Abstract
Our previous work showed that immunization of rabbits with 4-hydroxy 2-nonenal-modified Ro60 (HNE-Ro60) accelerates autoimmunity. We extended this model into mice, hypothesizing that the severity of autoimmunity would be dependent on degree of HNE-modification of Ro60. Five groups of BALB/c mice (ten/group) were used. Group I was immunized with Ro60. Groups II to IV were immunized with Ro60 modified with 0.4 mM (low), 2 mM (medium) and 10 mM (high) HNE respectively. Group V controls received Freund's adjuvant. A rapid abrogation of tolerance to Ro60/La antigens occurred in mice immunized with HNE-modified Ro60, especially in the low and medium HNE-Ro60 groups. Lymphocytic infiltration and significantly high decrement in salivary flow (37%) compared to controls was observed only in high HNE-Ro60 group, suggesting induction of a SS-like condition in this group. Anti-dsDNA occurred only in mice immunized with medium HNE-Ro60. This group did not have significant decrement in salivary flow, suggesting induction of SLE-like manifestation in this group. Significantly high antibodies to Ro60 were found in saliva of mice in low and medium HNE-Ro60, and Ro60 groups as well as anti-HNE Ro60 in low and medium HNE-Ro60 groups. Understanding the mechanism of this differential induction may help delineate between these two autoimmune diseases.
Keywords: Sjögren's syndrome, SLE, autoimmunity, epitope spreading, autoantibodies, antigens, 4-hydroxy-2-nonenal, oxidative damage
Introduction
Sjögren's syndrome (SS) is a chronic, inflammatory autoimmune disorder characterized by diminished lacrimal and salivary gland secretion (sicca complex) resulting in keratoconjunctivitis sicca and xerostomia. SS is the second most common autoimmune disorder after rheumatoid arthritis, affecting about 1% of the population. There is a female predilection in this disorder, being more commonly found in women than men, with a ratio of 9:1. Extreme fatigue, dryness of the eyes and mouth, lymphocytic infiltration of the salivary glands and autoantibodies occur characteristically in SS.
Autoantibodies to self antigens is a common factor in SS. Antibodies target the Ro RNP particle, containing a 60 kDa protein (60 kDa Ro or SS-A) non-covalently associated with at least one of four short uridine rich RNAs (the hY RNAs). These hY RNAs are also, at least transiently, associated with the 48,000 MW La (or SSB) autoantigen [1-6]. Antibodies to Ro60 occur in greater than 70% of SS patients. There are other autoantibodies found in sera of SS patients, such as anti-muscarinic receptors antibodies, which may be involved in the pathogenesis of glandular dysfunction and are tissue specific [7].
This disorder is classified as primary when only SS is present. However, secondary SS can also occur along with other inflammatory autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), primary biliary cirrhosis, polymyositis or scleroderma [1,2,8-11].
SLE is also a chronic inflammatory and autoimmune disorder associated with the production of antibodies to self-constituents, including targeting of the Ro60 antigen. Anti-Ro60 occurs in 25-40% of patients with SLE, while anti-La is found in substantially fewer patients [10-13]. The presence of anti-Ro is associated with subacute cutaneous lupus, photosensitive skin rash, deficiency of early complement components, renal disease, neonatal lupus, lymphopenia and neutropenia [14-16]. Anti-Ro60 is associated with interaction of HLA-DQα, HLA-DQβ, and the T cell receptor β [17-22].
The mechanism of the loss of tolerance in SLE or SS patients, resulting in the targeting of the Ro RNP complex, is unknown. Investigations have revealed that if tolerance is broken to one component of an in vivo complex, the immune response can then generalize and expand, so that an entire complex is no longer recognized as self by the immune system [23-26]. This phenomenon of acquiring new autoreactivity as the disease matures is referred to as epitope spreading. When the antigen specific autoimmune response spreads to different epitopes within one protein, then it is referred to as intramolecular epitope spreading. The term intermolecular epitope spreading is applied when the response spreads to epitopes located on other structural/functional proteins.
Oxygen radicals have been shown to be involved in the pathogenesis of several diseases, including SLE [27-32]. Products of oxidative damage have been shown to form adducts with lysine, histidine, cysteine targets [33-37]. HNE (4-hydroxy-2-nonenal) is one of the most common reactive lipid peroxidation by-products [38]. Elevated levels of proteins modified by HNE have been detected in the sera of children with autoimmune diseases [29]. HNE-protein adducts are potential neoantigens, and therefore could be involved in the pathogenesis of autoimmune diseases.
Therefore, we hypothesized that oxidative by-products, like HNE, would cross link with Ro60 and help initiate autoimmunity. To test this hypothesis we immunized rabbits with either the HNE-modified Ro or the unmodified Ro. Our results demonstrated that autoimmunity is established faster and more vigorously in the animals that were immunized with HNE modified Ro60 [39]. Specific and vigorous intra- and inter-molecular epitope spreading occurred when the animal was immunized with the HNE-modified Ro and not with unmodified Ro.
We undertook this study to carry out these studies in mice, where genetic manipulation is possible and to determine whether varying degrees of HNE modification gave differing outcomes.
Materials and methods
Materials
γ-irradiated mouse chow was from Picolab Rodent Diet 20, LabDiet, St. Louis, MO. Ro60 antigen was purchased from Immunovision, Springdale, AK. Avertin, isoproterenol and amyl alcohol were from Sigma Chemical Co, St. Louis, MO. Non-heparinized capillary tubes for saliva collection was from Fisher Scientific, St. Louis, MO. 4-hydroxy-2-nonenal was from Cayman Scientific, Ann Arbor, MI. Crithidia lucillae immunofluorescent anti-nDNA and ANA test kits were from Binding Site, San Diego, CA/Inova Diagnostics, San Diego, CA. Anti-rabbit IgG fluoroisothiocyanate was from Jackson Laboratories, Bar Harbor, ME. All other chemicals were of reagent grade.
Animals
Four week old female BALB/c mice were purchased from the Jackson Laboratory, Bar Harbor, Maine. The animals were housed and acclimatized at the Laboratory Animal Resource Facility at the Oklahoma Medical Research Foundation on a 12 h light/dark cycle. Mice were fed standard γ-irradiated mouse chow and acidified water ad libitum. All experimental designs were approved by the OMRF and University of Oklahoma Health Sciences Center (OUHSC) Institutional Animal Care and Use Committee according to established guidelines.
Preparation of antigen for immunization
Purified Ro60 (1 mg/ml) was modified by the addition of either 400 μM (low), 2 mM (medium) or 10 mM (high) HNE at room temperature for 24 hour in the presence of 1 mM sodium cyanoborohydride. The modified proteins were then dialyzed against 0.1M NaCl using a 10, 000 molecular weight cut-off membrane. Ro60 treated with sodium cyanoborohydride and dialyzed simultaneously with the HNE-modified Ro60, served as the control [39]. Aggregates were seen in Ro60 sample modified with 10 mM HNE.
HNE modification of bovine serum albumin
One mg of BSA was incubated with 5 mM Tris-HCl, pH 8.3 and 5 mM of HNE for 7 hours at 37 °C. The unbound HNE was removed by centrifuging the sample with a 10,000 molecular weight cut-off spin microfuge from Millipore, Billerica, MA. HNE-modification was verified by immunoblotting, using a commercial anti-HNE antibody. The HNE-modified BSA was used as the solid phase antigen in ELISA to determine anti-HNE antibodies in the mice sera.
Ro60 Immunization
Female BALB/c mice (6 weeks of age) were immunized as previously described [24]. Briefly, mice were immunized intraperitoneally with 50 μg of the unmodified Ro60 or the Ro60 modified with different amounts of HNE in complete Freund's adjuvant. Subcutaneous boosting was performed with the antigens suspended incomplete Freund's adjuvant on days 14, 35, 51 and 63. Animals were bled on days 21, 42, 58, 70 and sera obtained were stored at -20° C until use. All animals had pre-immunization serum collected.
Enzyme linked immunosorbant assay (ELISA)
A solid phase ELISA for Ro60, HNE-Ro60, Sm and nRNP, anti-HNE were performed as previously described [4,12]. In the case of anti-HNE ELISA, the primary sera were diluted 1:500 in 1% BSA containing 0.05% Tween-20 to block possible anti-BSA antibodies.
SDS PAGE and immunoblotting
Gradient (4-20 %) and 10 % SDS PAGE were carried out according to Laemmli [40,41] and the gel transferred to nitrocellulose. Standard immunoblotting was carried out according to Towbin et al. [42,43].
