Abstract
Transporters in the choroid plexus (CP) regulate transport of numerous compounds of physiological and therapeutic interest between blood and CSF and thus likely play a key role in determining CNS levels of drugs, toxins and metabolites. Here, high CP expression was noted for the organic anion transporters, Oat1 (SLC22A6 or NKT) and Oat3 (SLC22A8) which are also the principal Oats in the renal proximal tubule, as well as SLC22A17, believed to be involved in iron transport. Because Oat1 and Oat3 have overlapping substrate specificity, ex vivo preparations of CP from Oat1(−/−) and Oat3(−/−) mice were used to isolate the individual transport function of each, respectively. Tissue from either knockout mouse mediated the probenecid-inhibitable transport of the Oat substrate, 6-carboxyfluorescein (6CF), confirming the presence of Oat1 and Oat3 function. Because many antiviral medications are Oat substrates, including those crucial in the treatment of HIV infections, the interaction of the antivirals zidovudine, acyclovir, tenofovir, lamivudine, and stavudine, with Oat1 and Oat3 in CP, was investigated by determining the inhibition of 6CF uptake. All the antivirals tested manifested significant interaction with both Oat1 and Oat3, with the exception of stavudine which did not significantly affect Oat1 function. These results could have important implications for antiretroviral (and other drugs) penetration into or retention within the CNS, a major reservoir for virus during HIV infection. Apart from any effect at the blood brain barrier (BBB), designing specific inhibitors of Oat1 and Oat3 may be helpful in altering CNS drug levels by blocking organic anion transporters in the CP. The ability of Oats to regulate the movement of small molecules across the BBB, CP, proximal tubule and other tissues may also be important for their hypothesized role in remote sensing and signaling (Ahn and Nigam, 2009; Wu et al., 2011).
Keywords: organic anion transporter 1/NKT/Slc22a6, Choroid plexus, Probenecid, Cerebrospinal fluid, Blood brain barrier, Antivirals, Remote sensing and signaling hypothesis
1. Introduction
Localization of organic anion and cation transporters in the brain and choroid plexus (CP), which functions in the removal of toxins, drugs and metabolites from the cerebrospinal fluid (CSF), has been previously described [10, 17]. The organic anion transporters (Oats) located in the CP handle a wide variety of organic anions, including para-aminohippurate (PAH), estrone sulfate, benzylpenicillin [9], in a mechanism similar to that present in the kidney, although it is functionally reversed [14]: Oat1/Slc22a6 and Oat3/SLC22a8 which are found on the basolateral surface of the proximal tubule [10], function in clearing endogenous metabolites, toxins and drugs from blood to the urine utilizing a tertiary active transport mechanism and a similar mechanism mediates movement of organic anions across the apical membrane of the CP [18]. This secretory epithelial tissue is found in the ventricles of the brain where it is believed to regulate the flow of drugs and toxins in and out of brain tissue, the CSF, and central nervous system (CNS). While transport in the CP has remained relatively unexplored due to its smaller size and comparative inaccessibility [14], the generation of knockouts for Oat1 and Oat3 has provided in vivo evidence for Oat-mediated drug transport in this tissue [6, 17].
Antiretroviral drug availability in all areas of the CNS is essential for the efficacy of treatment in HIV patients [3]. Drug distribution and effects are directly related to antiretroviral uptake into and removal from the CNS at the CP. Previously, we demonstrated the interaction of HIV antiretrovirals with Oat1 and Oat3 [19]. A similar transport mechanism of nucleoside analogs has also been described in the CP [15]. In the present study, we have investigated the interaction of various antiretrovirals with the organic anion uptake system using a known tracer for these Oats, 6-carboxyfluorescein (6-CF), in CP derived from mice deficient for either Oat1/Slc22a6 or Oat3/slc22a8.
