Abstract
Purpose
To investigate the effects of motion following repair with a modified Kessler core suture and 5 different epitendinous suture designs on the gliding resistance, breaking strength, 2-mm gap force, and stiffness of flexor digitorum profundus tendons in a human in vitro model.
Methods
The flexor digitorum profundus tendons of the index, middle, ring, and little fingers of 50 human cadavers were transected and repaired with a 2-strand modified Kessler suture and assigned to 5 groups based on type of epitendinous suture design. The 5 epitendinous designs tested were a simple, running epitendinous suture whose knot was outside the repair (simple running KO); a simple, running epitendinous suture whose knot was inside the repair (simple running KI); a cross-stitch epitendinous suture; an interlocking, horizontal mattress (IHM) epitendinous suture; and a running–locking epitendinous suture. The tendon repair strength and 2-mm gap force were measured after 1,000 cycles of tendon motion. The resistance to gap formation, a measure of repair stiffness, was obtained from the force versus gap data.
Results
None of the repairs showed any gap formation after 1,000 cycles of tendon motion. The cross-stitch epitendinous suture, IHM epitendinous suture, and running–locking epitendinous suture all had significantly lower gliding resistance than the simple running KO epitendinous suture after 1 cycle. The simple running KI epitendinous suture had significantly lower gliding resistance than the simple running KO epitendinous suture after 100 cycles and 1,000 cycles. The differences for gap force at 2 mm and stiffness of the repaired tendon evaluation were not statistically significant. The cross-stitch epitendinous suture, IHM epitendinous suture, and running–locking epitendinous suture all had significantly higher maximal failure strength after 1,000 cycles than the simple running KI epitendinous suture.
Conclusions
The cross-stitch, IHM, and running–locking epitendinous sutures had the best combination of higher strength and lower gliding resistance in this study. Although these findings suggest a potential for these suture types to be preferred as epitendinous sutures, these repairs should first be investigated in vivo to address their effect on tendon healing and adhesion formation.
Keywords: Epitendinous suture, gliding resistance, suture, tendon, tendon repair
The effect of suture materials and suture design on the strength and gliding resistance of tendon core sutures has been well described.1–5 There is also information regarding the strength of peripheral sutures used to tidy the tendon repair. In the simple running epitendinous stitch, first described by Kleinert et al. in 1973, the sutures are added circumferentially in a simple over-and-over pattern around the repair site.6 The running–locking peripheral suture technique as introduced by Lin et al.7 in 1988 places the knot inside the repair. Lin et al. reported that this suture technique was 377% as strong as the traditional simple running suture, when both running stitches were used without a core stitch.7 In the cross-stitch suture technique, introduced by Silfverskiold and Andersson in 1993, a continuous strand encircles the repaired tendon in a crisscrossing pattern, with the knot outside the repair. They reported that the cross-stitch suture was 117% as strong as a conventional epitendinous suture when used in association with a modified Kessler repair.8 In the interlocking horizontal mattress (IHM) suture technique, introduced by Dona et al.9 in 2003, the suture strands are locked and run more longitudinally than in the cross-stitch, with the knot outside the repair. They reported that the IHM was 171% as strong as the conventional epitendinous repair and 126% as strong as the cross-stitch when not reinforced by a core stitch.9
In contrast, less is known about the effect of the running peripheral stitch on tendon gliding resistance, even though such stitches often provide the bulk of suture material on the tendon surface. This has potential clinical importance, because high-friction repairs are associated with greater adhesion formation.10 A high-friction repair can more than offset the advantage gained in strength, by increasing gliding resistance and causing abrasion of the tendon sheath.10 For example, the knots of commonly used epitendinous sutures are commonly placed outside the repair site, on the surface of the flexor tendon. If the knot is located inside the repair side, its friction would be lower.2,11,12 In addition, little is known about the effect of motion cycles on either gliding resistance or the strength of epitendinous sutures. Previous studies have measured the strength and stiffness of epitendinous suture immediately after repair, not after 1,000 cycles.7–9
The purpose of this study was therefore to investigate the effects of 5 different epitendinous suture designs on tendon gliding resistance and strength, using a human cadaver model of zone 2 repair, a standard modified Kessler core repair, and 1,000 cycles of tendon motion.
