Abstract
Background
While increasing evidence suggests that cannabis use may play a role in the development of schizophrenia in some young people, less is known about the strength and specificity of its relationship to latent schizophrenia liability, i.e., schizotypal personality disorder traits.
Aims
Determine the predictive value of cannabis use during childhood and early adolescence on schizotypal personality disorder (SPD) symptoms projecting into adulthood, using a community-based longitudinal cohort from upstate New York.
Method
Prospective data from 804 participants was used to determine associations between early cannabis use and later schizotypal symptoms, accounting for important potential confounds (e.g., adolescent schizotypal symptoms).
Results
Cannabis use with onset prior to age 14 strongly predicted SPD symptoms in adulthood, independent of early adolescent SPD symptoms, major depression, anxiety disorder, other drug use, and cigarette use. There was no interaction effect of early cannabis use and early adolescent SPD symptoms on SPD symptoms into adulthood.
Conclusions
Our data provide further support for a strong association of early cannabis use with the development of symptoms characteristic of schizophrenia spectrum disorders. As with studies in schizophrenia, early SPD symptoms could not fully explain the association of early cannabis use with later schizotypal symptoms. The mechanisms that underlie the association of cannabis use and schizotypal symptoms in a developmental context deserve further exploration.
1. Introduction
Accumulating evidence suggests that cannabis use can lead to the development of schizophrenia in some young people (Arseneault et al., 2004; Fergusson et al., 2006; Moore et al., 2007; Murray et al., 2007; Large et al., 2011). However, definitive causality between cannabis use and the development of schizophrenia has not been established, as there is still some debate regarding possible confounders, including an underlying predisposition to psychosis or schizophrenia that may also increase the likelihood of cannabis use (Vedoux et al., 2003). Latent schizophrenia liability (i.e. schizotypy) includes disturbances which can be subtle signs of schizophrenia without overt psychosis (American Psychiatric Association, 2001), which may be influenced by or may influence cannabis use (Williams et al., 1996; Skosnik et al., 2001; Schiffman et al., 2004; Compton et al., 2007; Barkus and Lewis, 2008; Esterberg et al., 2009; Cohen et al., 2011; Genetic Risk and Outcomes (GROUP) Investigators, 2011; Rossler et al., 2011; Fridberg et al., 2011; Kuepper at al. 2011). A significant relationship between cannabis use, a risk factor implicated in schizophrenia, and schizotypal characteristics enhances the extent to which cannabis use is implicated as a risk factor in the schizophrenia spectrum. For example, Esterberg et al. (2009) demonstrated schizotypal symptoms were positively related to lifetime and recent cannabis use and Barkus and Lewis (2008) demonstrated college students high on schizotypy reported more psychotic-like symptoms before and after using cannabis.
The purpose of the present study is to determine the specificity and strength of the relationship between cannabis use and schizotypal traits and to determine whether this relationship persists into adulthood using a prospective longitudinal design. We addressed the main purpose of the study by focusing on the following three major objectives: 1) examine whether early cannabis use has predictive value for schizotypal symptoms (SPD) into adulthood, as opposed to having effects restricted to a specific developmental period (i.e. age-specific effects); 2) determine whether the relationship between adolescent cannabis use and schizotypal symptoms in adulthood is specific to cannabis users exhibiting higher levels of schizotypal symptoms in younger years and/or explained by early schizotypal symptoms; and 3) determine if associations between cannabis use and schizotypal symptoms could be accounted for by other factors, such as sex, anxiety and depression (Brooks et al., 1998; Verdoux et al. 2003), or use of other drugs, including tobacco (Zammitt et al, 2002; Macleod et al., 2004).
