Abstract
There have been substantial advances in cancer diagnostics and therapies in the past decade. Besides chemotherapeutic agents and radiation therapy, approaches now include targeting cancer cell–intrinsic mediators linked to genetic aberrations in cancer cells, in addition to cancer cell–extrinsic pathways, especially those regulating vascular programming of solid tumors. More recently, immunotherapeutics have entered the clinic largely on the basis of the recognition that several immune cell subsets, when chronically activated, foster tumor development. Here, we discuss clinical and experimental studies delineating protumorigenic roles for immune cell subsets that are players in cancer-associated inflammation. Some of these cells can be targeted to reprogram their function, leading to resolution, or at least neutralization, of cancer-promoting chronic inflammation, thereby facilitating cancer rejection.
Inflammation is a hallmark of cancer wherein diverse immune cells exert either pro- or antitumor properties (1, 2) and affect therapeutic resistance (3). Although Virchow first hypothesized that cancer occurred at sites of chronic inflammation, postulating that immune cells release factors stimulating proliferation (of would-be tumor cells) (4), Coley successfully treated sarcomas with bacterial mixtures, for example, Coley’s toxins, leading to tumor regression, now known to be mediated by acutely activated cytotoxic immune cells (5). These paradoxical properties of leukocytes owe in part to functional plasticity of myeloid- and lymphoid-lineage cells. Macrophages, for example, when exposed to type 2 cytokines like interleukin-4 (IL-4), express vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) and thereby enhance angiogenesis and mammary carcinoma metastasis, respectively (6). These are variably referred to as M2, alternatively activated, or type 2 macrophages. In contrast, macrophages activated through the tumor necrosis factor (TNF) receptor superfamily member CD40 become tumoricidal and deplete tumor stroma, thus enabling access by other immune cells and cytotoxic drugs and resulting in pancreatic tumor regression (7). Experimental and clinical data indicate that plasticity is a common property of most leukocyte subtypes and thus can be leveraged therapeutically. The immune armamentarium involved in cancer-associated inflammation encompasses a broad spectrum of immune cells and products. Critiqued below are the laboratory- and clinical-based studies providing insight into these issues and identifying potential targets for therapeutic intervention.
Tumor-Promoting Inflammation
The majority of malignant tumors (95%) have been linked to somatic (as opposed to germline) mutations in genes encoding proteins regulating critical aspects of cell cycle progression and/or death (8). Epidemiological studies have provided etiologic insight into many of these mutations, thus revealing that 30% of human malignancies are linked to tobacco use, 35% to diet, 14 to 20% to obesity, 18% to infectious agents, and 7% to radiation or environmental pollutants (9). Besides directly “initiating” the formation of cancerous cells, these factors might also act as tumor promoters by triggering acute activation of immune effector programs leading to infiltration of “initiated” tissues by immune cells (10, 11). When sustained over long periods without resolution, these tissue assaults become chronic and, by various mechanisms, provide the underpinnings for tumor development (12, 13). Adding fuel to the fire, age-related cellular senescence can also act as a tumor promoter by initiating several inflammatory programs (14), possibly explaining the higher incidence of malignancy in aged populations.
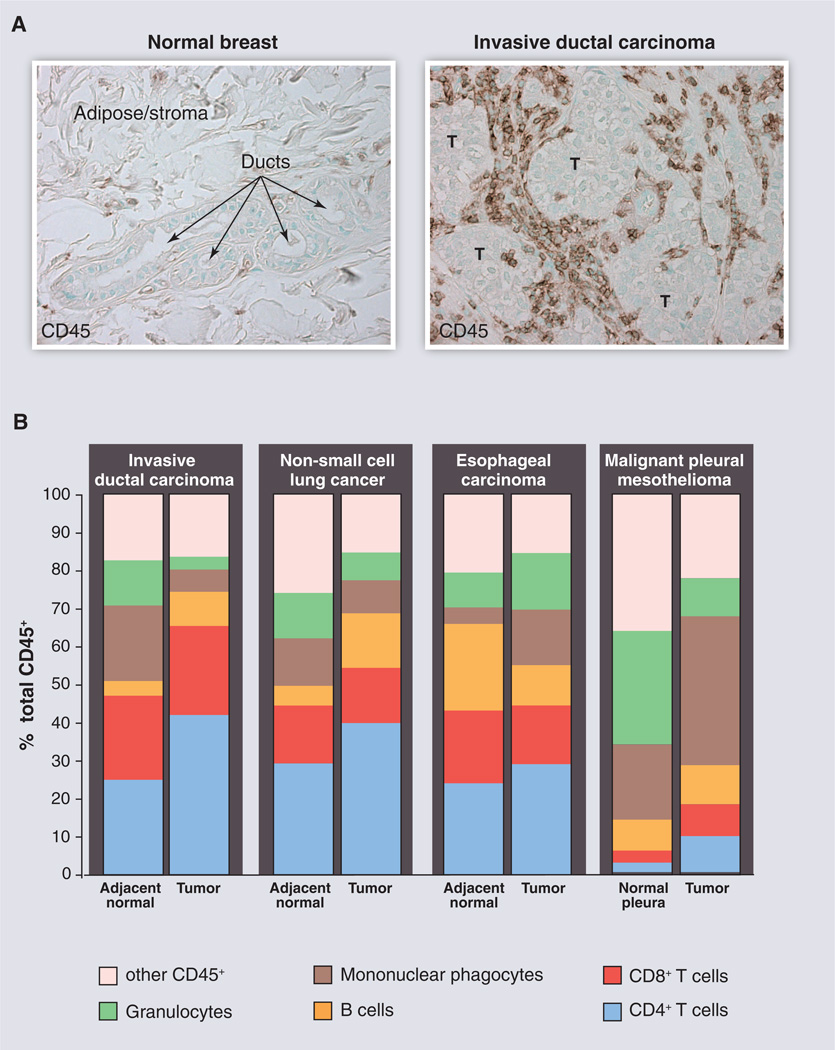
Nevertheless, several questions arise as to which subsets of immune cells directly or indirectly promote malignancy, which of these can be reprogrammed based on their functional plasticity to instead combat cancer, and to what degree these properties are generic or tissue-specific. Although most adult solid tumors (carcinomas most notably) contain infiltrates of diverse leukocyte subsets (15) (Fig. 1), flow cytometric analysis of solid tumors with distinct genetic anomalies (breast, lung, mesothelioma) indicates that leukocyte complexity varies depending on the tissue or organ location and stage of malignancy, suggesting that immune-based therapies will need to reflect these nuances and be more personalized.