MATERIALS AND METHODS
Specimen preparation
After institutional review board approval, 4 right hands and 9 left hands of 13 human cadavers were obtained, including 6 men and 7 women, with an average age of 66.1 years (range, 45–96). One hand of each of 13 unique cadavers was used. Altogether, 50 flexor digitorum profundus (FDP) tendons from 12 index, 12 middle, 13 ring, and 13 little fingers were used for this study. Each finger was disarticulated at the metacarpophalangeal joint level, preserving the flexor tendons at the level of the wrist. The FDP tendons were accessed through a transverse incision in the flexor sheath, just distal to the A2 pulley, and marked at that level, both at full passive extension and at full flexion, with a 4.9-N load attached to the proximal FDP tendon to maintain tendon tension. The distance between these 2 marks represented the FDP tendon excursion during full finger motion.
After measurement of gliding resistance in the intact tendons (see method later), a transverse tendon laceration was made 12 mm distal to the proximal FDP tendon mark. This level of transection was chosen in order to ensure movement of the repair site through the pulley system during testing. The laceration level was proximal to the A2 pulley, and thus it was possible to perform the repair without incision of the pulley.
All core tendon repairs were made with a coated, braided polyester (Ethibond; Ethicon, Somerville, NJ) 3-0 suture, using a grasping, modified Kessler suture technique.13 All core suture knots were made inside the repair site, to minimize the frictional force.
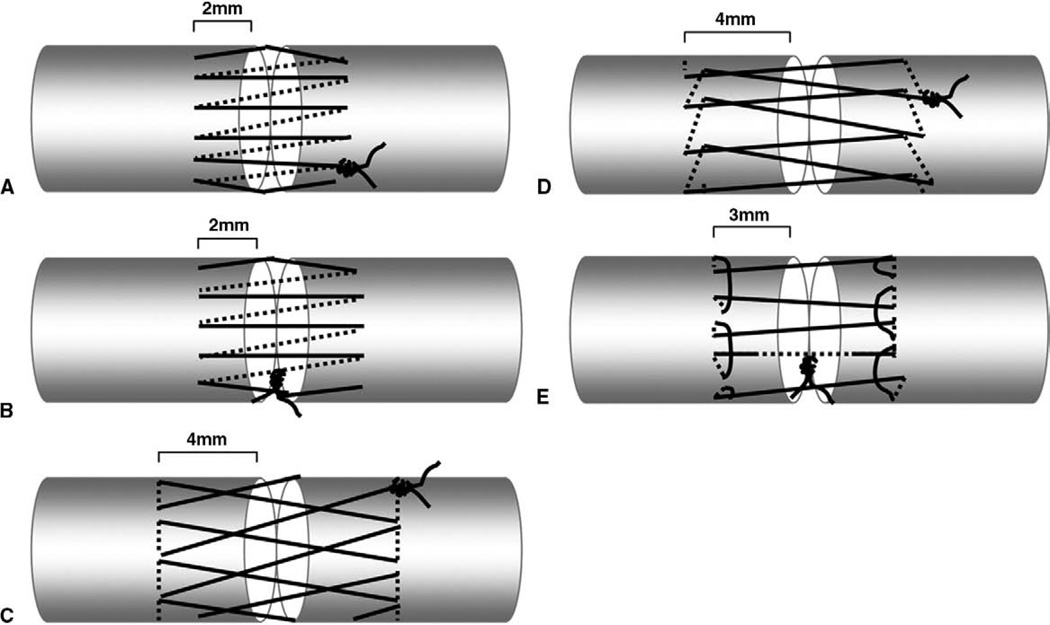
The repaired tendons were then randomly allocated into 5 groups of 10 tendons each. The first group was repaired using a simple, running epitendinous suture whose knot was outside the repair (simple running KO) with the knot on the anterolateral surface of the tendon (Fig. 1A); the second group was repaired using a simple, running epitendinous suture whose knot was inside the repair (simple running KI) (Fig. 1B); the third group was repaired using a cross-stitch suture (cross-stitch) (Fig. 1C);8 the fourth group was repaired using a interlocking horizontal mattress suture (IHM) (Fig. 1D);9 and the fifth group was repaired using a running–locking suture (Fig. 1E).7 All epitendinous tendon repairs were made using a monofilament polypropylene (Prolene; Ethicon, Somerville, NJ) 6-0 suture with a cutting needle. The simple, running epitendinous throws were approximately 2 mm from the lacerated tendon ends, with 1 mm between each pair of stitches. The number of epitendinous throws ranged from 12 to 16, depending on the tendon size.14 The cross-stitch throws were approximately 4 mm from the lacerated tendon ends, with 2mm between each pair of stitches. The number of epitendinous throws ranged from 6 to 8, depending on the tendon size.8 The IHM throws were approximately 4 mm from the lacerated tendon ends, with 2 mm between each pair of stitches. The number of epitendinous throws ranged from 6 to 8, depending on the tendon size.9 The running–locking throws were approximately 3 mm from the lacerated tendon ends, with 2 mm between each pair of stitches. The number of epitendinous throws ranged from 6 to 8, depending on the tendon size.7
FIGURE 1.
