Abstract
A century of research has described the development of walking based on periodic gait over a straight, uniform path. The current study provides the first corpus of natural infant locomotion based on spontaneous activity during free play. Locomotor experience was immense: 12- to 19-month-olds averaged 2368 steps and fell 17 times/hour. Novice walkers traveled farther faster than expert crawlers, but fall rates were comparable, suggesting that increased efficiency without increased cost motivates expert crawlers to transition to walking. After walking onset, natural locomotion dramatically improved: Infants took more steps, traveled farther distances, and fell less. Walking was distributed in short bouts with variable paths—frequently too short or irregular to qualify as periodic gait. Nonetheless, measures of periodic gait and natural locomotion were correlated, indicating that better walkers spontaneously walk more and fall less. Immense amounts of time-distributed, variable practice constitute the natural practice regimen for learning to walk.
How do infants learn to walk? For more than 100 years, researchers have described developmental antecedents of walking, improvements in the kinematics of walking gait, and changes in the neurophysiological correlates of walking (Adolph & Robinson, in press). However, a century of research has proceeded without a natural ecology of infant locomotion. We know nothing about how much infants crawl and walk and how their activity is distributed over time, how far they travel and where they go, how frequently they fall and what motivates them to persevere, and how natural locomotion changes with development. Lack of such descriptive data is a serious omission, unique to motor development. In other areas, descriptions of infants’ natural activity have been instrumental for constraining theory, guiding clinical interventions, and motivating new research: language acquisition (Hart & Risley, 1995; Hurtado, Marchman, & Fernald, 2008; MacWhinney, 2000), cognitive development (Piaget, 1952), social/emotional development (Barker & Wright, 1951; Messinger, Ruvolo, Ekas, & Fogel, 2010), symbolic play (Tamis-LeMonda & Bornstein, 1996), sleep (Kleitman & Engelmann, 1953), and natural vision (Cicchino, Aslin, & Rakison, 2011; Franchak, Kretch, Soska, & Adolph, 2011; Smith, Yu, & Pereira, 2011). But theories about the development of locomotion and therapies designed to redress atypical development are not connected to data on infants’ real-world experiences with locomotion.
Why are natural descriptions so conspicuously absent from the literature on infant locomotion? One reason for the absence of data is the traditional emphasis on neuromuscular maturation. The long-held assumption that locomotion develops as a universal series of increasingly erect stages led researchers to focus on the formal structure of prone crawling postures en route to upright walking (Gesell, 1946). Similarly, the search for locomotor “primitives” led to formal comparisons between alternating leg movements in newborn stepping, treadmill-elicited stepping, and independent walking (Dominici et al., 2011; Forssberg, 1985; McGraw, 1945; Thelen, 1986; Zelazo, 1983). But age-related sequences in the topography of locomotion dodge the question of why crawlers ever bother to walk. That is, why would expert crawlers abandon a presumably stable, quadrupedal posture that took months to master in order to move in a precarious, upright posture where falling is rampant? In fact, the question of why children persist in acquiring new skills that are initially less functional than the skills already in their repertoires is a central but unanswered question in developmental psychology (Miller & Seier, 1994; Siegler, 2000).
A related reason for the lack of data on natural locomotion is that researchers historically measure aspects of periodic gait—consecutive, regular steps over open ground—not natural locomotion in a cluttered environment where deviations from periodic gait can be adaptive and functional. Since the 1930s, researchers have described infants’ movements as they take a series of continuous steps over a straight, uniform path (Bril & Breniere, 1993; Dominici et al., 2011; Hallemans, De Clercq, & Aerts, 2006; McGraw, 1945; Shirley, 1931). With the standard paradigm, it is imperative that infants walk as quickly and as straight as possible because speed and straightness affect measures such as step length. But the first thing that researchers discover as they try to coax infants along a straight, continuous path is that infants do not readily walk this way. Instead, they stop after a few steps, speed up and slow down, swerve and change direction, and misstep or fall. Typically, such deviations from periodic gait are ignored because they invalidate standard skill measures and thus trials must be repeated. However, in natural locomotion, modifications in step length, speed, and direction are necessary to cope with variable terrain (Patla, 1997). Without a corpus of natural infant locomotion, we cannot know whether standard skill measures such as step length and speed during periodic gait are related to functional skill measures in the everyday environment such as how much infants crawl or walk, how many steps they take, how far they travel, and how frequently they fall.