Peptide mass fingerprinting
Peptide mass fingerprinting for the identification of salivary proteins was conducted as described before [16,44]. Briefly, a protein band of Coomassie blue-stained SDS-PAGE gel was excised and destained with 50% methyl cyanide (CH3CN)/100 mM ammonium hydrogen carbonate (NH4HCO3) for 16 h. The gel pieces were dried, digested with 0.005 % tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Promega, Madison, WI) for 4 h, and the peptide solution was recovered. The remaining gel piece was further extracted by shaking with 50 % CH3CN/0.5 % trifluoroacetic acid (TFA) for 30 min, and the peptide solution was recovered. Both peptide solutions were combined and concentrated on a SpeedVac concentrator (Thermo Electron Corporation, Waltham, MA) for 90 min. Peptides were dissolved in 5 μl of 0.2 % TFA; and 0.5 μl of aliquots were mixed with 0.5 μl of matrix solution containing 1 % a-cyano-4-hydroxycinnamic acid, 50 % CH3CN, and 0.1% TFA. The peptide/matrix solution was applied to a target plate. Mass spectra were obtained using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF-MS) (Voyger Elite, Applied BioSystems, Foster City, CA). The MS spectra were analyzed in the positive ion mode and the mass peaks were assigned by PerSeptive GRAMS/386 v3.02. The assigned peak values of peptide masses were searched against the nonredundant database at the National Center for Biotechnology Information (NCBInr) by using the Mascot program (http://www.matrixscience.com) and probability-based MOWSE (Molecular Weight Search) scores [45] were obtained. The score was expressed as −10*log(P), where P is the probability that the observed match is a random event. Individual MOWSE scores of more than 62 (p<0.05) for Mus musculus indicate identity or extensive homology.
Detection of antibodies to double stranded DNA by CLIF
Anti-dsDNA assays using Crithidia lucillae immunofluorescence (CLIF) dsDNA kit was carried out according to manufacturer's instructions (Binding Site, San Diego, CA).
Anti-nuclear antibody (ANA) testing
ANA using HEp-2 cells was carried out following the instructions of the manufacturer (ANOVA, San Diego, CA).
Histopathology
Salivary glands were excised from mice on day 126 of the immunization protocol. The gland from each animal was fixed immediately in 10% formalin and embedded in paraffin. Thin sections were cut and stained with H&E. Lymphocytic infiltrates (>50 cells each) were scored by an observer who was blinded to the immunization group of individual mouse.
Results
Antibodies to Ro60
Antibodies to Ro60 and HNE-modified Ro60 in sera were determined by ELISA using either unmodified Ro60 or Ro60 modified by low, moderate or high HNE as solid phase antigens. In general, a rapid abrogation of tolerance to Ro60 was observed in the mice immunized with HNE-modified Ro60. Mice immunized with Freund's alone did not make antibodies to Ro60 or other antigens studied.
i. Using Ro60 as solid phase antigen
Mice immunized with high-HNE Ro60, did not show a strong anti-Ro60 response compared to Ro60 alone, low HNE Ro60 or moderate HNE Ro 60 immunized mice when Ro60 was used as the solid phase substrate (Figure 1). Anti-Ro60 levels in the high HNE Ro60 immunized group continued to remain at levels that were half that of the Ro60 immunized mice up to the 8th bleed (data not shown). On the other hand antibodies to Ro60 were found to be induced twice as fast by the first bleed in mice that were immunized with moderate-HNE Ro60 compared to unmodified Ro60 immunized group (Figure 1). However, by the fourth bleed the Ro60, low HNE Ro60 and moderate HNE Ro60 immunized mice showed similar antibody response.
Figure 1.
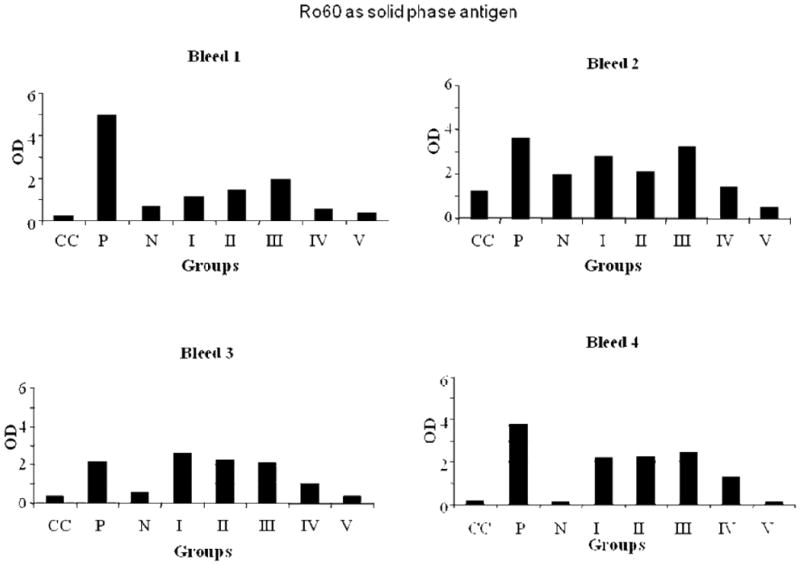
ELISA results of Ro60, used as solid phase antigen, bound by mice bleeds 1-4. Group I-mice immunized with unmodified Ro60; Group II-mice immunized with low HNE Ro60; Group III-mice immunized with moderate HNE Ro60; Group IV-mice immunized with high HNE Ro60; Group V-mice immunized with Freund's alone. CC refers to conjugate control. P refers to positive control and N refers to normal control.
ii. Using low, moderate or high HNE Ro60 as the solid phase antigen
Antibodies to HNE Ro60 were found to be present at high levels in mice immunized with low, moderate or high HNE Ro60 (Figures 1-4). Antibodies to Ro60 in Ro60 immunized mice were significantly lower than low, moderate or high HNE Ro60 immunized mice when either low, moderate or high HNE-modified Ro60 was used as the solid phase antigen in ELISA (Figures 2-4). Moderate-HNE modified Ro60 immunized group had the highest levels of anti-Ro60 antibody levels when either Ro60 or the HNE-modified Ro60 antigens were used as the solid phase antigen (Figures 2-4). Anti-Ro60 levels in Ro60 immunized mice and anti-HNE Ro levels in low HNE Ro60 and moderate HNE Ro60 immunized mice remain high till the 8th bleed (data not shown).
Figure 4.
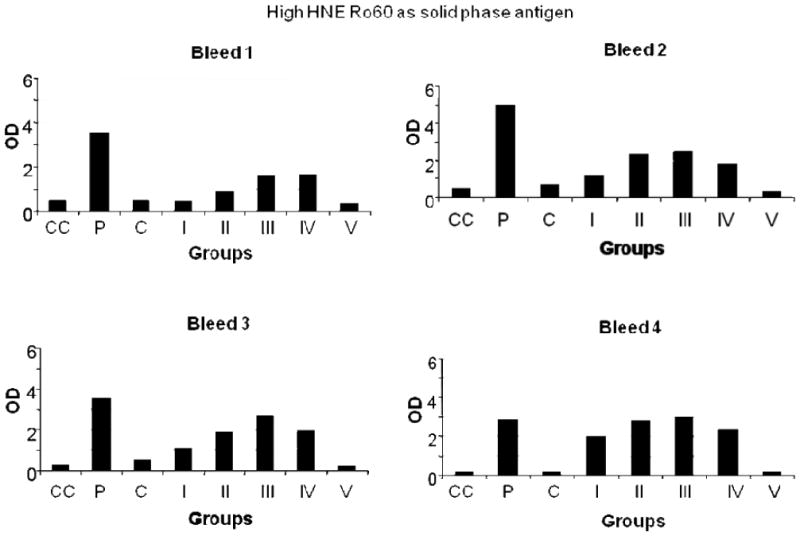
ELISA results of high HNE Ro60, used as solid phase antigen, bound by mice bleeds 1-4. Group I-mice immunized with unmodified Ro60; Group II-mice immunized with low HNE Ro60; Group III-mice immunized with moderate HNE Ro60; Group IV-mice immunized with high HNE Ro60; Group V-mice immunized with Freund's alone. CC refers to conjugate control. P refers to positive control and N refers to normal control.
Figure 2.
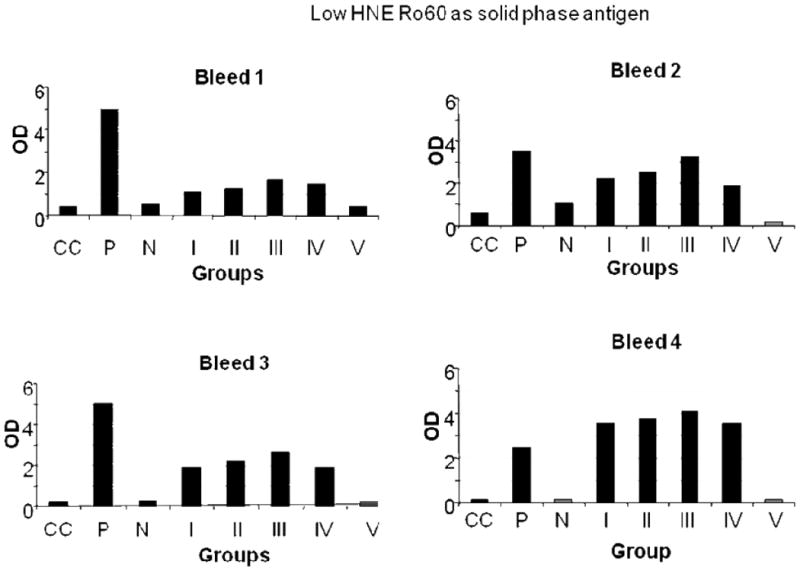
ELISA results of low HNE Ro60, used as solid phase antigen, bound by mice bleeds 1-4. Group I-mice immunized with unmodified Ro60; Group II-mice immunized with low HNE Ro60; Group III-mice immunized with moderate HNE Ro60; Group IV-mice immunized with high HNE Ro60; Group V-mice immunized with Freund's alone. CC refers to conjugate control. P refers to positive control and N refers to normal control.
