Abstract
Purpose
We reviewed the current literature on mechanisms involved in the pathogenesis of prostatitis/chronic pelvic pain syndrome (CPPS).
Materials and Methods
A literature review for the years 1966 to 2003 was performed using the MEDLINE database of the United States National Library of Medicine.
Results
National Institutes of Health categories I and II prostatitis result from identifiable prostatic infections, whereas patients with category IV are asymptomatic. The majority of symptomatic cases are category III or chronic prostatitis (CP)/CPPS. The etiology of CP/CPPS is unknown. The traditional marker of inflammation, namely white blood cells in prostatic fluids, does not correlate with the predominant symptom of pelvic pain. An imbalance toward increased proinflammatory and decreased anti-inflammatory cytokines has been implicated and a few studies have shown some correlation of this with pelvic pain. The imbalance in some men may result from polymorphisms at the cytokine loci. An autoimmune process may be involved and experimental evidence indicates that this can be under hormonal influence. Recent findings include possible defects in the androgen receptor. The prostate may not even be the source of the symptoms. Pelvic pain also correlates with the neurotrophin nerve growth factor implicated in neurogenic inflammation and central sensitization. Finally, psychological stress may produce measurable biochemical changes and influence the other processes. The role of normal prostatic bacterial flora in inciting the inflammatory response has also been reconsidered.
Conclusions
The symptoms of CP/CPPS appear to result from an interplay between psychological factors and dysfunction in the immune, neurological and endocrine systems.
Keywords: prostate, prostatitis, pelvic pain, cytokines, nerve growth factor
The most recent National Institutes of Health (NIH) classification of prostatitis adopted in 1995 includes several clinical entities, ranging from acute or chronic bacterial infections, chronic pelvic pain syndrome (CPPS) and even asymptomatic inflammation of the prostate.1 The traditional classification of prostatitis included acute prostatitis, chronic bacterial prostatitis, chronic nonbacterial prostatitis and prostatodynia.2 In the current system categories I and II prostatitis reflect acute and chronic bacterial prostatitis, respectively. Together the 2 categories account for no more than 5% to 10% of all cases.3 These cases are clearly associated with bacterial infection and they have a urine culture that yields uropathogens. Acute prostatitis is characterized by the sudden onset of fever and dysuria, whereas chronic bacterial prostatitis typically involves relapsing episodes of urinary tract infections, usually with the same organism seen on urine cultures. These patients are usually asymptomatic between infections. Category IV prostatitis refers to asymptomatic inflammatory prostatitis that is diagnosed incidentally during evaluation for infertility, elevated prostate specific antigen or benign prostatic hypertrophy.
The most common type of prostatitis is category III, also known as chronic prostatitis (CP)/CPPS. The current NIH definition of CP/CPPS includes genitourinary pain with or without voiding symptoms in the absence of uropathogenic bacteria, as detected by standard microbiological methods, or another identifiable cause such as malignancy.1 The accepted research definition is that of chronic pelvic pain for at least 3 of the preceding 6 months in the absence of other identifiable causes.4 CP/CPPS is divided into IIIA and IIIB. IIIA refers to the presence of any number white blood cells in the semen, post-prostate massage urine specimen (VB3) or expressed prostatic secretions (EPS). This corresponds to the previously used classification of nonbacterial prostatitis. Category IIIB is comparable to the formerly used term prostatodynia and it refers to patients with pelvic pain but no evidence of inflammation in the semen, VB3 or EPS.
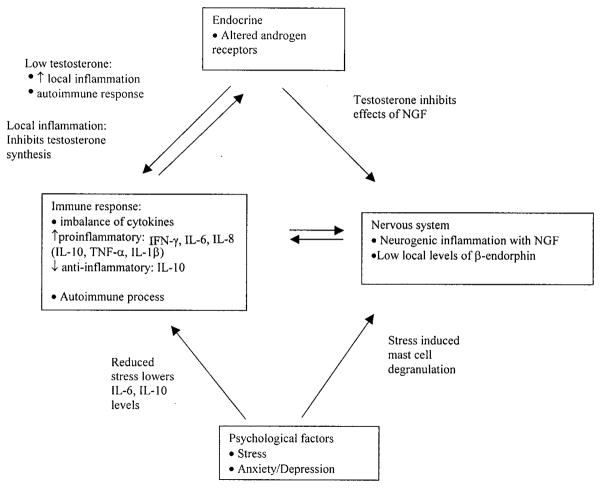
Many different etiologies and mechanisms of pathogenesis of CP/CPPS have been proposed. They suggest a role for immunological, neurological, endocrine and psychological factors. We examined the data supporting the role of each of these areas and also examined the possible interrelationship of these factors in producing the symptoms of CP/CPPS. The figure shows the factors involved and their possible relationships. The impact of these findings on normal prostatic bacterial flora in inciting the inflammatory response is also considered. Taken as a whole the published literature does not directly implicate the prostate as the only source of symptoms in CP/CPPS.
Figure.
Interplay of immunological, endocrine, neurological and psychological factors in development of CP/CPPS and proposed mechanisms
MATERIALS AND METHODS
A literature review for 1966 to 2003 was performed using the MEDLINE database of the United States National Library of Medicine. The keyword prostatitis was cross-referenced to the key words cytokines, inflammation, brady-kinins, prostaglandins, substance P, pain, pelvic pain, complex regional pain syndromes, calcitonin gene-related peptide, neural plasticity, neuralgia, nerve growth factor and autoimmunity. Priority was given to articles that correlated specific molecular and cellular abnormalities to clinical symptoms.