A third factor contributing to our ignorance about infants’ natural experiences with locomotion is that researchers (including the current authors) routinely represent experience as the number of days that have elapsed since an onset date. We report walking experience as the number of days between the first day of walking and the day of testing. However, this definition is misleading: New walkers walk intermittently, vacillating between days when they walk and days they do not (Adolph, Robinson, Young, & Gill-Alvarez, 2008). More important, this definition is a conceptual misrepresentation of experience. The passage of time is only a proxy for the events that infants actually experience (Adolph & Robinson, in press; Wohlwill, 1970). Although walking experience reliably predicts improvements in standard skill measures such as step length and step width (Adolph, Vereijken, & Shrout, 2003; Bril & Breniere, 1993) and perceptual-motor tasks such as perceiving affordances of slopes (Adolph, 1997), the number of days since walking onset carries little more meaning than test age (the number of days since birth). Indeed, some researchers refer to the number of days since onset as “walking age” (Clark, Whitall, & Phillips, 1988). Possibly, sheer practice indexed by accumulated number of steps facilitates improvements in gait. Alternatively, particular experiences such as surfaces encountered or falls teach infants to walk. Without a natural corpus of infant locomotion, we have no empirical basis for hypothesizing about underlying learning mechanisms.
Current Study
The current study provides the first data on natural infant locomotion—time in motion and distribution of activity over time, variety of locomotor paths, and accumulated steps, distance, and falls. We had three aims. First, we compared natural locomotion in experienced crawlers and novice walkers to gain purchase on the question of why crawlers are motivated to walk. Second, we asked whether functional measures of walking skill such as number of steps and falls per hour improve with test age and walking age as do standard skill measures such as step length and step width. Third, we investigated relations between standard and functional measures of walking skill.
Presumably, most spontaneous walking occurs while infants explore the environment and interact with caregivers. Accordingly, data were collected while infants played freely under caregivers’ supervision. We videotaped infants rather than relying on step-counters or parent informants—two methods that proved problematic in earlier attempts to quantify infants’ natural locomotion (Adolph, 2002). Because video coding was intensely detailed and laborious, we collected representative (15- to 60-min) samples of activity, as is customary in studies of language acquisition (e.g., Hurtado et al., 2008). Most samples were collected in a laboratory playroom to maximize recording quality and to eliminate individual differences in home environments. We also observed infants in their homes to ensure the validity of the laboratory data as an estimate of natural activity. We focused on 12- to 14-month-old novice walkers where improvements in standard skill measures are most dramatic, but included a sample of older, more experienced 19-month-olds where skill measures have begun to asymptote (Adolph et al., 2003; Bril & Breniere, 1993; Clark et al., 1988; Hallemans et al., 2006). We also observed a comparison group of 12-month-old expert crawlers.
Method
Participants and Procedure
We collected 15–60 minutes of spontaneous activity in 151 infants (72 girls, 79 boys) from the New York City area (Figure 1AB). Most families were middle-class and 73% were white. Data from 5 additional infants were excluded due to fussiness or technical problems. We observed 20 crawlers (11.8 to 12.2 months of age) and 116 walkers (11.8 to 19.3 months) in a laboratory playroom (8.66 m × 6.10 m) filled with furniture, varied ground surfaces, and toys (Figure 2A). Infants could move freely throughout the room (Figure 2B). To ensure that playroom observations were representative of natural locomotion, we also observed 15 12.8- to 13.8-month-old walkers in their homes. Caregivers were instructed to interact normally with their infants and to mind their safety. In both settings, an experimenter recorded infants’ movements with a hand-held camera. In the laboratory, two additional fixed cameras recorded side and overhead views to aid coding.
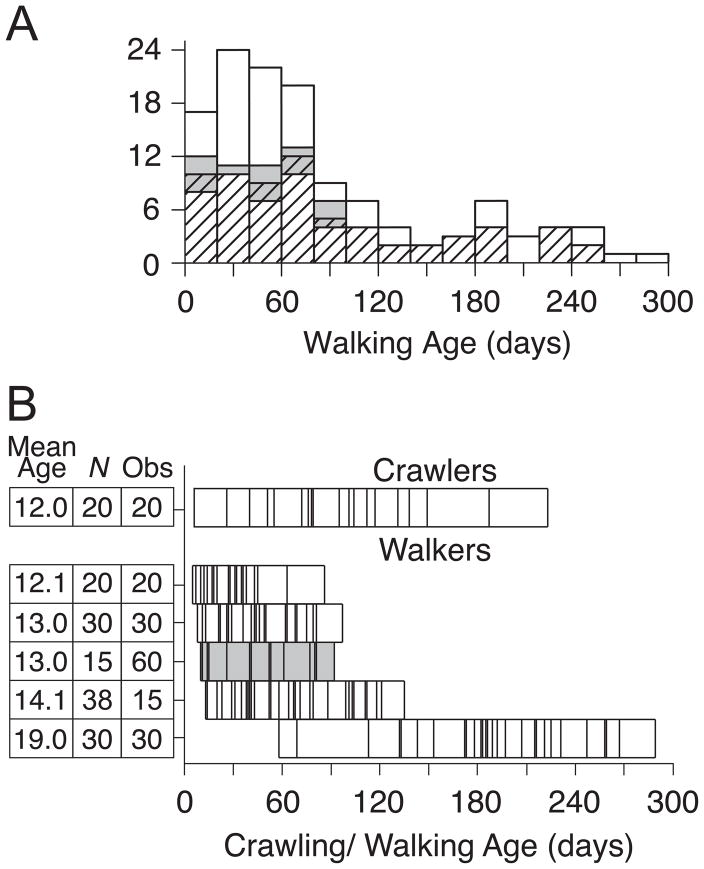
Figure 1.