Antibodies to La
A rapid loss of tolerance to La was observed in the sera of mice immunized with HNE-modified Ro60. Mice immunized with Freund's alone did not make antibodies to La or other antigens studied. Antibodies to the La autoantigen were detected as early as the first bleed in mice immunized with low-and medium-HNE modified Ro60 antigens (Figures 5,6). However, by the second bleed all mice groups except high HNE Ro60 and Freund's only control groups made antibodies to La (Figures 5,6).
Figure 5.
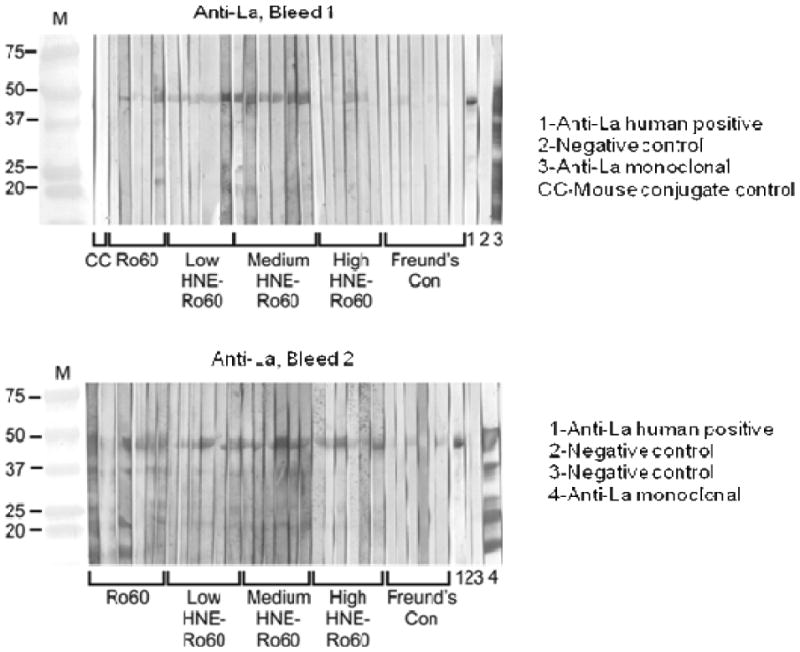
Immunoblot analysis of intermolecular epitope spreading to La autoantigen in the Ro60, Freund's adjuvant and low, medium or high HNE-modified Ro60 immunized animals. Immunoblotting was carried as mentioned in “Materials and methods”. Top panel-Bleed 1; Bottom panel-Bleed 2.
Figure 6.
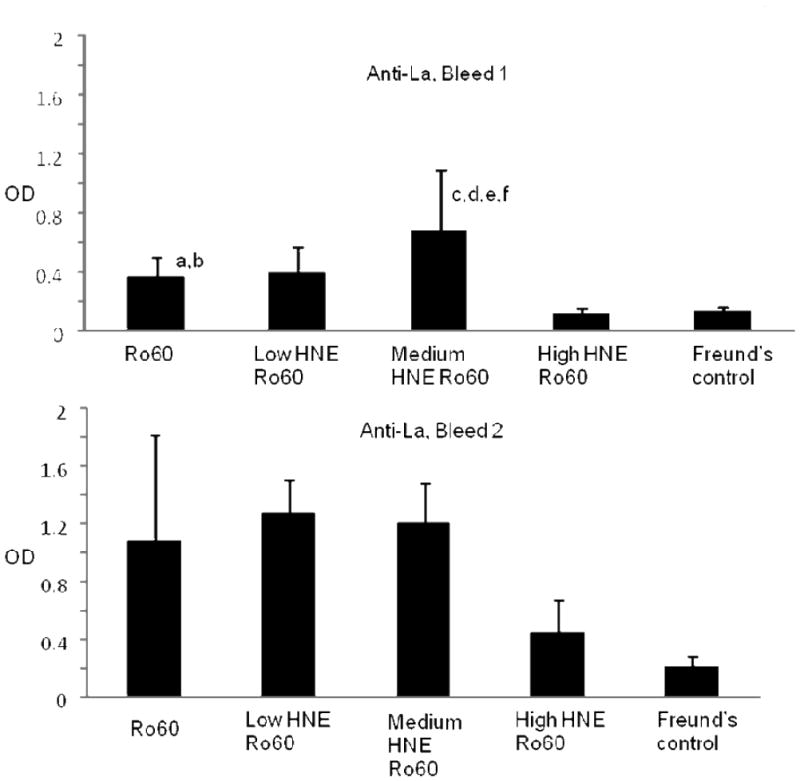
ELISA analysis of intermolecular epitope spreading to La autoantigen in the Ro60, Freund's adjuvant and low, medium or high HNE-modified Ro60 immunized animals. Top panel-Bleed 1; Bottom panel-Bleed 2. ELISA was carried out as mentioned in “Materials and methods”. Values are means ± standard deviation (8 animals in Ro 60 group and Freund's control; 10 animals each in the other three groups), a= p<0.000046 compared to high HNE Ro60 group, b= p< 0.00021 compared to Freund's control group, c= p< 0.053 compared to Ro60 group, d=p< 0.056 compared to low HNE group, e= p< 0.00006 compared to high HNE group, f= p< 0.000158 compared to Freund's control group
Antibodies to double stranded DNA and anti-nuclear antibody (ANA)
Antibodies to dsDNA, determined using CLIF, were observed only in the sera of mice immunized with moderate HNE-modified Ro60 (bleeds 6, 7) (4/4 mice in each bleed) (Figure 7). Mice immunized with unmodified Ro60, low HNE-modified Ro60, high HNE-modified Ro60 or Freund's did not have anti-dsDNA antibodies in these bleeds (Figures 7), suggesting that immunization with moderate HNE Ro60 induces a lupus like condition in BALB/c mice. ANA indirect immunoflourescence mainly showed speckled patterns in the sera of Ro60, low and medium HNE Ro60 immunized mice. Freund's control sera did not have positive ANAs (Table 1).
Figure 7.
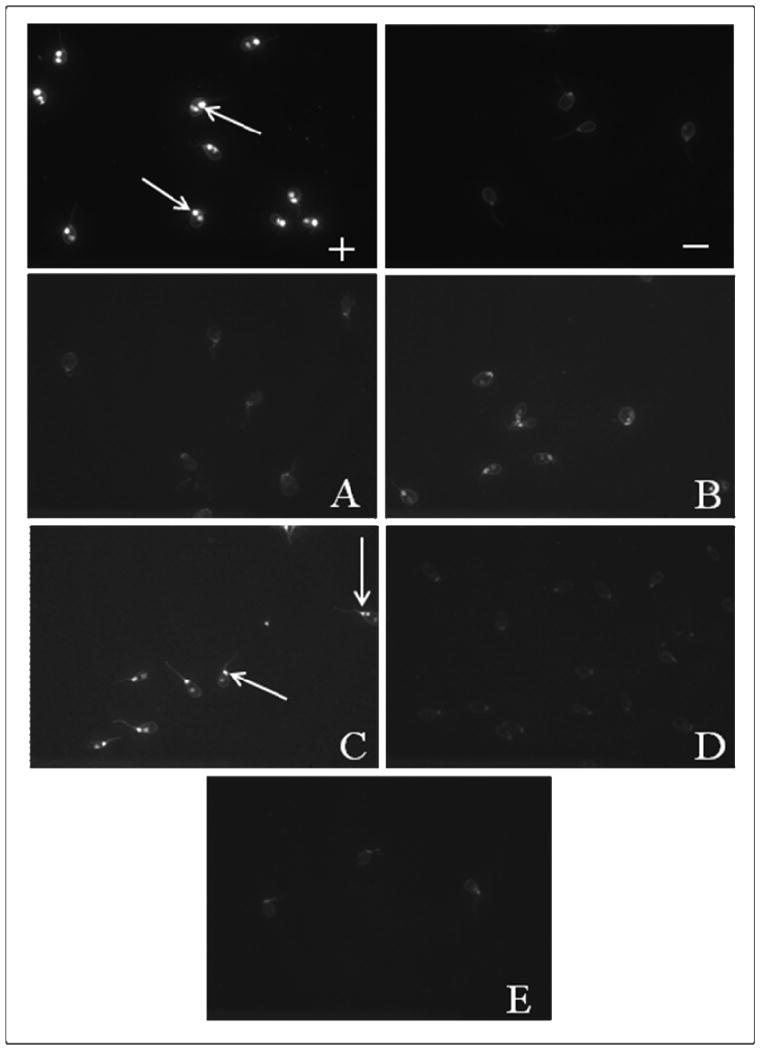
Antibodies to dsDNA detected by CLIF assay in mice immunized with Ro60, Freund's adjuvant and low, medium or high HNE-modified Ro60. “+” refers to human positive control, and “-” to human negative control supplied in the kit. A: Ro60 immunized group; B: Low HNE Ro60 group. C: moderate-HNE Ro60 group; D: high-HNE Ro60 group. E: Freund's control
Table 1.
