Abstract
Recent evidence suggests that liking and wanting of food rewards can be experimentally dissociated (e.g., Berridge, 1996); this dissociation extends to attenuated neophobia in the present study. Rats tend to eat less of a novel food than a familiar food, a phenomenon called neophobia. The present experiments evaluated whether attenuation of neophobia by prior exposure reflects enhanced liking of the flavor using the Taste Reactivity (TR) test. In Experiment 1, rats given five 10 sec TR trials with water or various concentrations of saccharin solution (0.1%, 0.2%, 0.5%) did not show a change in the number of hedonic reactions displayed across trials. However, in a subsequent consumption test from a bottle containing 0.25% saccharin solution, rats with no prior saccharin exposure (group water) consumed less than rats with prior saccharin exposure; that is they displayed neophobia. In Experiment 2, whether rats received five 10 sec TR trials with water or 0.5% saccharin solution, they did not display a difference in hedonic reactions to 0.25% saccharin solution in two 5 min TR test trials. These results suggest that the attenuation of neophobia is evidenced as an increase in the tendency to approach a bottle containing the flavored solution (wanting), but not as an enhanced liking of that solution.
Keywords: Taste reactivity, neophobia, palatability, taste, latent inhibition, rat
Rats and mice are unable to vomit; therefore, it is especially important for rodents to determine which foods are safe to eat (e.g., Rozin, 1968). Spoiled foods can be identified using olfactory cues and taste can prevent food intake when the food is sour or bitter. Vomiting is an additional line of defence against poisoning in species capable of expelling foods eaten. It has been suggested that the rat has adapted to its lack of vomiting reflex by displaying the behavior of neophobia, an extreme wariness of new foods (e.g., Barnett, 1956; 1958; Rzoska, 1953). Neophobia may serve as the first line of defence in the rat (i.e., smell, taste). This wariness of new foods is extremely highly developed in the rat and perhaps as a result, the defence against toxins produced by vomiting has become redundant and is now non-functional (Andrews & Horn, 2006). In the laboratory rat, it is known that gastric afferents respond to physical and chemical stimulation that precedes vomiting in emetic species, such as the ferret (Blackshaw & Grundy, 1993). In the rat, the detection mechanism is present, but the vomiting response is absent (Andrews & Horn, 2006). In fact, there are anatomical differences in the abdominal oesophagus of emetic species relative to the non-emetic rat; that is the oesophagus is comparatively wider and shorter in emetic species (Andrews, 1995), providing greater propulsive power for expelling gastric contents.
A rat’s tendency to be wary of novel tastes and its strong capacity to learn to avoid tastes paired with illness serve it well in survival. Rats develop much stronger aversions to novel tastes than to familiar tastes (Revusky & Bedarf, 1967; Lubow, 2009). In fact, if a taste is novel, rats learn to avoid that taste despite delays of several hours between the taste and illness in a single trial (Revusky, 1968). According toGarcia et al. (1972), the palatability of a novel flavor is modified by the visceral state of the animal about an hour or two after the meal. If the rat is made ill during this period, the flavor becomes unpalatable. However, if the animal ingests a novel food and no noxious visceral state occurs, then the palatability of that flavor is increased. Therefore,Garcia et al. (1972) suggest that the attenuation of neophobia is reflected in enhancement of the palatability of the flavor—it tastes better to the rat. However, the assessment of neophobia attenuation has consistently been evaluated by a bottle consumption test that includes both the appetitive responding of approach to the bottle and consummatory responding of licking (e.g., Barot & Bernstein, 2005; Green & Parker, 1975; Lin, Roman, St. Andre & Reilly, 2009; Miller & Holzman, 1981; Morón & Gallo, 2006). Not only palatability of the fluid, but also other motivational factors can influence a rat’s tendency to approach a bottle and drink.
The Taste Reactivity (TR) test (Grill & Norgren, 1978) provides a direct measure of the palatability of a taste. The novel flavor is intraorally infused into the rat’s mouth via a permanently implanted cannula for a specified time. In this test the experimenter controls exposure to the flavored solution, and the rat reacts with only the consummatory responses, which reflect the palatability of the solution. The palatability of the solution is measured by the orofacial reactions that the rat displays in the TR test (Grill & Norgren, 1978). A sweet taste elicits the hedonic reactions of tongue protrusions visible at the front of the mouth and distinctive lateral tongue protrusions that emerge at the side of the mouth and sweep back along the lips and prolonged lapping at the target. They are discernible and recognizable patterns (Berridge, 1996). If attenuated neophobia reflects an increase in the palatability of the flavored solution as suggested byGarcia et al. (1972), then the rat should show more hedonic reactions (lateral tongue protrusions, forward tongue protrusions) on subsequent exposures.
According to Berridge (1996), a consumption test measures both liking and wanting, however the TR test only measures liking. Berridge (1996) has shown that wanting and liking are independent processes; liking is a reflection of the palatability of the solution, whereas wanting reflects the likelihood that the rat will reach out to get the solution by approaching the bottle and drinking. Wanting and liking have separate underlying brain substrates (Berridge, 1996). To distinguish between appetite and palatability means to distinguish between the disposition to eat and the sensory “pleasure” of actually eating. The TR test isolates consummatory responses in absence of the approach; measuring the rats “liking” of the taste.