Schematic drawings of the 5 epitendinous suture methods. A The simple running KO suture. B The simple running KI suture. C The cross-stitch suture. D The IHM suture. E The running–locking suture.
All core and epitendinous suture knots were tied with 4 square knots, and the tension of all core and epitendinous sutures was set to coapt without causing bunching of the repair site. All repairs were performed by the same investigator (T.M.) to ensure consistency of technique. The A2 pulleys and flexor digitorum superficialis tendons were kept intact during the experiment.
Measurement of friction force
The friction between tendon and pulley was measured using the method of Uchiyama et al.15,16 Briefly, the proximal and middle phalanges, A2 pulley, flexor digitorum superficialis, parietal membrane of A2 pulley, and visceral membrane of FDP were preserved, and the remaining tendon sheath, pulleys, and the distal phalanx were removed. A K-wire was used to fix the proximal interphalangeal joint in the extended position. Each specimen was mounted on the custom testing device with the palmar side upward. To maintain tension in the flexor digitorum superficialis tendon, a 2-N weight was attached to its proximal end. The magnitude of this weight was determined based on previous in vivo tendon research on the magnitude of load experienced by normal tendons in situ.17,18 The measurement system consisted of 1 mechanical actuator with a linear potentiometer, 2 tensile load transducers, and a mechanical pulley. The load transducers were attached to the proximal and distal ends of the FDP tendon. The distal transducer was connected to a 4.9-N weight to simulate passive mobilization of the finger, again based on previous tendon research.17,18 The proximal load transducer was connected to the mechanical actuator.
Based on the experience of previous studies, a set arc of contact, 30° and 20° between the horizontal plane of the phalanx and the proximal and distal transducer cables, respectively, is sufficient to provide adequate measurement of the gliding resistance.11,19 For each cycle of testing, the tendon was pulled proximally by the actuator against the 4.9-N weight at a rate of 2 mm/s. Excursion was limited to the distance between the 2 FDP tendon markers, thus reproducing the tendon excursion from full digit flexion to full digit extension. The forces at the proximal and distal tendon ends and the tendon excursion were recorded. The specimens were kept moist throughout testing by immersion in a saline bath. The force differential between the proximal and distal tendon ends represents the gliding resistance. The mean gliding resistance over the excursion range was calculated for both flexion and extension motion. The flexion and extension means were then averaged and reported as the mean gliding resistance.
The gliding resistance for the intact FDP tendon was initially recorded for 1 cycle. The tendon was then transected and repaired. After repair of the tendon, the gliding resistance was recorded after 1 cycle, and then after every 50 cycles up to 500 cycles, and then after every 100 cycles up to 1,000 cycles.
Tendon repair strength
After gliding resistance testing, the repaired tendon strength was measured using a servohydraulic testing machine (MTS, Minneapolis, MN). The repaired tendon was secured in the testing machine and distracted to failure at a rate of 20 mm/min. A differential, variable reluctance transducer (DVRT; Microstrain, Williston, VT) was attached to the tendon, spanning the repair site, to measure gap formation during testing. Tensile force, grip-to-grip displacement, and gap displacement measured by the DVRT were collected at a rate of 20 Hz. Throughout testing, the tendons were kept moist by spraying with physiologic saline. Maximum breaking force and the force to produce a 2-mm gap were recorded. In addition, a regression line was fit to the linear region of the force versus gap formation (as measured by the DVRT) to measure the resistance to gap formation. The resistance to gap formation, a measure of repair stiffness, in units of N/mm, was obtained from the force versus gap relationship.
Gap measurement
During the 1,000 cycles of tendon motion, we observed for any gap formation. As the repair appeared proximal to the A2 pulley at the beginning of each cycle, gaps could be readily noted. If a visible gap formed, the gap was measured by a caliper, and the related motion cycle number was recorded.