(A) Frequency histogram of walking age across the entire sample. Striped bars denote girls. Gray bars denote home observations. (B) Table on the left of the figure shows mean test age, N, and length of observations. Bars to the right of the figure show distribution of crawling/walking age for each test age. Each vertical line represents one infant. Gray bar denotes home observations.
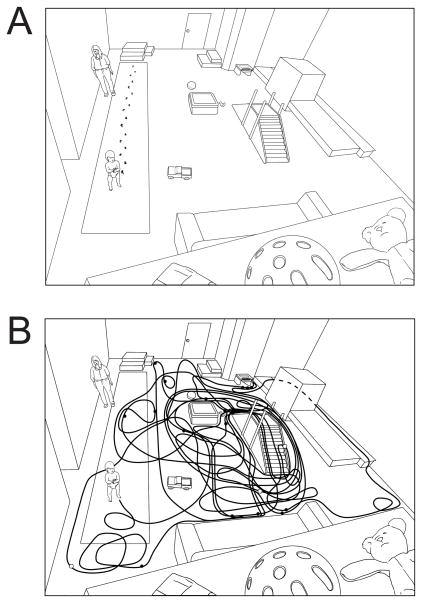
Figure 2.
(A) Layout of the laboratory playroom. Large rectangle on the left shows the gait carpet and one representative walking path. Dimensions are drawn to scale. The playroom also contained a couch, padded square pedestal, slide and small stairs, narrow catwalk behind a wooden barrier, large steps at ends of the catwalk, set of carpeted stairs, set of wooden stairs, a standing activity table, and a wall lined with shelves of toys. (B) Line superimposed over diagram shows the natural walking path of one typical 13-month-old during the first 10 minutes of spontaneous play. Overlapping lines indicate revisits to the same location. Filled circles represent the location of rest periods longer than 5 sec; open circles denote falls.
Crawling and walking age were determined from parental reports based on the first day that infants traveled 10 feet across a room without stopping. Walking age was unavailable for 5 infants. Figure 1A shows a frequency distribution of walking age and sex, and whether infants were observed in the home or playroom. Figure 1B shows the number of infants sampled at each test age, the observation time for each sample, and the overlap in crawling/walking age among samples. Notably, in the 12-month-olds, crawling age (M = 97.6 days) was considerably larger than walking age (M = 29.7 days), t(38) = 5.41, p < .01 (top two rows of Figure 1B). Across the entire sample, walking age ranged from 5 to 289 days. Walking age overlapped among the 12- to 14-month-olds, and there was no difference between 13-month-olds observed at home (M = 47.4 days) and lab (M = 45.9 days), p > .10.
At the end of the laboratory sessions, we collected two standard measures of walking skill as infants walked a straight path over a pressure-sensitive mat (3.6 × 0.89 m; www.gaitrite.com) as illustrated in Figure 2A: step length (front-to-back distance between consecutive footfalls) and step width (side-to-side distance between feet). We estimated crawlers’ average step length (distance between consecutive knee contacts) based on the number of steps to crawl a 3.6 m path. Three walkers and one crawler did not contribute useable data.
Data Coding
A primary coder scored 100% of the video data for the duration of time crawling or walking, number of crawling or walking steps, and falls. Time crawling or walking reflected a single step or series of steps flanked by rest periods of at least 0.5 s; onsets were scored from the video frame when walkers’ foot (or crawlers’ knee) left the floor until the frame when the foot/knee touched the floor in the last step of the series. Coders did not score time in motion for the home observations because they could not determine bout onsets and offsets reliably. A step was considered any up and down motion of a leg that changed infants’ location on the floor. Falls were scored when infants lost balance while crawling or walking and their bodies dropped to the floor unsupported. A second coder independently scored 25% of each infant’s data. Inter-rater agreement was high for time crawling or walking, number of steps, and falls, rs > .95, ps < .01.
To characterize overall amount of natural locomotion, we calculated the accumulated time crawling or walking, number of steps, and falls for each infant and then expressed the data as proportions or hourly rates to allow comparisons across different observation times. We estimated the total distance that infants walked, as if stringing their steps together end-to-end, by multiplying infants’ total step number by their average step length.