The present experiments are aimed at determining if attenuation of neophobia is accompanied by a change in the ‘liking’ of the taste in the absence of approach using the TR test. If the novel flavour becomes more palatable as it becomes familiar, then rats should display an increase in hedonic reactions over subsequent exposures.
Experiment 1
Subgroups of rats received a brief intraoral infusion of one of four concentrations of saccharin solution (0% [water], 0.1%, 0.25% or 0.5%) on each of five successive days. To ensure that post-ingestive consequences during each daily trial did not change reactivity to the taste, non-deprived rats received a total of 100 µl over 10 sec (see Grill & Norgren, 1978). Different concentrations of saccharin were employed because the neophobic reactions are greater (and their attenuation therefore more profound) in consumption tests with higher concentrations of saccharin (Gilley & Franchina, 1985). The hedonic reactions of rhythmic tongue protrusions (visible in the front of the mouth) and lateral tongue protrusions (emerging at the side of the mouth and sweeping back along the lips) were measured in the TR. There were very few occasions of paw licking (another hedonic reaction displayed during longer TR tests) or any aversive TR reaction on any trial.
Following the final TR test trial, the rats were water deprived over night (to ensure approach to the bottle) and presented with a graduated tube containing 0.25% saccharin solution with intake measures taken at 5 min and 24 hr to assess whether prior taste exposure by intraoral infusion would attenuate neophobia when tested by bottle. A single concentration between 0.1% and 0.5% was used because attenuated neophobia has been shown to generalize across concentrations of saccharin (Gilley & Franchina, 1985). Neophobia attenuation has been reported to occur within 4–6 hr (Green & Parker, 1975; Nachman and Ashe, 1974) following initial exposure to the taste in a consumption test.
Method
Subjects
Male Sprague-Dawley rats weighing 250–335 g, on the first day of testing served as subjects. The animals were obtained from Charles River Laboratories in St. Constant, Quebec. They were individually housed in shoebox cages. The rats had ad-lib access to Purina rat chow and water during the experiment, except as indicated. The rats were maintained on a reversed light/dark cycle (lights off at 07:00 h), at room temperature of 20–22° C and humidity of 60–70%. All conditioning and testing occurred between the hours of 07:45 and 14:00, in the dark cycle. All procedures employed in this experiment were approved by the University of Guelph Institutional Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
Intraoral cannulation
Three days after arriving in the laboratory, the rats were implanted with an intraoral cannula (as previously described, e.g., Rock et al, 2009). The rats were anaesthetized with isofluorane followed by subcutaneous (sc) injection of anafen (7 mg/kg; Merial, Duluth, GA). A thin-walled 15-gauge stainless steel needle was inserted at the back of the neck and directed subcutaneously around the ear and brought out behind the first molar inside the mouth. Intramedic Polyethylene tubing with an inner diameter of 0.86 mm and an outer diameter of 1.27 mm was run through the needle and the needle is removed. A circular rubber adaptor with a diameter of 3.00 mm was then attached to the exposed tubing at the back of the neck. The tubing was held secure in the oral cavity by an O-ring slipped over the tubing just at the back of the neck. Post-operative treatment included intraoral infusions of Nolvadent oral cleansing solution and application of Savlon topically, for a period of 3 days. For conditioning and testing, the cannula was connected to an infusion pump for delivery of a taste by slipping the exposed portion of the cannula into tightly fitted additional tubing attached to the infusion pump.
Procedure
Taste Reactivity Test
Following three days of recovery from intraoral cannulation surgery, on Day 1 and 2 of training, the rats received an adaptation trial in the TR chamber. The rats were individually placed in a Plexiglas TR chamber (22.5 cm X 26.0 cm X 20.0 cm) and their cannula was attached to an infusion pump for fluid delivery. During each adaptation trial, the rats received an infusion of 100 µl of water over 10 sec period after which they were returned to their home cage. All rats received the adaptation for two consecutive days. On training days 3 through 7 the rats were randomly assigned into groups (n = 8/group), which each received a different concentration of saccharin solution (0.0% [water], 0.1%, 0.25%, and 0.5%) mixed in reverse osmosis water at the rate of 100 µl over 10 sec. For each of the five training days the rats received the same concentration of saccharin solution at the same rate of infusion. Orofacial reactions were recorded using a digital video camcorder directed at a mirror at a 45° C angle below the TR chamber.
Bottle Consumption Test
At 4 p.m. on Day 7, the water bottles were removed from the rats home cages. The following day they were presented with a single graduated tube containing 0.25% saccharin solution on the home cage and the amount consumed was recorded after 5 min and 24 hr.
Behavioral measures
The videotapes were scored using The Observer (Noldus Information Technology, Sterling, VA) event recording system. The frequency of forward tongue protrusions (extensions of the tongue out of the front of the mouth) and lateral tongue protrusions (extension of the tongue out of the sides of the mouth, sweeping back along the lips) were scored frame by frame. These were also combined to provide a summed hedonic reaction score. The behaviors were scored by two raters and interrater correlation was assessed (with r’s > .96). The amount of saccharin solution consumed in milliliters (ml) during the one-bottle test provided the measure of the consumption test.
