Abstract
For both tendon allografts and autografts, the surface, initially optimized for gliding, may not be ideal to facilitate tissue integration for graft healing to host tendon or bone. As a prelude to studying tendon-bone integration, we investigated the effect of surface treatments with trypsin or mechanical abrasion on cell attachment to the tendon surface in a canine ex vivo intrasynovial tendon tissue culture model. Intrasynovial tendon allograft surfaces were seeded with cells after the following treatments: 1) no treatment, 2) mechanical abrasion, 3) trypsin, 4) abrasion and trypsin. The area covered by cells was determined using confocal laser microscopy at one and two weeks. Results were compared to untreated extrasynovial tendon. Additional tendons were characterized with scanning electron microscopy. Tendons with trypsin treatment had significantly more surface coverage with cells than the other groups, after both one and two weeks of culture. In terms of the cellular shape and size, cells on tendons with trypsin treatment spread more and were more polygonal in shape, whereas tendons with mechanical abrasion with/without trypsin treatment contained smaller, more spindle-like cells. Surface roughening can affect cell behavior with topographical stimulation. Trypsin surface digestion exposes a mesh-like structure on the tendon surface, which could enhance cell adherence and, possibly, tendon/bone healing.
Keywords: Tendon Allograft, Lyophilized Tendon, Surface Roughening, Surface Topography, Cell Spreading, Cell Proliferation
1. INTRODUCTION
Tendon allografts are used in many musculoskeletal reconstructive procedures, including knee ligament repair,1,2 shoulder rotator cuff repair3,4 and hand flexor tendon reconstruction.5,6 The advantages of allografts include ready availability, absence of donor site morbidity, and reduced operating time.7 In addition, allografts are especially useful in intrasynovial applications, such as in the knee, shoulder, or hand, because intrasynovial autografts are usually not available. Allografts can also be used in an extrasynovial environment, such as the proximal attachment of a tendon allograft to the host muscletendon unit. However, while allograft remodeling and incorporation into bone and host tendon are similar qualitatively to autografts,8 they progress much more slowly than in autografts, which can compromise results.9–13 Enhancement of allograft incorporation could improve clinical outcomes.
In addition to basic issues of host-graft tissue integration, tendon/bone healing is also problematic because of the difficulty of restoring the normal fibrocartilaginous transitional zone of the normal tendon/bone interface.13–16 This is even more difficult in allograft/bone healing,9 since allografts do not harbor live cells and can elicit an immunogenic response.11 Thus, when allografts are used, the tendon/bone interface healing mainly relies on the cells from surrounding tissue that migrate into the graft for tissue regeneration. Finally, for both tendon allografts and autografts, a surface initially optimized for gliding may not be ideal to facilitate tissue integration. In addition to other compounds on the surface of gliding tendons, lubricin, a lubricating glycoprotein present on the tendon surface,17,18 is known to inhibit cellular adhesion.19 When the tendon surface is modified by trypsin digestion, a procedure which removes lubricin and other proteins, the tendon surface becomes visibly rougher, and friction increases.18 Since lubricin also has anti-adhesive effects, removing lubricin from the graft surface might improve graft incorporation. The roughened surface might also provide a greater surface area for cellular adhesion.
With regard to surface topography, the effects of roughness on cellular behavior have been examined in various artificial materials, such as metals and polymers.20,21 However, little is known about the effect of roughening of native tissue on the topography of cell attachment.
Our overall goal is to enhance allograft tendon-bone healing. As noted above, one aspect of this process is cellular migration from the living bone host into the recipient allograft tendon. As a prelude to definitive in vivo studies, we wished to study methods that might make the allograft tendon surface more hospitable to migrating cells. Therefore, the purpose of this study was to investigate the effect of roughening the tendon surface, either chemically (with trypsin digestion), physically (by mechanical abrasion), or both, on bone marrow stromal cell attachment to the tendon surface in a canine ex vivo intrasynovial tendon model.
2. MATERIALS AND METHODS
Ninety canine FDP tendons of the 2nd, 3rd, 4th and 5th digits from four paws of six dogs were used. FDP tendons and bone marrow were harvested from mixed-breed dogs which were euthanized for other IACUC approved studies that did not involve or affect the tendons. The tendons were prepared as they would be for allograft use, by applying a repetitive freeze-thawing procedure that destroys the native cells,22 followed by lyophilization, a common procedure used to facilitate allograft storage. The tendons were divided into five groups. (Table 1) The tendons used for confocal microscopy were further subdivided into two subgroups, differentiated by culture with bone marrow stromal cells for either one or two weeks. To minimize post mortem effects, the bone marrow cells were harvested while the dogs were anesthetized and tendons were harvested immediately after euthanasia.
Table 1.