2. Methods
2.1. Participants and Procedures
Data for the present study was based on individuals from an epidemiological cohort of children (i.e., Children in the Community (CIC) study) randomly sampled from families living in upstate New York in 1975, who were subsequently repeatedly assessed for Axis I and II disorders. The first assessment of Axis I and II disorders was during the first follow-up in 1983 at mean age 13 (SD 2.6, age range 9–18). The present analyses are based on 804 youth who were followed up again at least twice in 1985–86 (mean age 16.4, SD=2.8); 1991–93 (mean age 22.4, SD=2.7); or in 2001–2004 (mean age 33.2, SD=2.8), and on whom we had self-reports of age of onset of cannabis use. This sample is 91% Caucasian and 51% male. Detailed information about this community sample is provided in several previous reports (e.g., Cohen and Cohen, 1996; Kasen et al. 2007; Skodol et al., 2007) and on the study Web site (http://nyspi.org/childcom). Consent was obtained for all interviews and a National Institute of Health (NIH) Certificate of Confidentiality exists for these data.
2.2. Assessments
Axis I symptoms and disorders of youth were determined using the Diagnostic Interview Schedule for Children (DISC; Costello et al., 1984), which was altered to increase appropriateness for adult respondents in the 1991–93 (mean age 22.4, SD=2.7) assessment (Cohen et al., 2007). In the 2001–4 assessment (mean age 33.2), the Structured Clinical Interview for DSM–IV (SCID-I/ NP; First et al., 1996) was employed. With regard to Axis II disorders, when disorders were first assessed in 1983, no standardized measure of personality disorders existed for the child and adolescent developmental stages. Accordingly, at that time, relevant items in the CIC study protocol were selected to correspond with DSM-III (American Psychiatric Association, 1980) criteria for Axis II disorders. Additional items taken from the Personality Diagnostic Questionnaire (PDQ; Hyler et al., 1990) and the Structured Clinical Interview for Personality Disorders (Spitzer and Williams, 1986) were adapted to make them age-appropriate and subsequently added to the protocol (Bernstein et al., 1993) (for a detailed history of personality disorder symptom scale development see (Crawford et al., 2005).
2.3. Measures
Schizotypal Personality Disorder Symptoms were assessed using the Children in the Community-Self-Report (CIC-SR) Schizotypal Personality Disorder (SPD) symptom scale (Crawford et al., 2005). This 16-item scale, which is based on self-report items in the CIC protocol, was created to measure the best possible dimensional estimates of schizotypal personality disorder in the CIC sample across all follow-up assessments. The 16 items in the scale provide full coverage of the DSM-IV criteria for schizotypal personality disorder and are measured on 4-point Likert scales. The internal consistency of the CIC-SR SPD symptom scale at mean age 33.2 was .65.
The convergent validity of the dimensional scores on the CIC-SR SPD symptom scale to diagnoses obtained using the SCID–II Personality Disorder Schizotypal Screen (First et al., 1995) was good (r = .47, p<.01). The predictive validity of the CIC-SR SPD symptom scale at mean age 22 to SCID-II clinical schizotypal diagnoses obtained at mean age 33 was fair (r = .26, p<.01; Crawford et al., 2005). The development of the scale is provided in detail elsewhere (Crawford et al., 2005). For the present study, the CIC-SR SPD symptom scale has been standardized over the trajectory of repeated assessments to enhance interpretability of effect sizes including annual mean changes. The scale for the 1983 assessment when the mean age for participants was 13.7 was used as an early adolescent control of schizotypal symptoms.
Cannabis use was first assessed at mean age 13.7 in 1983 and subsequently at mean ages 16.4, 22.4, and 33.2. As part of the DISC-I (Costello et al., 1984), youth participants were asked about prior marijuana use and age at first use. Responses to subsequent questions indicating at least monthly use were categorized as cannabis “users,” with those who have only experimented once or twice or who never used categorized as “non-users.”
The present analyses focus on youth-reported early cannabis use defined as onset before age 14 as reported in the initial, second, or third follow-up assessments. We chose onset prior to age 14 given previous findings that cannabis use occurring earlier during adolescence (e.g., <15; Arseneault et al., 2002) has a stronger predictive value for the development of schizophreniform disorder than use with later adolescent onset.
