Abstract
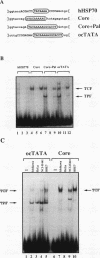
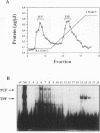
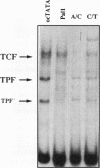
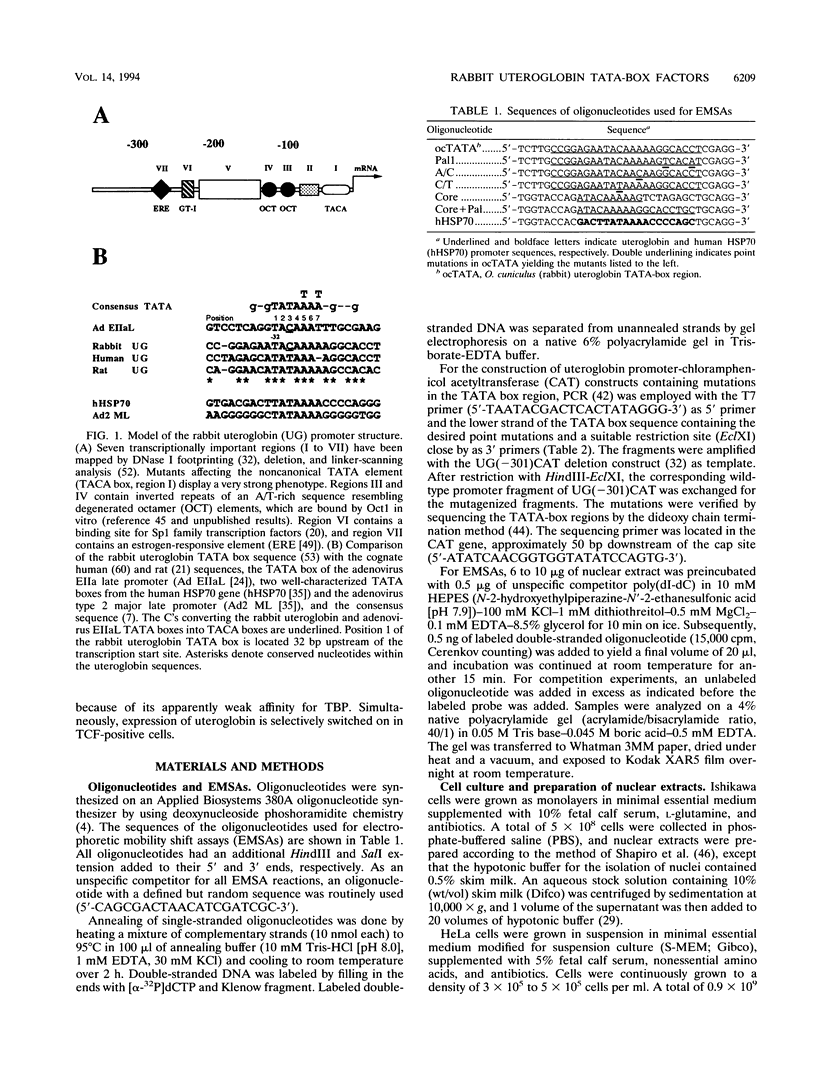
The rabbit uteroglobin gene is expressed in a variety of epithelial cell types like the lung Clara cells and the glandular and luminal epithelial cells of the endometrium. Expression in Clara cells is on a high constitutive level, whereas expression in the rabbit endometrium is under tight hormonal control. One important element of the rabbit uteroglobin gene mediating its efficient transcription in two epithelial cell lines from human endometrium (Ishikawa) and lung (NCI-H441) is its noncanonical TATA box (TACA). Here, we show that two factors (TATA core factor [TCF] and TATA palindrome factor [TPF]) different from the TATA-box binding protein bind to the DNA major groove at two adjacent sites within the uteroglobin TATA-box region and that one of them (TCF) is specifically expressed in cell lines derived from uteroglobin-expressing tissues. The binding sites for TCF and TPF, respectively, are both required for efficient transcription in Ishikawa and NCI-H441 cells. Mutation of the TACA box, which we show is a poor TATA box in functional terms, to a canonical TATA motif does not affect TCF and TPF binding. Therefore, we suggest that the function of the unusual cytosine could be to reduce rabbit uteroglobin expression in cells lacking TCF and that the interaction of TATA-box binding protein with the weak TACA site is facilitated in TCF- and TPF-positive cells.