Study Design (Grouping, Treatment, Measurement and Sample Size)
| Group | Tendon Origin | Treatment | Number of Tendons used for Confocal Microscopy |
Number of Tendons used for SEM |
|
|---|---|---|---|---|---|
| Mechanical Abrasion |
Trypsin | ||||
| A | Intrasynovial Tendon | − | − | 16 | 2 |
| B | Intrasynovial Tendon | + | − | 16 | 2 |
| C | Intrasynovial Tendon | − | + | 16 | 2 |
| D | Intrasynovial Tendon | + | + | 16 | 2 |
| E | Extrasynovial Tendon | − | − | 16 | 2 |
2.1. Bone Marrow Stromal Cells (BMSCs) Harvest and Culture
From each dog, 8.0 mL of tibial bone marrow were aspirated into a 20 mL syringe containing 2.0 mL of heparin solution. The aspirates were centrifuged at 1500 rpm for 5 minutes at room temperature to remove heparin, and then divided into three equal aliquots. Each aliquot was placed in a 100-mm culture dish with 10 mL of minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY, USA), 10% fetal calf serum, and 1% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY, USA). The bone marrow cells were incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After 3 days, the medium containing floating cells was removed and new medium was added to the remaining adherent cells. These adherent cells were defined as BMSCs.23 The medium was changed every 3 days. After the adherent cells reached confluence, they were released with trypsin from porcine pancreas (Sigma, T-0303, 0.25%) and seeded in new dishes.
2.2. Tendon Harvesting, Decellularization and Lyophilization
Bilateral fore and hind paws were shaved, scrubbed with povidone iodine, and sterilely draped. The 2nd through 5th digit FDP tendons were harvested. After harvest, the tendons were immersed immediately in liquid nitrogen for 1 minute and then thawed in warmed saline solution at 37°C for 5 minutes. This procedure was repeated five times. This procedure, which kills over 97% of the cells in tendon or ligament tissue,22 also helps to reduce the ability of the graft to incite an immune response in the host. After completing the freeze-thaw cycles, the tendons were frozen in liquid nitrogen and placed into a custom-made lyophilizer for 24 hours. Individual tendons were sealed in a plastic bag and stored at room temperature. All tendons were transferred to special plastic bags for gas sterilization one week before use. The tendons were rehydrated in a 0.9% NaCl bath in a closed, sterilized container for 24 hours in a 37°C incubator immediately prior to use.
2.3. Surface Treatment
In groups B and D, before rehydration, the lyophilized tendons were abraded 15 times with 180 grit abrasive cloths (3M, St. Paul, MN, USA) in parallel direction to the tendon. After rehydration, tendons in group C and D were treated with 0.25% EDTA-trypsin solution for 2 hours at 37°C with 5% CO2 and 95% air at 100% humidity.18
2.4. BMSCs Seeding of Decellularized Tendons and Culture
Each tendon was rehydrated with saline for 24 hours at 37°C, cut into a 1-cm long portion that was just proximal to distal vincula attachment, and then immersed in minimal essential medium with Earle’s salts 10% fetal calf serum, and 1% antibiotics in coculture with BMSCs at a density of 1×105 cells/mL, and incubated at 37°C in a humidified tissue culture chamber with 5% CO2 for one or two weeks. Culture medium was changed every three days. In clinical scenario, the tendon graft buried within bone tunnel for distal tendon/bone attachment repair is roughly 1cm in length. Thus we used 1-cm segments for this study.
2.5. Observation of Cell Attachment
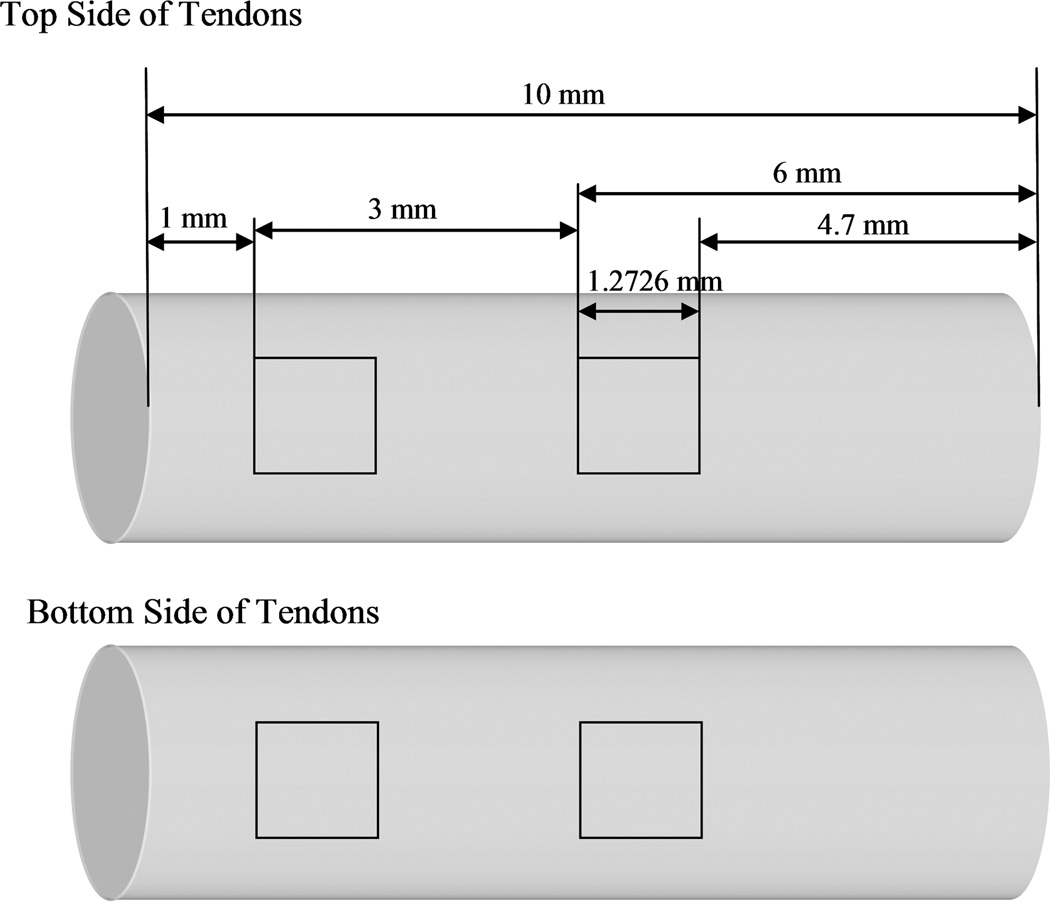