Statistical analysis
A previous study showed that the average gliding resistance of a human FDP tendon before and after repair using 3-0 Ethibond was 0.29 N and 1.12 N, respectively, with a standard deviation of 0.13 N and 0.46 N.6 Using this as a guide, with a sample size of 10 we have 80% power at a significance level of p<.05 to detect a difference in gliding resistance of 0.22 N (20% decrease).
Results of the gliding resistance were presented as mean ± standard deviation (SD). Analysis of variance was used to evaluate the effect of suture technique and testing cycle on gliding resistance; both 1-factor and 2-factor analysis of variance models were used. Because up to 4 digits from 1 hand of each of 13 unique cadavers was used, it was necessary to account for the within-cadaver correlation among the digits in the analysis. This was accomplished by using generalized estimating equations in a generalized, linear models framework. Because of the complexity of the 2-factor model (suture type and cycle number), separate analyses were conducted to evaluate suture technique within each level of testing cycle. For each model, contrast statements were generated to perform pairwise testing of each level of the independent variable (suture technique or testing cycle). The threshold of statistical significance was set at α = 0.05.
RESULTS
Measurement of friction force
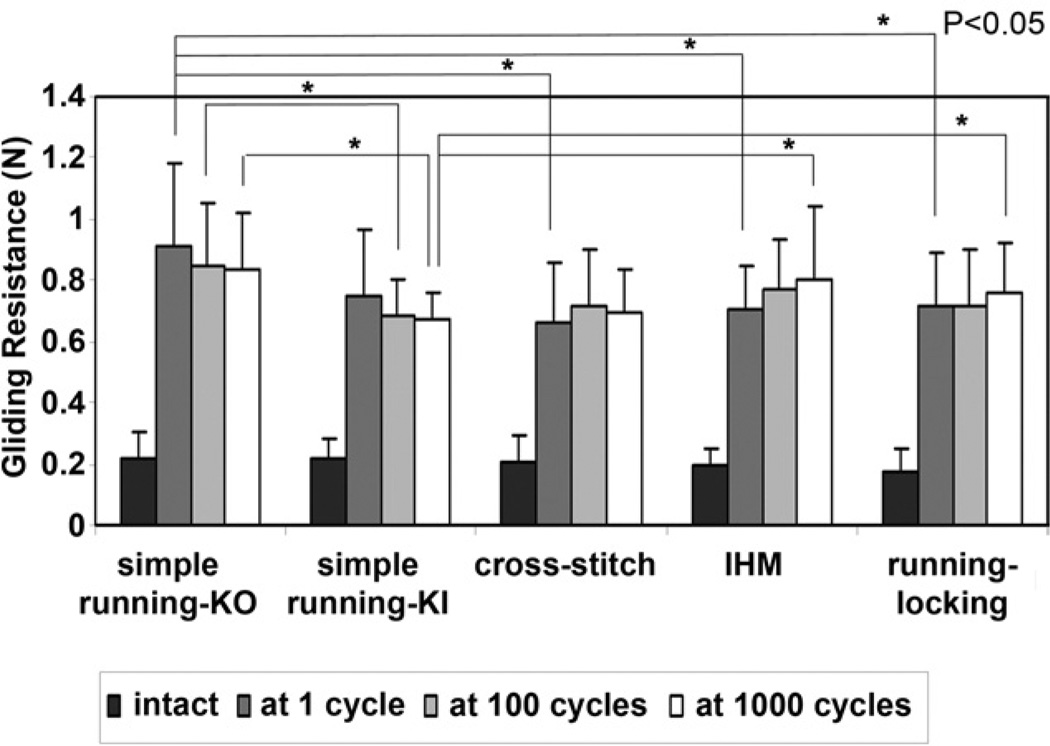
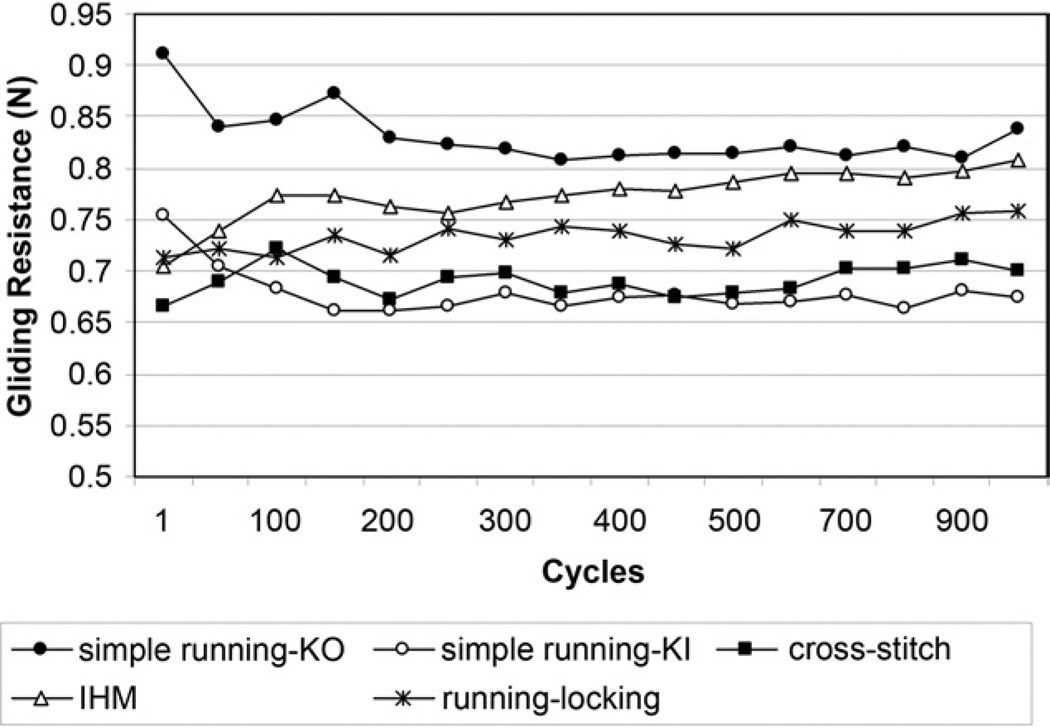
There was no significant difference in the gliding resistance of the intact FDP tendon among the 5 groups (Fig. 2). The gliding resistances during 1,000 cycles of tendon motion are shown in Figure 3. The cross-stitch suture (p=.038), IHM suture (p=.036), and running–locking suture (p= 0.023) had significantly lower gliding resistance than the simple running KO suture at 1 cycle. The simple running KI suture had significantly lower gliding resistance than the simple running KO suture at 100 cycles (p=.039) and 1,000 cycles (p=.021). The simple running KI suture had significantly lower gliding resistance than the IHM suture (p=.039) and running–locking suture (p=.034) at 1,000 cycles (Fig. 2).
FIGURE 2.
Mean gliding resistance of the FDP tendons in the 5 groups for intact tendons and repaired tendons at 1 cycle, 100 cycles, and 1,000 cycles. The error bars represent standard deviations. There was no difference among the intact tendon groups.
FIGURE 3.