IMMUNOLOGICAL/INFLAMMATION
One of the difficulties in determining the mechanisms of prostatitis is that, whereas categories I and II prostatitis are caused by bacteria, including Escherichia coli, Klebsiella, Enterobacter and Pseudomonas,5 the majority of cases are category III, for which the etiology and pathogenesis are unknown. Studies to date have failed to identify an ongoing infection in these men from any sexually transmitted organisms, including Chlamydia trachomatis, Ureaplasma urea-lyticum, Mycoplasma hominis or Trichomonas vaginalis.5 The use of molecular techniques to look for uropathogenic bacteria in CPPS was recently summarized.6 The starting point for investigation into the pathogenesis of CP/CPPS has been inflammation. Traditionally white blood cells (WBCs) in prostatic fluids have been studied and thought to be markers for an inflammatory process that contributes to the symptoms of prostatitis. The use of WBCs as markers of inflammation is limited for several reasons. WBCs can be found in the prostatic fluid or seminal plasma of asymptomatic men as well as in that of men with pelvic pain.7, 8 Also, in symptomatic men none of the measures of the NIH-CP Symptom Index, including subsets for pain, urinary and quality of life, show any correlation with WBCs in EPS, VB3 or seminal plasma.9 Another argument against an association between inflammation and symptoms is that patients with category IIIB have symptoms but no inflammation and conversely those with category IV have inflammation but no symptoms.
Proinflammatory cytokines
Given the lack of correlation between WBCs and symptoms, other inflammatory markers and mediators have been studied. Cytokines are soluble signaling molecules that are produced from leukocytes as well as endothelial, epithelial and several other cell types. They act locally over short cellular distances as initiators and modulators of immune and inflammatory responses. In regard to proinflammatory cytokines increased concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-1β were reported in seminal plasma in 2 studies,10, 11 while another study showed no significant differences in men with CP/CPPS compared with controls.12 IL-1β is a proximal cytokine acting on leukocytes to augment the production of additional cytokines, while TNF-α is induced by bacterial proteins, viruses and fungal antigens, providing host defense.13 IL-1α, which has only 26% homology to IL-1β but activates the same high affinity receptor, has not been shown to be greater in the seminal fluid of men with CPPS vs that of controls.12, 14 Increased concentrations of TNF-α and IL-1 β have been reported in EPS in category IIIA as opposed to IIIB cases and controls. EPS in patients with category IV prostatitis, or asymptomatic inflammation, also contain increased concentrations of these 2 cytokines.15
Concentrations of IL-2 are not detectable in patients with CP/CPPS.11 IL-2 is secreted by T lymphocytes stimulated by antigen activated antigen presenting cells, resulting in T-cell clonal proliferation.16 IL-6 is involved in T-cell activation, growth and differentiation, and it also induces IL-2 receptor expression in T cells. IL-6 has been reported to be significantly increased in seminal plasma in IIIa and IIIb cases of CPPS compared with the control group.11 Interferon (IFN)-γ is also elevated in seminal plasma in men with CPPS compared with controls.17 IL-8 was measured and found to be significantly higher in concentration in men with CP/CPPS in seminal plasma11, 12 and EPS18 compared with controls, while 1 study showed no difference in IL-8 in seminal plasma between these groups.17 EPS concentrations of epithelial neutrophil activating factor-78 (ENA-78) were also significantly increased, not only in men with CP/CPPS, but also in those with category IV compared with controls.18 IL-8 and ENA-78 are chemotactic factors involved in the recruitment and activation of neutrophils at an inflammatory site and the 2 cytokines correlate with the number of WBCs in EPS. IL-8 also recruits and activates lymphocytes.19
One important test for the role of proinflammatory cytokines in the pathogenesis of CP/CPPS is the correlation of their concentrations to symptoms. In other systems proinflammatory cytokines are involved in nociception.20 In 1 study of category IIIb cases serum and seminal plasma IL-6 increased initially and then decreased, correlating with the release of clinical symptoms.14 In another study IL-6 correlated inversely with pain.17 Static measurements of TNF-α, IL-1β, IL-8 and ENA-78 do not correlate with symptoms. However, proinflammatory cytokine concentrations in EPS can change in association with changes in symptoms and concentrations can also decrease after antibiotic therapy regardless of changes in symptoms or inflammatory status.21 A consideration in this finding is that antibiotics can directly decrease cytokine levels independent of their antibacterial actions.22 These findings raise some doubt about the association of proinflammatory cytokine concentrations and the processes producing pelvic pain.
Another way to try to establish the role of proinflammatory cytokines in men with CP/CPPS and the role of inflammation is to compare their levels to those in men with category IIIA and IIIB, and asymptomatic control subjects. Tables 1 and 2 show such comparisons. About half of the proinflammatory cytokines measured in various fluids are reported to be significantly greater in concentration in patients with category IIIA vs IIIB (table 1). Several cytokines studied have been found to be significantly elevated in 1 fluid such as EPS but not in another such as semen or vice versa. When comparing patients with IIIA and asymptomatic patients with prostatic inflammation (category IV) proinflammatory cytokines have not been found to be increased in patients with inflammation and symptoms compared with those with inflammation only. About half of the proinflammatory cytokines were reported to be increased in patients with category IIIB compared with asymptomatic controls (table 2). Overall this analysis indicates that proinflammatory cytokines may be associated with inflammation but they may also be present in patients without WBCs in prostatic fluids and they do not distinguish between symptomatic and asymptomatic individuals.
Table 1.