Full text
PDF

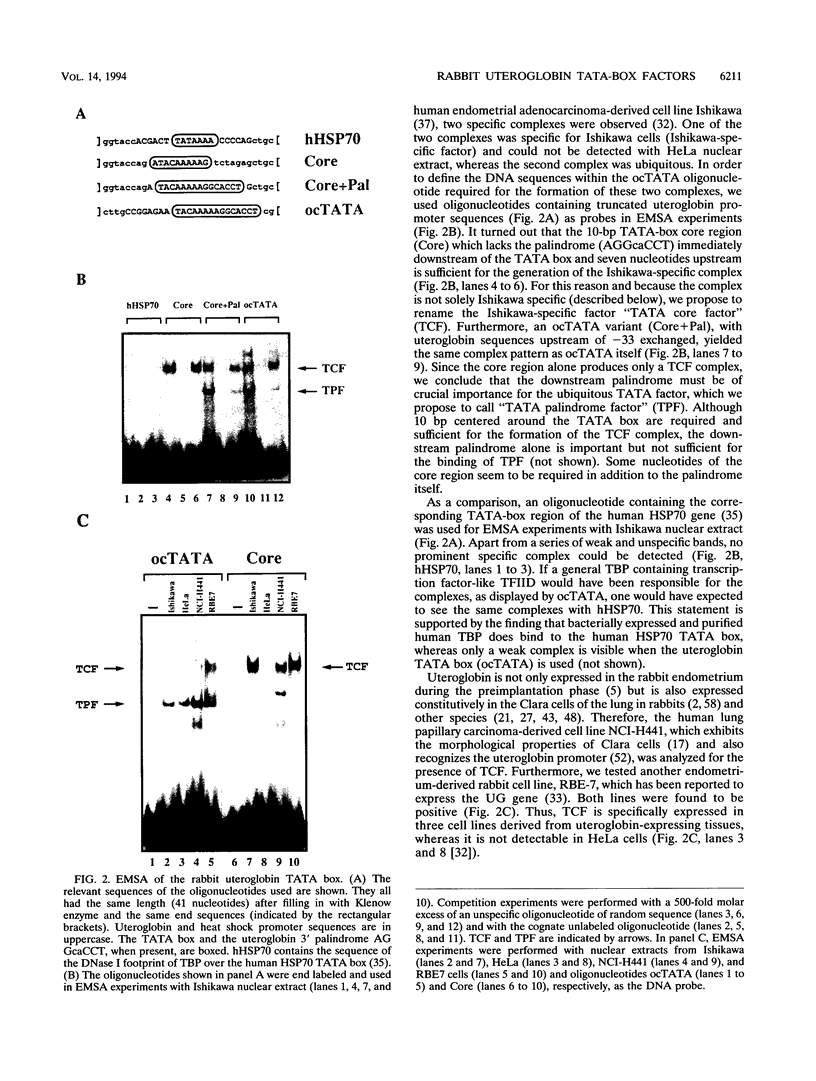

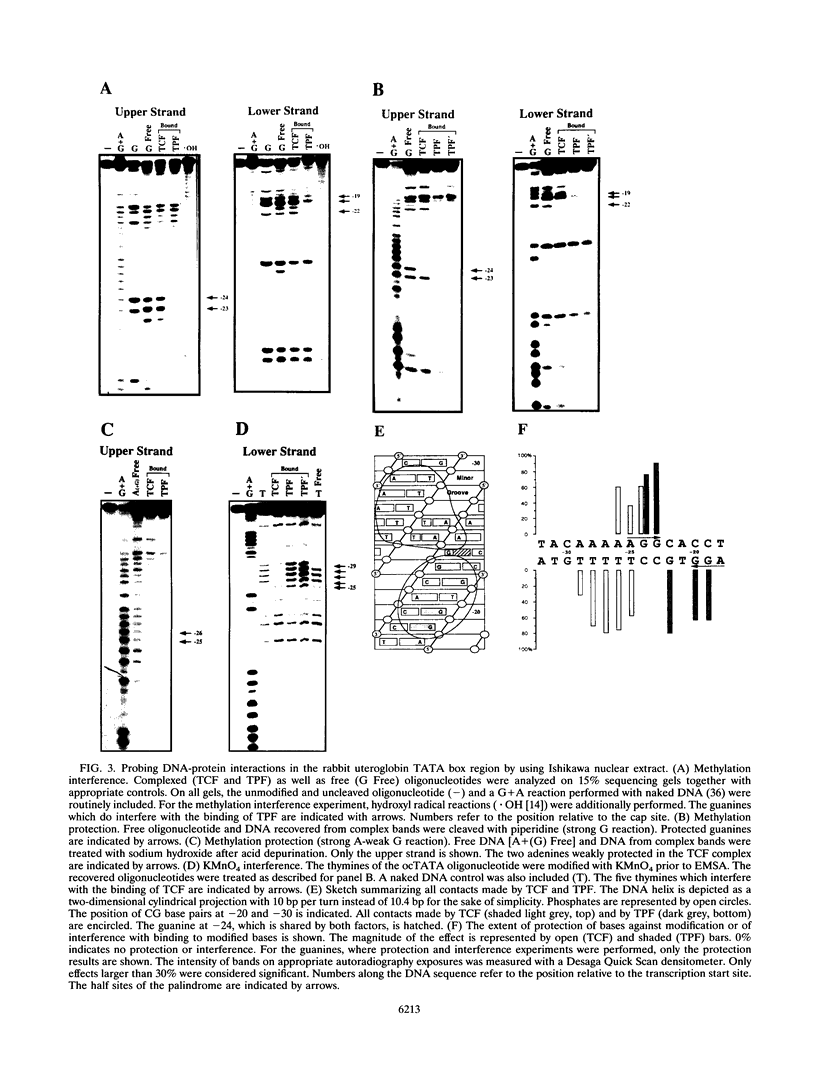
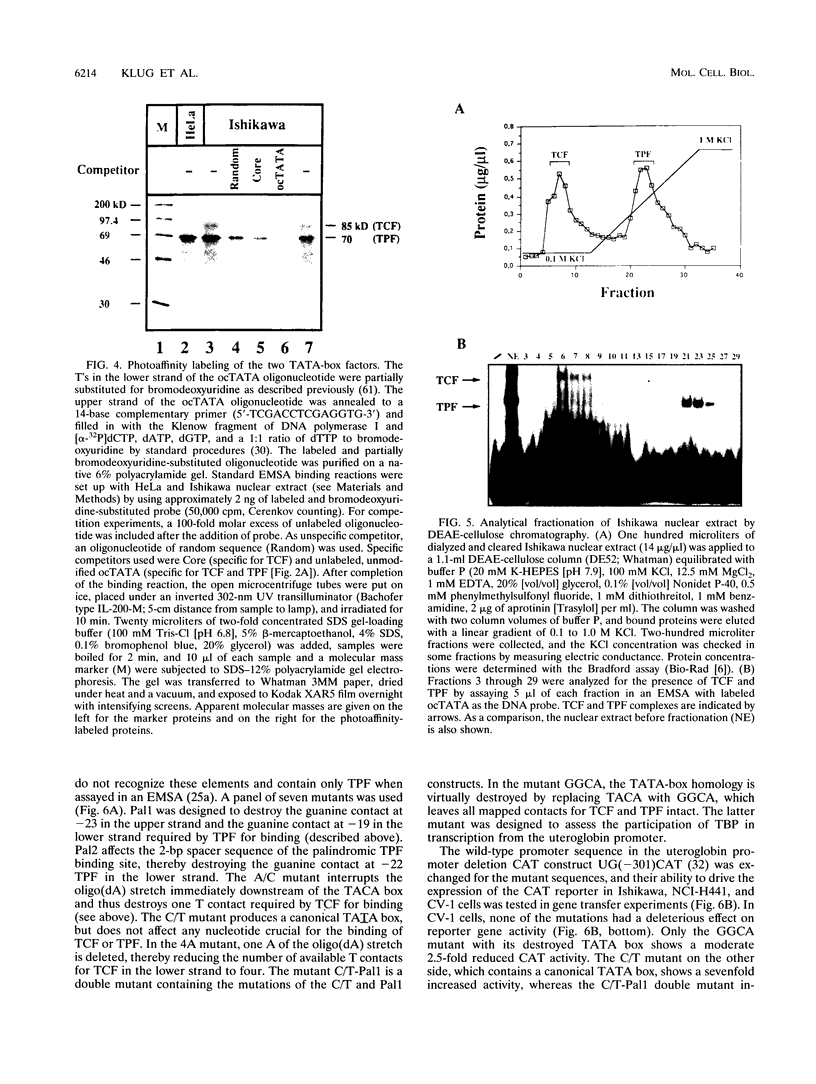
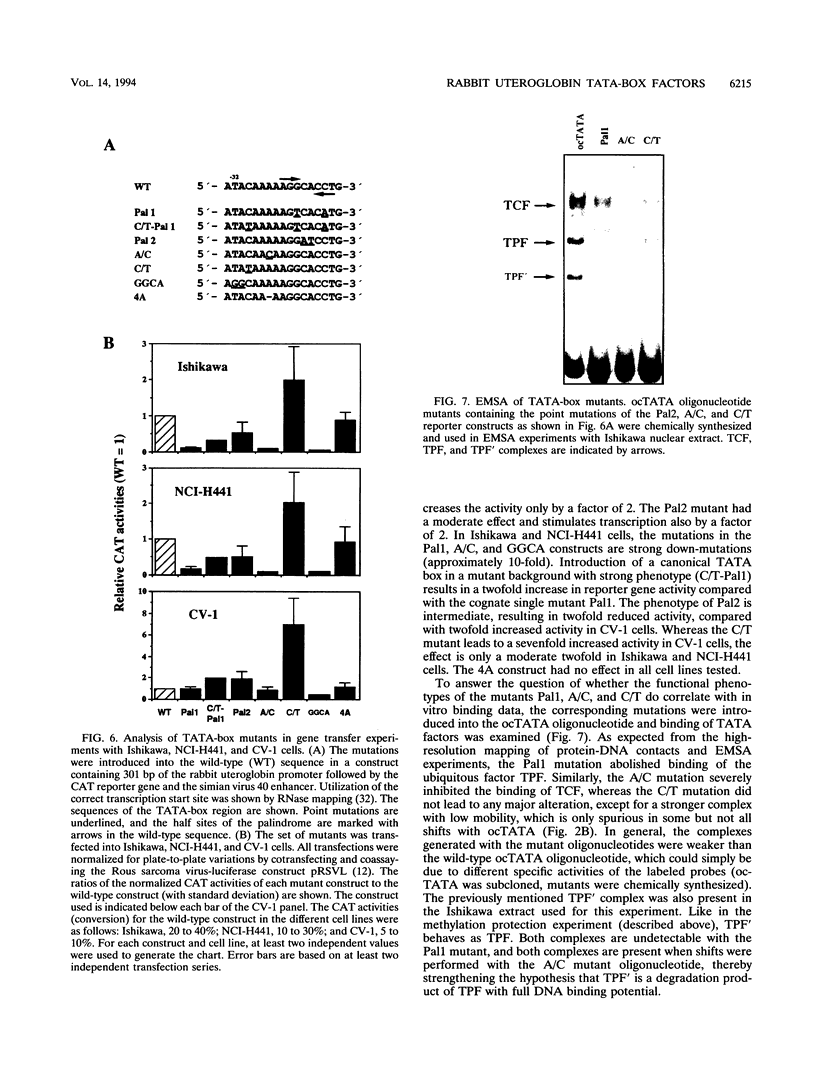



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumüller G., Seitz J., Heyns W., Kirchner C. Ultrastructural localization of uteroglobin immunoreactivity in rabbit lung and endometrium, and rat ventral prostate. Histochemistry. 1985;83(5):413–417. doi: 10.1007/BF00509202. [DOI] [PubMed] [Google Scholar]
- Beier H. M. Uteroglobin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochim Biophys Acta. 1968 Jun 26;160(2):289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cairns C., Gustafsson J. A., Carlstedt-Duke J. Identification of protein contact sites within the glucocorticoid/progestin response element. Mol Endocrinol. 1991 Apr;5(4):598–604. doi: 10.1210/mend-5-4-598. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Conaway J. W. General initiation factors for RNA polymerase II. Annu Rev Biochem. 1993;62:161–190. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Hoey T., Liu J. K., Jan Y. N., Jan L. Y., Tjian R. A new factor related to TATA-binding protein has highly restricted expression patterns in Drosophila. Nature. 1993 Feb 11;361(6412):557–561. doi: 10.1038/361557a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. J., Hayes J. J., Levin J. R., Weidner M. F., Dombroski B. A., Tullius T. D. Hydroxyl radical footprinting. Methods Enzymol. 1991;208:380–413. doi: 10.1016/0076-6879(91)08021-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Renau D., Lombardero M., Nieto A. Glucocorticoid-dependent uteroglobin synthesis and uteroglobulin mRNA levels in rabbit lung explants cultured in vitro. Eur J Biochem. 1984 Nov 2;144(3):523–527. doi: 10.1111/j.1432-1033.1984.tb08497.x. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Ou S. H., Wu F., Lusis A. J., Sparkes R. S., Gaynor R. B. Cloning and chromosomal mapping of a human immunodeficiency virus 1 "TATA" element modulatory factor. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9372–9376. doi: 10.1073/pnas.89.20.