2. Materials and Methods
2.1. General
Water-soluble probenecid was purchased from Molecular Probes (Carlsbad, CA); 6-carboxyfluoroscein was obtained from Sigma-Aldrich (St. Louis, MO). Stavudine (d4T), lamivudine (3TC), tenofovir, zidovudine (AZT) and acyclovir were purchased from either Moravek Biochemicals (Brea, CA) or Sigma-Aldrich. Flk antibody was purchased from Sigma-Aldrich. Knockout (KO) and wild type (WT) mice were generated as previously described [6]. Oat1 KO mice were back-crossed for 7 generations to C57BL/6J; Oat3 KO mice were back-crossed for 4 generations to C57BL/6J, and C57BL/6J mice were used as wild-type (WT) controls. All experiments were performed on age matched adult female (Oat1 and Oat3 KO) mice between 12–20 weeks of age.
2.2. Adult CP uptake assay, imaging, and PCR
Isolation of the choroid plexus was performed as previously described [17, 18]. For uptake assays, each CP was cut into 2 pieces and incubated for 1 hour at RT in 1µM 6CF with or without probenecid (2mM), stavudine (2mM), acyclovir (2mM), tenofovir (2mM), zidovudine (2mM) or lamivudine (2mM), respectively. Tissues were then washed with ice cold PBS 4–5 times, frozen in OCT compound and cryosectioned. The 30 µM sections were immediately mounted on slides and imaged with a Nikon confocal microscope. For PCR, CP was isolated from wild type C57BL6 mice, and PCR products of Slc22 genes were resolved on agarose gel by electrophoresis.
2.3. Image acquisition and analysis
30 minutes prior to data collection the confocal microscope was turned on and allowed to equilibrate. All imaging parameters including lens, aperture and gains were manually set to predetermined values to generate image intensities that fell in the middle of the dynamic range. Digital images were obtained and signal intensity was than measured by averaging 15 points of intensity in well-focused portions along the choroid plexus sample image using NIH image processing software ImageJ.
2.4. Ethics Statement
All work was done in accordance with animal protocols approved by the Institutional Animal Care and Use Committee of the UCSD. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
3. Results
3.1. Isolation of CP
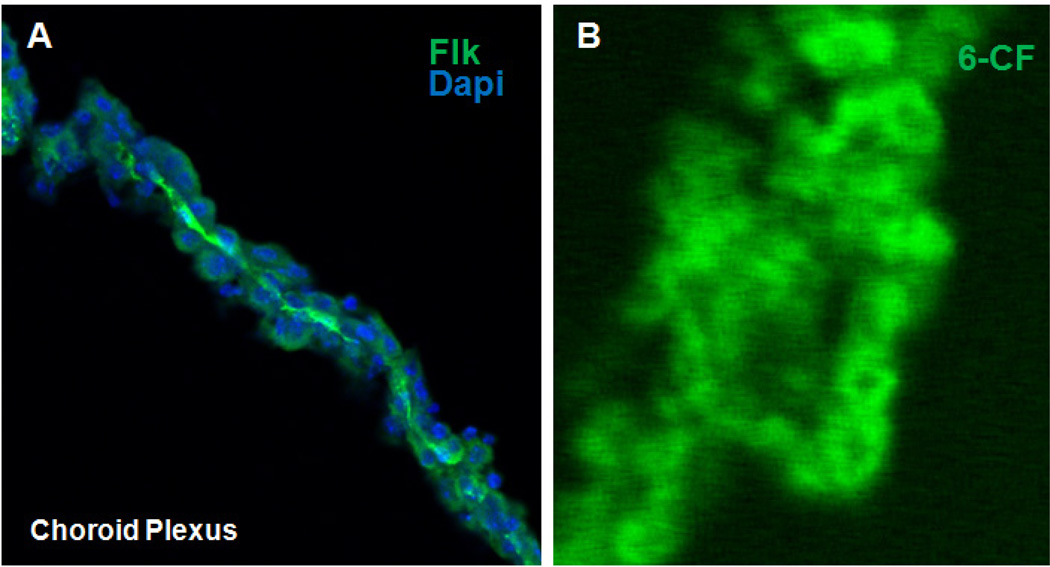
To establish whether the tissue isolated from the brain is indeed CP, immunohistochemical staining was carried out with primary antibody against a receptor for vascular endothelial growth factor A, Flk, followed by probing with a fluorescein conjugated secondary antibody. The tissues were also stained with DAPI for visualization of nuclei. Consistent with its expression in endothelial cells, strong Flk staining was observed at the surface of the CP epithelial cells facing the blood, which is on the opposite side of the surface facing the CSF where the Oats are expressed (Figure 1A).