Covariates
Other Psychopathology used for these analyses included adolescent DSM-III-R anxiety disorders (overanxious, social phobia, and/or separation anxiety, N = 211) and major depressive disorder (N = 40). These disorders were derived from the parent and youth reports on the DISC-I. Other Drug Use included youth reports of nonmedical use of amphetamines, barbiturates, cocaine, heroin, LSD or other psychedelics, quaaludes, or tranquilizers of at least once a month (N = 40). Cigarette Use was determined with an affirmative youth response regarding amount of cigarette usage (N = 338).
Demographic control variables
Based on significantly higher mean schizotypal symptoms in males and in children with lower family socioeconomic status (SES) (Anglin et al., 2008), we controlled for sex and SES in all analyses. An index of SES which has been used in numerous previous CIC studies (e.g., Skodol et al., 2007) was computed as the standardized sum of standardized measures of years of maternal and paternal education, occupational status, and family income.
2.4. Data Analyses
Hierarchical linear regression (SAS PROC MIXED) was used to estimate the random (trajectory differences between participants) effects and the fixed (average) effects of predictors on these trajectories of schizotypal personality disorder (SPD) symptoms from ages 11 to 35 in 804 members of the cohort. Age was centered at age 23 (the mean age over the three follow-up assessments at mean ages 16.4, 22.4, and 33.2): thus the final equation intercept equals the standardized mean level of symptoms estimated at that age under the circumstances when all predictors in the equation are also centered. Initial analyses examined the prediction by early cannabis use of this trajectory. Subsequent analyses examined potential variables that may confound or modify these estimates.
3. Results
3.1. Descriptive Analyses
About 70% of participants (n=567) reported ever using cannabis at any point during the first three follow up assessments (mean ages 16.4, 22.4, and 33.2), of whom the majority reported experimental or rare use. In contrast, early cannabis use, defined as onset before age 14 with a regular frequency of use, was evident among only 17.1% (n=97) of all participants who ever tried cannabis at any age.
Early cannabis users were compared to the rest of the sample on relevant demographic and clinical variables. There was no significant difference between the number of males (58%) and females (42%) who were early cannabis users (X2=2.23, p>.05). Early users were significantly more likely than non-users to use other drugs (18.6% vs. 2.0%; X2=59.45, p<.001) and to smoke cigarettes (85.6% vs. 36%; X2=85.72, p<.001). Early cannabis users were also more likely than non-users to have had major depression (9.3% vs. 4.2%; X2=4.75, p<.05), but not an anxiety disorder (22.7% vs. 26.5%; X2=.65, p>0.05). A major issue for consideration is the extent to which schizotypal symptoms were already present among the early users. There was a trend difference between early cannabis users and non-users on SPD symptoms assessed at mean age 13.7 (p=.08). Early cannabis users exhibited a slightly higher mean level of SPD symptoms than non-users.
3.2. Descriptive Findings from Multi-level Analyses
The base model that gives annual age changes in standardized SPD symptoms was significantly negative but small per year over the nearly 2 decades covered, and linear. (See Table 2 for mean SPD symptom scores). No variable, including cannabis use significantly predicted differential annual age changes on schizotypal symptoms. Inspection of the level one data revealed that the across-participant covariance between the means and slopes of the SPD trajectory was not statistically significant, indicating that those with high averages over the years showed about the same pattern of decline as did those with lower averages. Inspection of the level two data, i.e. fixed effects, revealed that the mean level of SPD symptoms declined with increasing age per year (regression coefficient (b) = −.01, SE=.002, p<.001) (See Table 1). This finding was fairly consistent in all multilevel models and is not reported further.
Table 2.