Results
TR Trials
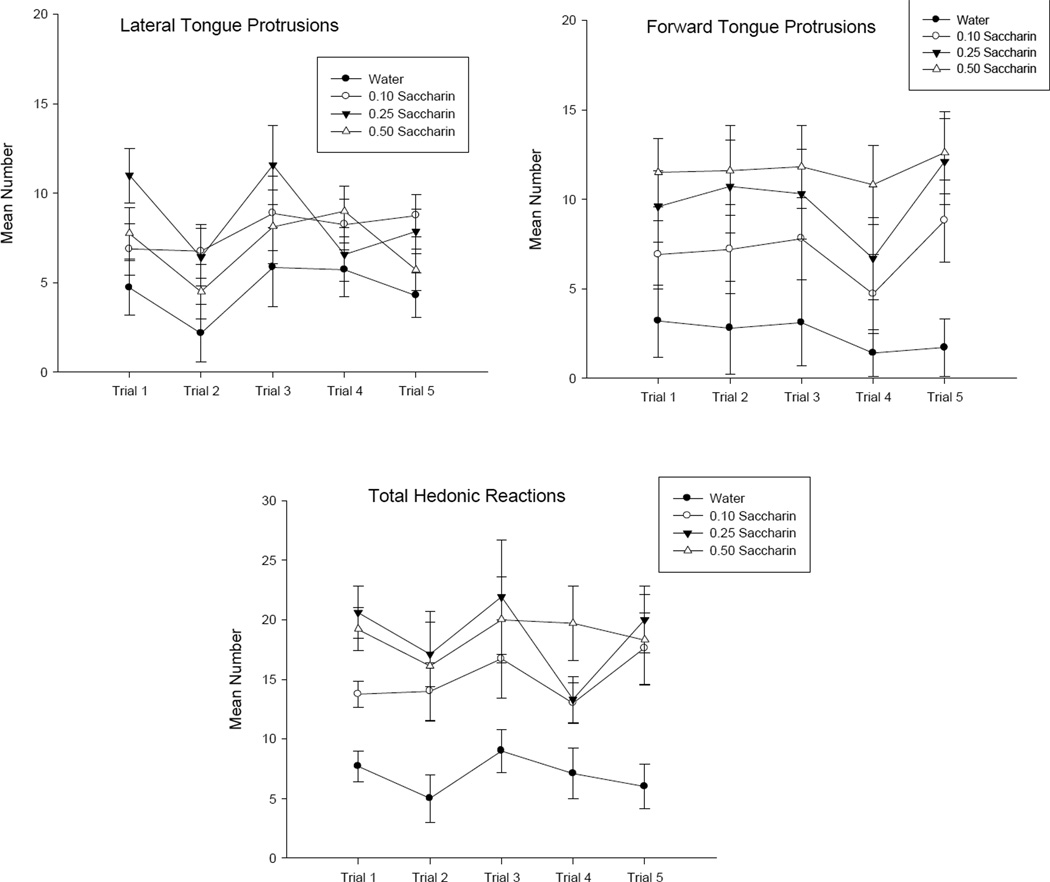
Group Water, the control condition, displayed fewer hedonic reactions than the saccharin groups for each of the reactions scored. Furthermore, there was no evidence of systematic increases in the hedonic responding (attenuation of neophobia) across trials for any group. Figure 1 presents each of the TR hedonic reactions as well as the total hedonic reactions displayed by the various groups. Each of the behaviors and the total hedonic reactions were entered into a 4 by 5 mixed factors analysis of variance (ANOVA) with the between groups factor of concentration and the within groups factor of trials. For the reaction of lateral tongue protrusions (upper right section), the analysis revealed a significant main effect of concentration, F(3, 26) = 8.2; p < .001. Subsequent Bonferroni post-hoc comparison tests revealed that the rats infused with water displayed significantly fewer lateral tongue protrusions than did any other group (p’s < .01), but no other groups differed. The Concentration by Trials interaction was not significant (p >.20) For the reaction of forward tongue protrusions (upper left section), there was also a significant main effect of concentration, F(3, 26) = 5.1; p < .01; rats infused with water displayed significantly fewer forward tongue protrusions than rats infused with either 0.25% or 0.5% saccharin solution (p’s < .05), but they only approached (p = .07) a difference from rats infused with 0.1% saccharin. Neither of the trials effect nor the Concentration by Trials effect was significant (p >.20). Finally, for the total hedonic reaction score (lower section), there was a significant main effect of concentration, F(3,26) = 7.6; p < .001, with rats infused with water showing fewer hedonic reactions than all other groups (p’s < .01). However, the Concentration by Trials effect was not significant (p > .20).
Figure 1.
