine D2-receptors than the (+)-form [21]. Several in vitro studies have shown another feature of racemic Tz.
The compound is concentrated in human macrophages and different tissue types, such as pulmonary epithelial cells.
Racemic Tz has a great potentiality for the therapy of MDR-tuberculosis since this compound is concentrated 100-fold in the human macrophages where the tubercle bacilli multiply and remain viable and where antibiotics fail to enter [22]. Furthermore racemic Tz has been shown to possess the capacity to lower the invasion ability of Gram positive and Gram negative bacteria in human epithelial cell lines [23]. Moreover racemic Tz proved to be highly efficient in disintegrating the invading cells of Salmonella enterica serover Typhimurium in mice at rather low levels [21]. The present study aims to define the specific antibacterial properties of the enantiomeric forms of thioridazine, e.g. the racemic, the (+)- and the (–)- compounds and clarify, whether there is a difference in the efficacy of the drug based on its stereoisomeric profile. In order to achieve this goal, we performed comparative in vitro and in vivo studies with two enantiomers along with the racemic compound available commercially (Sigma Chemicals, Denmark).
Materials and Methods
Bacteria
A total of 55 different bacteria belonging to both Gram positive and Gram negative types were taken for this study (Table 1).
Table 1. Minimum inhibitory concentration (MIC) of three optical forms of Tz with respect to different bacteria.
| BACTERIA | MIC(µg/ml) | ||
| Racemic | (+) | (−) | |
| S. aureus NCTC 6571, V. cholerae 569B, V. cholerae 1023 | 25 | 25 | 25 |
| S. aureus NCTC 8530, S. aureus ATCC 25923, S. dysenteriae 7 NCTC 519, Sh. boydii NCTC 254, V.cholerae ATCC 14033,ATCC 14035, V. cholerae DN7 | 50 | 100 | 50 |
| Sh. flexneri 4a NCTC 24, Sh. sonnei NCTC 9774, V.cholerae 713, 820 | 100 | 100 | 100 |
| S. aureus ML 16, ML 152, ML 329, ML 358, S. typhi NCTC 59, S. choleraesuis NCTC 36, 37, L. monocytogenes NCTC 7973,NCTC 10351, NCTC 11994 | 200 | 200 | 100 |
| B. subtilis ATCC 6633, B. pumilus NCTC 8241, S. aureus ML 266, ML 358, ML 422, E. coli K12 Row, E. coli C600, S. berta NCTC 69,S. abony NCTC 6017 | 200 | 200 | 100 |
| B. polymyxa NCTC 4747, B. licheniformis NCTC 10341, S. London NCTC 76, S. enterica serovar Typhimurium NCTC 11, NCTC 74 | 500 | 500 | 500 |
| S. aureus ML 277, V. cholerae 137/62 | 1000 | 1000 | 1000 |
| K. pneumoniae ATCC 10031, K. oxytoca ATCC 130988 | 2000 | 2000 | 2000 |
| L. monocytogenes AMRI 3, A. boumannii KPC 470, 517, P. aeruginosa ATCC 27853, ATCC 25619, C/1/5, Kr/8/89, BVC 1,2,3,4,5, APC1. | >2000 | >2000 | >2000 |
Drugs
Racemic thioridazine (Sigma Chemicals, Denmark). The two enantiomers of thioridazine were prepared according to the procedure of Bourquin et al [22].
Media
Liquid media were nutrient broth (NB; Oxoid), peptone water (PW) containing 1.0% peptone (Oxoid) and 0.5% NaCl and Mueller Hinton broth (MHB, Oxoid). Solid media were nutrient agar (NA, Oxoid), brain heart infusion agar (BHA), Oxoid and Mueller Hinton agar (MHA), Oxoid); pH was always maintained at 7.2 to 7.4.
Inoculum
Each bacterium was grown in NA/MHA at 37°C, harvested at stationary phase and suspended in 5 ml sterile distilled water. Turbidity of each suspension was matched against 0.5 McFarland standard [14] along with a spectrophotometer at 625 nm corresponding to 2.4×105 colony forming unit (CFU)/ml.