Cytokine levels in categories IIIA vs IIIB and vs IV
| Fluid | Significantly Increased (p <0.05) | References |
|---|---|---|
| IIIA vs IIIB | ||
| IL-1β: | ||
| VB3 | Yes | Nishimura et al23 |
| Semen | No | Orhan et al11 |
| EPS | Yes | John et al14 |
| IL-1ra VB3 | No | Nishimura et al23 |
| IL-6 Semen | Yes | Orhan et al11 |
| IL-8: | ||
| Semen | No | Orhan et al11 |
| EPS | Yes | Miller et al17 |
| TNF-α: | ||
| Semen | No | Orhan et al11 |
| EPS | Yes | John et al14 |
| ENA-78 EPS | No | Miller et al17 |
| IIIA vs IV | ||
| IL-1β EPS | No | John et al14 |
| IL-8 EPS | No | Miller et al17 |
| TNF-α EPS | No | John et al14 |
| ENA-78 EPS | No | Miller et al17 |
Table 2.
Cytokine levels in category IIIB vs asymptomatic controls
| Fluid | IIIB vs Controls Significantly Increased (p <0.05) | References |
|---|---|---|
| IL-1ra VB3 | Yes | Nishimura et al23 |
| IL-1α semen | No | Opree and Kress20 |
| IL-1β: | ||
| Semen | Yes | Orhan et al11 |
| VB3 | No | Nishimura et al23 |
| EPS | No | John et al14 |
| IL-2 semen | No | Opree and Kress20 |
| IL-6: | ||
| Semen | Yes | Opree and Kress20 |
| Semen | No | Orhan et al11 |
| IL-8: | ||
| Semen | Yes | Orhan et al11 |
| EPS | No | Miller et al17 |
| TNF-α: | ||
| Semen | Yes | Orhan et al11 |
| EPS | No | John et al14 |
| ENA-78 EPS | No | Miller et al17 |
Anti-inflammatory cytokines
Instead of increases in proinflammatory cytokines another proposed mechanism is low concentrations of endogenous cytokine inhibitors. IL-1 receptor antagonist (IL-1ra) binds with high affinity to IL-1 receptors and it is an endogenous, competitive inhibitor of IL-1. In CP/CPPS IL-1 and IL-1ra concentrations in EPS are elevated in category III prostatitis but there is no evidence of low IL-1ra in relation to IL-1 to contribute to ongoing inflammation.23 Recent evidence points to IL-10 regulation as a potential contributing factor in CPPS. IL-10 is produced by monocytes, macrophages, T0, T1, T2 and B lymphocytes, mast cells, eosinophils, keratinocytes and various tumor cells. Although it is generally considered to be anti-inflammatory, IL-10 is pleiotropic and it can show immunosuppressive effects, such as decreased TNFα, IL-1α, IL-2, IL-6, IL-8 and IFN-γ, or immunostimulatory effects, such as increased B-cell proliferation and antibody production, increased proliferation, activation and chemotaxis of CD8+ T cells, and increased IL-2 induced natural killer cell cytotoxicity.24
Differences in the DNA sequence, or polymorphisms, have been identified in the promoter regions of several cytokines. These differences in DNA sequence of the promoter region result in different degrees of cytokine production in response to a given stimulus.25 The IL-10 AA genotype is associated with low IL-10 production.26 In a recent study significantly more men with CPPS expressed the IL-10 AA genotype compared with controls (11 of 36 or 31% vs 33 of 272 or 12%, p = 0.007).27 All 8 patients with IIIa had the low TNF-α production genotype. There was no difference in the TNF-α genotype in 22 patients with IIIb vs 272 controls but all 8 patients with IIIa had the low TNF-α genotype. Cytokine polymorphisms also correlated with the clinical response to treatment with the antioxidant quercetin. All 11 of the 28 patients with type III treated with quercitin in whom therapy failed had the low TNF-α genotype vs 5 of the 17 (29%) with a favorable clinical response to quercetin therapy (p = 0.0003). Only 1 of the 11 patients who failed treatment had the low IL-10 genotype vs 8 of the 17 (47%) patients who had a beneficial therapeutic response to quercetin therapy (p = 0.04). Another report showed that the seminal plasma IL-10 concentration is increased 2.4 fold in men with CP/CPPS compared with asymptomatic controls and these concentrations correlate with pain severity.17
These findings at first appear to be at odds with each other with one indicating a lower capacity to produce IL-1027 and the other indicating increased local concentrations of IL-10.17 However, they may be consistent with several pathways in which inflammation could be generated in men with CP/CPPS. Given the findings of alleles for IL-10 associated with low production of this cytokine, some men with CPPS may have a blunted IL-10 response to inflammation. TNF-α induces IL-10 production,28 and so in men with category IIIa prostatitis low concentrations of TNFα may lead to decreased IL-10, which would lead to less inhibition of IL-8 and, thus, increased WBC chemotaxis. Some men may have low IL-10 primarily based on the allele for IL-10 irrespective of TNF-α levels. Low systemic IL-10 concentrations may have several effects, including failure to suppress inflammation. Sustained proinflammatory cytokines could lead to tissue injury. IL-10 is a powerful suppressant of monocyte-macrophage function, inhibiting the production of proinflammatory cytokines such as TNF-α, IL-1, IL-6 and IL-8, and down-regulating macrophage production of nitric oxide.24 IL-10 also inhibits monocyte prostaglandin H synthase, which is needed for monocyte matrix metalloproteinase activity, which has been implicated in the extensive tissue destruction seen in chronic inflammation.24 IL-10 down-regulates the production of the IFN-γ, IL-2 and TNF-β produced by T lymphocytes, thereby, suppressing cell mediated and delayed type hypersensitivity reactions, and inhibiting T-cell proliferation.29 The concept of a lack of immune suppression leading to chronic inflammation and pain in these men is supported by the observation that a man with CP/CPPS who was immunosuppressed after renal transplantation experienced the resolution of pelvic pain.30 The effect of low levels of anti-inflammatory factors is seen in polymyalgia rheumatica, in which increased serum IL-10 correlates with a lower incidence of symptoms.31
The findings of high IL-10 concentrations in seminal plasma as opposed to serum may be a marker of local inflammation.17 In seminal plasma IL-10 concentrations correlate with concentrations of IFN-γ, which is involved in chronic inflammation.17 High IL-10 concentrations can also enhance B-cell proliferation, differentiation and antibody production32 and, therefore, could also contribute to a local prostate tissue autoimmune response. High concentrations of IL-10 have been seen in some other poorly explained pain syndromes believed to have an autoimmune component, including myasthenia gravis, Graves’ disease, Sjögren’s syndrome, polymyositis, rheumatoid arthritis and dermatomyositis.24
A limitation to measuring cytokines may be the fluids in which they are measured. Assays for cytokines that have been validated in fluids such as serum and tissue culture medium are being used to assay cytokine concentrations in other fluids such as expressed prostatic secretions and seminal plasma. To date none of these reports have provided a validation of the assay method in EPS or seminal plasma. At the minimum this would entail adding the purified cytokine to the clinical specimen and verifying that the assayed concentration increases accordingly. Until this is done it is possible that cytokine determinations in these fluids may be falsely high or falsely low. Variation in patient symptoms from day to day may also be reflected in variations in cytokine levels in that individual: Another limitation may be an inability to collect certain specimens such as EPS or semen from a given patient.