Figure 1.
(A) Confocal fluorescent photomicrograph of isolated adult mouse CP indicating presence of Flk1 (green) in the endothelium of the CP. Dapi (blue) staining indicating nuclei of surrounding CP epithelial cells. (B) Fluorescent photomicrograph demonstrating the uptake of 6-CF into isolated adult wild-type mouse CP.
3.2. Uptake of a known organic anion transporter substrate (6-CF) in CP of Wt mice
To determine if the epithelial cells of CP can take up the known Oat specific substrate 6-CF, isolated CP from adult WT mice were analyzed using a well-characterized cellular uptake assay. Image analysis revealed accumulation of fluorescence in the cells of CP derived from wild-type mice (Figure 1B) which, as expected, was inhibited by more than 90% by 2 mM probenecid (data not shown). At high magnification, the fluorescence appears to accumulate in the cytoplasm of the cell, as observed using the same assay in the kidney tubule cell [12].
3.3. Both Oat1 and Oat3 are expressed in the CP
To profile the expression of multispecific Slc22 drug transporters in CP, a subset of Slc22 family genes was analyzed by PCR with tissues derived from wild-type C57LB6 mice. Two multispecific organic anion drug transporters, Slc22a6/Oat1 and Slc22a8/OAT3, were found to be expressed in the mouse CP (Figure 2A). In addition, Slc22a17, believed to be a facilitator of iron transport in the brain [5], and Slc22a12 (URAT1/Rst) were also expressed in the CP. Examination of an EST database derived from a pooled mouse brain cDNA library was largely consistent with the PCR data (Figure 2B), except for Slc22a4 which is expressed in the brain (Figure 2A) [2]. Searching for Slc22 localization data (with in situ hybridization data and immunohistochemistry data) confirmed CP localization of the Slc22 gene products as well as Slc22a5 at lower levels, and several other Slc22 family genes.