Results
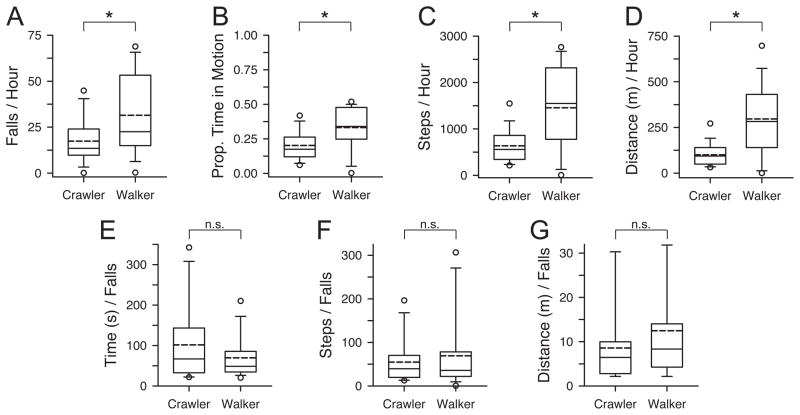
How did functional skill measures compare between 12-month-old crawlers and walkers? As expected, novice walkers fell more per hour (M = 31.5) than expert crawlers (M = 17.4), t(38) = 2.52, p < .02 (Figure 3A), although the prevalence of falls in expert crawlers was unexpected. However, walkers walked more than crawlers crawled (Figure 3B–D): Walkers spent more time in motion (M = 33.1%) than crawlers (M = 20.3%), t(38) = 3.04, p < .01; walkers accumulated more steps/hour (M = 1456.1) than crawlers (M = 635.9), t(38) = 3.78, p < .01; and walkers traveled farther distances/hour (M = 296.9 m) than crawlers (M = 100.4 m), t(36) = 4.05, p < .01. When we reconsidered falls normalized by the differences in activity between crawlers and walkers, differences in fall rate disappeared Figure 3E–G: Walkers were in motion for M = 1.2 minutes before a fall and crawlers were in motion for M = 1.7 minutes; walkers took M = 69.2 steps before a fall and crawlers took M = 54.7 steps; walkers traveled M = 12.5 m before a fall and crawlers traveled M = 8.6 m; all ps > .10.
Figure 3.
Comparisons between 12-month-old expert crawlers and novice walkers: (A) falls/hour, (B) proportion of time in motion, (C) steps/hour, (D) distance/hour, (E) time in motion before each fall, (F) steps before each fall, (G) distance traveled before each fall. Solid lines on box plots denote medians and dashed lines denote means; circles denote outliers beyond the 5th and 95th percentiles.
Averaged across the entire data set, walking infants took 2367.6 steps/hour, traveled 701.2 m/hour, and fell 17.4 times/hour. However, like periodic gait, natural walking develops (Figure S1 in the Supplemental Material available online). As shown in the top two rows of Table 1A, test age and walking age were significantly correlated with standard (step length, step width) and functional measures of walking skill (proportion of time walking, steps/hour, distance/hour, falls/hour): Infants took longer, narrower steps during periodic gait over the gait carpet, and during free play they spontaneously spent more time walking, took more steps, traveled farther distances, and fell less frequently, all ps < .01. These significant correlations remained, even when time in motion was partialled out (Table 1B, rows 1–2), all ps < .01, meaning that functional skill measures reflect more than overall activity level. Figures S1D and S1E also show that infants observed in their homes (crosses) appear similar to infants observed in the laboratory playroom (circles); t-tests comparing home (N = 15) and lab observations (N = 70) of infants with equivalent walking age showed no differences for steps/hr or falls/hr, all ps > .10.
Table 1A.
Correlations Between Test Age, Walking Age, Standard Skill Measures (Step Length, Step Width), and Functional Skill Measures (Time Walking, Steps/hr, Distance/hr, Falls/hr)
| Measures
|
|||||||
|---|---|---|---|---|---|---|---|
| Walking age | Step length | Step width | Time walking | Steps/hr | Distance/hr | Falls/hr | |
| Test age | .86** (124) | .71** (111) | −.60** (111) | .20* (114) | .46** (129) | .65** (111) | −.35** (129) |
| Walking age | .74** (106) | −.68** (106) | .28** (109) | .48** (124) | .68** (106) | −.33** (124) | |
| Step length | −.65** (111) | .28** (111) | .51** (111) | .76** (111) | −.28** (111) | ||
| Step width | −.24* (111) | −.42** (111) | −.55** (111) | .32** (111) | |||
| Time walking | .85** (114) | .72**(111) | .14 (114) | ||||
| Steps/hr | .92** (111) | −.09 (129) | |||||
| Distance/hr | −.17 (111) | ||||||
p< .05,
p< .01
Note, df shown in parentheses
Table 1B.