Mean (±SE) number of lateral tongue protrusions, forward tongue protrusions, and total hedonic reactions elicited by groups exposed to water (filled circles), 0.1% saccharin (unfilled circles), 0.25% saccharin (filled inverted triangle) and 0.5% saccharin (unfilled triangle) during 10-second infusions in the TR trials of Experiment 1.
Bottle Test
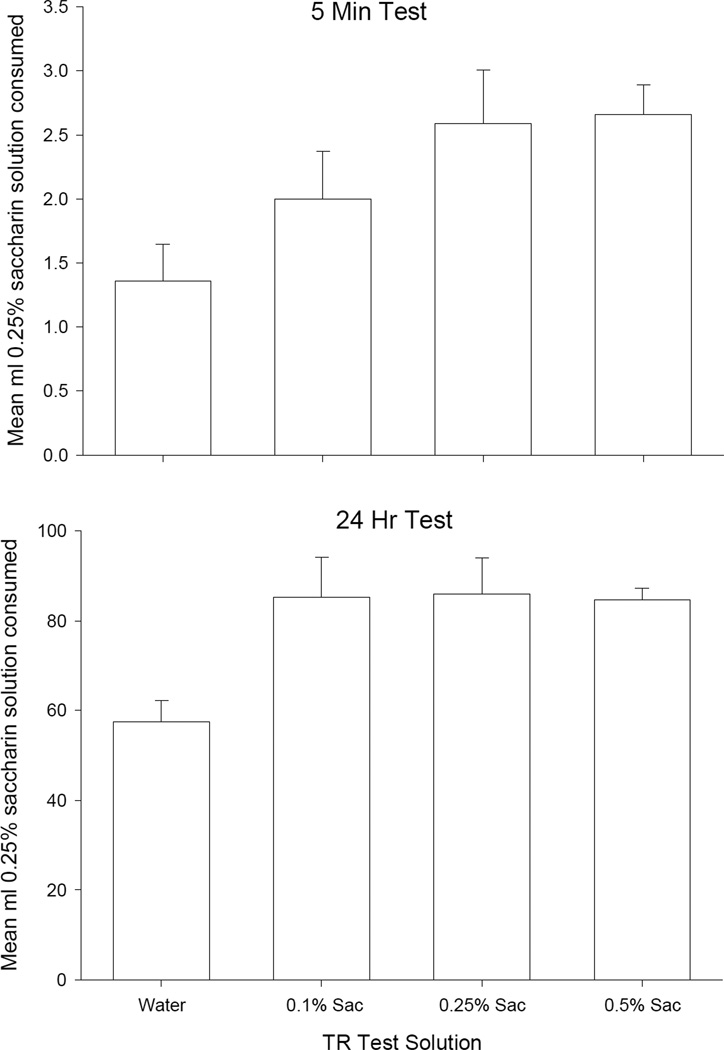
When given either a 5 min test or 24 h test, only rats treated with water during the TR trials displayed neophobia for the 0.25% saccharin solution. The mean ml of 0.25% saccharin solution consumed by the different TR concentration groups after 5 min of drinking (top section) and after 24 h of drinking (bottom section) is presented in Figure 2. A single factor ANOVA revealed a significant effect of TR test concentration for the 5 min test, F(3, 26) = 3.2; p < .05, and for the 24 h test, F(3, 26) = 3.6; p < .05. For the 5 min test, Bonferroni post-hoc comparison tests revealed that rats with no previous saccharin experience (Group Water) drank significantly less saccharin than those rats exposed to 0.25% or 0.5% saccharin solution during the TR tests (p’s < .025). For the 24 h test, rats infused with water in the TR tests drank less saccharin solution than all groups infused with saccharin in the TR tests (p’s < .01).
Figure 2.
Mean (±SE) amount of 0.25% saccharin solution consumed in ml by rats with prior exposure in the TR test to water, 0.1% saccharin, 0.25% saccharin, and 0.5% saccharin during the one-bottle test at 5 min and 24 hr.
Discussion
In Experiment 1, when intraorally infused with various concentrations of saccharin solution across trials, rats did not show any evidence of enhanced hedonic reactions in the TR test. On the other hand, when rats were presented with a 5 min and 24 h bottle test containing 0.25% saccharin solution, prior exposure to saccharin solution by intraoral infusion in the TR test resulted in enhanced consumption. During the 5 min test, rats that had prior exposure to 0.25% and 0.5% saccharin solution in the TR test drank significantly more than those with no saccharin experience. In addition, after 24 hrs, rats with prior exposure to any saccharin solution in the TR test drank significantly more than those with no experience with the taste. These results suggest that the display of attenuated neophobia does not require enhancement of palatability of the taste. On the other hand, the bottle test provided a longer period of flavour exposure (at least 5 min), providing a greater opportunity to display enhanced wanting or liking of the solution than the brief TR test.