Schizotypal Personality Disorder (SPD) Symptom Mean Scores (SD) at the Three Time Points in the Trajectory
| Predictor Variables | SPD T3 (Mean Age=16.4) | SPD T4 (Mean Age=22.4) | SPD T6 (Mean Age= 33.2) |
|---|---|---|---|
| Male | 25.75 (8.53) | 24.58 (9.19) | 24.14 (8.51) |
| Female | 20.86 (9.52) | 20.22 (8.56) | 19.10 (9.54) |
| Cannabis Use | 25.41 (9.24) | 24.19 (9.07) | 25.13 (10.5) |
| Non-Use | 22.99 (9.36) | 22.05 (9.15) | 20.82 (9.05) |
| Anxiety Disorder | 24.91 (8.98) | 23.59 (9.21) | 22.97 (10.26) |
| No Anxiety Disorder | 22.76 (9.42) | 21.82 (9.05) | 20.87 (8.99) |
| MDD | 26.41 (9.77) | 23.79 (9.80) | 25.45 (9.25) |
| No MDD | 23.16 (9.31) | 22.22 (9.10) | 21.26 (9.40) |
| Cigarette Use | 23.41 (9.84) | 21.33 (9.25) | 22.50 (9.67) |
| Non-Use | 23.12 (8.88) | 22.65 (9.02) | 20.35 (9.11) |
| Other Drug Use | 27.11 (11.56) | 23.61 (10.35) | 23.61 (12.02) |
| Non-Use | 23.05 (9.16) | 21.97 (9.08) | 21.23 (9.25) |
Table 1.
HLM Regression Estimates of Effects of Early Cannabis Use on the trajectory of Schizotypal Personality Disorder Symptoms over 20 years
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variables | b | s.e. | b | s.e. | b | s.e. | b | s.e. |
| Mean Schizotypal symptoms at trajectory | ||||||||
| mean age 23*** | −.28 | .04 | −.25 | .04 | −.36 | .04 | −.27 | .05 |
| Average annual change in schizotypal sxs*** | −.01 | .002 | −.01 | .002 | −.01 | .002 | −.01 | .002 |
| Sex*** | .53 | .06 | .48 | .05 | .56 | .06 | .49 | .05 |
| SES*** | −.20 | .03 | −.14 | .03 | −.19 | .03 | −.14 | .03 |
| Early Cannabis use | .24** | .08 | .20* | .08 | .23** | .08 | .18* | .09 |
| Early Schizotypal*** | .28 | .03 | -- | -- | .27 | .03 | ||
| Early Depression | .26* | .13 | .20 | .12 | ||||
| Early Anxiety | .19** | .06 | .05 | .06 | ||||
| Other Drug Use | .16 | .14 | ||||||
| Cigarette Use | −.02 | .06 | ||||||
p <.05,
p<.01,
p<.0001
Note: Non-significant interactions discussed in the text are not included in the table. The effects for variables noted with 3 stars remained significant throughout every model at the p<.0001 level.
Main Effects in Level 2 (Fixed Effects) Multi-level Models
Model 1 in Table 1 demonstrates the estimated effects of early cannabis use on average over the schizotypal symptoms trajectory, independent of age, gender, and SES effects. Early cannabis users demonstrated higher levels of SPD symptoms (.24 SD unit difference, Cohen’s d, p<0.01) than non-users over the trajectory. On average over the trajectory, SPD symptoms in males were higher than in females (Cohen’s d = 0.53, p<0.001), and children with lower family socioeconomic status (SES) reported more SPD symptoms than those from higher SES backgrounds (Cohen’s d = 0.20, p<.001).