ANA results of anti-Ro and anti-HNE Ro antibodies in experimental mice binding to HEp-2 cell substrate. Ro60, low and medium HNE Ro60 mice bind strongly, while high HNE Ro60 immunized mice binds only moderately. Freund's control do not bind at all. ‘↑’ refers to high binding; ‘→’ refers to moderate binding; ‘↓’ refers to no binding.
| Bleed 1 (1:80) | ANA Intensity | Samples Tested |
|---|---|---|
| Ro60 | ↑↑↑ | 3 |
| Low HNE-Ro60 | ↑↑↑→ | 4 |
| Med HNE-Ro60 | ↑↑→→ | 4 |
| High HNE-Ro60 | ↑→→→ | 4 |
| Freund's Con | ↓→ | 2 |
| Bleed 1 (1:40) | ANA Intensity | Samples Tested |
| Ro60 | ↑↑↑ | 3 |
| Low HNE-Ro60 | ↑↑↑→ | 4 |
| Med HNE-Ro60 | ↑↑↑→ | 4 |
| High HNE-Ro60 | ↑↑↑→→ | 5 |
| Freund's Con | →→ | 2 |
| Bleed 2 (1:40) | ANA Intensity | Samples Tested |
| Ro60 | ↑↑→→ | 4 |
| Low HNE-Ro60 | ↑↑→→ | 4 |
| Med HNE-Ro60 | ↑↑→→ | 4 |
| High HNE-Ro60 | ↑→→→ | 4 |
| Freund's Con | ↑↓↓ | 3 |
| Bleed 6 (1:40) | ANA Intensity | Samples Tested |
| Ro60 | ↑↑↑↑ | 4 |
| Low HNE-Ro60 | ↑↑↑→→ | 5 |
| Med HNE-Ro60 | ↑↑→→ | 4 |
| High HNE-Ro60 | ↑→→→ | 4 |
| Freund's Con | ↑→↓↓ | 4 |
Salivary flow and salivary infiltrates
Salivary flow, collected 120 days following immunization, decreased significantly (37%) (p<0.05, Kruskal-Wallis rank order testing) only in the mice immunized with high HNE modified Ro60 compared to Freund's (Figure 8).
Figure 8.
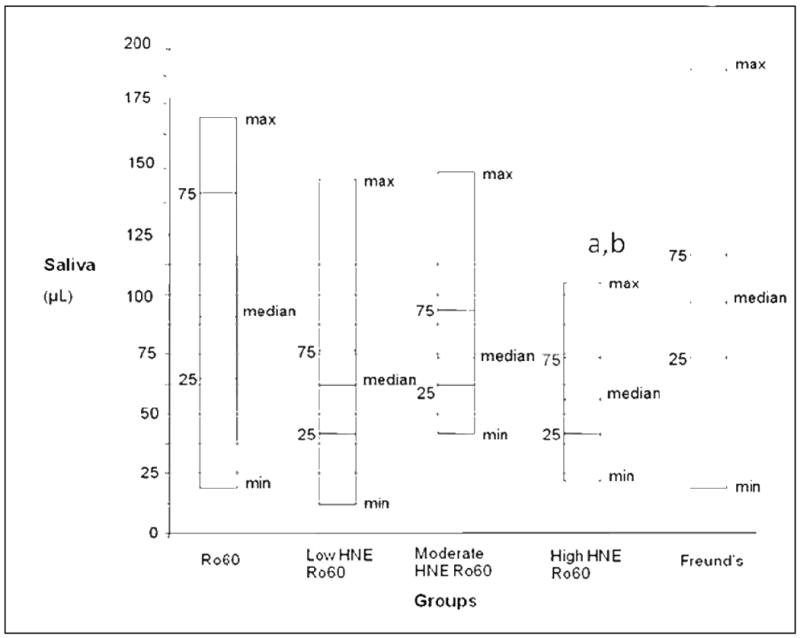
Salivary flow in mice immunized with unmodified Ro60, low HNE Ro60, medium HNE Ro 60, high HNE Ro60 and Freund's adjuvant only. Minimum, 25%tile, median, 75%tile and maximum are shown with 10 animals in each group. Salivary flow is measured as mentioned in “Materials and methods”., a= p<0.05 compared to Ro60 group, Kruskal-Wallis rank order testing, b= p< 0.05 compared to Freund's control group, Kruskal-Wallis rank order testing
Lymphocytic infiltration was observed in the high HNE modified Ro60 group but not in Freund's control (Figure 9), suggesting that immunization with high HNE Ro60 brings about a SS-like condition in these mice.
Figure 9.
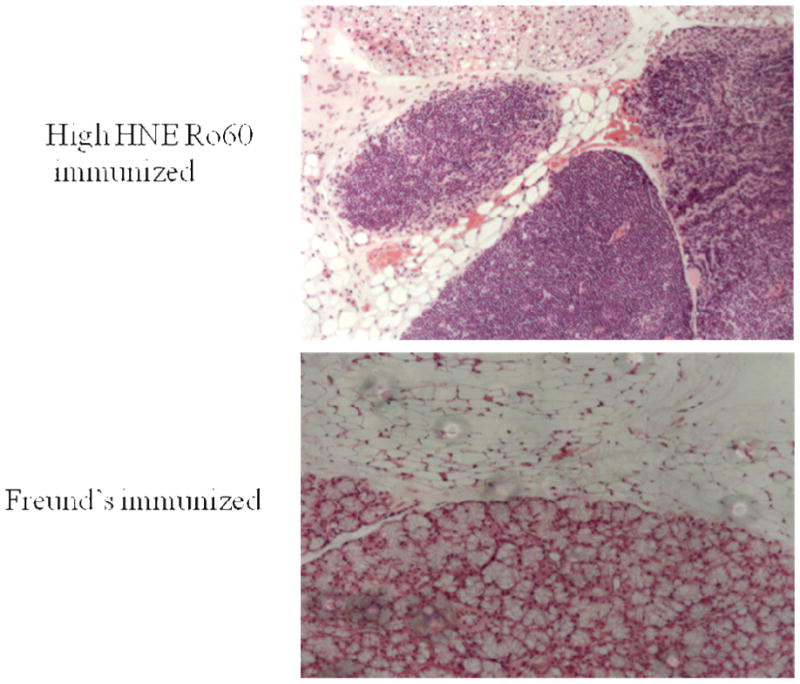
Pathology of salivary glands from animals immunized with high HNE Ro60 or Freund's only. Salivary lymphocytic infiltrates in an animal immunized with high HNE-modified Ro60 (Top Panel) and in an animal immunized with Freund's (Bottom panel).
Identification of salivary proteins by tryptic digestion and MALDI-TOF Mass spectrometry
Saliva collected from the different groups of mice was subjected to SDS-PAGE and Coomassie Brilliant Blue staining [46,47]. The band that showed differences in protein intensity was excised and subjected to peptide mass fingerprinting as described in Materials and Methods. The tryptic fragments produced by the antigen matched those produced from Mus musculus alpha-amylase with Mowse score of 95, corresponding to p=0.000025. Alpha-amylase was found to be decreased in all the groups, particularly the group immunized with high-HNE Ro60, compared to Freund's only immunized group (data not shown).
Antibodies to Ro60 and HNE-Ro60 in saliva of Ro60, HNE-Ro60 and Freund's control mice
Significantly high levels of anti-Ro60 antibodies were found in the saliva of mice in the first three groups (p=0.017; p=0.002; p<0.001 respectively) but not in the group immunized with the high HNE modified Ro60 and Freund's. Significantly high levels of anti-HNE Ro60 antibodies (p<0.0001) were observed only in the saliva of mice immunized with low, medium and high HNE modified Ro60 antigens. (Figures 10,11). There was no anti-Sm or anti-La in the saliva of the immunized groups (data not shown).
Figure 10.