Determination of Minimum Inhibitory Concentration (MIC) of the Enantiomers and the Racemate of Tz
This was carried out according to the guidelines of Clinical and Laboratory Standards Institute (CLSI) [24] by spotting 105 CFU contained in a 2 mm loop from diluted 18 h broth cultures on plates containing 0 (control), 50, 100, 200,500, 1000 and 2000 µg/ml of a drug. The plates were incubated at 370 C, observed for appearance of growth after 24 h and again after 72 h.
Spectrophotometry
A stock solution containing 1 mg/ml of thioridazine HCl (Sigma), racemic mixture of thioridazine HCl, dextrorotatory thioridazine HCl (+) and levorotatory thioridazine HCl (−) were prepared by dissolving these separately in methanol. Each stock solution was further diluted to 10µg/ml of methanol. Aliquots of this solution were taken in a quartz cell and scanned for λmax in the range of 200–600 nm using methanol as the blank in a double beam UV spectrophotometer (Jasco-V630). The maximum absorbance was determined by using Spectra manager (Version-2.05.03).
HPLC Parameter
Name of HPLC: Jasco.
Column: C8, 250×4.6 mm, 5 µ particle size.
Flow Rate: 1.0 ml/min.
Loop size: 50 µ lit.
UV Absorption: 264 nm.
Mobile Phase: Methanol: Water containing 0.1% v/v phosphoric acid.
Run Time: 8 mins.
Software used: Clarity lite (Version: 2.6.4.402)
All the samples were prepared as per the above method and each sample was diluted by methanol (HPLC Grade).Water was used for the analysis was MilliQ water.
Animal Experiments
Swiss albino male mice each weighing 18–20 gm were selected for this work. This study was approved by the Institutional Animal Ethics Committee (IAEC) of Jadavpur University and TAAB Biostudy Services. Animals were maintained under standard conditions of temperature (24±1°C) and relative humidity (50–60%) with a photoperiod of 14∶10 h of light:dark. Water and a dry pellet diet were provided ad libitum. The animals were checked regularly for their health and diet according to the rules and guidelines set by the Ethical Committee at definite intervals of time of 12 hr at 8 A.M. in the morning and 8 P.M. in the evening. The animals which showed symptoms of illness were carefully observed, identified and were separated from the healthy ones. These animals were not included in our experiments and were given proper treatment.
The intensity of virulence of infection caused by Salmonella enterica serovar Typhimurium 74 and the median lethal dose (MLD or LD50) of the mouse-passaged strain was as described earlier [14], [25]. Protective efficacies of the forms of Tz in mice infected with virulent S. enterica were carried out as described below. Four groups of animals with 60 mice in the control group and 20 mice each in the 6 experimental groups were taken. The control group received 0.1 ml of sterile saline while for each form of Tz there were 40 animals, in which 20 mice received 100 µg and the other 20 received 200 µg of the drug. After 3 h all the animals were infected with 50 MLD of virulent S.enterica 74 as described [25]. Protective capacity of all 3 forms of Tz was determined by recording the mortality of mice in the different groups upto 100 h after the challenge. The animals which survived after 100 hr of infection were euthanased with the help of cervical dislocation as suggested in the Ethical Committee. The end point for performing euthanasia was observation upto 100 hr with regular monitoring at 12 hr intervals. The animals which were euthanased prior to 100 hr were based on the condition of their health. Generally within 72 hr if they showed severe signs of illness, for example loss of appetite, weight loss, lack of movement, breathlessness, shivering etc. they were euthanased as advised by the veterinary doctor in the Ethical Committee The number of animals euthanased prior to 100 hr varied from 2–7 in each group. Assessment of the animals euthanized prior to 100 hr was monitored by the veterinary doctor after every 12 hr as mentioned 8 AM in the morning and 8 PM in the evening.
Determination of the Effect of Treatment by 3 Forms of Tz on CFU of S. enterica 74 from the Liver, Spleen and Heart Blood of Infected Mice
In a separate experiment 4 groups of 5 mice each were given the following treatment: Group 1 was administered 0.1 ml saline while the other 3 groups received 200 µg each of racemic or (+) or (−) forms of Tz. After 3 h all the animals were challenged with 50 MLD of the same organism. After 18 h mice in each of the 4 groups were sacrificed by cervical dislocation (as recommended by the Ethical Committee) and their spleens and livers were aseptically removed; heart blood was drawn directly from the heart with the help of a micro-syringe for determination of CFU. The spleens and livers were homogenized separately in a tissue homogenizer maintained at 4°C and each specimen was processed for CFU counts.