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Linnoila R. I., Kurita Y., Oie H. K., Mulshine J. L., Clark J. C., Whitsett J. A. Peripheral airway cell differentiation in human lung cancer cell lines. Cancer Res. 1990 Sep 1;50(17):5481–5487. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hagen G., Müller S., Beato M., Suske G. Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res. 1992 Nov 11;20(21):5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Wolf M., Katyal S. L., Singh G., Beato M., Suske G. Tissue-specific expression, hormonal regulation and 5'-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res. 1990 May 25;18(10):2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A., Irwin N., Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983 Feb;32(2):319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- Huang D. H., Horikoshi M., Roeder R. G. Activation of the adenovirus EIIa late promoter by a single-point mutation which enhances binding of transcription factor IID. J Biol Chem. 1988 Sep 5;263(25):12596–12601. [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Lee D. K., Horikoshi M., Roeder R. G. Interaction of TFIID in the minor groove of the TATA element. Cell. 1991 Dec 20;67(6):1241–1250. doi: 10.1016/0092-8674(91)90300-n. [DOI] [PubMed] [Google Scholar]
- López de Haro M. S., Nieto A. Glucocorticoids induce the expression of the uteroglobin gene in rabbit foetal lung explants cultured in vitro. Biochem J. 1985 Jan 1;225(1):255–258. doi: 10.1042/bj2250255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire P., Wuarin J., Schibler U. The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science. 1989 Apr 21;244(4902):343–346. doi: 10.1126/science.2711183. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misseyanni A., Klug J., Suske G., Beato M. Novel upstream elements and the TATA-box region mediate preferential transcription from the uteroglobin promoter in endometrial cells. Nucleic Acids Res. 1991 Jun 11;19(11):2849–2859. doi: 10.1093/nar/19.11.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. B., Murty L. C., Chou J. Y. Differentiation and uteroglobin gene expression by novel rabbit endometrial cell lines. Mol Cell Endocrinol. 1993 Aug;94(2):R15–R22. doi: 10.1016/0303-7207(93)90176-k. [DOI] [PubMed] [Google Scholar]