A confocal laser microscope (Zeiss 5 Live, Carl Zeiss, Thornwood, NY, USA) was used to assess cell distribution and estimate the cell count on the tendon surface. At the conclusion of the one or two week culture period, the culture medium was replaced with a new medium containing 5 µM calcein AM and 5 µM ethidium homodimer-1 (LIVE/DEAD Viability/Cytotoxicity Kit) (Molecular Probes, Eugene, OR, USA). After 30 minutes the tendons were removed from the medium and washed with PBS. Four surface photos in each tendon (using C-Apochromat 10X/0.45 W objective lens and 1.0 magnification zoom factor) were captured using the confocal microscope (excitation wavelength: 488 nm; emission wavelength: 505–550 nm for live cells, 568 nm and 635 nm for dead cells respectively) as shown in Figure 1. The percentage of the area covered by live cells was determined using image processing software (Zeiss KS400; Carl Zeiss, Thornwood, NY, USA). The mean percentage of four photos was calculated. We initially tried to detach all cells from tendon surfaces by trypsin treatment for cell quantification such as an Alamar Blue assay. However, we could not remove all the cells from the tendon surface by this method. Therefore, we used the mean percentage of cell coverage on the tendon surface to compare cell attachment between the groups. All cell counts were done by an individual who was blinded as to which group the tendons belonged to.
Figure 1.
Four areas of confocal microscopy. Each square equaled 1.6195 mm2 (1.2726 × 1.2726 mm). The first square was 1 mm away from the edge and the second was 4 mm on the anterior tendon surface. The third and fourth squares were placed in same manner on the posterior surface.
2.6. The Strength of Cell Attachment
After confocal microscopy, all tendons were treated immediately with 0.25% EDTA-trypsin solution for 10 minutes at 37°C with 5% CO2 and 95% air at 100% humidity. The percentage of cell coverage was determined again, and then the ratio of cell area before and after EDTA-trypsin treatment was calculated, as a measure of the strength of cell attachment.
2.7. Scanning Electron Microscopy (SEM)
Two tendons from each group were used for SEM analysis. One tendon was used to observe tendon surface topography and the other was used to observe cell shape after culture. Specimens were prepared for SEM as described previously.18
2.8. Statistics
Cell coverage areas for the five different treatment groups at the two time points were analyzed with multiple-way ANOVA and post hoc pair-wise comparisons using Tukey’s technique. After trypsinizing the tendon surfaces, the cell area ratios were evaluated using the same statistical analysis method. The five treatments were considered as a main effect, and the individual dog and source of the tendons (left or right, front and back paw) were considered as random or repeated factors in the analyses. A P-value < 0.05 for any statistical analysis was considered significant.
3. RESULTS
3.1. Cell Attachment
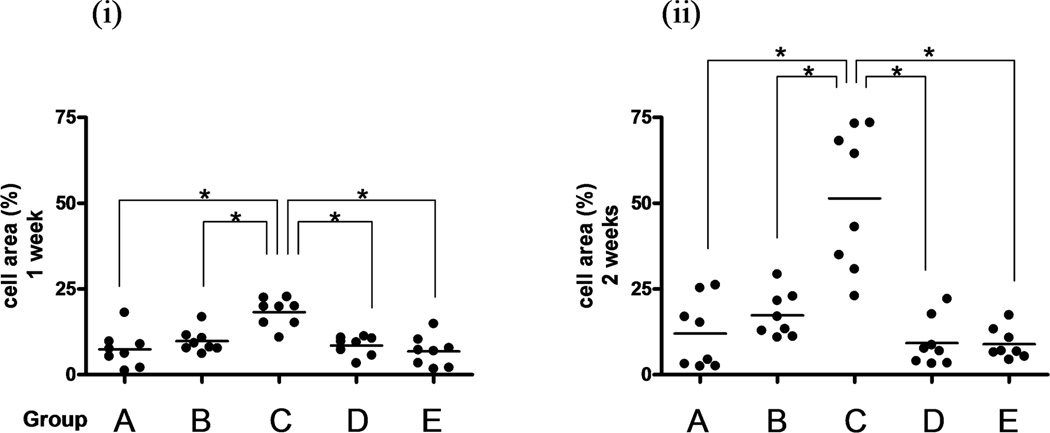
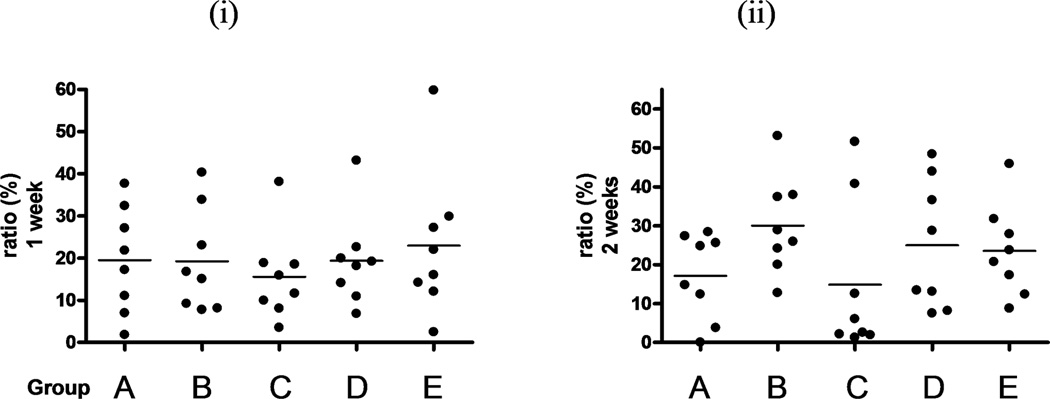
Tendons with trypsin treatment had a significantly larger coverage by BMSCs than any of the other tendon groups, after both one and two weeks of culture (p<0.05). (Figure 2A and B) Tendons with mechanical abrasion had more coverage than untreated intrasynovial tendons, but no significant difference was found. Interestingly, tendons with combined treatment had similar coverage to untreated intrasynovial tendons after one week and less coverage after two weeks. Extrasynovial tendons had less coverage than intrasynovial tendons, but no significant differences were found.