Partial Correlations Between Test Age, Walking Age, Standard Skill Measures (Step Length, Step Width), and Functional Skill Measures (Time Walking, Steps/hr, Distance/hr, Falls/hr) Controlling for Time Walking
| Measures
|
|||
|---|---|---|---|
| Steps/hr | Distance/hr | Falls/hr | |
| Test age | .55** (113) | .75** (110) | −.39** (113) |
| Walking age | .49** (108) | .71** (105) | −.39** (109) |
| Step length | .54** (110) | .84** (110) | −.33** (110) |
| Step width | −.43** (110) | −.57** (110) | .36** (110) |
| Steps/hr | .84** (110) | −.39** (113) | |
| Distance/hr | −.40** (110) | ||
p< .01
Note, df shown in parentheses
Better walkers on the gait carpet were also better walkers during free play: Standard and functional skill measures were significantly correlated (rows 3–4 of Table 1A) and these correlations remained after time in motion was partialled out (rows 3–4 of Table 1B). Time walking, steps/hour, and distance/hour were inherently intercorrelated because infants who took more steps had to cover more ground and spend more time in motion (rows 5–6 of Table 1A). However, falls/hour was not correlated with time walking, steps/hour, or distance/hour (Table 1A) because although infants who walked more had more opportunities to fall, they were also better walkers and thus fell less. When time in motion was partialled out, falls/hour was significantly negatively correlated with steps/hour and distance/hour (rows 5–6 of Table 1B) and all functional measures were consistent: Better walkers took more steps, traveled farther distances, and fell less frequently.
Although standard and functional skill measures were correlated, periodic gait on the gait carpet and natural locomotion during free play looked very different (Figure 2AB). Our impression from scoring the video files was that infants’ natural paths twisted through most of the open space in the room. We confirmed that impression in 7 randomly selected novice walkers (M walking age = 57.7 days) and 7 experienced walkers (M = 190.3 days) in the first 10 minutes of play. We superimposed 105 grid squares over the open areas of the playroom and scored each time infants entered each square. All infants rambled throughout the room and spontaneously played on the slide, pedestal, catwalk, carpeted stairs, wooden stairs, and near the couch. The number of different grid squares was similar between novices (M = 49) and experts (M = 57), but experts made more return trips to the same squares. Novices entered/reentered M = 128.3 grid squares and experts visited/revisted M = 205.9 grid squares, t(12) = 2.71, p < .05.
Although infants accumulated thousands of steps during the observation periods, they spent most of the time stationary. They were under no obligation to move, and one 12-month-old did not take any walking steps. On average, infants walked only 32.3% of the time. Walking was distributed over time in primarily short bursts of activity. The raster plot in Figure S2 in the Supplemental Material available online shows the even distribution of walking bouts for the 60 infants observed for 30 minutes, ranked by walking age. Raster plots of the other 56 infants for whom we scored bout duration showed similarly even distributions. On average, 46% of bouts consisted of 1–3 steps, and 23% consisted of a single step—too short to qualify as periodic gait and too short for calculating standard measures of walking skill. There was no difference in duration, step number, or step rate in walking bouts that ended in falls and those that did not, ps > .10.
Discussion
A remarkable thing about basic skills acquired during infancy is the apparent ease and rapidity of acquisition. Infants learn to walk, talk, think, play, and perceive objects and events in the course of natural activity. Thus, descriptions of natural activity play a critical role in guiding developmental research, theory, and application. The development of locomotion is a notable exception: Until now, research, theory, and clinical intervention have proceeded without a natural ecology of infant locomotion. By collecting such a corpus, the current study aimed to: address the question of why expert crawlers transition to walking, investigate developmental changes in natural locomotion vis-à-vis improvements on standard measures, and provide an empirical basis for hypothesizing about learning mechanisms.
Why Walk?
Our inclusion of a comparison group of expert crawlers provided some clues to the longstanding puzzle of why infants who are skilled crawlers would abandon it for a precarious, new, upright posture. To our surprise, expert crawlers were not more skilled than novice walkers. Functional measures of locomotor skill showed that crawlers crawled less than walkers walked, took fewer steps, and traveled shorter distances. Moreover, falling was common: All but one crawler fell. As expected, falling was far more common in novice walkers: One racked up 69 falls/hour. But when we normalized fall rate by the difference in activity between crawlers and walkers, the difference in fall rates disappeared and walkers were no longer at a disadvantage. In fact, when we reanalyzed standard measures of locomotor skill (crawling/walking over a straight, uniform path) in infants observed longitudinally (originally reported in Adolph, 1997), step length and speed increased steadily from infants’ first week of crawling to their nineteenth week of walking, and showed no decrement over the transition from crawling to walking (Adolph, 2008). In other words, based on both standard and functional skill measures, new walkers reap all the benefits of an upright posture without incurring additional risk of falling. Thus, part of the answer to “why walk?” is “why not?”