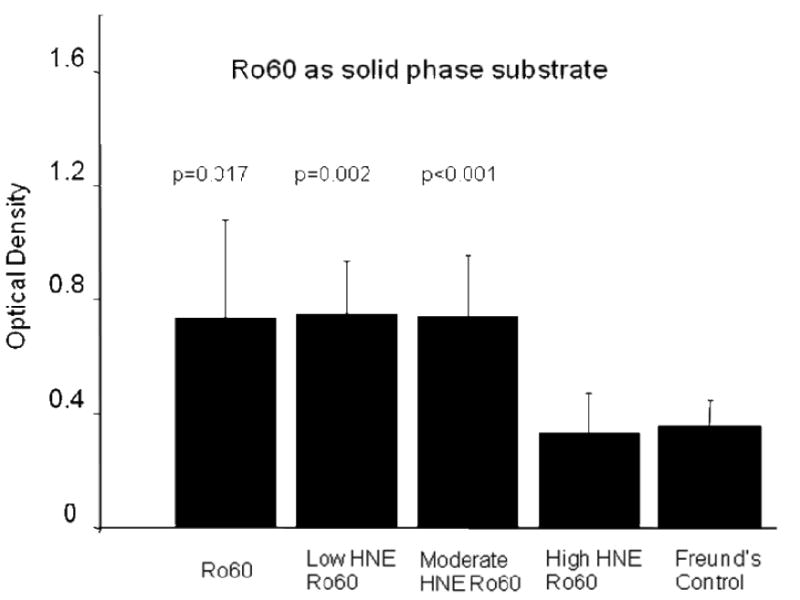
ELISA analysis of anti Ro60 antibodies in the saliva of mice immunized with Ro60, Freund's adjuvant and low, medium or high HNE-modified Ro60 (Groups I-V) using unmodified Ro60 as solid phase substrate. Group I: Mice immunized with unmodified Ro60; Group II: Mice immunized with Ro60 modified with 0.2 mM HNE (low HNE); Group III: Mice immunized Ro60 modified with 2 mM HNE (medium HNE); Group IV: Mice immunized Ro60 modified with 10 mM HNE (high HNE); Group V: Mice immunized with Freund's alone. Antibody response is evident in Ro, HNE 1 and 2 groups. Values are means ± SD for 4 determinations.
Figure 11.
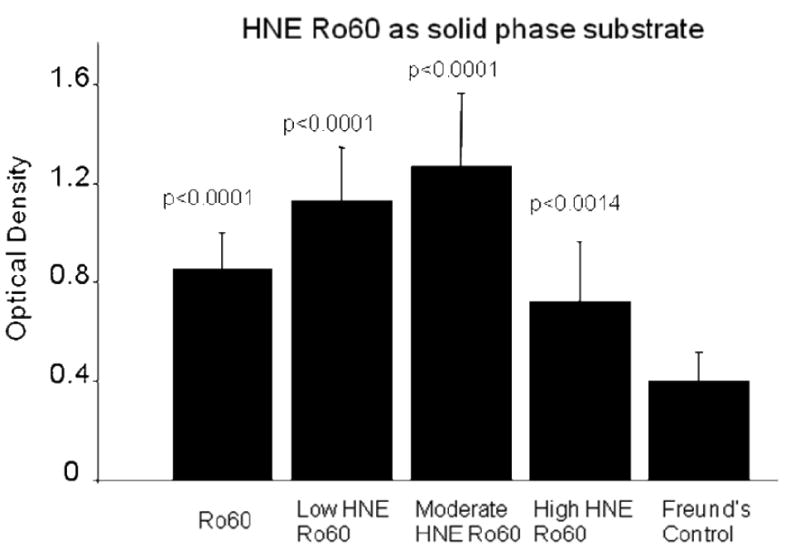
ELISA analysis of anti-HNE Ro60 antibodies in the saliva of mice immunized with Ro60, Freund's adjuvant and low, medium or high HNE-modified Ro60 (Groups I-V) using high HNE modified Ro60 as solid phase substrate. Group I: Mice immunized with unmodified Ro60; Group II: Mice immunized with Ro60 modified with 0.2 mM HNE (low HNE); Group III: Mice immunized Ro60 modified with 2 mM HNE (medium HNE); Group IV: Mice immunized Ro60 modified with 10 mM HNE (high HNE); Group V: Mice immunized with Freund's alone. Antibody response is evident in Ro, HNE 1 and 2 groups. Values are means ± SD for 4 determinations.
Antibodies to HNE in the sera of Ro60, HNE-Ro60 and Freund's control mice
Significantly high levels of anti-HNE antibodies were found in the low, medium and high HNE-Ro60 groups compared to control (Figures 12 and 13), in the first, second and sixth bleeds. Anti-HNE antibodies appeared in the first bleed itself, with the anti-HNE level being higher in the medium HNE Ro60 group compared to the low and high HNE Ro60 groups (Figure 12). However, only the difference in anti-HNE levels between medium and low HNE Ro60 groups was significant (p<0.004). Anti-HNE levels were only slightly higher in the medium HNE Ro60 immunized group compared to the low and high HNE groups in the second bleed and sixth bleeds (Figure 13).
Figure 12.
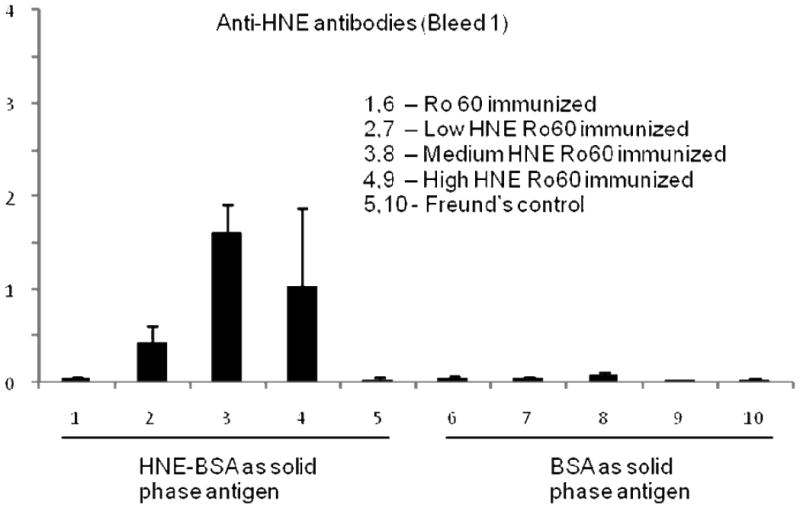
Anti-HNE levels in the first bleed of mice immunized with unmodified Ro60, low HNE Ro60, medium HNE Ro60, high HNE Ro60 and Freund's adjuvant. Values are means ± standard deviation (10 animals in Ro60 and Freund's control group; 3 animals in HNE Ro60 groups). p<0.004 –anti-HNE levels in medium HNE Ro60 group versus low HNE Ro60 group
Figure 13.
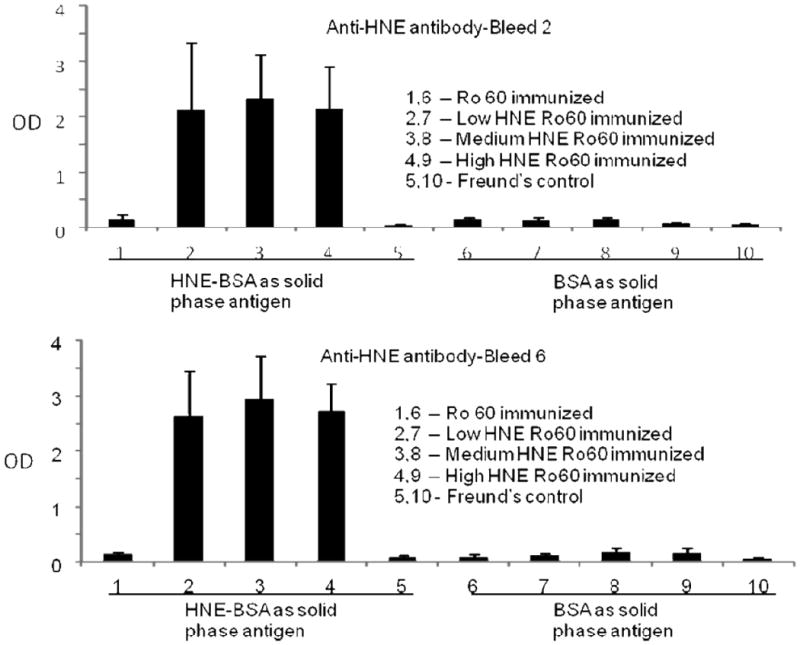
Anti-HNE levels in the first and seconds bleeds of mice immunized with unmodified Ro60, low HNE Ro60, medium HNE Ro60, high HNE Ro60 and Freund's adjuvant. Values are means ± standard deviation (8 animals in Ro 60 and Freund's control group; 10 animals in HNE Ro60 groups).
Discussion
The etiology of autoimmune disease is still not very clear even though genetic and environmental factors have been implicated. Several lines of evidence point to the association between oxidative modification of proteins and autoimmunity. Modification of proteins can alter their conformational structure such that they can behave like neoantigens. Such altered immunogenicity might lead to an autoimmune response by stimulating T cells (especially activation of Th1 cells) and inducing an accelerated autoantibody response.
SS and SLE are autoimmune diseases that can be distinguished from one another clinically without much difficulty in most cases. Keratoconjunctivitis sicca occurs in SS as the main symptom. However, kidney disease, abnormalities in the central nervous system and arthritis can occur in SS similar to that seen in SLE [48,49]. These complications are seen particularly in far-advanced cases of SS. SLE can occur as a mild disease, with non-life threatening manifestations like lymphopenia, arthritis and rash as well as a severe disease with manifestations such as glomerulonephritis, heart disease, neurological involvement or severe hematological disease. In addition, an overlap between the two diseases is seen frequently, with antibodies to both Ro60 and La [48]. ANA occurs in most patients with SS and in almost all patients with SLE while anti-dsDNA occurs specifically in SLE. Thus, the differential diagnosis of the two diseases can be confusing under some circumstances [50].