Figure 2.
The percentage of the area covered by live cells was determined with KS 400 after one week (i) or two weeks (ii) in tissue culture. The mean cell area of the four photos was calculated. Dots show each area and bars indicate averages. (A–E) are correspond to each group letter shown in Table 1. * indicates significant difference at p< 0.05.
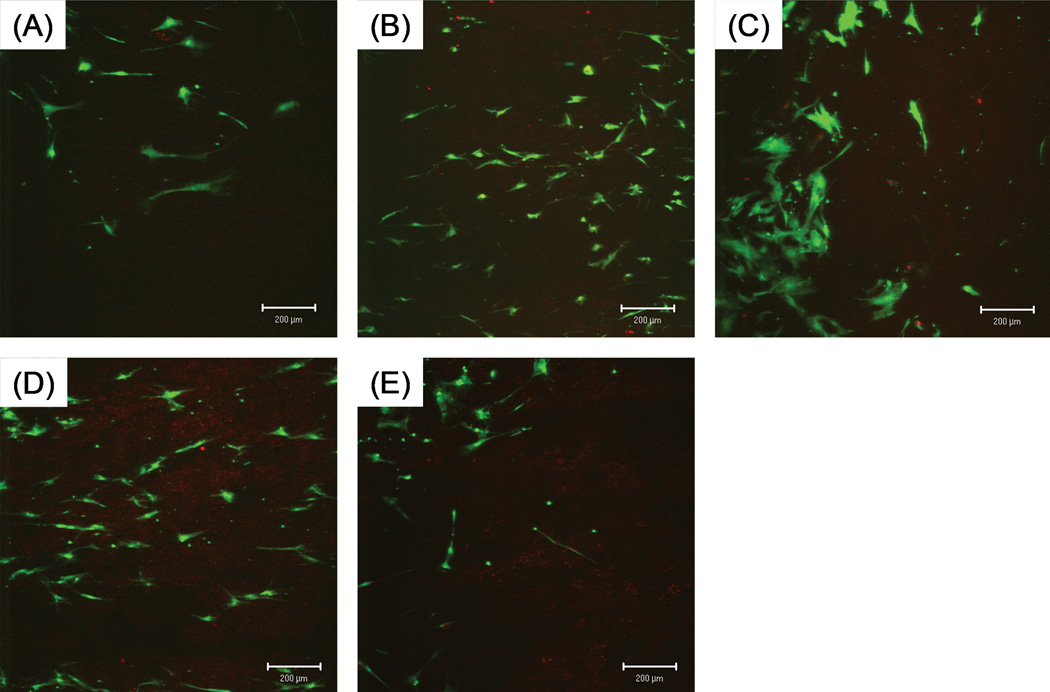
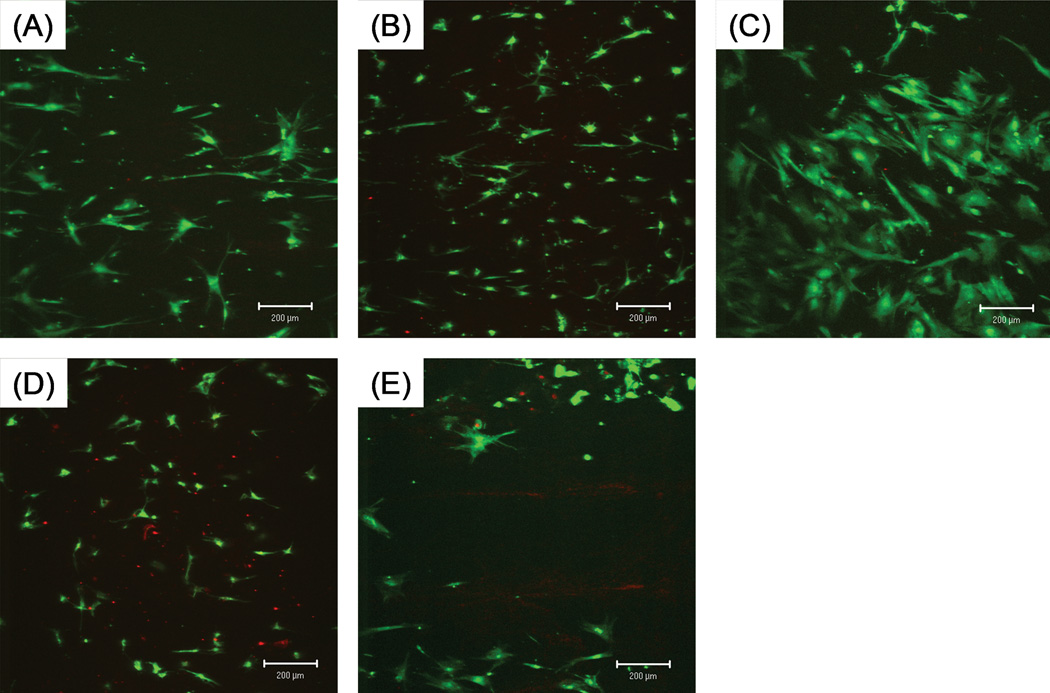
The shape and size of individual cells varied between groups. (Figures 3, 4) Cells on tendons with trypsin treatment spread more and had a more polygonal shape. Cells on tendon with mechanical abrasion or combined treatment spread less and looked more spindle-like. Cells on untreated tendons resembled each other and spread moderately.