Autoimmunity
An autoimmune basis for CP/CPPS is a prominent theory for the etiology/pathogenesis of CP/CPPS. Animal models have been used to look at autoimmunity. In rats immunization with male accessory gland, a mixed organ homogenate of ventral, dorsal and lateral prostate, and coagulating gland, promotes experimental autoimmune prostatitis.33 This is characterized by a specific cell mediated response with CD4+ and CD8+ T-cell infiltrates, no circulating antibodies and the expression of major histocompatibility complex class II molecules on prostatic epithelial cells, which are normally only expressed by lymphocytes.34 Male accessory gland sensitized T cells are capable of expanding and differentiating by themselves in response to homogenates of only the prostate35 and immunization with only rat ventral prostate produces lymphocytic infiltration of the stroma and periglandular region of the dorsal and lateral lobes only.36 There are parallel observations in humans to suggest a similar process. Markers for cytotoxic T cells are found in the EPS of men with CP/CPPS, a cell type not typical of antimicrobial immunity but more consistent with autoimmune inflammation or secondary remodeling of injured tissue.37 T cells from some men with CP/CPPS show proliferation responses to seminal antigens obtained from normal asymptomatic men or men with seminal vesicle atresia, while this was not seen in controls who donated seminal plasma.38 Further studies showed a proliferative response of T cells to prostate specific antigen in 5 of 14 patients and in none of the controls, whereas there was no response to β microseminoprotein and prostatic acid phosphatase.39 In addition to responding to seminal plasma from other individuals, men with CPPS also have greater a T-cell proliferation response to their seminal plasma than controls.40 These studies lend support to an autoimmune hypothesis. A 9% incidence of intraprostatic spermatozoa has been described at autopsy, mainly in the peripheral zone.41 Therefore, since nonprostatic substances such as spermatozoa can be present in the prostate, it is entirely possible that the target antigen of the autoimmune response may not even be of prostatic origin.
Prostate histology
It is helpful to look at histological specimens from biopsies of the prostate of men with CP/CPPS to see if findings confirm those in animal studies and human studies of inflammatory mediators in prostatic fluids. A correlation to date has been findings in transrectal biopsies of what would now be classified as patients with category IIIa CPPS, in whom mononuclear cell infiltrates were found in the periglandular and stromal tissue in 40 of 50 biopsied.42 The same inflammatory infiltration is seen in histological sections of rat models of autoimmune prostatitis.33 This correlation is limited somewhat because in the human study the diagnosis was not based on pelvic pain but on the presence of 10 leukocytes per high power field in EPS or 5 per high power field in VB3. Additional studies in a similarly selected group of patients also showed IgA and IgM deposition along with C3 and fibrinogen in patients with prostatitis but not in biopsies from a group of asymptomatic controls who also underwent biopsy.43 In IIIb cases biopsies have shown intra-acinar T cells dominated by cytotoxic T cells.14 Another biopsy study raises a different issue. Prostatic inflammation was found in only 33% of patients with CP/CPPS who underwent transperineal prostate biopsy.44 A limitation of the perineal approach is that the peripheral zone may not be accessed as it is in transrectal biopsies. Nonetheless, the findings raise the question of whether the prostate is even actually involved in the symptoms of CP/CPPS.
ENDOCRINE
An important factor in the development of prostatitis may be sex hormones. Male Wistar rats have spontaneous auto-immune inflammation of the lateral prostate with age, which is hormone dependent. In this model all old Wistar rats treated with estrogen for 30 days had histological evidence of lateral prostate inflammation.45 Some mechanisms of the estrogen effect are an increase in mRNA transcripts of TNF-α, IL-1β, IL-6, MIP-2 and inducible nitric oxide synthase.46 Studies have also shown that co-administration of testosterone with estradiol prevented estrogen induced prostatitis, while dihydrotestosterone (DHT) was less effective.45 This suggests that testosterone must exert an independent role on the prostate aside from conversion to DHT.