Statistical Analysis
The results were statistically evaluated by students ‘t’ test and χ2 test wherever applicable, Using freely available statistical software GraphPad (www.graphpad.com).
Results
Bacterial Inhibitory Spectra of 3 Different Forms of Tz
A total of 55 different Gram positive and Gram negative bacteria when tested against the 3 forms of Tz, racemic, (+) and (−), it was found that S. aureus NCTC 6571, V. cholerae 569B, 1023 could be inhibited at 25 µg/ml of each agent (Table 1). Among others it was found that strains of S. aureus, V. cholerae and shigellae were also sensitive to these agents MIC of racemic, (+) and (−) forms of Tz produced almost identical type of inhibition in such organisms. However, (+) variety was less inhibitory than the other two. Strains of S. enterica serovar Typhimurium were inhibited at 500 µg/ml of all the compounds. L. monocytogenes NCTC 7973, NCTC 10351, NCTC 11994 were inhibited at 200µg/ml of racemic and (+) forms and at 100µg/ml of (−) form. The strains of klebsiellae, P. aeruginosa and L. monocytogenes AMRI 3 were highly resistant to all the compounds.
UV Spectrophotometer analysis
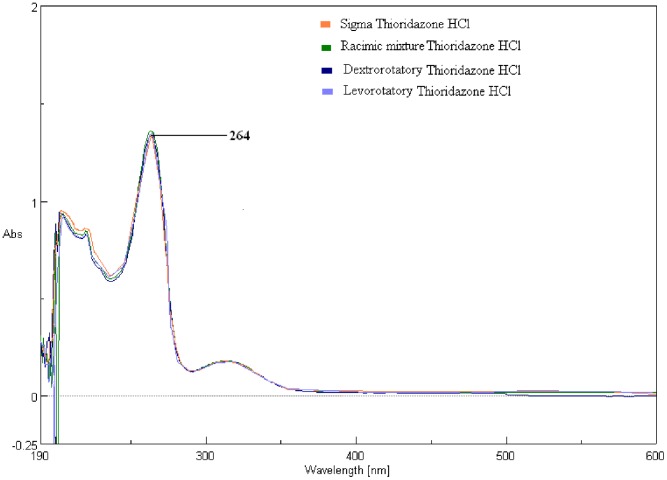
Maximum absorbance (λmax) of the 3 forms of Tz and the reference thioridazine (Sigma) were found to be 264 nm. All the four analytes had the same absorbance as well as identical absorption spectra (Fig. 1).
Figure 1. The spectrophotometric scanning results show that all four compounds had the same λmax (264 nm). HPLC result:
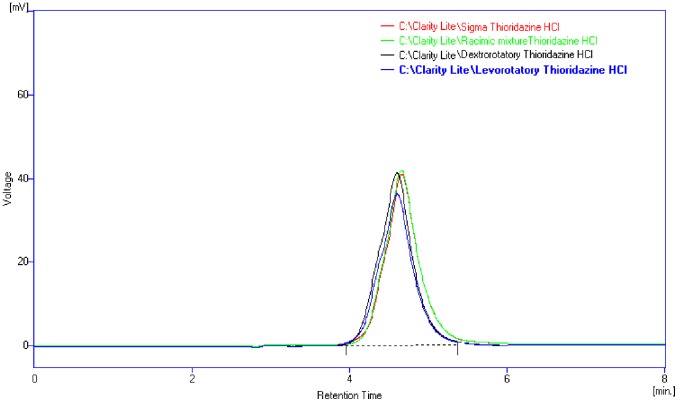
The chromatogram of the four different samples having the same retention time about 4.6 mints indicating that the compounds are identical except for chirality.
HPLC analysis
The chromatogram of the three forms of Tz and the reference Tz from Sigma had the same retention time (4.6 min). All the chromatograms were identical showing peaks at the same concentration (Fig. 2).
Figure 2. The chromatogram showing that all four different samples had the same retention time (about 4.6 min).