Experiment 2
In Experiment 1, the bottle test lasted a minimum of 5 min, but the TR tests were only 10 sec in duration. Therefore, it is possible that the longer opportunity to consume from the bottle revealed the effect of prior experience with saccharin (attenuated neophobia). Therefore, in Experiment 2, rats were given 5, 10-sec infusions of either water or 0.5% saccharin (as in Experiment 1), but instead of a subsequent 5 min bottle test with 0.25% saccharin solution, all rats received a 5 min infusion of 0.25% saccharin solution in the TR test on the subsequent day and again 24 hr later. If neophobia is based upon reluctance to approach the bottle, then the 5 min TR test should show no effect of prior saccharin exposure. If however, neophobia is reflected in the enhancement of the palatability of the flavor, rats will display a greater number of hedonic reactions across trials. The latter effect would suggest that the 10 sec infusions of saccharin solutions across the 5 trials were too brief for the display of increased liking.
Method
Experiment 2 was conducted as Experiment 1, except that during Days 3 – 7, rats received daily 10 sec infusions of water (n = 7) or 0.5% saccharin solution (n = 8). On Days 8 and 9, they were given 5-min (5 ml at the rate of 1 ml/min) intraoral infusions of 0.25% saccharin solution in a non-deprived state. These latter tests trials were scored at a 1/5 speed.
Results
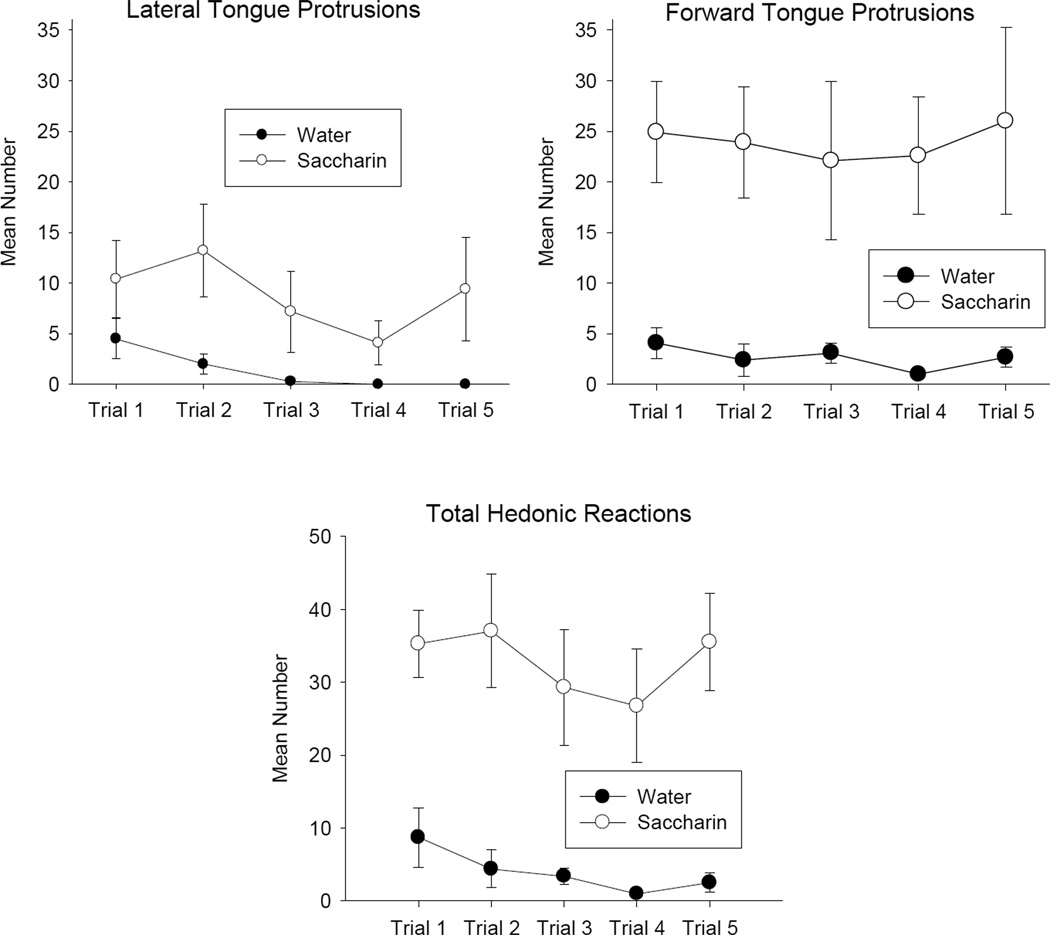
As in Experiment 1, rats in Group 0.5% Saccharin displayed more hedonic reactions than rats in Group Water during the five TR training trials. As well, there was no evidence that saccharin palatability increased as training proceeded. Figure 3 presents the mean (±SE) number of each of the hedonic reaction displayed during each 10 sec trial. As is apparent, the 2 by 5 repeated measures ANOVAs revealed only a significant group (water versus saccharin) effect for each of the responses of lateral tongue protrusions, F(1, 13) = 5.2, p < .05, forward tongue protrusions, F(1,13)= 30.1, p < .001, and total hedonic reactions, F(1, 13) = 24.0, p < .001.
Figure 3.
Mean (±SE) total number of lateral tongue protrusions, forward tongue protrusions, and summed total hedonic reactions elicited by groups exposed to water (filled circles) or 0.5% saccharin solution (open circles) during 10 second infusions in the TR trials of Experiment 2.