Steroid hormones may be also be affected locally by the inflammation. In models of autoimmunity there is evidence of chemotactic cytokines such as TNF-α and IFN-γ altering the surface of endothelial cells, favoring mononuclear cell homing and infiltration, thus, leading to decreased steroid hormone production.47 In the rat model of autoimmune prostatitis inflammation impairs 5α reductase activity and lowers the intraprostatic levels of DHT relative to testosterone.48 Given the effects of testosterone on prostate inflammation, this may be a mechanism to limit prostatic inflammation. This observation may be the basis of the possible beneficial clinical effect seen with the use of finasteride for CPPS, which inhibits the conversion of testosterone to DHT and may increase local testosterone levels.49 Recent findings on the genetics of patients with CPPS bolster the theory that there may be an underlying problem with androgens in prostatitis. Differences have been reported in the frequency of 3 alleles near the phosphoglycerate kinase gene between patients with CPPS and controls.50 The alleles differed in the number of short tandem repeats. The phosphoglycerate kinase 1 gene in the region assessed has been found to be associated with familial prostate cancer, hypospadias and androgen insensitivity. Another gene in the same region of the X chromosome, Xq11-Xq13, is the androgen receptor. This finding raises the possibility of androgen insensitivity or dysfunction in the pathogenesis of CPPS. Tandem repeats, such as these found in men with CP/CPPS, were found to function frequently in genes encoding membrane associated proteins, such as plasma membranes, synapses, mitochondrial membranes and nuclear envelopes.51 To our knowledge whether there is a defect in the interaction between androgen receptor and membranes such as the nuclear envelope in men with CP/CPPS remains to be determined. Overall these findings lead to the possibility that if testosterone protects against inflammation, as seen in animal models, androgen insensitivity may lead to prostatic inflammation.
NEUROLOGICAL
The fact that these men have pain indicates some neurological involvement on a local level or in the central nervous system. Inflammation alone may alter the local environment. It is known that immune cells produce β -endorphin, an endogenous opioid, at sites of inflammation or tissue injury to decrease pain and the inflammatory marker prostaglandin E2 (PGE2) is known to inhibit β -endorphin.52 In a group of men with CP/CPPS PGE2 concentrations in prostatic fluid were 4 to 6 times higher than in asymptomatic controls.53 After treatment with an antibiotic or antioxidant β-endorphin concentrations were significantly higher and PGE2 concentrations were significantly lower, indicating a role for oxidative stress.
The pain of CP may also be a result of neurogenic inflammation in the peripheral and central nervous systems. Experimental evidence for central remodeling is provided by the finding that chemical irritation of the rat prostate and bladder causes c-fos expression at spinal cord levels L6 and S1 along with plasma extravasation in the skin at the identical L6 and S1 dermatomes, underscoring the overlap of afferent nerve fiber distribution.54 One of the hallmarks of such remodeling or windup is neurogenic inflammation. In the Wistar rat model of spontaneous prostatitis with age increased nerve fiber density, the sensory neuropeptide calcitonin gene-related peptide and evidence of progressive mast cell degranulation are noted at progressive ages.55 One of the products released from activated mast cells is nerve growth factor (NGF).56
The importance of NGF is that it is one of the few factors that correlates with pain in CP/CPPS.57 NGF is a neurotrophin that has been found to have a role in the regulation of nociceptive nerves, and as a mediator and amplifier of neurogenic inflammation. NGF regulates the sensitivity of adult sensory neurons to capsaicin, which excites C-mechano heat receptors.58 These C-fibers are sensory nerves associated with pain transmission and they also innervate mast cells.56 NGF is also a potent stimulator of mast cells and it can cause their degranulation.59 Released substances lead to neurogenic inflammation and then sensitize C-fibers. After it is released NGF can also bind the receptor on sensory neurons to be transported retrograde to the cell body, where it can lead to a rapid and large increase in the production of neuropeptides, such as substance P and calcitonin gene-related peptide at the level of gene expression.60 Concentrations of NGF in damaged or inflamed tissue have been shown to increase many-fold above normal.61 Increased concentrations of NGF in a peripheral target can sensitize central neurons to afferent barrages from that target and through enhanced neurotransmission mediated by the NMDA receptor it can produce long lasting depolarizations.62 Given the role of NGF in neurogenic inflammation and nociception, and its correlation with pain in CPPS, it is likely that neurogenic inflammation is involved in the pathogenesis of CP/CPPS symptoms.
The endocrine and immune systems can also have a role in neurogenic inflammation. Testosterone can have a negative effect on NGF. All rat pelvic noradrenergic neurons express the NGF receptors trkA and p75.63 NGF induces neurite growth in these neurons. In vitro testosterone impeded the NGF induced growth of long neurites from pelvic ganglion cells cultured from adult male rats.64 Many cytokines and growth factors, including TNF-α, IL1-β, transforming growth factor-α and transforming growth factor-β 1 can up-regulate NGF production.65 Mast cells may also act as antigen presenting cells66 that degranulate after contact with T cells and, thus, activate nearby nerve fibers and release NGF. The immune system also has a role in mast cell survival. In the short term IL-10 and IL-4 promote mast cell survival but long-term exposure of mast cells to IL-3, IL-4 and IL-10 induces down-regulation of critical mast cell proteins, such as the stem cell factor receptor Kit and the high affinity IgE receptor Fc;RI, which is followed by mast cell apoptosis.67 Thus, in individuals with low IL-10 there may be decreased mast cell apoptosis, greater numbers of mast cells and a higher chance of neurogenic inflammation.
PSYCHOLOGICAL FACTORS
In addition to the interplay between the immune, endocrine and nervous systems, psychological factors also appear to be involved in producing symptoms in men with CP/CPPS. Psychological stress is a common finding in men with CPPS.68 When assessing quality of life with the 12 item short form instrument,69 the mental component summary score in patients with CPPS is lower than that observed in the most severe subgroups with congestive heart failure and diabetes.70 There are cellular and molecular changes induced by psychological difficulties and stress that may have a role in these men. In addition to many other stimuli such as cytokines, bacterial toxins and hypoxia, mast cells release their contents in response to stress.71 In patients with CPPS there appear to be some direct, measurable effects of stress or stress decreases on cytokine levels. In patients with category III prostatitis the degree of spousal concern and support as well as an effort to distract the patient from symptoms correlates with lower seminal plasma IL-6 and IL-10 concentrations.57 Also, patients with depression have been shown to have lower levels of IL-10 in peripheral blood mononuclear cells than controls.72
ROLE OF NORMAL PROSTATE BACTERIAL FLORA?