Mean gliding resistance of the FDP tendons in the 5 groups at different cycles of tendon motion.
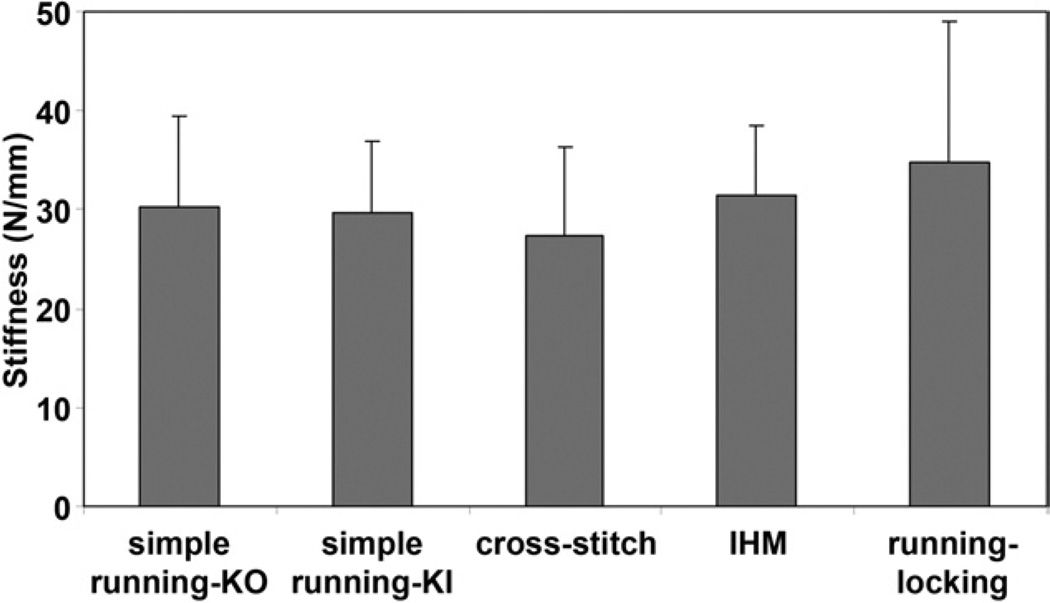
Tendon repair strength
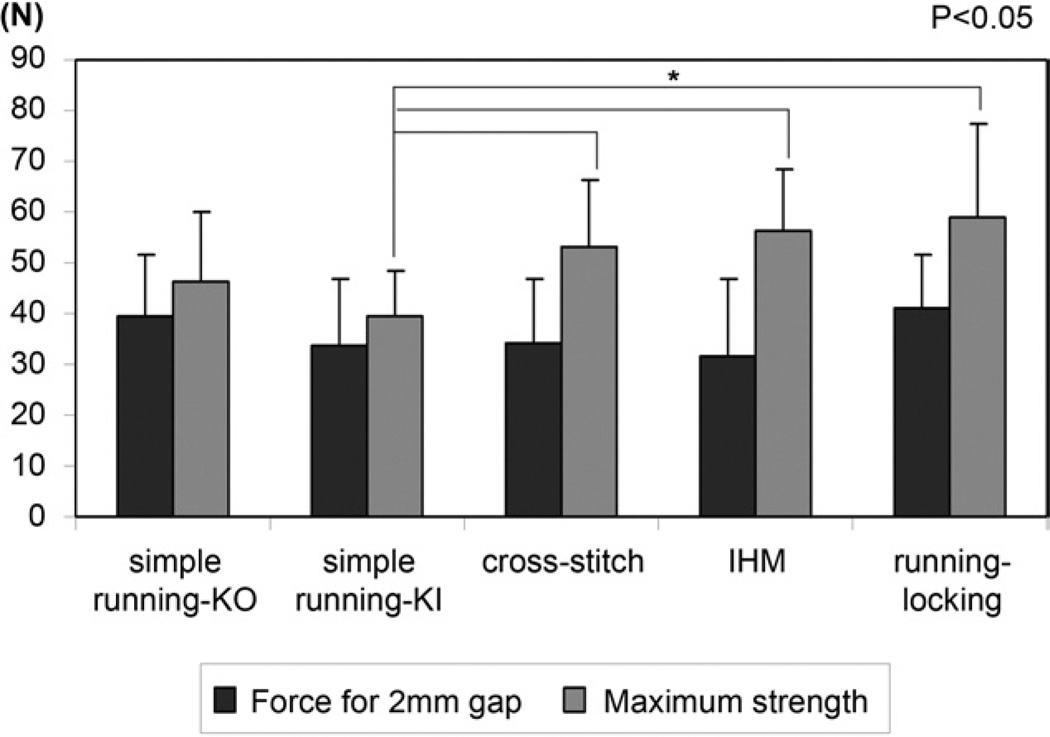
The differences in gap force at 2 mm and stiffness were not statistically significant among 5 groups (Figs. 4, 5). The simple running KI suture had significantly lower maximal failure strength after 1,000 cycles than the cross-stitch suture (p<.0001), IHM suture (p<.0001), and running–locking suture (p=.0012) (Fig. 4). Four repaired tendons in the simple running KO and simple running KI groups failed due to the epitendinous suture pulling out from the tendon stump. Six tendons in the simple running KO and simple running KI repairs and 10 tendons in the cross-stitch, IHM, and running–locking repairs failed by epitendinous suture breakage.
FIGURE 4.
Mean force for 2-mm gap and maximum strength in the 5 groups. The error bars represent standard deviations. There was no difference in force for 2-mm gap among the groups.
FIGURE 5.
Mean stiffness in the 5 groups. The error bars represent standard deviations. There was no difference in stiffness among the groups.
Gap formation
No gap formation was noted in any tendon.