Previously we have induced a SS like condition, with anti-Ro60, decreased salivary flow and salivary infiltrates representing serological, clinical and pathological manifestations in BALB/c mice by immunizing with a peptide from Ro60 [1]. We have also shown that immunization of rabbits with HNE (10 mM)-modified Ro60 antigen brought about accelerated epitope spreading and autoimmunity compared to rabbits immunized with unmodified Ro60. Based on these observations, we hypothesized that the degree of modification of the Ro60 autoantigen with HNE would determine severity of disease and possibly bring about differential induction of Sjögren's syndrome (SS) and SLE in BALB/c mice. The results of this work reveal a novel mechanism by which epitope spreading could be induced selectively by varying the degree of modification of an immunogen with a by-product of oxidative damage, namely HNE.
The progression of an autoimmune response from initial activation to a chronic state involving increased targeting of autoantigens by T cells and antibodies is referred to as epitope spreading. Abrogation of immune tolerance to one component results in diversification of B- and T-cell responses to other components of the macromolecule with the recognition of other epitopes in the intact particle (48). Studies based on immunization of non-autoimmune mice with self-peptides gives credence to the thought that the highly diverse B-and T-cell autoimmune responses in SLE might originate from a single protein or even a single cryptic self epitope without the need for foreign pathogens or molecular mimics [24, 51-54].
In the present study, immunization with Ro60 modified with medium HNE (2 mM) brought about accelerated epitope spreading, with an early induction of anti-Ro60 and anti-La antibodies compared to immunization with low and high HNE modified Ro60 antigen. Antibodies to double stranded DNA, highly specific for SLE [55], were observed only in mice immunized with medium HNE-modified Ro60. There was no significant decrease in salivary flow in this group compared to Freund's control. Thus, this group behaved more like an animal model for SLE.
Interestingly, mice immunized with high-HNE modified Ro60 behaved more like an animal model for SS. These mice showed the highest decrement in salivary flow, antibodies to Ro60, salivary lymphocytic infiltrates and complete lack of antibodies to double stranded DNA. However, low HNE group also showed a significant decrease in salivary flow compared to Freund's control, but salivary gland pathology is lacking for these animals.
Taken together, these results suggest that differential modification of Ro60 with HNE brings about separate pathways for induction of SLE and SS autoimmunity. Oxidative damage has been shown to occur in SLE patients by us and others [56], but there are no such data for SS. HNE-modified proteins have been observed in the sera of pediatric lupus patients [29]. Our previous data showed accelerated epitope spreading and induction of autoimmunity. Our results demonstrate a clear-cut difference in intermolecular epitope spreading depending upon the degree of modification of the immunogen with HNE. SLE and SS associated proteins, such as Ro60, could form chemical adducts consequent to modification with by-products of oxidative damage and then serve as neo-antigens that the immune system has probably not been exposed to before. Lysines, histidines and cysteines are natural substrates for modification with HNE. There are 47 lysines, 13 each of histidine and cysteine in the Ro60 autoantigen. In this study Ro60 autoantigen was modified in vitro with varying amounts of the lipid oxidation by-product HNE (0.4, 2 and 10 mM). Thus, the Ro60 antigens will be modified differentially depending upon the level of HNE used. That this may be the case is suggested by the observation of varying levels of anti-HNE obtained in the first bleed of mice in the low, medium and high HNE Ro60 immunized groups.
We hypothesize that the differential epitope spreading between mice with SLE-like manifestation and SS-like syndrome that we have observed in this study may be directly related to differential internalization of the neoantigens by antigen presenting cells such as dendritic cells, or macrophages. The Ro60 autoantigen modified with 10 mM HNE was observed to precipitate out of solution following modification. For immunization purposes Ro60 modified with 10 mM HNE had to be mixed well prior to immunization to distribute the antigen evenly. Modification with 10 mM HNE apparently results in maximal modification and thus renders a significant portion of the Ro60 insoluble. The insoluble high HNE modified Ro60 might not be amenable for easy internalization and presentation compared to the low and medium HNE modified Ro60 antigens. The internalization of the non-modified Ro60 might in turn be also different from the internalization of the modified Ro60 by antigen presenting cells.
The antigen presenting cells in turn present novel self peptides to T cells, most probably in a differential manner depending upon the degree of modification of the protein, to provide help to autoreactive B cells to induce differential epitope spreading. B cells, specific for either the differentially modified or unmodified Ro, could also differentially internalize these antigens, by means of cell surface Ig receptor. Selective epitopes from each of these different proteins could be then presented to naïve T cells, in the context of major histocompatibility complex Class II, resulting in a differential diversification of autoreactive T cells, which assist a diversified B cell response that can in turn recognize separate B-cell antigenic determinants from the different antigens resulting in autoreactivity.
Another possibility is that, depending upon the degree of modification the number of HNE molecules on Ro60 available for interaction with other molecules will be different. The alkene bond of HNE reacts with the three nucleophilic amino acids cysteine, histidine and lysine through Michael-type addition [57]. The product of this reaction is generally thought to be a cyclic hemiacetal, which equilibrates with an open-chain form of the protein adduct. The free aldehyde in the open-chain form of the alkenal adduct is expected to retain its electrophilic character, and therefore can react with a second lysine, histidine or cysteine. Thus, HNE can act as heterobifunctional crosslinking reagents.
The high modification of Ro60 by HNE resulted in aggregates, as mentioned in Materials and methods. Therefore, we hypothesize that there would be less number of HNE molecules to interact with other molecules like dsDNA, Ro60 or La. Thus, there will be limited epitope spreading in the mice immunized with high HNE Ro60. That there are low levels of HNE exposed in the Ro60 molecule in the high HNE Ro60 immunized group is borne out indirectly by the low anti-HNE levels in the saliva of this group compared to low HNE and moderate HNE Ro60 immunized groups (Figure 11). The differential induction of intra molecular and inter molecular epitope spreading could be a mechanism by which there is differential induction of SLE or SS upon immunization with differentially modified autoantigens.
Oxidatively modified Ro60 has been reported in human liver, which points to the fact that it is possible that Ro60 could be subject to modification in autoimmunity. Such a scenario envisages developing antibodies to Ro60 and thus autoimmunity to the entire Ro ribonucleoprotein particle after an initial immune response to oxidized Ro60. A similar effect was observed under experimental conditions when we immunized mice with Ro60 modified with different levels of HNE. Distinct intermolecular epitope spreading effects were seen in these animals, with a higher modification leading to a SS-like condition while the antigen with a medium modification with HNE led to a lupus-like scenario.
Reports show that oxidatively modified self-antigens are targeted by autoantibodies. Aldehyde-modified proteins have been demonstrated to be highly immunogenic and autoantibodies directed against epitopes in malonildialdehyde- (MDA) and HNE modified low-density lipoproteins (LDL) have been shown in the plasma of rabbits and mice immunized with oxidized LDL (ox-LDL) [57,58]. Antibodies against ox-LDL or MDA-LDL have been found in atherosclerotic plaques [59-62]. Itabe et al, 1998 [63] have shown that MDA-crosslinked proteins are actually present in vivo and that their production is stimulated by oxidative stress. Recently Chang et al, 1999 [64] have demonstrated oxidation-specific antigens on the surface of apoptotic cells. Antigens modified by oxidative by-products have been shown to induce immune responses in alcoholic liver disease [65]. In type 1 diabetes mellitus, autoantibodies have been shown to be present in patient sera that bind oxidatively modified islet cell proteins [66].
We found significant levels of anti-Ro60 in the saliva of mice immunized with Ro60 and HNE-Ro60. By comparing the average OD values of unmodified 60 kD Ro with low HNE modified Ro60 and medium HNE modified Ro60 group (Figure 10), it is evident that the OD is almost the same for all groups, showing that the same level of anti-Ro antibodies is present in these groups. But when HNE-modified Ro60 was used as solid phase substrate (Figure 11) the OD values were significantly elevated in low and medium HNE-Ro60 groups compared to unmodified Ro60 as the solid phase antigen (Figure 10), suggesting that a different antibody is responsible for this elevation, namely anti-HNE antibodies, or antibodies specific for HNE-Ro60 that do not also bind unmodified Ro60. At the same time, the level of antibodies in mice immunized with unmodified Ro remained the same (Figures 10 and 11), indicating that only anti Ro60 antibodies are present in that group, regardless of the use of unmodified Ro or HNE-modified Ro as solid phase substrate. Therefore, we hypothesize that different antibodies were introduced to the two coated antigens: (a) when unmodified 60-kD Ro was used as solid phase substrate, only the anti-Ro antibodies were introduced to the antigens, which is similar for unmodified Ro, HNE 1 and HNE 2 (Figure 10) but (b) when HNE-modified Ro60 was used as solid phase substrate, anti-HNE antibodies are introduced to the antigens (Figure 11).
In summary, highly significant anti-Ro and anti-HNE Ro antibodies were observed in the saliva of HNE groups of mice compared to unmodified Ro60 immunized group. Further, immunization with Ro60 undergoing the moderate level of oxidative modification gave an SLE-like illness, while the low and high levels of modification resulted in a SS-like picture in the immunized mice. High HNE-modified mice did not have salivary autoantibodies; however, the main etiology of antibodies in saliva of immunized mice is unknown. Decreased levels of salivary amylase have been reported in SS patients (2). The highly significant decrement in the levels of alpha-amylase in high HNE-modified Ro60 immunized group of mice (Figure 10) gives more credence to the presence of SS-like condition in this group.