Given available data on the possible molecular mechanisms involved in CP/CPPS, it is interesting to revisit the question of the role of bacteria in this disorder. It is unlikely that there is ongoing acute infection. Given the findings of cultures localizing uropathogenic bacteria to the prostate in 8% of asymptomatic men and prostatic localization of what are considered to be nonuropathogens in 74%,8 a compelling question becomes, why is there not an ongoing prostate infection in the majority of men? An interesting analogy is in the gastrointestinal system. IL-10 deficient mice have chronic enterocolitis by age 2 to 3 months. This is an aberrant immune response to ordinary enteric antigens due to an uncontrolled, cell mediated immune response.73 Patients with irritable bowel syndrome have a lower incidence of the high producer genotype for IL-10 than controls.74 In men with CPPS does dysregulation of proinflammatory and anti-inflammatory cytokines lead to inflammation from otherwise normal prostate bacteria? Another marker of inflammation is reactive oxygen species (ROS). Neutrophils release ROS-free radicals (O2·, HO· and water2) in response to antigenic stimulation. As a marker of tissue injury secondary to pathogenic bacterial infection, oxidative stress in EPS was studied with the premise that infection by gram-positive bacteria in category IIIa, not just prostatic colonization, results in oxidative stress since tissue injury by definition follows infection and not colonization. Thus, after antibiotic treatment for infection oxidative stress would be decreased, proving gram-positive bacteria to be true pathogens. Less ROS minimizes tissue injury and ultimately leads to less pain. Elevated concentrations of ROS were found in the EPS of patients with CPPS with less subsequently detected after clinically successful treatment with oral antibiotics or the antioxidant quercetin, supporting the hypothesis.37 The presence of cytotoxic T cells in patient EPS also led to speculation that bacterial superantigens may have a role. Superantigens are proteins that form complexes with major histocompatibility complex class II molecules on antigen presenting cells, leading to T-cell proliferation.75 Those found on gram-positive bacteria can directly stimulate the clonal expansion of cytotoxic T cells.75
CONCLUSIONS
A significant limitation to defining the molecular mechanisms of CP/CPPS is that the cause of the condition is largely unknown. Current thinking points to an inciting event, be it infection or trauma, which leads to inflammation that becomes chronic. The event may be an abnormal response to otherwise normal flora in the prostate. The standard of WBCs as a marker for inflammation appears limited given the preponderance in symptomatic and asymptomatic individuals. However, other molecules involved in inflammation appear to be more sensitive markers to determine clinical status. The inflammatory reaction appears to involve the interplay between immunological, endocrine, neurological and psychological factors to produce pelvic pain and associated symptoms. The figure shows this relationship among possible mechanisms. To what degree these factors interact in a given patient and to what degree there is a common pathway or several pathways that lead to the end point of pelvic pain remains to be determined.
References
- 1.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 2.Drach GW, Fair WR, Meares EM, Stamey TA. Classification of benign diseases associated with prostatic pain: prostatitis or prostatodynia? (letter to the editor) J Urol. 1978;120:266. doi: 10.1016/s0022-5347(17)57135-9. [DOI] [PubMed] [Google Scholar]
- 3.de la Rosette JJ, Hubregtse MR, Meuleman EJ, Stolk-Engelaar MV, Debruyne FM. Diagnosis and treatment of 409 patients with prostatitis syndromes. Urology. 1993;41:301. doi: 10.1016/0090-4295(93)90584-w. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology. 1999;54:229. doi: 10.1016/s0090-4295(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 5.Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991;19:S119. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- 6.Krieger JN, Riley DE. Bacteria in the chronic prostatitis-chronic pelvic pain syndrome: molecular approaches to critical research questions. J Urol. 2002;167:2574. [PubMed] [Google Scholar]
- 7.Schaeffer AJ, Wendel EF, Dunn JK, Grayhack JT. Prevalence and significance of prostatic inflammation. J Urol. 1981;125:215. doi: 10.1016/s0022-5347(17)54976-9. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003;170:818. doi: 10.1097/01.ju.0000082252.49374.e9. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer AJ, Knauss JS, Landis JR, Propert KJ, Alexander RB, Litwin MS, et al. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J Urol. 2002;168:1048. doi: 10.1016/S0022-5347(05)64572-7. [DOI] [PubMed] [Google Scholar]
- 10.Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52:744. doi: 10.1016/s0090-4295(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 11.Orhan I, Onur R, Ilhan N, Ardicoglu A. Seminal plasma cytokine levels in the diagnosis of chronic pelvic pain syndrome. Int J Urol. 2001;8:495. doi: 10.1046/j.1442-2042.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruggieri MR, Sr, Braverman AS, Filer-Martin S, Gaughan JP, Pontari MA the NIH Chronic Prostatitis Clinical Research Network. Biochemical markers for inflammation and glands that contribute to the semen in chronic prostatitis patients. J Urol. 2000;163(suppl):26, abstract 112. [Google Scholar]