Figure 2.
(A) RT-PCR showing the expression of Slc22 family genes in wild type CP (Gapdh control). B. Numbers of ESTs of Slc22 genes in mouse brain with data derived from the cancer genome anatomy database.
3.4. Interaction of antivirals with Oat1 and Oat3 in adult CP
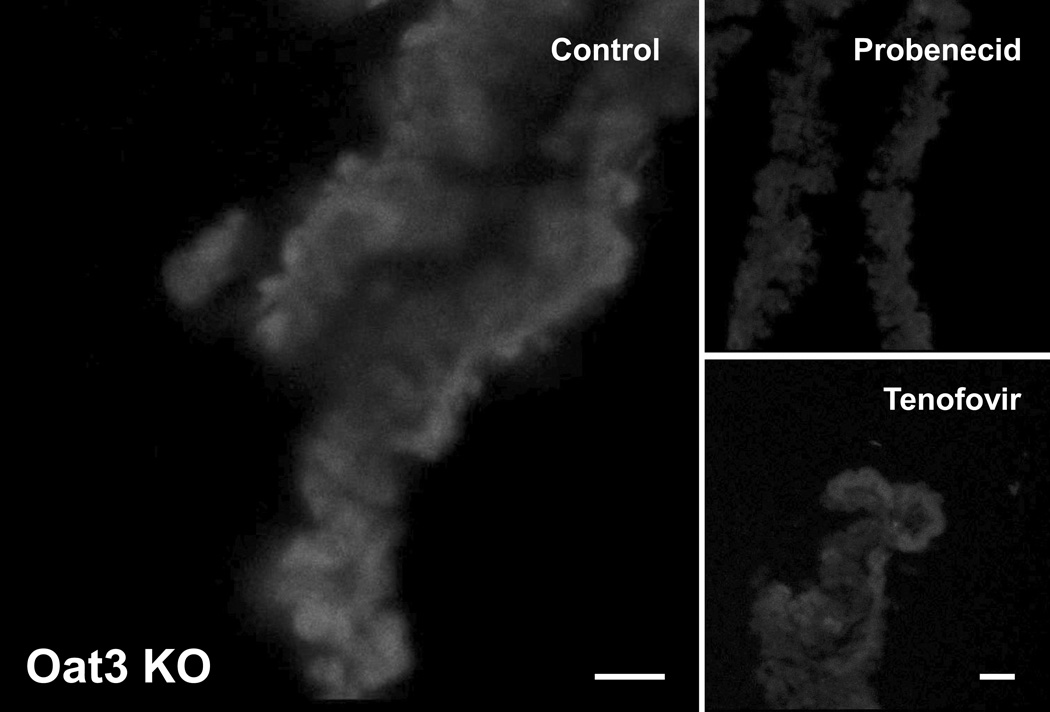
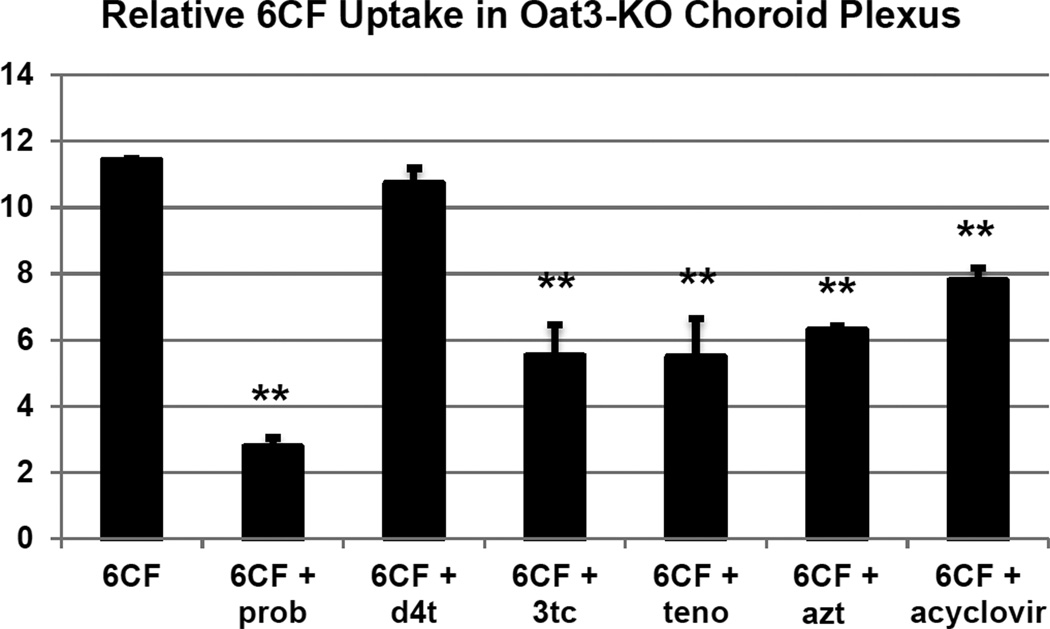
Oat1 and Oat3 are two closely related organic anion transporters with overlapping specificity, and both can mediate cellular uptake of 6-CF. Here we investigated the inhibitory effects on 6-CF uptake of the aforementioned antivirals in adult CP tissue derived from mice deficient for Slc22a6/Oat1 or Slc22a8/Oat3 [6, 17]. As previously reported in our studies on embryonic kidney [12, 16], the interaction of the antivirals with the Oats was assessed by determining the degree of inhibition of 6-CF uptake in the presence of the antiviral drugs. CP derived from the Slc22a6/Oat1 and Slc22a8/Oat3 knockouts were used to evaluate Oat3- and Oat1-mediated transport, respectively. As a control, probenecid, which can block both Oat1- and Oat3-mediated transport, was used and found to inhibit the uptake of 6-CF in CP derived from Oat1 KO (Figure 3 and 4) as well as Oat3 KO mice (Figure 5 and 6). Co-incubation of 6-CF with the individual antivirals, stavudine, lamivudine, tenofovir, zidovudine and acyclovir, reduced 6-CF uptake in the CP of Oat1 deficient mice (Figure 4) individually. In Oat3 deficient mice, uptake of 6-CF was reduced in the presence of lamivudine, tenofovir, zidovudine and acyclovir, but not stavudine (Figure 6).