DISCUSSION
Our results show that the simple running KO suture has significantly higher gliding resistance than the simple running KI suture at 100 cycles and 1,000 cycles. This means that even a knot of 6-0 Prolene on the volar surface of the tendon affects the gliding resistance. Momose et al also found that 1 volar knot had a higher gliding resistance than 1 lateral knot.20 We therefore agree with Momose et al. that if knots are placed outside the tendon, they should be placed laterally, not anteriorly. Our result regarding the effect of knot location might also explain why the friction of the running–locking suture is not higher than the otherwise similar cross-stitch and IHM designs; its knot was inside the repair, whereas the knots of the cross-stitch and IHM were on the surface of the tendon. It is possible that the cross-stitch suture and the IHM suture would have lower friction if the knots were inside the repair.
In contrast to the location of the knot, the number of throws did not seem to have an important effect. The repair with the most throws had either the highest or lowest gliding resistance, depending on whether the knot was inside or outside the repair site. The purchase of the throws might have a separate effect, but we could not analyze this separately from the complexity of the surface geometry of the suture, because the simple running repair had a 2-mm purchase and the more complex repairs all had 3- or 4-mm purchases.
The simple running KO also had significantly higher gliding resistance than the cross-stitch, IHM, and running–locking repairs at 1 cycle. We expected the opposite result: that the cross-stitch, IHM, and running–locking repairs would have higher gliding resistance than simple running repairs because their sutures cross complicatedly on the surface of tendon. It is possible that simple running KO does not sufficiently tidy and compress the repair site and that these more complex running sutures act as something of a girdle at the repair site. We did not measure the cross-sectional volume of the repair region, however, and so we cannot document this impression.
In our study, the maximum strength of the cross-stitch suture was 115% as strong as the simple running KO suture. Kim et al.21 reported that ultimate tensile strength of cross-stitch was 245% as strong as simple running KO when both were used to reinforce a modified Kessler repair in a canine model. They fixed 10 strands of epitendinous suture material across the gap for both epitendinous repairs, but we chose 12 to 16 strands of epitendinous suture material across the gap, depending on the tendon size, for both epitendinous repairs. Also, they tested immediately after repair, whereas we tested after 1,000 cycles, so the test conditions were different.21 The maximum strength of IHM and running–locking sutures were 121% and 128% as strong as the simple running KO suture. Again, whereas previous studies reported 171%9 and 377% of the simple running suture strength,7 respectively, they tested immediately after repair, whereas we tested after 1,000 cycles and did not use a core suture, so the test conditions were different.
This study has several limitations. First and most obviously, it was done in vitro, and results may be different in vivo. The DVRT device that we used to measure gap formation might also have affected breaking strength. However, the device was inserted into an intact part of the tendon above and below the suture site and beyond the bite of the sutures themselves. Thus, although the device might damage some tendon fascicles, it does not weaken the repair construct in any way. The DVRT also might have displaced during testing, affecting the gap recordings. However, we directly observed each tendon during testing and found no such displacement. Our testing method was not anatomic, in that the tendon glided against a segment of an annular pulley and not against the intact digital sheath. Thus, the resistance recorded here was merely frictional force, and angles of loading on the tendon were not changed, as they would have been in an intact digit. Second, these peripheral sutures had different throws and different distances between each pair of stitches. These were based on published descriptions, but they could certainly have affected the results, as noted earlier. Third, although every attempt was made to repair the tendons with similar tension, ultimately these are hand made and could vary from tendon to tendon. However, we believe that these effects should be random and should not affect 1 repair type more than another. Fourth, the linear breaking strength, the resistance to gapping, and the stiffness measurements reflected a combination of the peripheral suture strength plus the modified Kessler core suture strength. Because the core suture was the stronger of the 2 elements, its strength might mask differences in strength between the epitendinous suture designs. Finally, we kept all A2 pulleys intact during the experiment. Partial release, “venting” the sheath laterally, and using a pulley plasty are known to improve tendon gliding.22–25 If we had adopted these techniques, the gliding resistance might have been lower with all methods.
In this in vitro test model, the cross-stitch, IHM, and running–locking epitendinous sutures had the best combination of higher strength and lower gliding resistance. Although these findings suggest a potential for these suture types to be preferred as epitendinous sutures, these repairs should first be investigated in vivo to address their effect on tendon healing and adhesion formation.
Acknowledgments
The authors sincerely thank Dirk Larson and Melissa C. Larson for their assistance with the statistical methods and data analysis.
This study was supported by a grant from the Orthopedic Research Review Committee of Mayo Clinic.