- 13.Mestan J, Digel W, Mittnacht S, Hillen H, Blohm D, Moller A, et al. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature. 1986;323:816. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- 14.John H, Barghorn A, Funke G, Sulser T, Hailemariam S, Hauri D, et al. Noninflammatory chronic pelvic pain syndrome: immunological study in blood, ejaculate and prostate tissue. Eur Urol. 2001;39:72. doi: 10.1159/000052415. [DOI] [PubMed] [Google Scholar]
- 15.Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, et al. IL-1β and TNF-α in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000;164:214. [PubMed] [Google Scholar]
- 16.Gesbert F, Delespine-Carmagnat M, Bertoglio J. Recent advances in the understanding of interleukin-2 signal transduction. J Clin Immunol. 1998;18:307. doi: 10.1023/a:1023223614407. [DOI] [PubMed] [Google Scholar]
- 17.Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, et al. Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. J Urol. 2002;167:753. doi: 10.1016/S0022-5347(01)69139-0. [DOI] [PubMed] [Google Scholar]
- 18.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, et al. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56:1025. doi: 10.1016/s0090-4295(00)00844-x. [DOI] [PubMed] [Google Scholar]
- 19.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Yarnold PR, Schaeffer AJ. Diagnostic value of serial cytokine changes in expressed prostatic secretions. J Urol. 2000;163(suppl):24, abstract 105. [Google Scholar]
- 22.Galley HF, Dhillon JK, Paterson RL, Webster NR. Effect of ciprofloxacin on the activation of the transcription factors nuclear factor kappaB, activator protein-1 and nuclear factor-interleukin-6, and interleukin-6 and interleukin-8 mRNA expression in a human endothelial cell line. Clin Sci. 2000;99:405. [PubMed] [Google Scholar]
- 23.Nishimura T, Abe H, Ito H, Ikeda K, Oka F, Yamamoto M. IL-1ra versus IL-1 levels in prostatic fluid from prostatitis patients. Urol Int. 1998;60:92. doi: 10.1159/000030218. [DOI] [PubMed] [Google Scholar]
- 24.Lalani I, Bhol K, Ahmed AR. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- 25.Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 26.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Shoskes DA, Albakri Q, Thomas K, Cook D. Cytokine polymorphisms in men with chronic prostatitis/chronic pelvic pain syndrome: association with diagnosis and treatment response. J Urol. 2002;168:331. [PubMed] [Google Scholar]
- 28.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853. [PubMed] [Google Scholar]
- 29.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palapattu GS, Shoskes DA. Resolution of the chronic pelvic pain syndrome after renal transplantation. J Urol. 2000;164:127. [PubMed] [Google Scholar]
- 31.Straub RH, Herfarth HH, Rinkes B, Konecna L, Gluck T, von Landenberg P, et al. Favorable role of interleukin 10 in patients with polymyalgia rheumatica. J Rheumatol. 1999;26:1318. [PubMed] [Google Scholar]
- 32.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacheco-Rupil B, Depiante-Depaoli M, Casadio B. Experimental autoimmune damage to rat male accessory glands. II. T cell requirement in adoptive transfer of specific tissue damage. Am J Reprod Immunol. 1984;5:15. doi: 10.1111/j.1600-0897.1984.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 34.Donadio AC, Depiante-Depaoli M. Inflammatory cells and MHC class II antigens expression in prostate during time-course experimental autoimmune prostatitis development. Clin Immunol Immunopathol. 1997;85:158. doi: 10.1006/clin.1997.4427. [DOI] [PubMed] [Google Scholar]
- 35.Casas-Ingaramo A, Depiante-Depaoli M, Pacheco-Rupil B. Activation of cytotoxic cells by syngeneic prostate antigens in experimental autoimmune vesiculo-prostatitis. Autoimmunity. 1991;9:151. doi: 10.3109/08916939109006751. [DOI] [PubMed] [Google Scholar]
- 36.Keetch DW, Humphrey P, Ratliff TL. Development of a mouse model for nonbacterial prostatitis. J Urol. 1994;152:247. doi: 10.1016/s0022-5347(17)32871-9. [DOI] [PubMed] [Google Scholar]
- 37.Shahed AR, Shoskes DA. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: correlation with gram positive bacterial growth and treatment response. J Androl. 2000;21:669. [PubMed] [Google Scholar]
- 38.Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50:893. doi: 10.1016/S0090-4295(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 39.Ponniah S, Arah I, Alexander RB. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate. 2000;44:49. doi: 10.1002/1097-0045(20000615)44:1<49::aid-pros7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Batstone GR, Doble A, Gaston JS. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS) Clin Exp Immunol. 2002;128:302. doi: 10.1046/j.1365-2249.2002.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson G, Culberson DE, Gardner WA., Jr Intraprostatic spermatozoa. Hum Pathol. 1988;19:541. doi: 10.1016/s0046-8177(88)80201-6. [DOI] [PubMed] [Google Scholar]
- 42.Doble A, Thomas BJ, Walker MM, Harris JRW, Witherow R, O’ N, Taylor-Robinson D. The role of Chlamydia trachomatis in chronic abacterial prostatitis: a study using ultrasound guided biopsy. J Urol. 1989;141:332. doi: 10.1016/s0022-5347(17)40758-0. [DOI] [PubMed] [Google Scholar]
- 43.Doble A, Walker MM, Harris JR, Taylor-Robinson D, Withrow RO. Intraprostatic antibody deposition in chronic abacterial prostatitis. Br J Urol. 1990;65:598. doi: 10.1111/j.1464-410x.1990.tb14827.x. [DOI] [PubMed] [Google Scholar]
- 44.True LD, Berger RE, Rothman I, Ross SO, Krieger JN. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J Urol. 1999;162:2014. doi: 10.1016/S0022-5347(05)68090-1. [DOI] [PubMed] [Google Scholar]