3.3. Tests of Potential Confounders
In Model 2, we added schizotypal symptoms at mean age 13.7 to the predictors. On average over the trajectory, youth who reported more SPD symptoms in early adolescence continued to report higher levels of SPD symptoms thereafter. However, the effect of early cannabis use on the SPD trajectory remained statistically significant in this model, only decreasing .04 SD units. We found that the effect of early cannabis use on SPD symptoms into adulthood was not specific to those exhibiting higher levels of SPD symptoms in 1983 (results not shown in Table 1), as the interaction term was not significant. Thus, the effect of early cannabis use on SPD symptoms into adulthood was not restricted to those already exhibiting significant levels of these symptoms. In Model 3, we tested the effect of early cannabis use in the presence of other adolescent psychopathology. Those with a probable anxiety disorder (.19 SD unit difference) and a probable major depressive disorder (.26 SD unit difference) reported higher levels of SPD symptoms into adulthood compared to those without the respective disorders. However, these effects did not explain the effect of early cannabis use on SPD symptoms, as this remained significant in Model 3 (p<.01). In Model 4 (Table 1), those smoking cigarettes and those using other drugs were not significantly more likely to report SPD symptoms into adulthood (p=.76 and p=.26, respectively) in this model.
4. Discussion
4.1. The relationship between early cannabis use and SPD symptoms
The present investigation demonstrated that early adolescent cannabis use predicted the subsequent trajectory for schizotypal personality disorder (SPD) symptoms from mean age 13 to 35. SPD symptoms are characterized by attenuated psychotic symptoms that include unusual perceptual experiences and beliefs, and odd and withdrawn behavior. Overall, the average level of these types of symptoms decreased with increasing age in our community sample. In general, declines in mean levels of personality disorder symptoms over time can be expected (e.g., Bernstein et al., 1993; Lenzenweger, 1999; Johnson et al., 2000; Kasen et al., 2000). Using cannabis regularly in adolescence predicted higher self-reported levels of these symptoms over the next decades. This is fairly consistent with evidence from studies examining cannabis and schizophrenia (e.g., Andreasson et al, 1987; Aresneault et al, 2002; Zammit et al., 2002; Large et al., 2011).
The effect of cannabis use on SPD symptoms into adulthood was not completely explained by key potential confounding factors identified in the literature such as symptoms that may precede or co-occur with early cannabis use. Arseneault et al. (2002) found that once the presence of psychotic-like symptoms at age 11 was controlled, the effect of starting cannabis use by age 15 was reduced by about 31% and no longer significant in predicting schizophrenia or schizophrenia-like symptoms, although the magnitude of the effect remained high (OR=3.12). Here we also found part of the effect of adolescent cannabis use on SPD symptoms into adulthood was explained by pre-existing or co-occurring SPD symptoms, which were strongly related to SPD symptoms into adulthood. That said, the effect of cannabis use was still evident and remained statistically significant for early regular use. Furthermore, the cannabis use effect was not exclusive to those already exhibiting higher levels of these SPD symptoms. Our findings were also consistent with previous work (e.g. Arseneault et al., 2002) that found other drug use and cigarette use did not explain the effects of cannabis use on schizophrenia-spectrum outcomes.
D’Souza and colleagues (2005) found that intravenous delta 9THC, the principal psychoactive substance in cannabis, can induce not only psychotic symptoms, but also anxiety, cognitive deficits, and other symptoms in patients with schizophrenia and in healthy individuals. In our study, early cannabis users were more likely to have a probable anxiety or depressive disorder during adolescence, and adolescents with these disorders were more likely to exhibit SPD symptoms over the life course. However, the independent effects of these disorders did not explain why early regular cannabis use predicted higher self-reported trajectories of SPD symptoms into adulthood. Thus, it is not likely that underlying anxiety or depression among adolescents, who may end up using cannabis to cope with distressing psychopathology, explains why levels of schizophrenia-like symptoms are elevated over the life course in these cannabis users.
4.2. Limitations and Future Directions
The low prevalence of schizotypal personality disorder (SPD) did not permit us to examine the clinical diagnosis of SPD as an outcome variable in the sample. Thus, although the community sample improves the generalizability of the findings, the sample size did not provide adequate statistical power to make firm conclusions about the diagnostic level of SPD symptoms. The age range of our cohort limits the precision to which we can assert a one-way temporal relationship between cannabis use and schizotypal symptoms. The use of a community sample and attainment of the cannabis exposure information during adolescence minimizes the risk of other biases.