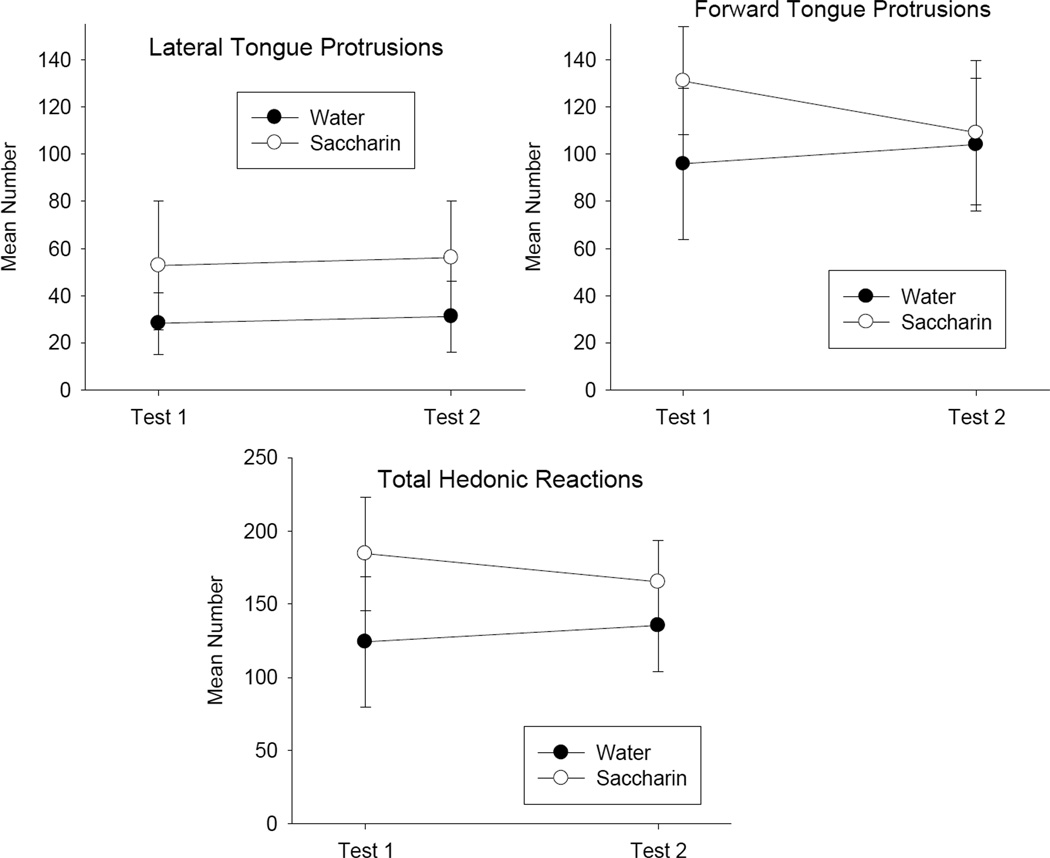
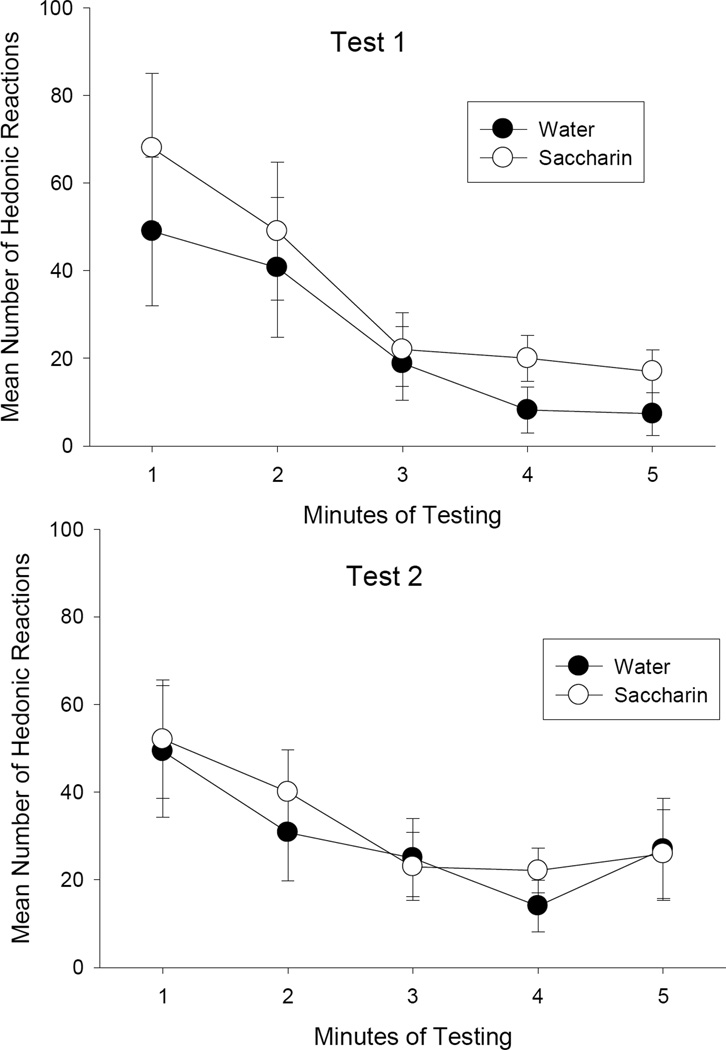
Rats having received 5 prior 10 sec infusions of 0.5% saccharin solution did not show enhanced liking of a .25% saccharin solution in either of two subsequent 5 min infusions. Figure 4 presents the mean (±SE) number of hedonic reactions displayed during the 5 min tests on Test trials 1 (Day 10) and Test Trial 2 (Day 11). The Group (history with water or saccharin) X Trials mixed factors ANOVA revealed no significant effects (p’s > .20). Additionally, to determine whether the initial reaction to saccharin was affected by prior exposure, the number of hedonic reactions was analyzed across five-1 min bins on Test Days 1 and 2 (Day 10 and 11). As depicted in Figure 5, the groups did not significantly differ (p > .20). The 2 by 2 by 5 mixed factors ANOVA revealed only a significant effect of minutes of testing, F(4, 48) = 12.8, p < .001. Although the rats displayed fewer hedonic reactions during the later minutes of testing (possibly a satiation effect) than the earlier minutes of testing, the groups did not differ on the basis of previous experience with saccharin.