- 45.Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- 46.Harris MT, Feldberg RS, Lau KM, Lazarus NH, Cochrane DE. Expression of proinflammatory genes during estrogen-induced inflammation of the rat prostate. Prostate. 2000;44:19. doi: 10.1002/1097-0045(20000615)44:1<19::aid-pros3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 47.Diserio GP, Nowotny E. Experimental autoimmune prostatitis: in vivo induction of the autoimmune response to lymphocytic soluble factors. Alterations at the endocrine metabolism level. Am J Reprod Immunol. 1998;39:226. doi: 10.1111/j.1600-0897.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 48.Diserio GP, Carrizo AE, Pacheco-Rupil B, Nowotny E. Effect of male accessory glands autoaggression on androgenic cytosolic and nuclear receptors of rat prostate. Cell Mol Biol. 1992;38:201. [PubMed] [Google Scholar]
- 49.Nickel JC, Downey J, Pontari MA, Shoskes D, Zeitlin SI. A randomized placebo-controlled multicentre study to evaluate the safety and efficacy of finasteride for male chronic pelvic pain syndrome (category IIIA chronic nonbacterial prostatitis) BJU Int. 2004;93:991. doi: 10.1111/j.1464-410X.2003.04766.x. [DOI] [PubMed] [Google Scholar]
- 50.Riley DE, Krieger JN. X Chromosomal short tandem repeat polymorphisms near the phosphoglycerate kinase gene in men with chronic prostatitis. Biochim Biophys Acta. 2002;1586:99. doi: 10.1016/s0925-4439(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 51.Riley DE, Krieger JN. Diverse eukaryotic transcripts suggest short tandem repeats have cellular functions. Biochem Biophys Res Comm. 2002;298:581. doi: 10.1016/s0006-291x(02)02509-3. [DOI] [PubMed] [Google Scholar]
- 52.Vlaskovska M, Hertting G, Knepel W. Adrenocorticotropin and beta-endorphin release from rat adenohypophysis in vitro: inhibition by prostaglandin E2 formed locally in response to vasopressin and corticotropin-releasing factor. Endocrinology. 1984;115:895. doi: 10.1210/endo-115-3-895. [DOI] [PubMed] [Google Scholar]
- 53.Shahed AR, Shoskes DA. Correlation of β -endorphin and PGE2 levels in prostatic fluid of chronic prostatitis patients with diagnosis and treatment response. J Urol. 2001;166:1738. [PubMed] [Google Scholar]
- 54.Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA. Similarity of distributions of spinal c-fos and plasma extravasation after acute chemical irritation of the bladder and the prostate. J Urol. 2000;164:1751. [PubMed] [Google Scholar]
- 55.Keith IM, Jin J, Neal D, Jr, Teunissen BD, Moon TD. Cell relationship in a Wistar rat model of spontaneous prostatitis. J Urol. 2001;166:323. [PubMed] [Google Scholar]
- 56.Skaper SD. Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol. 2001;24:183. doi: 10.1385/MN:24:1-3:183. [DOI] [PubMed] [Google Scholar]
- 57.Miller LJ, Fischer KA, Goralnick SJ, Litt M, Burleson JA, Albertsen P, et al. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:603. doi: 10.1016/s0090-4295(01)01597-7. [DOI] [PubMed] [Google Scholar]
- 58.Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- 59.Mazurek N, Weskamp G, Erne P, Otten U. Nerve growth factor induces mast cell degranulation without changing intracellular calcium levels. FEBS Lett. 1986;198:315. doi: 10.1016/0014-5793(86)80428-8. [DOI] [PubMed] [Google Scholar]
- 60.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 61.Varilek GW, Weinstock JV, Pantazis NJ. Isolated hepatic granulomas from mice infected with Schistosoma mansoni contain nerve growth factor. Infect Immun. 1991;59:4443. doi: 10.1128/iai.59.12.4443-4449.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 63.Keast JR, Kepper ME. Differential regulation of trkA and p75 in noradrenergic pelvic autonomic ganglion cells after deafferentation of their cholinergic neighbours. Eur J Neurosci. 2001;13:211. [PubMed] [Google Scholar]
- 64.Meusburger SM, Keast JR. Testosterone and nerve growth factor have distinct but interacting effects on structure and neurotransmitter expression of adult pelvic ganglion cells in vitro. Neuroscience. 2001;108:331. doi: 10.1016/s0306-4522(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida K, Gage FH. Cooperative regulation of nerve growth factor synthesis and secretion in fibroblasts and astrocytes by fibroblast growth factor and other cytokines. Brain Res. 1992;569:14. doi: 10.1016/0006-8993(92)90364-f. [DOI] [PubMed] [Google Scholar]
- 66.Foreman JC. Peptides and neurogenic inflammation. Br Med Bull. 1987;43:386. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- 67.Shelburne CP, Ryan JJ. The role of Th2 cytokines in mast cell homeostasis. Immunol Rev. 2001;179:82. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 68.Mehik A, Hellstrom P, Sarpola A, Lukkarinen O, Jarvelin MR. Fears, sexual disturbances and personality features in men with prostatitis: a population-based cross-sectional study in Finland. BJU Int. 2001;88:35. doi: 10.1046/j.1464-410x.2001.02259.x. [DOI] [PubMed] [Google Scholar]
- 69.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 70.McNaughton Collins M, Pontari MA, O’Leary MP, Calhoun EA, Santanna J, Landis JR, et al. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16:656. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spanos C, Pang X, Ligris K, Letourneau R, Alferes L, Alexacos N, et al. Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol. 1997;157:669. [PubMed] [Google Scholar]
- 72.Borish L, Schmaling K, DiClementi JD, Streib J, Negri J, Jones JF. Chronic fatigue syndrome: identification of distinct subgroups on the basis of allergy and psychologic variables. J Allergy Clin Immunol. 1998;102:222. doi: 10.1016/s0091-6749(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 73.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 74.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kotb M. Superantigens of gram-positive bacteria: structure-function analyses and their implications for biological activity. Curr Opin Microbiol. 1998;1:56. doi: 10.1016/s1369-5274(98)80143-4. [DOI] [PubMed] [Google Scholar]