In order to maintain consistency of measurement of SPD into middle adulthood we were limited to self-report. This limitation may not be problematic because other information suggests that self-report is as strongly predictive of subsequent dysfunction as is combined maternal-youth report (Skodol et al., 1991; Crawford et al., 2005). While we cannot draw a definitive conclusion that there may be a characteristic that increases both the risk of engaging in cannabis use and in exhibiting SPD symptoms, we were able to adjust for SPD symptoms during childhood and early adolescence.
4.3. Conclusion
Numerous studies have demonstrated that cannabis use is temporally associated with psychotic-like symptoms (e.g., Corcoran et al, 2008), and fewer have done so with schizotypy (e.g., Cohen et al., 2011; Esterberg et al., 2009; Skosnik et al., 2001; Williams et al., 1996). The current study demonstrated prospectively that early use of this substance is related to later schizotypal symptoms. This is the first longitudinal cohort study of cannabis and schizotypy to examine potential interaction. Our findings suggest cannabis may lead to the development of schizotypal symptoms, and not simply maintain persistent symptoms. While our findings do not completely rule out the possibility that the effects of cannabis use could serve as a trigger in already vulnerable individuals predisposed to oddness, they do suggest that cannabis use may contribute to the etiopathogenesis of schizophrenia-related symptoms. Future research should determine whether these effects of using cannabis on SPD symptoms vary with duration of use throughout the lifecourse. Thus it is still unclear whether the effects of early cannabis are minimized among those who stop smoking at some other developmentally critical point (e.g., in their early 20’s or even later).
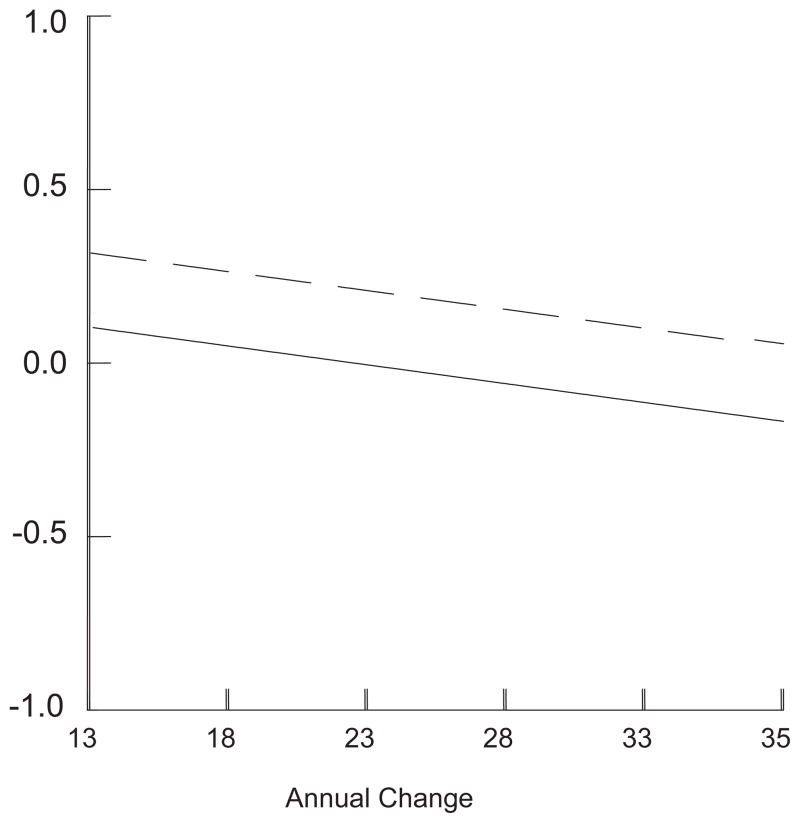
Figure 1.