Figure 3.
Attachment of BMSCs at one week in culture. Cultured tendons were incubated with LIVE/DEAD Viability/Cytotoxicity Kit that labeled live cells with green under fluorescent microscope. Note spreading of cells on group-C tendons and smaller and more spindle-like cells on group-B and D tendons. Scale bar: 200 µm.
Figure 4.
Attachment of BMSCs at two weeks. Surface coverage is greater and cell shape is similar to those at one week. Scale bar: 200 µm.
3.2. The Strength of Cell Attachment
Digestion of the tendon surface with trypsin for ten minutes detached some cells from the tendon surface in both one-week and two-week cultured tendons. In most tendons, more than two-thirds of cells detached from the surface. Cells tended to more easily detach from tendons with initial trypsin treatment, but no significant differences were found between any of these groups. (Figure 5)
Figure 5.
Adhesion strength (i) at one week of culture; (ii) at two weeks. After confocal microscopy, tendons were treated with trypsin, and then observed again. The ratio of the cell area of the second observation to the first one was calculated and considered to reflect the adhesion strength. Dots show the ratios of each tendon and bars indicate the averages. No significant difference was found, but cells on tendon with trypsin treatment seemed to detach easily.
3.3. SEM
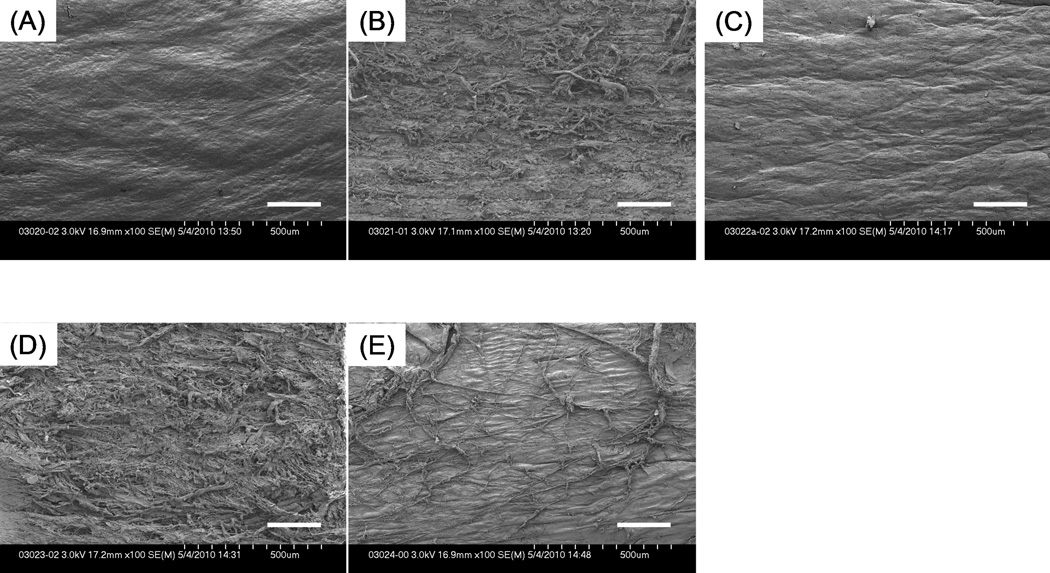
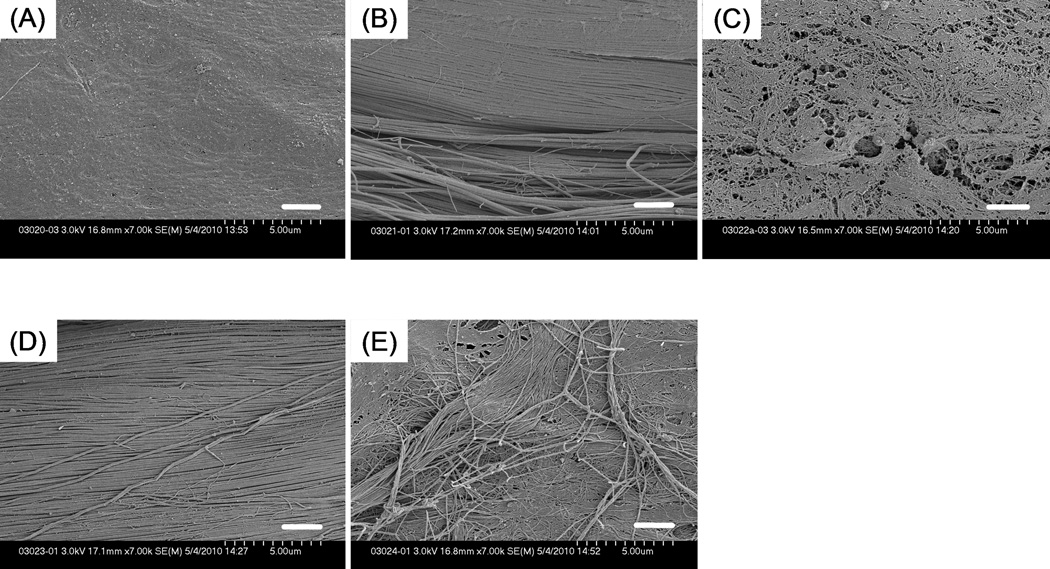
The surface of untreated intrasynovial tendons looked smooth and was uniformly covered with an epitenon layer as described in a previous study on frozen tendons. (Figure 6) 18 Both mechanical abrasion and combined treatment produced a visibly rougher surface than the other treatments. Tendons with trypsin