Footnotes
No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
REFERENCES
- 1.Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An KN. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg. 2004;29A:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Momose T, Amadio PC, Zhao C, Zobitz ME, Couvreur PJ, An KN. Suture techniques with high breaking strength and low gliding resistance: experiments in the dog flexor digitorum profundus tendon. Acta Orthop Scand. 2001;72:635–641. doi: 10.1080/000164701317269085. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg. 2004;86A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Wade PJ, Muir IF, Hutcheon LL. Primary flexor tendon repair: the mechanical limitations of the modified Kessler technique. J Hand Surg. 1986;11B:71–76. doi: 10.1016/0266-7681(86)90018-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Amadio PC, Tanaka T, Kutsumi K, Tsubone T, Zobitz ME, et al. Effect of gap size on gliding resistance after flexor tendon repair. J Bone Joint Surg. 2004;86A:2482–2488. doi: 10.2106/00004623-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Silva JM, Zhao C, An KN, Zobitz ME, Amadio PC. Gliding resistance and strength of composite sutures in human flexor digitorum profundus tendon repair: an in vitro biomechanical study. J Hand Surg. 2009;34A:87–92. doi: 10.1016/j.jhsa.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin GT, An KN, Amadio PC, Cooney WP., III Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg. 1988;13A:553–558. doi: 10.1016/s0363-5023(88)80094-7. [DOI] [PubMed] [Google Scholar]
- 8.Silfverskiold KL, Andersson CH. Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg. 1993;18A:58–65. doi: 10.1016/0363-5023(93)90246-Y. [DOI] [PubMed] [Google Scholar]
- 9.Dona E, Turner AW, Gianoutsos MP, Walsh WR. Biomechanical properties of four circumferential flexor tendon suture techniques. J Hand Surg. 2003;28A:824–831. doi: 10.1016/s0363-5023(03)00305-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Gliding resistance after repair of partially lacerated human flexor digitorum profundus tendon in vitro. Clin Biomech (Bristol, Avon) 2001;16:696–701. doi: 10.1016/s0268-0033(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 13.Kleinert HE, Schepel S, Gill T. Flexor tendon injuries. Surg Clin North Am. 1981;61:267–286. doi: 10.1016/s0039-6109(16)42381-9. [DOI] [PubMed] [Google Scholar]
- 14.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–876. [PubMed] [Google Scholar]
- 15.Uchiyama S, Amadio PC, Ishikawa J, An KN. Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg. 1997;79A:213–218. [PubMed] [Google Scholar]
- 16.Coert JH, Uchiyama S, Amadio PC, Berglund LJ, An KN. Flexor tendon-pulley interaction after tendon repair. A biomechanical study. J Hand Surg. 1995;20B:573–577. doi: 10.1016/s0266-7681(05)80113-5. [DOI] [PubMed] [Google Scholar]
- 17.Lieber RL, Amiel D, Kaufman KR, Whitney J, Gelberman RH. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg. 1996;21A:957–962. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 18.Schuind F, Garcia-Elias M, Cooney WP, III, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg. 1992;17A:291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg. 1997;79A:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Momose T, Amadio PC, Zhao C, Zobitz ME, An KN. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res. 2000;53:806–811. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Kim PT, Aoki M, Tokita F, Ishii S. Tensile strength of cross-stitch epitenon suture. J Hand Surg. 1996;21B:821–823. doi: 10.1016/s0266-7681(96)80200-2. [DOI] [PubMed] [Google Scholar]
- 22.Strickland JW. Flexor tendon injuries. Part 2. Flexor tendon repair. Orthop Rev. 1986;15:701–721. [PubMed] [Google Scholar]
- 23.Kwai Ben I, Elliiot D. “Venting” or partial release of the A2 and A4 pulleys after repair of zone 2 flexor tendon injuries. J Hand Surg. 1998;23B:649–654. doi: 10.1016/s0266-7681(98)80020-x. [DOI] [PubMed] [Google Scholar]
- 24.Dona E, Walsh WR. Flexor tendon pulley V-Y plasty: an alternative to pulley venting or resection. J Hand Surg. 2006;31B:133–137. doi: 10.1016/j.jhsb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Bakhach J, Sentucq-Rigal J, Mouton P, Boileau R, Panconi B, Guimberteau JC. The Omega “Omega” pulley plasty. A new technique to increase the diameter of the annular flexor digital pulleys [in French] Ann Chir Plast Esthet. 2005;50:705–714. doi: 10.1016/j.anplas.2005.06.002. [DOI] [PubMed] [Google Scholar]