Standardized schizotypal personality disorder symptoms from age 13 to 35 for participants with (Dashed Line) and without (Solid Line) early cannabis use
Acknowledgments
Role of funding source
This study was funded by grants MH-36971, MH-38916, MH-49191, and MH-60911 (Dr. Cohen), KO2-MH65422 (Dr. Brown), from the National Institute of Mental Health (Dr Cohen), MH-066279 (Dr. Corcoran), and DA-03188 from the National Institute of Drug Abuse (Dr Brook).
The funding agencies had no further role in the study design; in the collection and analysis of data; in the writing of the report; and in the decision to submit the paper for publication.
We thank the members of the Children in the Community (CIC) study team who have been integral in the execution of this prospective longitudinal study.
Footnotes
Conflict of Interest
All authors declare no actual or potential conflict of interest.
Contributors
Drs. Cohen and Brook were involved in the study design and wrote the protocol. Drs. Cohen, Chen, and Anglin undertook the statistical analyses. Quenesha Lighty was involved in entering data and coding variables. Dr. Anglin was involved with conducting literature searches and wrote the first draft of the manuscript. Drs. Cohen, Corcoran and Brown contributed to multiple revisions of the first draft. All authors contributed to and have approved the final manuscript.
This paper was presented at the poster session of the annual meeting for the American Psychopathological Association held from March 5–7, 2009 in NY, NY.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D. C: 2001. Revised) (DSM-IV-TR) [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, D. C: 1980. (DSM-III) [Google Scholar]
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Anglin DM, Cohen PR, Chen H. Duration of early maternal separation and prediction of schizotypal symptoms from early adolescence to midlife. Schizophr Res. 2008;103:143–150. doi: 10.1016/j.schres.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus E, Lewis S. Schizotypy and psychosis-like experiences from recreational cannabis in a non-clinical sample. Psychol Med. 2008;38:1267–1276. doi: 10.1017/S0033291707002619. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Cohen P, Velez CN, Schwab-Stone M, Siever LJ, Shinsato L. The prevalence and stability of the DSM-III-R personality disorders in a community-based survey of adolescents. Am J Psychiatry. 1993;150:1237–1243. doi: 10.1176/ajp.150.8.1237. [DOI] [PubMed] [Google Scholar]
- Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry. 1998;37:322–330. doi: 10.1097/00004583-199803000-00018. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Buckner JD, Najolia GM, Steward DW. Cannabis and psychometrically-defined schizotypy: use, problems and treatment considerations. J Psychiatr Res. 2011;45:548–554. doi: 10.1016/j.jpsychires.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J. Life Values and Adolescent Mental Health. Lawrence Erlbaum Associates; Mahwah, NJ: 1996. [Google Scholar]
- Cohen P, Chen H, Crawford TN, Brook JS, Gordon K. Personality disorders in early adolescence and the development of later substance use disorders in the general population. Drug Alcohol Depend. 2007;88:S71–84. doi: 10.1016/j.drugalcdep.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Goulding SM, Walker EF. Cannabis use, first-episode psychosis, and schizotypy: a summary and synthesis of recent literature Curr. Psychiatr Rev. 2007;3:161–171. [Google Scholar]
- Corcoran CM, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, Schobel S, Harkavy-Friedman J, Goetz R, Colibazzi T, Cressman V, Malaspina D. Temporal association of cannabis use with the symptoms in individuals at clinical high risk for psychosis. Schizoph Res. 2008;106:286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Edelbrock CS, Duncan MK, Kalas R. Testing of the NIMH Diagnostic Interview Schedule for Children (DISC) in a Clinical Population: Final Report to the Center for Epidemiological Studies, NIMH. University of Pittsburgh; Pittsburg, PA: 1984. [Google Scholar]