There are limitations to this study. First, we do not know the status of renal disease in these animals, especially those that demonstrate SLE-like manifestations. Even though the presence of anti-dsDNA antibodies is a strong indicator of the presence of lupus it would be of interest to know the severity of disease by investigating kidney damage in these mice. Further studies our group is pursuing may shed some light in this regard.
As far as we know this is the first report demonstrating differential development of SS and SLE in mice following immunization with an oxidatively modified autoantigen. The preferential targeting of dsDNA in these mice as well as induction of salivary flow decrement based on immunization with Ro60 modified to different levels with HNE suggests distinct pathways of anti-Ro60 autoimmunity in mice with either SLE- or SS-like disease. Recent work shows preferential targeting of a Ro60 apotope found on early apoptotic cells by autoantibodies found in lupus patients but not in SS patients. The preferential targeting was seen even in a subset of SLE patients with anti-Ro and no anti-La [67,68]. In our current study, the preferential targeting of specific proteins and dsDNA implies disease-specific pathways for the induction of anti–Ro60 autoimmunity. Our report also underscores the importance of an hitherto unappreciated mechanism for epitope spreading, which may have clinical implications in SS and SLE. There may be differential modification of various autoantigens in the clinical setting in these autoimmune diseases.
Since oxidative processes are important factors that induce autoimmunity in the clinical scenario, it may be possible to prevent the development of autoimmunity in genetically susceptible individuals by administration of antioxidants or other dietary manipulations to decrease formation of HNE/oxidative by-products.
Figure 3.
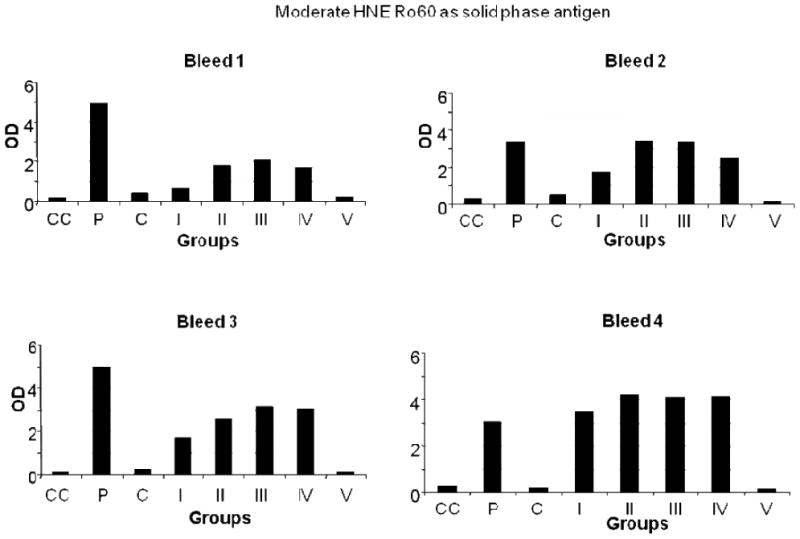
ELISA results of moderate HNE Ro60, used as solid phase antigen, bound by mice bleeds 1-4. Group I-mice immunized with unmodified Ro60; Group II-mice immunized with low HNE Ro60; Group III-mice immunized with moderate HNE Ro60; Group IV-mice immunized with high HNE Ro60; Group V-mice immunized with Freund's alone. CC refers to conjugate control. P refers to positive control and N refers to normal control.
Acknowledgments
Supported by NIH grant 1RO1 ARO53294 to RHS. We thank Ms. Kristen Matthias, Oklahoma School of Science and Mathematics, Oklahoma City, OK, for her assistance in the characterization of HNE-modified BSA using SDS-PAGE and immunoblots.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scofield RH, Asfa S, Obeso D, Jonsson R, Kurien BT. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren's syndrome. J Immunol. 2005;175:8409–14. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- 2.Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjögren's syndrome. J Dent Res. 2008;87:308–18. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 3.Farris AD, Gross JK, Hanas JS, Harley JB. Genes for murine Y1 and Y3 Ro RNAs have class 3 RNA polymerase III promoter structures and are unlinked on mouse chromosome 6. Gene. 1996;174:35–42. doi: 10.1016/0378-1119(96)00279-x. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien CA, Wolin SL. A possible role for the 60-kDa Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 5.Scofield RH, Kurien BT, Zhang F, Mehta P, Kaufman K, Gross T, Bachmann M, Gordon T, Harley JB. Protein-protein interaction of the Ro-ribonucleoprotein particle using multiple antigenic peptides. Mol Immunol. 1999;36:1093–106. doi: 10.1016/s0161-5890(99)00095-4. [DOI] [PubMed] [Google Scholar]
- 6.Scofield RH, Kurien BT, Gross JK, Reed NB, Taylor AK, Dominguez N, Mehta P, Guthridge JM, Bachmann M. Interaction of calcium and Ro60: increase of antigenicity. Mol Immunol. 2004;41:809–16. doi: 10.1016/j.molimm.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Gordon TP, Bolstad AI, Rischmueller M, Jonsson R, Waterman SA. Autoantibodies in primary Sjogren's syndrome: new insights into mechanisms of autoantibody diversification and disease pathogenesis. Autoimmunity. 2001;34:123–32. doi: 10.3109/08916930109001960. [DOI] [PubMed] [Google Scholar]
- 8.Scofield RH. Genetics of systemic lupus erythematosus and Sjögren's syndrome. Curr Opin Rheumatol. 2009;21:448–53. doi: 10.1097/BOR.0b013e32832f0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manoussakis MN, Moutsopoulos HM. Sjogren's syndrome: current concepts. Adv Intern Med. 2001;47:191–217. [PubMed] [Google Scholar]
- 10.Scofield RH, Kurien BT, Reichlin M. Immunologically restricted and inhibitory anti-Ro/SSA in monozygotic twins. Lupus. 1997;6:395–8. doi: 10.1177/096120339700600409. [DOI] [PubMed] [Google Scholar]
- 11.Bolstad AI, Haga HJ, Wassmuth R, Jonsson R. Monozygotic twins with primary Sjögren's syndrome. J Rheumatol. 2000;27:2264–6. [PubMed] [Google Scholar]
- 12.Huang SC, Scofield RH, Kurien BT, Harley JB. Human anti-Ro autoantibodies bind multiple conformational epitopes of 60-kD Ro autoantigen. Clin Immunol. 1997;17:212–19. doi: 10.1023/a:1027354327344. [DOI] [PubMed] [Google Scholar]
- 13.Scofield RH, Zhang FC, Kurien BT, Harley JB. Anti-Ro fine specificity defined by multiple antigenic peptides identifies components of tertiary epitopes. Clin Exp Immunol. 1997;109:480–87. doi: 10.1046/j.1365-2249.1997.4791374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren's syndrome and systemic lupus erythematosus. Rheum Dis Clin N Am. 1992;18:337–58. [PubMed] [Google Scholar]
- 15.Harley JB, Sestak AS, Willis LG, Fu SM, Hansen JA, Reichlin MA. model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies and lymphopenia or renal disease. Arthritis Rheum. 1989;32:826–36. [PubMed] [Google Scholar]
- 16.Kurien BT, Newland JG, Paczkowski C, Moore KL, Scofield RH. Anti-Ro mediates neutropenia in systemic lupus erythematosus by binding a 60,000 MW antigen on the surface of neutrophils. Clin Exp Immunol. 2000;120:209–17. doi: 10.1046/j.1365-2249.2000.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanagasegar S, Cimaz R, Kurien BT, Brucato A, Scofield RH. Neonatal lupus manifests as isolated neutropenia and mildly abnormal liver functions. J Rheumatol. 2002;29:187–91. [PubMed] [Google Scholar]
- 18.Fujisaku A, Frank MB, Neas B, Reichlin M, Harley JB. HLA-DQ gene complement and other histocompatibility relationships in man with the Anti-Ro/SSA autoantibody response in systemic lupus erythematosus. J Clin Invest. 1989;86:606–11. doi: 10.1172/JCI114751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scofield RH, Frank MB, Neas BR, Horowitz RM, Hardgrave KL, Fujisaku A, McArthur R, Harley JB. Cooperative association of T cell beta receptor and HLA-DQ alleles in the production of anti-Ro in systemic lupus erythematosus. Clin Immunol Immunopathol. 1994;72:335–41. doi: 10.1006/clin.1994.1150. [DOI] [PubMed] [Google Scholar]
- 20.Scofield RH, Dickey WD, Hardgrave KL, Neas BR, Horowitz RM, McArthur RA, Fujisaku A, Frank MB, Harley JB. Immunogenetics of epitopes of the carboxyl terminus of the human 60-kDa Ro autoantigen. Clin Exp Immunol. 1995;99:256–61. doi: 10.1111/j.1365-2249.1995.tb05542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reveille JD, MaCLeod MJ, Whittington K, Arnett FC. Specific amino acid residues in the second hypervariable region of HLA-DQA1 and DQB1 chain genes promote the Ro (SS-A)/La (SS-B) autoantibody responses. J Immunol. 1991;146:3871–6. [PubMed] [Google Scholar]
- 22.Scofield RH, Harley JB. Association of anti-Ro (SS-A) autoantibodies with glutamine in position 34 of DQA1 and leucine in position 26 of DQB1. Arthritis Rheum. 1994;37:961–2. doi: 10.1002/art.1780370630. [DOI] [PubMed] [Google Scholar]
- 23.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides derived from the systemic lupus erythematosus associated 60 kDa Ro autoantigen results in autoimmunity directed towards the Ro ribonucleoprotein. J Immunol. 1996;156:4059–66. [PubMed] [Google Scholar]