- Müller H., Beato M. RNA synthesis in rabbit endometrial nuclei. Hormonal regulation of transcription of the uteroglobin gene. Eur J Biochem. 1980 Nov;112(2):235–241. doi: 10.1111/j.1432-1033.1980.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri R., Costanzo G., Di Mauro E. A single-reaction method for DNA sequence determination. Anal Biochem. 1991 Sep 2;197(2):389–395. doi: 10.1016/0003-2697(91)90409-m. [DOI] [PubMed] [Google Scholar]
- Nishida M., Kasahara K., Kaneko M., Iwasaki H., Hayashi K. [Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors]. Nihon Sanka Fujinka Gakkai Zasshi. 1985 Jul;37(7):1103–1111. [PubMed] [Google Scholar]
- Papavassiliou A. G. Localisation of DNA-protein contact points by DMS resistance of complexes resolved in gel retardation assays. Nucleic Acids Res. 1993 Feb 11;21(3):757–758. doi: 10.1093/nar/21.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penotti F. E. Human DNA TATA boxes and transcription initiation sites. A statistical study. J Mol Biol. 1990 May 5;213(1):37–52. doi: 10.1016/S0022-2836(05)80120-2. [DOI] [PubMed] [Google Scholar]
- Pothier F., Ouellet M., Julien J. P., Guérin S. L. An improved CAT assay for promoter analysis in either transgenic mice or tissue culture cells. DNA Cell Biol. 1992 Jan-Feb;11(1):83–90. doi: 10.1089/dna.1992.11.83. [DOI] [PubMed] [Google Scholar]
- Rigby P. W. Three in one and one in three: it all depends on TBP. Cell. 1993 Jan 15;72(1):7–10. doi: 10.1016/0092-8674(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sandmöller A., Voss A. K., Hahn J., Redemann-Fibi B., Suske G., Beato M. Cell-specific, developmentally and hormonally regulated expression of the rabbit uteroglobin transgene and the endogenous mouse uteroglobin gene in transgenic mice. Mech Dev. 1991 Mar;34(1):57–67. doi: 10.1016/0925-4773(91)90091-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya P. L., Stripp B. R., Whitsett J. A., Luse D. S. The lung-specific CC10 gene is regulated by transcription factors from the AP-1, octamer, and hepatocyte nuclear factor 3 families. Mol Cell Biol. 1993 Jul;13(7):3860–3871. doi: 10.1128/mcb.13.