Development of Natural Locomotion
After 100 years of studying the development of walking by coercing infants to walk at a steady pace along a straight, uniform path, researchers can say with certitude that standard measures of walking skill (e.g., step length and step width) improve with test age and walking age. We replicated that century-old finding. More newsworthy, we showed that natural locomotion also improves: Functional measures of walking skill obtained from spontaneous locomotion during free play (steps, distance, and falls/hour) improve with test age and walking age. These findings held up after statistically adjusting for time walking, meaning that older, more experienced walkers not only walk more, they walk better. Like intercorrelations among standard skill measures, functional skill measures were highly consistent. With time walking partialled out to statistically adjust for activity, infants who took more steps and traveled farther distances fell less frequently.
Moreover, we found that standard and functional skill measures were significantly correlated. Thus, for the first time, we have construct validity for standard skill measures in terms of natural infant walking. This set of findings is remarkable because periodic gait (Figure 2A) looks notably different from natural locomotion (Figure 2B).
Possible Learning Mechanisms
We need to reconsider the long-held tradition of using walking age to represent walking experience. Walking age signifies only the elapsed time since walking onset. Like test age, walking age is a robust predictor of various developmental outcomes, but it is not an explanatory variable. In other areas of developmental research, descriptions of natural activity have informed understanding about learning mechanisms. For example, in language acquisition, the sheer number of utterances and word tokens in mothers’ natural talk to infants at 18 months of age (estimated from 12 minutes of mother-infant free play in a laboratory playroom) influences rate of vocabulary growth and language processing speed at 24 months (Hurtado et al., 2008). In contrast, diversity of language (number of word types) is not predictive. In conceptual development, event type rather than sheer quantity of input affects learning about causal agency: Viewing agentive events during natural activity (estimated from one hour of video collected with a head camera at 3, 8 and 12 months of age) influences generalization about causal agency at 10–14 months of age in habituation tasks (Cicchino et al., 2011). Similarly, a corpus of natural locomotion allows researchers to investigate possible learning mechanisms by analyzing specific measures of locomotor experience. The current study suggests that quantity, distribution, and variety of experiences are viable candidates as factors affecting learning to walk.
Although most people would assume that infants walk and fall a lot, few would guess that the average toddler takes 2368 steps, travels 701 m—the length of 7.7 American football fields—and falls 17 times per hour. Hourly rates provide only a tantalizing window into the amounts of practice that likely accumulate over a day. For example, a multiplier of 6 hours (approximately half of infants’ waking day) would indicate a daily rate of about 14,000 steps, 46 football fields, and 100 falls. Estimates of natural activity are equally enormous for other skills. Middle-class infants hear 2,150 words/hour, more than 30 million words by 3 years (Hart & Risley, 1995). Eleven- to 13-month-olds spend more than 30 min/hour engaged with objects during everyday activity (Karasik, Tamis-LeMonda, & Adolph, 2011). By 2 months of age, infants have executed over 2.5 million eye movements (Johnson, Amso, & Slemmer, 2003), and by 3.5 months, they have performed 3–6 million.
To put these immense numbers into perspective, concert musicians and professional athletes require approximately 4 hours of practice per day to train and fine-tune their perceptual-motor systems (Ericsson, Krampe, & Tesch-Romer, 1993). The consensus in the literature on expertise is that large amounts of regular practice, accumulated over years of training, promote expert performance (Ericsson & Ward, 2007). The same principle could apply to acquiring expertise in walking.
Natural walking was distributed in time and occurred in variable patterns and contexts. Short bursts of walking were separated by longer stationary periods. Walking bouts were frequently too short to qualify as periodic gait: 1–3 steps in length. Moreover, infants started and stopped at will, traveled in winding paths over varying surfaces, took sideways and backward steps, varied walking speed, switched from upright to other postures, and misstepped and fell. They visited multiple locations and engaged in different activities therein.