- 24.Scofield RH, Kaufman KM, Baber U, James JA, Harley JB, Kurien BT. Immunization of mouse with 60 kDa Ro peptides results in epitope spreading if peptides are highly homologous between man and mouse. Arthritis Rheum. 1999;42:1017–24. doi: 10.1002/1529-0131(199905)42:5<1017::AID-ANR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Scofield RH, Pierce PG, James JA, Kaufman KM, Kurien BT. Immunization with peptides from 60 kDa Ro in diverse mouse strains. Scan J Immunol. 2002;56:477–83. doi: 10.1046/j.1365-3083.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 26.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel P, Eggert W, Albrecht-Nebe H, Grune T. Increased lipid peroxidation in children with autoimmune diseases. Acta Paediatr. 1997;86:609–12. doi: 10.1111/j.1651-2227.1997.tb08943.x. [DOI] [PubMed] [Google Scholar]
- 28.Serban MG, Tanaseanu S. Lipid peroxidation in autoimmune systemic vasculitides. Effect of corticoid treatment on lipid peroxidation. Antioxidant protection with vitamin E Rom. J Intern Med. 1994;32:137–42. [PubMed] [Google Scholar]
- 29.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med. 1997;23:357–60. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 30.Turi S, Nemeth I, Torkos A, Saghy L, Vargha I, Matcovics B, Nagy J. Oxidative stress and antioxidant defense mechanism in glomerular diseases. Free Radic Biol Med. 1997;22:161–8. doi: 10.1016/s0891-5849(96)00284-5. [DOI] [PubMed] [Google Scholar]
- 31.Serban MG, Tanaseanu S, Bara C. Oxidant stress and antioxidant protection in lupus nephropathy. Rom J Intern Med. 1996;34:105–9. [PubMed] [Google Scholar]
- 32.Kurien TB, Selvam R. Induction of lipid-peroxidation in calcium-oxalate stone formation. Ind J Exp Biol. 1989;27:450–53. [PubMed] [Google Scholar]
- 33.Jurgens G, Hoff HB, Chisolm GM, Esterbauer H. Modification of human serum low density lipoprotein by oxidation: characterization and pathophysiological implications. Chem Phys Lipids. 1987;45:315–36. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Witztum JL. Immune response to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis. J Intern Med. 2000;247:371–80. doi: 10.1046/j.1365-2796.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 35.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Luis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation and genetics. Circulation. 1995;91:2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg D. Oxidative modification of LDL and atherosclerosis. Circulation. 1997;95:1062–71. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Kanamatsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation. Formation of free acrolein and its conjugation with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–66. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 38.Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic Res. 1998;28:623–35. doi: 10.3109/10715769809065818. Review. [DOI] [PubMed] [Google Scholar]
- 39.Scofield RH, Kurien BT, Ganick S, McClain MT, Pye Q, James JA, Schneider RI, Broyles RH, Bachmann M, Hensley K. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radic Biol Med. 2005;38:719–28. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Dorri Y, Kurien BT, Scofield RH. A Simpler and Faster Version of Two-Dimensional Gel Electrophoresis Using Vertical, Mini SDS-PAGE Apparatus. Iran J Chem Chem Eng. 2009;28:51–56. [Google Scholar]
- 42.Towbin H, Staehelin T, Gordon T. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurien BT, Scofield RH. Protein blotting. A Review. J Immunol Methods. 2003;274:1–15. doi: 10.1016/s0022-1759(02)00523-9. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto H, Komori N. Ocular proteomics: cataloging photoreceptor proteins by two-dimensional gel electrophoresis and mass spectrometry. Methods Enzymol. 2000;316:492–511. doi: 10.1016/s0076-6879(00)16745-x. [DOI] [PubMed] [Google Scholar]
- 45.Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 46.Kurien BT, Scofield RH. Heat mediated quick Coomassie blue protein staining and destaining of SDS-PAGE gels. Indian J Biochem Biophys. 1998;35:385–389. [PubMed] [Google Scholar]
- 47.Dorri Y, Kurien BT. Environmentally safe removal/disposal of Coomassie brilliant blue from gel destain and used gel stain. Anal Biochem. 2010;404:193–196. doi: 10.1016/j.ab.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Alexander EL, Malinow K, Lejewski JE, Jerdan MS, Provost TT, Alexander GE. Primary Sjögren's syndrome with central nervous system disease mimicking multiple sclerosis. Ann Intern Med. 1986;104:323–30. doi: 10.7326/0003-4819-104-3-323. [DOI] [PubMed] [Google Scholar]
- 49.Alexander EL. Neurologic disease in Sjögren's syndrome: mononuclear inflammatory vasculopathy affecting central/peripheral nervous system and muscle. A clinical reviewand update of immunopathogenesis. Rheum Dis Clin North Am. 1993;19:869–908. [PubMed] [Google Scholar]
- 50.Scofield AN, Kurien BT, Gordon TP, Scofield RH. Can B cell epitopes of 60 kD Ro distinguish systemic lupus erythematosus from Sjogren's syndrome. Lupus. 2001;10:547–53. doi: 10.1191/096120301701549679. [DOI] [PubMed] [Google Scholar]
- 51.Mamula MJ. Epitope spreading: the role of self peptides and autoantigen processing by B lymphocytes. Immunol Rev. 1998;164:231–9. doi: 10.1111/j.1600-065x.1998.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 52.Topfer F, Gordon T, McCluskey J. Intra- and inter-molecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawahata K, Misaki Y, Komagata Y, Setoguchi K, Tsunekawa S, Yoshikawa Y, Miyazaki J, Yamamoto K. Altered expression level of a systemic nuclear autoantigen determines the fate of immune response to self. J Immunol. 1999;162:6482–91. [PubMed] [Google Scholar]
- 54.Deshmukh US, Lewis JE, Gaskin F, Kannapell CC, Waters ST, Lou YH, Tung KS, Fu SM. Immune response to Ro60 and its peptides in mice. I. The nature of the immunogen and endogenous autoantigen determine the specificities of the induced antibodies. J Exp Med. 1999;189:531–40. doi: 10.1084/jem.189.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227–35. doi: 10.1111/j.1365-3083.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 56.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73:1655. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 57.Esterbauer H, Schaur RG, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 58.Palinski W, Witztum JL. Immune response to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis. J Intern Med. 2000;247:371–80. doi: 10.1046/j.1365-2796.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 59.Maggi E, Chiesa R, Melisano G, Castellano R, Astore G, Grossi A, Finardi G, Bellomo G. LDL oxidation in patients with atherosclerosis: a study of in vitro and in vivo oxidation markers. Arterioscler Thromb Vasc Biol. 1995;14:1892. doi: 10.1161/01.atv.14.12.1892. [DOI] [PubMed] [Google Scholar]
- 60.Bergmark C, Wu R, de Faire U, Lefvert AK, Swedenborg J. Patients with early onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:441. doi: 10.1161/01.atv.15.4.441. [DOI] [PubMed] [Google Scholar]
- 61.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdyhyde-modified LDL in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla-Herttuala S, Luoma JS, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Arterioscler Thromb Vasc Biol. 1990;19:23. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 63.Itabe H, Jimi S, Kamimura S, Suzuki K, Uesugi N, Imanaka T, Shijo H, Tanako T. Appearance of cross linked proteins in human atheroma and rat pre-fibrotic liver detected by a new monoclonal antibody. Biochim Biophys Acta. 1998;1406:28. doi: 10.1016/s0925-4439(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 64.Chang MK, Bergmark C, Laurila A, Horkko S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicites macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti MG, Vidali M, Sartori M, Rigamonti C, Day CP, Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Rad Biol Med. 2002;32:38. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 66.Trigwell SM, Radford PM, Page SR, Loweth AC, James RF, Morgan NG, Todd I. Islet glutamic acid decarboxylase modified by reactive oxygen species is recognized by antibodies from patients with type 1 diabetes mellitus. Clin Exp Immunol. 2001;126:242. doi: 10.1046/j.1365-2249.2001.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reed JH, Jackson MW, Gordon TP. A B cell apotope of Ro 60 in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1125–9. doi: 10.1002/art.23377. [DOI] [PubMed] [Google Scholar]
- 68.Reed JH, Jackson MW, Gordon TP. Lupus. 2010;19:107–8. doi: 10.1177/0961203309106764. [DOI] [PubMed] [Google Scholar]