Figure 4.
Mean (±SE) number of lateral tongue protrusions, forward tongue protrusions, and total hedonic reactions elicited by 0.25% saccharin solution during each 5 min TR Test Trial (24 hr and 48 hr after last 10 sec infusion) following prior exposure to water or 0.5% saccharin solution.
Figure 5.
Mean (±SE) number of total hedonic reactions elicited by 0.25% saccharin solution during each min of testing during each 5 min test trial (24 hr and 48 hr after last 10 sec infusion) following prior exposure to either water or 0.5% saccharin solution.
General Discussion
Prior brief (10 sec) intraoral exposures to a concentrated saccharin solution enhanced subsequent consumption (over 5 min and over 24 hr) of that flavored solution when presented by bottle in Experiment 1. On the other hand, prior brief exposures to saccharin solution did not enhance subsequent liking of that flavoured solution when presented by intraoral infusion in each of two 5-min TR Tests in Experiment 2. In fact, there was no evidence of enhanced liking of saccharin solution across TR trials when infused in either experiment. The failure to see enhanced hedonic reactions across TR trials in Experiment 1 was not simply the result of the brief duration (10 sec) of the taste exposure, because in Experiment 2, in the two subsequent 5 min TR test trials, rats with prior saccharin exposure did not show enhanced hedonic reactions. Therefore, the results reported here do not support the suggestion by Garcia et al (1972) that attenuated neophobia is the result of enhanced palatability of the flavor.
Reilly and colleagues (see Reilly & Bornovalova 2005, for review; Nachman & Ashe, 1974; Roman et al, 2009; St Andre & Reilly, 2007) have argued that the deficit in conditioned taste avoidance learning evident in rats with lesions of the basolateral amygdala (BLA) or the insular cortex is actually a deficit in their capacity to identify novel foods; that is, they do not display neophobia. If the display of neophobia relies upon the approach component of responding and does not simply reflect an enhanced ‘liking’ of the flavor, then the deficit in conditioned taste avoidance may also represent a deficit is approach rather than a deficit in palatability processing. In support of such an interpretation, Rana and Parker (2008) recently reported that lesions of the BLA interfered with lithium-induced conditioned taste avoidance, but not with lithium-induced conditioned disgust reactions, reflecting ‘dislike” for the taste. Furthermore, in support of the present findings, Rana and Parker (2008) reported no evidence of enhanced liking of the novel flavour of saccharin (0.1%) over subsequent TR exposures among saline conditioned lesioned or sham rats.
In order to ensure that rats would approach the drinking spout during the bottle test of Experiment 1, it was necessary to water deprive the rats overnight. However, the rats were not water deprived during any of the TR tests. One might, therefore, argue that differential water deprivation between tests produced the display of neophobia to the novel saccharin (in Group Water) in the bottle test, but not by TR test. However, the heightened motivation to drink produced by water deprivation would more likely interfere with the display of neophobia than promote the display of this hesitancy to drink. Therefore, it is unlikely that water deprivation (although it might also produce greater stress) prior to the 5-min TR test in Experiment 2 would have suppressed hedonic reactions elicited by novel 0.25% saccharin solution in Group Water.