Figure 3.
Fluorescent photomicrographs of 6-CF uptake in isolated adult Oat1(−/−) CP in the absence (control) or in the presence of either probenecid or a representative antiviral (tenofovir). All images are representative of triplicate sections from the same experiment.
Figure 4.
Quantitative image analysis of fluorescent signal strength indicating relative uptake of 6CF in isolated adult Oat1(−/−) CP in the absence (6CF) or presence of either probenecid (6CF + prob) or various antivirals. Signal strength analyses were derived from triplicate sections. p values were derived from comparison with controls: *, p<0.05; **, p< 0.01.
Figure 5.
Fluorescent photomicrographs of 6-CF uptake in isolated adult Oat3(−/−) CP in the absence (control) or in the presence of either probenecid or a representative antiviral (tenofovir). All images are representative of triplicate sections from the same experiment.
Figure 6.
Quantitative image analysis of fluorescent signal strength indicating relative uptake of 6CF in isolated adult Oat3(−/−) CP in the absence (6CF) or presence of either probenecid (6CF + prob) or various antivirals. Signal strength analyses were derived from triplicate sections. P values were derived from comparison with controls: *, p<0.05; **, p< 0.01.
4. Discussion
Ex vivo preparations of choroid plexus (CP) isolated from Oat1(−/−) and Oat3(−/−) mice were used to determine the ability of the transporters to interact with antivirals commonly utilized in the treatment of HIV infection in this critical CNS epithelial barrier. The results of this study found that: 1) the CP expresses a number of Slc22 family members, including Oat1, Oat3 and Slc22a17; 2) CP from either Oat1(−/−) or Oat3(−/−) animals retains the ability to mediate probenecid-inhibitable transport of 6CF indicating the presence of functional organic anion transporters in knockout tissues; and 3) the anti-HIV antiretrovirals tested manifested significant interaction with both CP-expressed transporters, except for stavudine which did not significantly inhibit Oat1-mediated uptake of 6CF.
Together with the blood-brain barrier, the CP represents the major interface between the blood and CSF. The CP protrudes into the ventricles and consists of modified cuboidal epithelial cells that not only produce the CSF, but also represent a major barrier to the movement of solutes (such as antiviral drugs) from the blood to the CSF [4]. The inability of drugs to penetrate the blood-brain and blood-CP barriers has led to the belief that the CNS represents an important viral sanctuary site [20]. However it is becoming increasingly clear that this is only partly due to the ability of the blood-brain and/or blood-CSF barriers to hinder the penetration of most antiviral drugs [13]. The CP-specific expression of transporters mediating the rapid efflux of many small molecule drugs such antivirals out of the CNS greatly complicate antiviral pharmacotherapies [7]. Our analysis of the interaction of antivirals with Oat1 and/or Oat3 in the murine CP in transporter knockout mice suggest that both of these Slc22 family members contribute to the capacity of antiviral handling in the CP and efflux from the CSF (this is likely different from the blood-brain barrier where the expression of Oat3 appears to be dominant [11]). Consistent with our previous finding [19], of the antivirals tested in this paper, d4t has the least affinity for either Oat1 or Oat3. Nevertheless, these results potentially have important implications for antiretroviral (and other drug) retention within the CNS. For example, specific inhibitors of these transporters could increase the retention (and thus half-life) of drugs within the CSF by decreasing or blocking efflux mediated by Oats expressed in the CP.