Laboratory studies with older children and adults indicate that time-distributed, variable practice is beneficial for motor learning (Gentile, 2000; Schmidt & Lee, 1999). Time-distributed practice is more effective than massed practice because intermittent rest periods allow learning to be consolidated, relieve fatigue, and renew motivation. Variable practice leads to greater flexibility and broader transfer than blocked practice because executing a variety of movements in a variety of contexts helps learners to identify the relevant parameters and their allowable settings. Recent efforts to teach robots to walk provide additional support for variable practice. The traditional approach is to train robots to walk as fast as possible in a straight line—essentially, to train robots on periodic gait (Kohl & Stone, 2004). But training robots with omnidirectional gait on variable paths—similar to infants’ natural locomotion—led to more adaptive, functional locomotor skill. After 15,000 runs through an obstacle course, robots showed decreased falls, increased step number, distance, and speed, and in a test not possible with infants, elite performance in robot soccer: With a variable training regimen, the UT Austin Villa team won all 24 games in the 2011 RoboCup 3D simulation competition, scoring 136 goals and conceding none (Macalpine, Urieli, Barrett, Vu, & Stone, 2011; Urieli, MacAlpine, Kalyanakrishnan, Bentor, & Stone, 2011).
Conclusion
How do infants learn to walk? This corpus of natural locomotion indicates that infants accumulate massive amounts of time-distributed, variable practice. With each day of walking, they take more steps, travel farther distances, and fall less; and they may be motivated to walk in the first place because it takes them farther faster than crawling without increased risk of falling. Traditional studies of infant locomotion during periodic gait could not have revealed these findings.
Supplementary Material
Scatterplots of walking age by (A–B) standard measures of walking skill obtained during periodic gait over the gait carpet and (C–F) functional measures of walking skill obtained during natural locomotion during free play. Circles denote infants observed in the laboratory playroom, crosses denote infants observed at home.
Raster plot of distribution of walking bouts in 60 infants observed over 30 minutes of natural walking. Each row represents one infant. Rows were ordered by walking age.
The width of each vertical line represents the duration of the walking bout. The thinnest lines denote bout that were ≤ 6 s in duration (minimum bout duration that could be represented given the resolution of the image).
Acknowledgments
This research was supported by NICHD Grant R37-HD033486 to KEA. We thank Mark Blumberg, Judy DeLoache, Peter Gordon, Rachel Keen, Patrick MacAlpine, and Peter Stone for helpful comments, Samira Iravani for line drawings, and John Franchak for help with figures.
References
- Adolph KE. Learning in the development of infant locomotion. Monographs of the Society for Research in Child Development. 1997;62(3) Serial No. 251. [PubMed] [Google Scholar]
- Adolph KE. Learning to keep balance. In: Kail R, editor. Advances in child development and behavior. Vol. 30. Amsterdam: Elsevier Science; 2002. pp. 1–40. [PubMed] [Google Scholar]
- Adolph KE. The growing body in action: What infant locomotion tells us about perceptually guided action. In: Klatzy R, Behrmann M, MacWhinney B, editors. Embodiment, ego-space, and action. Mahwah: Erlbaum; 2008. pp. 275–321. [Google Scholar]
- Adolph KE, Robinson SR. The road to walking: What learning to walk tells us about development. In: Zelazo P, editor. Oxford handbook of developmental psychology. NY: Oxford University Press; (in press) [Google Scholar]
- Adolph KE, Robinson SR, Young JW, Gill-Alvarez F. What is the shape of developmental change? Psychological Review. 2008;115:527–543. doi: 10.1037/0033-295X.115.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Development. 2003;74:474–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- Barker RG, Wright HF. One boy’s day: A specimen record of behavior. New York: Harper Brothers; 1951. [Google Scholar]
- Bril B, Breniere Y. Posture and independent locomotion in early childhood: Learning to walk or learning dynamic postural control? In: Savelsbergh GJP, editor. The development of coordination in infancy. North–Holland, The Netherlands: Elsevier; 1993. pp. 337–358. [Google Scholar]
- Cicchino JB, Aslin RN, Rakison DH. Correspondences between what infants see and know about causal and self-propelled motion. Cognition. 2011;118:171–192. doi: 10.1016/j.cognition.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JE, Whitall J, Phillips SJ. Human interlimb coordination: The first 6 months of independent walking. Developmental Psychobiology. 1988;21:445–456. doi: 10.1002/dev.420210504. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, D’Avella A, Mondi V, Cicchese M, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science. 2011;334:997–999. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychological Review. 1993;100:363–406. [Google Scholar]