treatment appeared smooth at lower magnification, but a mesh-like structure could be seen at higher magnification. (Figure 7) Trypsin treated tendons were moderately rougher than untreated intrasynovial tendons. Untreated extrasynovial tendons, on which surface the residues of paratenon and capillary vessels lie, looked rougher than untreated intrasynovial and trypsin-treated tendons.
Figure 6.
SEM images (× 100) showing tendon surface that underwent each surface treatment. (A) untreated tendon; (B) tendon with mechanical abrasion; (C) tendon with trypsin treatment; (D) tendon with combined treatment; (E) untreated extrasynovial tendon. Scale Bar: 200 µm.
Figure 7.
SEM images at higher magnification (× 7000) showing tendon surface that underwent each surface treatment. (A) untreated tendon; (B) tendon with mechanical abrasion; (C) tendon with trypsin treatment; (D) tendon with combined treatment; (E) untreated extrasynovial tendon. The mesh-like structure of epitenon layer is exposed in trypsinized tendon. Scale Bar: 2 µm.
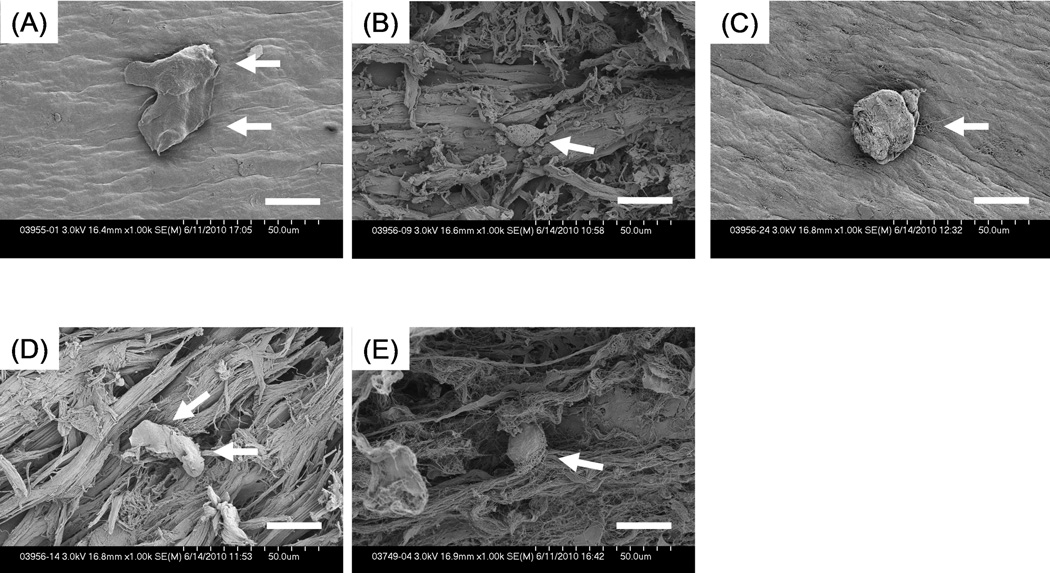
Figure 8 shows that cells appeared to alter their shape in response to surface morphology. Cells on the mesh-like topography of trypsin-treated tendons spread the most. But those on the rougher surface of tendons with mechanical abrasion and combined treatment became smaller and more spindle-like in shape.
Figure 8.
SEM images (× 1000) showing cell spreading on each tendon surface. (A) untreated tendon; (B) tendon with mechanical abrasion; (C) tendon with trypsin treatment; (D) tendon with combined treatment; (E) untreated extrasynovial tendon. Cells (white arrows) on normal and trypsinized tendons spread more than those on abraded tendons. Scale Bar: 20 µm.