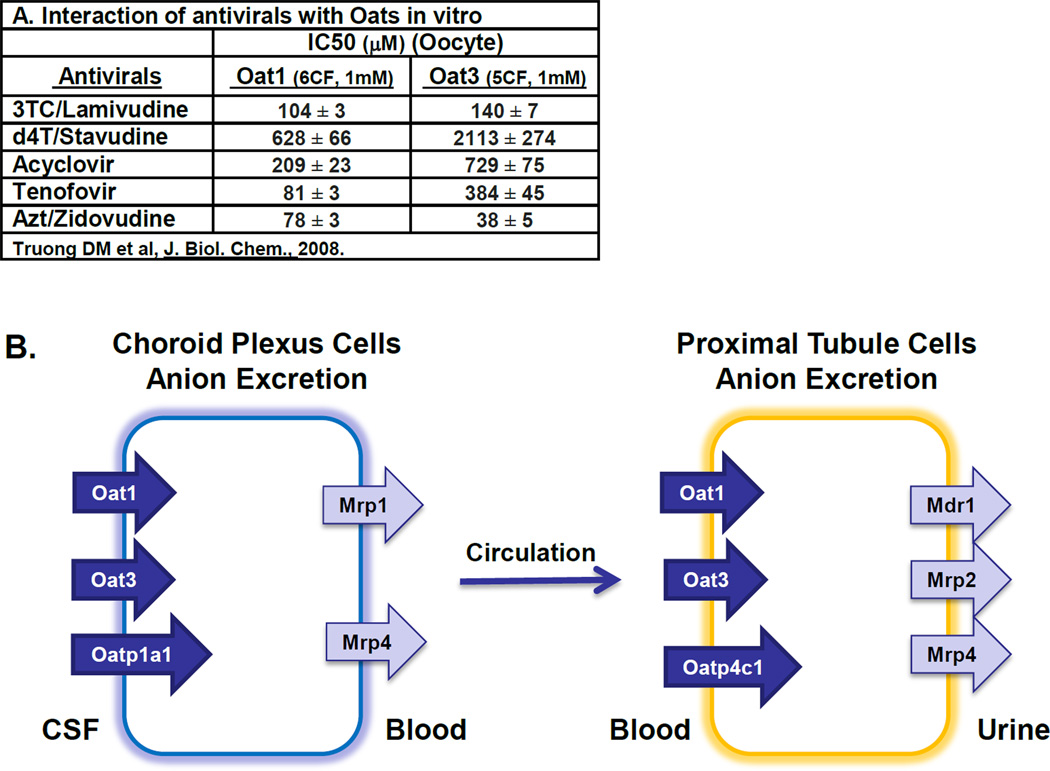
Furthermore, these same two organic anion transporters are also found on the basolateral surface of the proximal tubule cells mediating the initial steps of excretion of organic solutes from blood to urine. This raises the possibility that the same set of transporters may mediate the movement of cerebral metabolites, toxins, and drugs from brain/CSF through blood and finally their excretion into urine (Figure 7). This potential movement of metabolites between brain, CSF, blood and urine may be interesting in light of the remote sensing and signaling hypothesis that suggests the existence of a transporter-mediated communication network between cells, organs and organisms which together with other regulatory systems (such as the endocrine and autonomic nervous system) is proposed to function in maintaining homeostasis [1, 8, 21].
Figure 7.
Accumulating data suggests transporter networks may mediate the movement of antivirals through various body compartments. A, Antivirals can interact with Oat1 and Oat3 in vitro (Truong et al., JBC, 2008) [12, 19]; B, Diagram depicting the transporter-mediated movement of Oat substrates, including antivirals, from brain (via CP) to urine (via kidney).