- Ericsson KA, Ward P. Capturing the naturally occurring superior performance of experts in the laboratory: Toward a science of exceptional performance. Current Directions in Psychological Science. 2007;16:346–350. [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion, and transition to independent locomotion. Experimental Brain Research. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- Franchak JM, Kretch KS, Soska KC, Adolph KE. Head-mounted eye tracking: A new method to describe infant looking. Child Development. 2011;82:1738–1750. doi: 10.1111/j.1467-8624.2011.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile AM. Skill acquisition: Action, movement, and neuromotor processes. In: Carr J, Shepard R, editors. Movement science: Foundations for physical therapy in rehabilitation. 2. New York: Aspen Press; 2000. pp. 111–187. [Google Scholar]
- Gesell A. The ontogenesis of infant behavior. In: Carmichael L, editor. Manual of child psychology. New York: John Wiley; 1946. pp. 295–331. [Google Scholar]
- Hallemans A, De Clercq D, Aerts P. Changes in 3d joint dynamics during the first 5 months after the onset of independent walking: A longitudinal follow-up study. Gait & Posture. 2006;24:270–279. doi: 10.1016/j.gaitpost.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hart B, Risley TD. Meaningful differences in the everyday experiences of young American children. Baltimore: Paul H. Brookes; 1995. [Google Scholar]
- Hurtado N, Marchman VA, Fernald A. Does input influence uptake? Links between maternal talk, processing speed and vocabulary size in Spanish-learning children. Developmental Science. 2008;11:F31–F39. doi: 10.1111/j.1467-7687.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SP, Amso D, Slemmer JA. Development of object concepts in infancy: Evidence for early learning in an eye tracking paradigm. Proceedings of the National Academy of Sciences. 2003;100:10568–10573. doi: 10.1073/pnas.1630655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. The transition from crawling to walking and infants’ actions with objects and people. Child Development. 2011;82:1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N, Engelmann TG. Sleep characteristics of infants. Journal of Applied Physiology. 1953;6:269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- Kohl N, Stone P. Policy gradient reinforcement learning for fast quadrupedal locomotion. Proceedings of the IEEE International Conference on Robotics and Automation; 2004. pp. 2619–2624. [Google Scholar]
- Macalpine P, Urieli D, Barrett S, Vu V, Stone P. Design and optimization of an omnidirectional humanoid walk: A winning approach at the RoboCup 2011 3D simulation competition. 2011. In preparation. [Google Scholar]
- MacWhinney B. The CHILDES project: Tools for analyzing talk. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- McGraw MB. The neuromuscular maturation of the human infant. New York: Columbia University Press; 1945. [Google Scholar]
- Messinger D, Ruvolo P, Ekas N, Fogel A. Applying machine learning to infant interaction: The development is in the details. Neural Networks. 2010;23:1004–1016. doi: 10.1016/j.neunet.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PH, Seier W. Strategy utilization deficiencies in children: When, where, and why. In: Reese H, editor. Advances in child development and behavior. Vol. 25. New York: Academic Press; 1994. pp. 107–156. [DOI] [PubMed] [Google Scholar]
- Patla AE. Understanding the role of vision in the control of human locomotion. Gait and Posture. 1997;5:54–69. [Google Scholar]
- Piaget J. The origins of intelligence in children. New York: International Universities Press; 1952. [Google Scholar]
- Schmidt RA, Lee TD. Motor control and learning: A behavioral emphasis. 3. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- Shirley MM. The first two years: A study of twenty-five babies. Westport, CT: Greenwood Press; 1931. [Google Scholar]
- Siegler RS. The rebirth of children’s learning. Child Development. 2000;71:26–35. doi: 10.1111/1467-8624.00115. [DOI] [PubMed] [Google Scholar]
- Smith LB, Yu C, Pereira AF. Not your mother’s view: the dynamics of toddler visual experience. Developmental Science. 2011;14:9–17. doi: 10.1111/j.1467-7687.2009.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH. Variation in children’s exploratory, nonsymbolic, and symbolic play: An explanatory multidimentional framework. In: Rovee-Collier CK, Lipsitt LR, editors. Advances in infancy research. Vol. 10. Westport, CT: Ablex; 1996. pp. 37–78. [Google Scholar]
- Thelen E. Treadmill-elicited stepping in seven-month-old infants. Child Development. 1986;57:1498–1506. [PubMed] [Google Scholar]
- Urieli D, MacAlpine P, Kalyanakrishnan S, Bentor Y, Stone P. On optimizing interdependent skills: A case study in simulated 3D humanoid robot soccer. The 10th International Conference on Autonomous Agents and Multiagent Systems. 2011;2:769–776. [Google Scholar]
- Wohlwill JP. The age variable in psychological research. Psychological Review. 1970;77:49–64. [Google Scholar]
- Zelazo PR. The development of walking: New findings on old assumptions. Journal of Motor Behavior. 1983;2:99–137. doi: 10.1080/00222895.1983.10735292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplots of walking age by (A–B) standard measures of walking skill obtained during periodic gait over the gait carpet and (C–F) functional measures of walking skill obtained during natural locomotion during free play. Circles denote infants observed in the laboratory playroom, crosses denote infants observed at home.
Raster plot of distribution of walking bouts in 60 infants observed over 30 minutes of natural walking. Each row represents one infant. Rows were ordered by walking age.
The width of each vertical line represents the duration of the walking bout. The thinnest lines denote bout that were ≤ 6 s in duration (minimum bout duration that could be represented given the resolution of the image).