4. DISCUSSION
This study has shown that tendon surface treatment can affect cell attachment and cell behavior. Trypsin digestion can increase the coverage of BMSCs on the tendon surface, as confirmed with both confocal microscopy and SEM. Such cell shape changes could be caused by either topographical or chemical stimulation of the cell substrate21,24,25 in this case the tendon surface collagen and associated matrix macromolecules.
When cells spread to exceed a threshold, cell proliferation can also be promoted.26,27 While overlap of cells precluded accurate cell counting in this study, we believe that both cell spreading and cell proliferation were responsible for the significant increase in cell coverage area for trypsinized tendons in this study. This interpretation is in keeping with the results reported by Goodman et al, who showed that bovine aortic endothelial cells cultured on ECM replicas spread more rapidly than those on an untextured surface.28 Similarly, Gigante et al. investigated the effect of collagen fiber orientation on cell behavior and showed significant differences in cell proliferation rate between cells seeded onto oriented and nonoriented fibers.29 Taken together, these results and those of other studies suggest that surfaces mimicking native, healthy tissue or native tissue itself would provide an ideal anchorage site for cells.30
In contrast, the results of this study suggest that the effect of the method that we chose for mechanical abrasion is negative with regard to cell spreading, regardless of whether surface proteins are removed chemically or not. Rougher surfaces should have more available surface for cell attachment than smoother surfaces. On the rougher surfaces created by our mechanical abrasion treatment, cells spread less; such cells are also, as noted above, less likely to proliferate.26 Thus a lack of proliferation may explain why no significant increases in cell coverage area were found in these tendons. Similarly, surface roughness has been shown to negatively affect cell spreading and proliferation on some artificial materials, which also support the observations of this study. Richards et al. reported that cells on smoother titanium surfaces spread more than those on surfaces roughened with abrasive paper.31 Chen et al. and Milner et al. reported an inhibition of cell spreading due to an array of dot-like protrusions32,33 that negatively affected the formation and maturation of focal contact between cell and the substrate. However, Gristina et al. showed that a rougher surface created by a fluorocarbon nanofiber coating increased cell attachment and growth.34 Our results suggest that tendon surfaces roughened mechanically in this study might be too rough and unsuitable for cell spreading and proliferation due to decreased focal contact.
What also seems clear from these studies is that the details of the surface matter, and that while some topographies may facilitate cell attachment and spreading, others may not. In addition, surface chemistry appears to play an important role in cell behavior. When cells adhere to matrices or substratum, molecular signals can be transduced through focal adhesion. Many proteins on tendon surfaces can affect cellular behavior. For example, lubricin, an anti-adhesive protein that inhibits synovial cell overgrowth in vitro and in vivo, 19,35 is known to be present on the surface of intrasynovial tendons.17 While we did not measure lubricin or any other matrix macromolecule either before or after trypsin treatment, our results suggest that trypsin treatment alters the molecular environment on the tendon surface in a way that does enhance coverage of the surface with BMSCs.
Adhesion strength was examined with a short-duration trypsin digestion that can hydrolyze cell adhesion molecules. No significant difference was found in this chemical strength test. Other methods, such as the micropipette aspiration technique36 or jet impingement,37 may also be used to determine attachment strength.
A limitation of our study is that we did not assess the entire surface of the tendons through confocal microscopy. While we believe that our sampling method was accurate, irregular cell distribution could potentially cause some error. In addition, due to cell overlap, we were unable to get accurate cell counts, and so limited the analysis to cell shape and coverage. We only used one abrasive technique, with 180 grit abrasive cloth. Other mechanical abrasion methods may have different effects. Finally, our model did not directly address tendon/bone interface healing, which is our overall goal. However, we believe that the attachment of BMSCs, either from the host bone tunnel or delivered exogenously onto the tendon surface, is the critical first step for tendon/bone healing, especially bone/allograft healing. Therefore, we chose this simple tendon-cell-attachment ex vivo model. An in vivo tendon/bone healing model is a necessary future study.
In conclusion, surface chemical treatment and roughening can affect cell attachment and cell shape. Our data suggest that trypsin treatment of the tendon end could be useful for the enhancement of tendon/bone healing. Further studies of the effects of the surface treatments, including in vivo study, are needed to investigate this possibility under more physiological conditions, which allow mechanical loading, inflammatory reaction and host-versus-graft reaction.