In vivo Experiments
Virulence of the infection produced by S. enterica NCTC 74 is being presented in Table 2. In a control group of 60 mice that received only the challenge the mortality was 86.7%. As the number of CFU of S.enterica 74 injected intraperitoneally into mice increased, the % mortality increased, becoming 100% with a dose of 0.95×109 CFU (Table 2). The protective capacity offered by the 3 different forms of Tz shows that there was 100% survival with 200µg/mouse with the (−) form. With the (+) variety the survival was 70% with 200 µg/mouse dose. However, racemic proved to be better than (+) as there was 95% survival with 200µg/mouse dose (Table 2). It may be mentioned here that the animals that received (−) form went to sleep within a few minutes after intraperitoneal injections. Animals of the other two forms went to sleep after 30–40 minutes of intraperitoneal injection of the compounds.
Table 2. Effects of 3 forms of thioridazine (Tz) [racemic, (+), (−)] on survival of mice challenged with Salmonella enterica serovar Typhimurium NCTC 74a.
| Control group (not receiving Tz) | Test groups (receiving Tz) | |||||
| Saline (ml/mouse) | No. of mice died (N = 60) | Mortality % | Tz (µg/mouse) | No.of mice died (N = 20) | % survived | |
| 0.1 | 52 | 86.7 | Racemic | 100 200* | 9 1 | 55 95 |
| (+) | 100 200 | 12 6 | 40 70 | |||
| (−) | 100 200* | 7 0 | 65 100 | |||
Mice received a challenge of 0.95×109 colony-forming units of S. enterica NCTC 74 in 0.5 ml of brain-heart infusion medium.
p<0.001 vs. controls (χ2 test).
The tests on the 3 forms of Tz in mice infected with S.enterica 74 revealed that 5 animals which received only saline and challenge had >108 live cells in liver, spleen and blood after 18 hr infection. However, the number of CFUs in all the organs were between 103 and 104, being much less in the test batches of mice that received one of the 3 drugs along with challenge. The data were statistically significant (Table 3).
Table 3. In vitro activity of sera obtained from blood and homogenates of liver and spleen of mice treated for Salmonella enterica 74.
| Group | Treatment | CFU/mlb | ||
| Sera | Homogenate | |||
| Heart blood | Liver | Spleen | ||
| Control | 0.1 ml of a sterile saline | 1.8×108 to 5.9×108 | 6.5×108 to 9.2×108 | 1.6×108 to 8.7×108 |
| I | 200 µg of Racemic form | 9.0×104 to 6.6×105 | 2.8×103 to 3.8×104 | 2.5×103 to 4.8×104 |
| II | 200 µg of (+) form | 3.6×104 to 9.6×105 | 2.2×104 to 5.0×105 | 7.8×103 to 2.8×105 |
| III | 200 µg of (−) form | 1.0×103 to 8.0×105 | 2.0×103 to 4.7×104 | 1.8×103 to 5.7×104 |
Mice received a challenge of 0.95×109 colony-forming units of S. enterica NCTC 74 in 0.5 ml of brain-heart infusion medium.
p<0.001 vs. controls (‘t’ test).
Discussion
Results obtained in the present study show that racemic, (+) and (−) forms of Tz did not have much difference in their in vitro action, only except that (−) form had shown slightly better inhibitory activity than the other two. Standard strains of L.monocytogenes, eg. NCTC 7973, 10351, 11994 could be inhibited at 500 µg/ml of the 3 forms of Tz, while L.monocytogenes AMRI 3 that was isolated from an acute systemic infection in Kolkata was highly resistant to all the drugs. Both the spectrophotometric and HPLC studies carried out with the 3 forms along with standard thioridazine from Sigma Chemicals, Denmark showed that there was no difference in the λmax and that absorption spectra were identical.
This study further revealed that administration of any form of Tz successfully protected the mice infected with virulent S.enterica from lethality. The protection offered by the drugs were also statistically significant as evidenced by the reduction in the viable cell count in the organs of infected mice compared to the animals that were not administered any drug.