Highlights.
-
➢
Transport of drugs by the choroid plexus (CP) is believed to be an important factor in regulating CNS drug levels.
-
➢
The organic anion transporters Oat1 (SLC22A6) and Oat3 (SLC22A8), as well as SLC22A17, are highly expressed in CP.
-
➢
Ex vivo transport of antivirals used for HIV and other diseases was studied in CP from Oat1 and Oat3 knockouts.
-
➢
Zidovudine, acyclovir, tenofovir and lamivudine were shown to interact with both Oat1 and Oat3.
-
➢
Specific inhibitors of Oat1 and Oat3 may be helpful in altering CNS drug levels by blocking Oat function in the CP.
Acknowledgments
The authors would like to thank Dr. Kevin T. Bush for his editorial assistance. This work was supported by the NIDDK (DK079784), the NIGMS (GM88824, GM098449) and the NHLBI (HL094728).
Abbreviations
- Oat1
organic anion transporter 1/NKT/Slc22a6
- Oat3
organic anion transporter 3/Slc22a8
- CP
Choroid plexus
- CSF
cerebrospinal fluid
- BBB
blood brain barrier
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
No conflict of interest was declared.
References
- 1.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76:481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- 3.Anthonypillai C, Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1. doi: 10.1186/1743-8454-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25:239–249. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- 5.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 7.Hermann DM, Kilic E, Spudich A, Kramer SD, Wunderli-Allenspach H, Bassetti CL. Role of drug efflux carriers in the healthy and diseased brain. Ann Neurol. 2006;60:489–498. doi: 10.1002/ana.21012. [DOI] [PubMed] [Google Scholar]
- 8.Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, Nigam SK. Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun. 2006;351:872–876. doi: 10.1016/j.bbrc.2006.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi R, Kusuhara H, Sugiyama D, Sugiyama Y. Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood-brain barrier. J Pharmacol Exp Ther. 2003;306:51–58. doi: 10.1124/jpet.103.049197. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem. 1997;272:6471–6478. doi: 10.1074/jbc.272.10.6471. [DOI] [PubMed] [Google Scholar]
- 11.Mori S, Ohtsuki S, Takanaga H, Kikkawa T, Kang YS, Terasaki T. Organic anion transporter 3 is involved in the brain-to-blood efflux transport of thiopurine nucleobase analogs. J Neurochem. 2004;90:931–941. doi: 10.1111/j.1471-4159.2004.02552.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. Analysis of Three-dimensional Systems for Developing and Mature Kidneys Clarifies the Role of OAT1 and OAT3 in Antiviral Handling. J Biol Chem. 2011;286:243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med. 2006;6:139–143. [PubMed] [Google Scholar]
- 14.Pritchard JB, Sweet DH, Miller DS, Walden R. Mechanism of organic anion transport across the apical membrane of choroid plexus. J Biol Chem. 1999;274:33382–33387. doi: 10.1074/jbc.274.47.33382. [DOI] [PubMed] [Google Scholar]
- 15.Strazielle N, Belin MF, Ghersi-Egea JF. Choroid plexus controls brain availability of anti-HIV nucleoside analogs via pharmacologically inhibitable organic anion transporters. AIDS. 2003;17:1473–1485. doi: 10.1097/00002030-200307040-00008. [DOI] [PubMed] [Google Scholar]
- 16.Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int. 2006;69:837–845. doi: 10.1038/sj.ki.5000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- 18.Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol. 2004;286:F972–F978. doi: 10.1152/ajprenal.00356.2003. [DOI] [PubMed] [Google Scholar]
- 19.Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. Multi-level analysis of organic anion transporters, 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem. 2008;283:8654–8663. doi: 10.1074/jbc.M708615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, Dnyanmote AV, Nigam SK. Remote Communication through Slc and Abc Drug Transporter Pathways: An Update on the Remote Sensing and Signaling Hypothesis. Mol Pharmacol. 2011;79:795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]