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Singer V. L., Wobbe C. R., Struhl K. A wide variety of DNA sequences can functionally replace a yeast TATA element for transcriptional activation. Genes Dev. 1990 Apr;4(4):636–645. doi: 10.1101/gad.4.4.636. [DOI] [PubMed] [Google Scholar]
- Singh G., Singh J., Katyal S. L., Brown W. E., Kramps J. A., Paradis I. L., Dauber J. H., Macpherson T. A., Squeglia N. Identification, cellular localization, isolation, and characterization of human Clara cell-specific 10 KD protein. J Histochem Cytochem. 1988 Jan;36(1):73–80. doi: 10.1177/36.1.3275712. [DOI] [PubMed] [Google Scholar]
- Slater E. P., Redeuihl G., Theis K., Suske G., Beato M. The uteroglobin promoter contains a noncanonical estrogen responsive element. Mol Endocrinol. 1990 Apr;4(4):604–610. doi: 10.1210/mend-4-4-604. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Soledad López de Haro M., Nieto A. Isolation and characterization of uteroglobin from the lung of the hare (Lepus capensis). Arch Biochem Biophys. 1983 Oct 15;226(2):539–547. doi: 10.1016/0003-9861(83)90323-5. [DOI] [PubMed] [Google Scholar]
- Starr D. B., Hawley D. K. TFIID binds in the minor groove of the TATA box. Cell. 1991 Dec 20;67(6):1231–1240. doi: 10.1016/0092-8674(91)90299-e. [DOI] [PubMed] [Google Scholar]
- Suske G., Lorenz W., Klug J., Gazdar A. F., Beato M. Elements of the rabbit uteroglobin promoter mediating its transcription in epithelial cells from the endometrium and lung. Gene Expr. 1992;2(4):339–352. [PMC free article] [PubMed] [Google Scholar]