Acknowledgment
This study was funded by grants from NIH/NIAMS (AR057745).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:661–681. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Amendola A, Stolley MP. What do we really know about allografts? Clin Sports Med. 2009;28:215–222. doi: 10.1016/j.csm.2008.10.002. vii-viii. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez R, Damacen H, Nyland J, Caborn D. Acromioclavicular joint reconstruction using peroneus brevis tendon allograft. Arthroscopy. 2007;23:788, e781–e784. doi: 10.1016/j.arthro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Ho JY, Miller SL. Allografts in the treatment of athletic injuries of the shoulder. Sports Med Arthrosc. 2007;15:149–157. doi: 10.1097/JSA.0b013e31805c08ed. [DOI] [PubMed] [Google Scholar]
- 5.Webster DA, Werner FW. Mechanical and functional properties of implanted freeze-dried flexor tendons. Clin Orthop Relat Res. 1983:301–309. [PubMed] [Google Scholar]
- 6.Hasslund S, Jacobson JA, Dadali T, et al. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res. 2008;26:824–833. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole DW, Ginn TA, Chen GJ, et al. Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy. 2005;21:786–790. doi: 10.1016/j.arthro.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 8.Malinin TI, Levitt RL, Bashore C, et al. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18:163–170. doi: 10.1053/jars.2002.30485. [DOI] [PubMed] [Google Scholar]
- 9.Zhang CL, Fan HB, Xu H, et al. Histological comparison of fate of ligamentous insertion after reconstruction of anterior cruciate ligament: autograft vs allograft. Chin J Traumatol. 2006;9:72–76. [PubMed] [Google Scholar]
- 10.Scheffler SU, Schmidt T, Gangey I, et al. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24:448–458. doi: 10.1016/j.arthro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Dustmann M, Schmidt T, Gangey I, et al. The extracellular remodeling of freesoft- tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16:360–369. doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 12.Gulotta LV, Rodeo SA. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:509–524. doi: 10.1016/j.csm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Dovan TT, Ritty T, Ditsios K, et al. Flexor digitorum profundus tendon to bone tunnel repair: a vascularization and histologic study in canines. J Hand Surg Am. 2005;30:246–257. doi: 10.1016/j.jhsa.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Whiston TB, Walmsley R. Some observations on the reactions of bone and tendon after tunnelling of bone and insertion of tendon. J Bone Joint Surg Br. 1960;42- B:377–386. doi: 10.1302/0301-620X.42B2.377. [DOI] [PubMed] [Google Scholar]
- 16.Liu SH, Panossian V, al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997:253–260. doi: 10.1097/00003086-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Berger EJ, Zhao C, et al. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res. 2006;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Chen MY, Zhao C, et al. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res. 2008;26:1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englert C, McGowan KB, Klein TJ, et al. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091–1099. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 20.Arnesen S, Mosler S, Larsen N, et al. The effects of collagen type I topography on myoblasts in vitro. Connect Tissue Res. 2004;45:238–247. doi: 10.1080/03008200490888424. [DOI] [PubMed] [Google Scholar]
- 21.Biggs MJ, Richards RG, Dalby MJ. Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomedicine. 2010;6:619–633. doi: 10.1016/j.nano.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tohyama H, Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122:594–599. doi: 10.1115/1.1319659. [DOI] [PubMed] [Google Scholar]
- 23.Ciapetti G, Ambrosio L, Marletta G, et al. Human bone marrow stromal cells: In vitro expansion and differentiation for bone engineering. Biomaterials. 2006;27:6150–6160. doi: 10.1016/j.biomaterials.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Choi YS, Choi BH, et al. Mussel adhesive protein fused with cell adhesion recognition motif triggers integrin-mediated adhesion and signaling for enhanced cell spreading, proliferation, and survival. J Biomed Mater Res A. 2010;94A:886–892. doi: 10.1002/jbm.a.32768. [DOI] [PubMed] [Google Scholar]
- 25.Flemming RG, Murphy CJ, Abrams GA, et al. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 27.Meredith DO, Eschbach L, Riehle MO, et al. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. J Orthop Res. 2007;25:1523–1533. doi: 10.1002/jor.20430. [DOI] [PubMed] [Google Scholar]
- 28.Goodman SL, Sims PA, Albrecht RM. Three-dimensional extracellular matrix textured biomaterials. Biomaterials. 1996;17:2087–2095. doi: 10.1016/0142-9612(96)00016-6. [DOI] [PubMed] [Google Scholar]
- 29.Gigante A, Cesari E, Busilacchi A, et al. Collagen I membranes for tendon repair: effect of collagen fiber orientation on cell behavior. J Orthop Res. 2009;27:826–832. doi: 10.1002/jor.20812. [DOI] [PubMed] [Google Scholar]
- 30.Sechler JL, Schwarzbauer JE. Control of cell cycle progression by fibronectin matrix architecture. J Biol Chem. 1998;273:25533–25536. doi: 10.1074/jbc.273.40.25533. [DOI] [PubMed] [Google Scholar]
- 31.Richards RG. The effect of surface roughness on fibroblast adhesion in vitro. Injury. 1996;27(Suppl 3):SC38–SC43. doi: 10.1016/0020-1383(96)89031-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Song W, Zhou F, et al. The effect of surface microtopography of poly(dimethylsiloxane) on protein adsorption, platelet and cell adhesion. Colloids Surf B Biointerfaces. 2009;71:275–281. doi: 10.1016/j.colsurfb.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Milner KR, Siedlecki CA. Submicron poly(L-lactic acid) pillars affect fibroblast adhesion and proliferation. J Biomed Mater Res A. 2007;82:80–91. doi: 10.1002/jbm.a.31049. [DOI] [PubMed] [Google Scholar]
- 34.Gristina R, D'Aloia E, Senesi GS, et al. Increasing cell adhesion on plasma deposited fluorocarbon coatings by changing the surface topography. J Biomed Mater Res B Appl Biomater. 2009;88:139–149. doi: 10.1002/jbm.b.31160. [DOI] [PubMed] [Google Scholar]
- 35.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin TW, Yang ZM, Wu ZZ, et al. Adhesion strength of human tenocytes to extracellular matrix component-modified poly(DL-lactide-co-glycolide) substrates. Biomaterials. 2005;26:6635–6642. doi: 10.1016/j.biomaterials.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Hallab NJ, Bundy KJ, O'Connor K, et al. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001;7:55–71. doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]