Our results suggest that attenuated neophobia is not simply an increase in a rats’ ‘liking’ of a flavored solution. Of course failure to observe a pattern of behavior is never definitive. Perhaps our measures were insensitive or the conditions of our experiment were inappropriate to detect a change in palatability of the solution. It is conceivable that longer exposures to the saccharin solution by bottle consumption would have enhanced the palatability of the saccharin solution in the TR tests. However, across a range of saccharin concentrations using both brief (10-sec) exposure tests and longer (5-min) exposure tests, rats showed no evidence of between trials modification in hedonic reactions to saccharin solution on the basis of prior experience with saccharin solution. Regardless of prior taste experience, within a session rats did, however, display suppressed hedonic reactions during the 5-min intraoral infusion of saccharin in Experiment 2 suggesting a satiation effect with prolonged exposure. A similar lack of modification of either ingestive responding or aversive responding between trials using the naturally aversive tastes of HCl and quinine hydrochloride has also been reported (Breslin et al., 1990), suggesting that the effect is not merely a function of the palatability of the flavored solution. Instead, the display of attenuation of neophobia in rats appears to rely upon a test that confounds appetitive and consummatory responding. When the approach component of responding is removed in the TR test, rats show no change in reactivity with prior experience with the tastant.
Acknowledgements
Karly Neath, Cheryl L. Limebeer and Linda A Parker, Department of Psychology, University of Guelph; Steve Reilly, Department of Psychology, University of Illinois at Chicago.
The research reported here was supported by a research grant from the Natural Sciences and Research Council of Canada to LAP and a grant from the National Institute of Deafness and Other Communication Disorders to SR.
References
- Andrews PLR. Why do some animals lack a vomiting reflex? Journal of Physiological Zoology. 1995;68:61. [Google Scholar]
- Andrews PLR, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Autonomic Neuroscience: Basic and Clinical. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. Behavior components in the feeding of wild and laboratory rats. Behavior. 1956;9:24–42. [Google Scholar]
- Barnett SA. Experiments on ‘neophobia’ in wild and laboratory rats. British Journal of Psychology. 1958;49:195–201. doi: 10.1111/j.2044-8295.1958.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Barot SK, Bernstein IL. Polycose taste pre-exposure fails to influence behavioral and neural indices of taste novelty. Behavioral Neuroscience. 2005;119:1640–1647. doi: 10.1037/0735-7044.119.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: Brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibers from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Breslin PAS, Davidson TL, Grill HJ. Conditioned reversal of reactions to normally avoided tastes. Physiology & Behavior. 1990;47:535–538. doi: 10.1016/0031-9384(90)90122-k. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankin WG, Robinson JH, Vogt JL. Bait shyness: tests of CS-US mediation. Physiology & Behavior. 1972;8:807–810. doi: 10.1016/0031-9384(72)90288-0. [DOI] [PubMed] [Google Scholar]
- Gilley DW, Franchina JJ. Effects of pre-exposure flavor concentration of conditioned aversion and neophobia. Behavioral & Neural Biology. 1985;44:503–508. doi: 10.1016/s0163-1047(85)91000-3. [DOI] [PubMed] [Google Scholar]
- Green KF, Parker LA. Gustatory memory: incubation and interference. Behavioral Biology. 1975;13:359–367. doi: 10.1016/s0091-6773(75)91416-9. [DOI] [PubMed] [Google Scholar]
- Grill HC, Norgren R. The taste reactivity test: I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Lin J-Y, Roman C, St. Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brian Research. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 37–57. [Google Scholar]
- Miller RR, Holzman AD. Neophobia: generality and function. Behavioral & Neural Biology. 1981;33:17–44. doi: 10.1016/s0163-1047(81)92202-0. [DOI] [PubMed] [Google Scholar]
- Morón I, Gallo M. Effect of previous taste experiences on taste neophobia in young-adult and aged rats. Physiology & Behavior. 2006;90:308–317. doi: 10.1016/j.physbeh.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. Journal of Comparative and Physiological Psychology. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- Rana SA, Parker LA. Differential effects of neurotoxin-induced lesions of the basolateral amygdala and central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behavioral Brain Research. 2008;189:284–297. doi: 10.1016/j.bbr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova M. Conditioned taste aversion and amygdala lesions in the rat: A critical review. Neuroscience and Biobehavioral Reviews. 2005;29:1067–1088. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Revusky SH. The role of interference in association over a delay. In: Honig W, James H, editors. Animal memory. New York: Academic Press; 1971. pp. 319–366. [Google Scholar]
- Revusky SH, Bedarf EW. Association of illness with prior ingestion of novel foods. Science. 1967;155:219–220. doi: 10.1126/science.155.3759.219. [DOI] [PubMed] [Google Scholar]
- Rock EM, Benzaquen J, Limebeer CL, Parker LA. Potential of the rat model of conditioned gaping to detect nausea produced by rolipram, a phosphodiesterase-4 (PDE4) inhibitor. Pharmacology, Biochemistry & Behavior. 2009;91:537–541. doi: 10.1016/j.pbb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Roman C, Lin J-Y, Reilly S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Research. 2009;1259:68–73. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P. Specific aversions and neophobia resulting from vitamin deficiency or poisoning in half-wild and domestic rats. Journal of Comparative and Physiological Psychology. 1968;66:82–88. doi: 10.1037/h0025974. [DOI] [PubMed] [Google Scholar]
- Rzoska J. Bait shyness, a study in rat behavior. British Journal of Animal Behavior. 1953;1:128–135. [Google Scholar]
- St. Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behavioral Neuroscience. 2007;121:90–99. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]