- Suske G., Wenz M., Cato A. C., Beato M. The uteroglobin gene region: hormonal regulation, repetitive elements and complete nucleotide sequence of the gene. Nucleic Acids Res. 1983 Apr 25;11(8):2257–2271. doi: 10.1093/nar/11.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Malhotra P., Manohar C. F., Dhar R., Thimmapaya B. Activation of a dual adenovirus promoter containing nonconsensus TATA motifs in Schizosaccharomyces pombe: role of TATA sequences in the efficiency of transcription. Nucleic Acids Res. 1993 Jun 11;21(11):2737–2746. doi: 10.1093/nar/21.11.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993 Aug;14(4):459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Beato M. Contacts between steroid hormone receptors and thymines in DNA: an interference method. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7180–7184. doi: 10.1073/pnas.87.18.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Slater E. P., Mader S., Beato M. Functional interaction of hybrid response elements with wild-type and mutant steroid hormone receptors. Mol Cell Biol. 1991 Jun;11(6):3247–3258. doi: 10.1128/mcb.11.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg M., Tranchant O., Atger M., Milgrom E. Uteroglobin messenger ribonucleic acid: localization in rabbit uterus and lung by in situ hybridization. Endocrinology. 1986 Oct;119(4):1632–1640. doi: 10.1210/endo-119-4-1632. [DOI] [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Mertz J. E. Functional binding of the "TATA" box binding component of transcription factor TFIID to the -30 region of TATA-less promoters. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5814–5818. doi: 10.1073/pnas.89.13.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Klug J., Hackenberg R., Gessler M., Grzeschik K. H., Beato M., Suske G. Human CC10, the homologue of rabbit uteroglobin: genomic cloning, chromosomal localization and expression in endometrial cell lines. Hum Mol Genet. 1992 Sep;1(6):371–378. doi: 10.1093/hmg/1.6.371. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- Yoza B. K., Roeder R. G. Identification of a novel factor that interacts with an immunoglobulin heavy-chain promoter and stimulates transcription in conjunction with the lymphoid cell-specific factor OTF2. Mol Cell Biol. 1990 May;10(5):2145–2153. doi: 10.1128/mcb.10.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]