Intraperitoneal infection by S.enterica in mice is likely to cause phagocytosis by neutrophils [24]. According to Gunn [26] salmonellae can efficiently resist the action of hydrolases due to the action of PmrA/B regulon responsible for inactivation of hydrolases. The MIC of all the compounds with respect to S.enterica 74 was 500 µg/ml and is equivalent to weight of water, The amount of Tz forms in a mouse receiving 200 µg dose each would be equivalent to 10 µg/ml, which is one-twentieth of the actual MIC value. Such a distinct protection by Tz forms in mice may be explained by the studies of Ordway et al [27].Since these authors could demonstrate that phenothiazines get concentrated 100 fold inside macrophages maintained in a suitable medium, it may be possible that the concentration takes place inside the lysozome leading to rupture of bacterial cell wall. Furthermore as the phenothiazines are known to promote loss of 55 kD protein [28], there may have been a significant reduction of virulence of bacterial cells in the phagolysozome and hence the lethality might have diminished distinctly. In absence of a direct proof regarding the actual mechanism of action of the different forms of Tz, the protection offered by these compounds remains an assumption. Although it may not be possible to recommend Tz alone against bacterial infections on the basis of our observation in the present study, it may be suggested that structural modifications of the original Tz molecule may open up an avenue on the possibilities of producing highly potent protective antibacterial agents in course of time.
Funding Statement
No current external funding sources was used for this study.
References
- 1. Dastidar SG, Saha PK, Sanyamat B, Chakrabarty AN (1976) Antibacterial activity of ambodryl and benadryl. J Appl Bacteriol 41: 209–214. [DOI] [PubMed] [Google Scholar]
- 2. Dastidar SG, Chaudhury A, Annadurai S, Roy S, Mookherjee M, et al. (1995) In vitro and in vivo antimicrobial action of fluphenazine, J. Chemotherapy. 7: 201–206. [DOI] [PubMed] [Google Scholar]
- 3. Dastidar SG, Jairaj J, Mookherjee M, Chakrabarty AN (1997) Studies on antimicrobial effect of the antihistaminic phenothiazine trimeprazine tartarate, Acta Microbiol. Immun. Hung. 44: 241–247. [PubMed] [Google Scholar]
- 4. Dastidar SG, Ganguly K, Chaudhury K, Chakrabarty AN (2000) The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis, International J. Antimicrobial Agents. 14: 249–251. [DOI] [PubMed] [Google Scholar]
- 5. Mazumdar R, Ganguly K, Dastidar SG, Chakrabarty AN (2001) Trifluoperazine: A broad-spectrum bactericide specially active on staphylococci and vibrios. International J. Antimicrob. Agents 18: 403–406. [DOI] [PubMed] [Google Scholar]
- 6. Kumar KA, Ganguly K, Mazumdar K, Dutta NK, Dastidar SG, et al. (2003) Amlodipine: a cardiovascular drug with powerful antimicrobial property, Acta Microbiol. Pol. 52: 285–292. [PubMed] [Google Scholar]
- 7. Dastidar SG, Debnath S, Mazumdar K, Ganguly K, Chakrabarty AN (2004) Triflupromazine: a microbicide non-antibiotic compound, Acta Microbiol. Immun. Hung. 51: 75–83. [PubMed] [Google Scholar]
- 8. Basu LR, Mazumdar K, Dutta NK, Karak P, Dastidar SG (2005) Antibacterial property of the antipsychotic agent prochlorperazine, and its synergism with methdilazine, Microbiol. Res. 160: 95–100. [DOI] [PubMed] [Google Scholar]
- 9. Jeyaseeli L, DasGupta A, Kumar KA, Mazumdar K, Dutta NK, et al. (2006) Antimicrobial potentiality of thioxanthene fluphenthixol through extensive in vitro and in vivo experiment, Int. J. Antimicrob. Agents 27: 58–62. [DOI] [PubMed] [Google Scholar]
- 10. Kristiansen JE (1992) The antimicrobial activity of non-antibiotics. Acta Path. Micro. Immun. Scand. 100: 7–14. [Google Scholar]
- 11. Kristiansen JE, Amaral L (1997) The potential management of resistant infections with non-antibiotics. J. Antimicrobial Chemother. 40: 319–327. [DOI] [PubMed] [Google Scholar]
- 12. Molnar J, Mandi Y, Kiraly J (1976) Antibacterial effect of some phenothiazine compounds and the R-factor elimination by chlorpromazine. Acta Microbiol. Acad. Sci. Hung. 23: 45–54. [PubMed] [Google Scholar]
- 13.Molnar J, Fischer J, Foldeak S, Gutmann F, Nakamura MJ (1992) Thiazines and structurally related compounds, ed. By Keyzer H, Eckert GM, Forrest IS, Gupta RR, Gutmann F et al. Krieger Publishing Company, Malabar, U.S.A. 197–202.