- Crawford TN, Cohen P, Johnson JG, Kasen S, First MB, Gordon K, Brook JS. Self-reported personality disorder in the children in the community sample: convergent and prospective validity in late adolescence and adulthood. Journal J Personal Disord. 2005;19:30–52. doi: 10.1521/pedi.19.1.30.62179. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Goulding SM, McClure-Tone EB, Compton MT. Schizotypy and nicotine, alcohol, and cannabis use in a non-psychiatric sample. Addict Behav. 2009;34:374–379. doi: 10.1016/j.addbeh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. Br J Psychiatry. 2006;332:172–175. doi: 10.1136/bmj.332.7534.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. User’s Guide for the Structured Clinical Interview for the DSM–IV Personality Disorders. American Psychiatric Press; Washington, D.C: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The structured clinical interview for DSM-III-R personality disorders (SCID-II), Part I: description. J Personal Disord. 1995;9:83–91. [Google Scholar]
- Fridberg D, Vollmer J, O'Donnell B, Skosnik P. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2011;186:46–52. doi: 10.1016/j.psychres.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Risk Outcome of Psychosis (GROUP) Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: An analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry. 2011;68:138–147. doi: 10.1001/archgenpsychiatry.2010.132. [DOI] [PubMed] [Google Scholar]
- Hyler SE, Skodol AE, Kellman HD, Oldham JM, Rosnick L. Validity of the Personality Diagnostic Questionnaire--revised: comparison with two structured interviews. Am J Psychiatry. 1990;147:1043–1048. doi: 10.1176/ajp.147.8.1043. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Skodol A, Brook J. Age–related change in personality disorder trait levels between early adolescence and adulthood: a community–based longitudinal investigation. Acta Psychiatr Scand. 2000;102:265–275. doi: 10.1034/j.1600-0447.2000.102004265.x. [DOI] [PubMed] [Google Scholar]
- Kasen S, Cohen P, Skodol A, First M, Johnson J, Brook JS, Oldham J. Comorbid personality disorder and treatment use in a community sample of youths: A 20-year follow-up. Acta Psychiatr Scand. 2007;115:56–65. doi: 10.1111/j.1600-0447.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van Os J, Lieb R, Wittchen H, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and early onset psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Stability and change in personality disorder features. Arch Gen Psychiatry. 1999;56:1009–1015. doi: 10.1001/archpsyc.56.11.1009. [DOI] [PubMed] [Google Scholar]
- Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, Oppenkowski R, Stokes-Lampard H, Smith GD. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363:1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- Moore THM, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, DiForti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Rössler W, Vetter S, Müller M, Gallo W, Haker H, Kawohl W, Lupi G, Ajdacic-Gross V. Risk factors at the low end of the psychosis continuum: much the same as at the upper end? Psychiatry Res. 2011;189:77–81. doi: 10.1016/j.psychres.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Nakamura B, Earleywine M, LaBrie J. Symptoms of schizotypy precede cannabis use. Psychiatry Res. 2004;134:37–42. doi: 10.1016/j.psychres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IIIR Personality Disorders. New York State Psychiatric Institute; New York, NY: 1986. [Google Scholar]
- Skodol AE, Oldham JM, Rosnick L, Kellman HD, Hyler SE. Diagnosis of DSM-III-R personality disorders: a comparison of two structured interviews. Int J Method Psych. 1991;1:13–26. [Google Scholar]
- Skodol A, Johnson J, Cohen P, Sneed J, Crawford T. Personality disorder and impaired functioning from adolescence to adulthood. Br J Psychiatry. 2007;190:415–420. doi: 10.1192/bjp.bp.105.019364. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res. 2001;48:83–92. doi: 10.1016/s0920-9964(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Gindre C, Sorbara F, Tournier M, Swendsen JD. Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Psychol Med. 2003;33:23–32. doi: 10.1017/s0033291702006384. [DOI] [PubMed] [Google Scholar]
- Williams JH, Wellman NA, Rawlins JN. Cannabis use correlates with schizotypy in healthy people. Addiction. 1996;91:869–877. [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]