- 14.Dasgupta A, Dastidar SG, Shiratki Y, Motohashi N (2004) Antibacterial activity of artificial phenothiazines and isoflavones from plants, In: Bioactive Heterocycles VI; Vol. 15, 67–132, Springer, Berlin/Heidelberg.
- 15. Chattopadhyay D, Dastidar SG, Chakrabarty AN (1988) Antimicrobial property of methdilazine and its synergism with antibiotics and some chemotherapeutic agents, Arzneimittelforschung (FRG). 38: 869–872. [PubMed] [Google Scholar]
- 16. Asok KK, Mazumdar K, Dutta NK, Karak P, Dastidar SG, et al. (2004) Evaluation of synergism between the aminoglycoside antibiotic streptomycin and the cardiovascular agent amlodipine, Biol. Pharm. Bull. 27: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 17. Dasgupta A, Chaki S, Mukherjee S, Jeyaseeli L, Mazumdar K, et al. (2010) Experimental analyses of synergistic combinations of antibiotics with a recently recognized antibacterial agent, lacidipine. Eur. J. Clin. Microb. Infect. Dis. 29: 239–243. [DOI] [PubMed] [Google Scholar]
- 18. Jeyaseeli L, Dasgupta A, Dastidar SG, Molnar J, Amaral L (2012) Evidences of Significant Synergism between Antibiotics and the Antipsychotic Antimicrobial Drug Flupenthixol. Eur. J. Clin. Microb. Infec. Dis. 31: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan V, Ganguly K, Ganguli M, Dastidar SG, Chakrabarty AN (1999) Potentiality of tricyclic compound thioridazine as an effective antibacterial and antiplasmid agent. Indian J. Exp. Biol. 671–675. [PubMed]
- 20. Jortani SA, Valentour JC, Poklis A (1994) Thioridazine enantiomeres in human tissues. Forensic Sci. Int. 64: 165–170. [DOI] [PubMed] [Google Scholar]
- 21. Svendsen CN, Froimowitz M, Hrbek C, Campbell A, Kula N, et al. (1988) Receptor affinity, neurochemistry and behavioral charactaristics of the enantiomeres of thioridazine: evidence for different stereoselectivities at D1 and D2 receptors in rat brain. Neuropharmacology 27: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 22. Ordway D, Viveiros M, Leandro C, Bettenocourt R, Almeida J, et al. (2003) Clinical concentration of thioridazine kill inracellular mltidrugresistant Mycobacterium tuberculosis. Antimicrob Agent Chemother. 47: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendricks O (2007) Antimicrobial effects of selected Non-antibiotics on sensivity and invasion of Gram positive bacteria; PhD Thesis, University of Southern Denmark.
- 24.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility testing of bacteria that grow aerobically. 7th ed., approved standard M7-A7.2009. Clinical and Laboratory Standards Institute, Wayne, PA.
- 25. Dasgupta A, Mukherjee S, Chaki S, Dastidar SG, Hendricks O, et al, 2010, Thioridazine protects the mouse from a virulent infection by Salmonella enterica serovar Typhimurium 74. Int. J. Antimicrob. Agents 35: 174–176. [DOI] [PubMed] [Google Scholar]
- 26. Gunn JS, 2008, The Salmonella PmrAB regulon: Lippolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 14: 225–229. [DOI] [PubMed] [Google Scholar]
- 27. Ordway D, Viveiros M, Leandro C, Arroz MJ, Amaral L, 2002, Intracellular activity of clinical concentrations of phenothiazines including thioridazine against phagocytosed Staphylococcus aureus. Int J Antimicrob Agents 20: 34–43. [DOI] [PubMed] [Google Scholar]
- 28. Amaral L, Kristiansen JE, Frølund Thomsen V, Markovich B, 2000, The effects of chlorpromazine on the outer cell wall of Salmonella typhimurium in ensuring resistance to drug. Int.J. Antimicrob. Agents 14: 225–229. [DOI] [PubMed] [Google Scholar]