Fig. 1.
Leukocyte infiltration and complexity in human cancers. (A) CD45+ leukocytes (brown staining) in normal human breast tissue compared with invasive ductal carcinoma. These images illustrate the substantial infiltration of leukocytes into neoplastic tissue compared with “normal” tissue counterparts. T indicates tumor nests or tumor cell clusters. (B) Immune cell complexity of adjacent normal tissues (or normal pleura) and the indicated tumors as revealed by polychromatic flow cytometry and expressed as a percentage of CD45+ cells. Colors indicate major categories of select immune cell lineages. [Images and data have not been published previously and are courtesy of the Coussens laboratory]
Players and Mechanisms
Myeloid cells
Under homeostatic conditions, leukocytes are charged with maintaining tissue health. Innate immune cells, including macrophages, granulocytes, mast cells, dendritic cells (DCs), innate lymphocytes, and natural killer (NK) cells, represent the first line of defense against pathogens and foreign agents. Perturbed tissue homeostasis, such as during an infection, activates tissue-resident macrophages and mast cells to secrete matrix-remodeling proteins, cytokines and chemokines, that collectively activate local stromal cells (fibroblasts, adipocytes, vascular cells, etc.) to recruit circulating leukocytes into damaged tissue (acute inflammation), leading to elimination of pathogenic agents (tissue damage) in situ. Response to a pathogen also involves DCs, a rare cell type that is one of the key cellular sensors of microbes. DCs are bone marrow–derived cells seeded in all tissues and are thereby linked to their environment through a wealth of molecular sensors that allow them to capture invading microbes (as well as tumor antigens) and to transmit the resulting information to lymphocytes; thus, DCs provide an essential link between the innate and adaptive immune responses (16), a critical step because T cells cannot recognize unprocessed antigens. Upon recognition of a foreign antigen, CD4+ and CD8+ T lymphocytes and B lymphocytes undergo clonal expansion and mount “adaptive” responses specific to the foreign agent. When compared with other antigen-presenting cells, such as macrophages, DCs are extremely efficient; very low numbers of DCs can elicit naïve T cells to respond. Once foreign agents have been eliminated (in the context of acute tissue damage), inflammation resolves and tissue homeostasis is restored.
In tumors, these well-orchestrated series of events fail to resolve and therefore lead to chronic inflammation of the “damaged” (neoplastic) tissue. Chronically activated leukocytes supply direct and indirect mitogenic growth factors that stimulate proliferation of cancer and stromal cells (12). Notable examples include EGF, transforming growth factor–β (TGFβ), TNFα, and fibroblast growth factors, as well as various ILs, chemokines, histamine, and heparins (12). In addition, several leukocyte subsets, predominantly macrophages, granulocytes, monocytes, and mast cells, secrete diverse classes of proteolytic enzymes that modify the structure and function of extracellular matrix (ECM), leading to uncaging of ECM-sequestered mitogenic agents (17). Although these are typical processes of tissue repair (15, 18), their chronic presence provides a survival advantage to evolving cancer cells by maintaining proliferative signaling; blunting cell death in response to matrix detachment; activation and maintenance of angiogenesis; facilitating cancer cell egress from primary tumors; and impairing antitumor cytotoxic cell–mediated killing of “damaged” (cancer) cells (2). Thus, chronically activated myeloid cells in neoplastic tissues support many of the hallmarks of cancer (2).
T cells
CD4+ T helper cells are key regulators of inflammatory processes in cancers. An expanding list of T helper (TH) subsets (TH1, 2, 9, 10, 17, and 22), specialized for promoting particular types of inflammation, function through their secretion of a restricted set of cytokines enabling immune responses (19), often tailored to the specific pathogen encountered. All of these distinct CD4+ T cell types can contribute to tumorigenesis in various ways, depending on context. For example, regulatory T cells (Tregs), an immunosuppressive subset of TH cells, inhibit cytotoxic functions of CD8+ T cells, thereby preventing tumor rejection (20). Although in general favoring tumor rejection, TH1 cells might contribute to tumor escape via secretion of interferon (IFN)–γ, which triggers expression of programmed cell death ligand (PDL)–1 that provides off signals to cytotoxic T cells (21). Furthermore, selective evolutionary pressure by IFN-γ may lead to tumor editing and selection of resistant clones, thereby facilitating tumor development (22). Such plasticity of outcomes is even further exemplified by the more recently identified TH17 cells (23) that exert either pro- or antitumor activity depending on the tissue environment in which they reside [reviewed in (24)]. Their major protumor effects are linked to angiogenesis, recruitment of myeloid cells, and in particular neutrophils that secrete elastase, a protumor mediator (24). However, IL-17 produced by TH17 cells can synergize with IFN-γ to induce secretion of the chemokines CXCL9 and CXCL10 by tumor cells, which in turn attract cytotoxic T cells (24). Such synergistic effects of IL-17 and IFN-γ could possibly be exploited for cancer therapy.
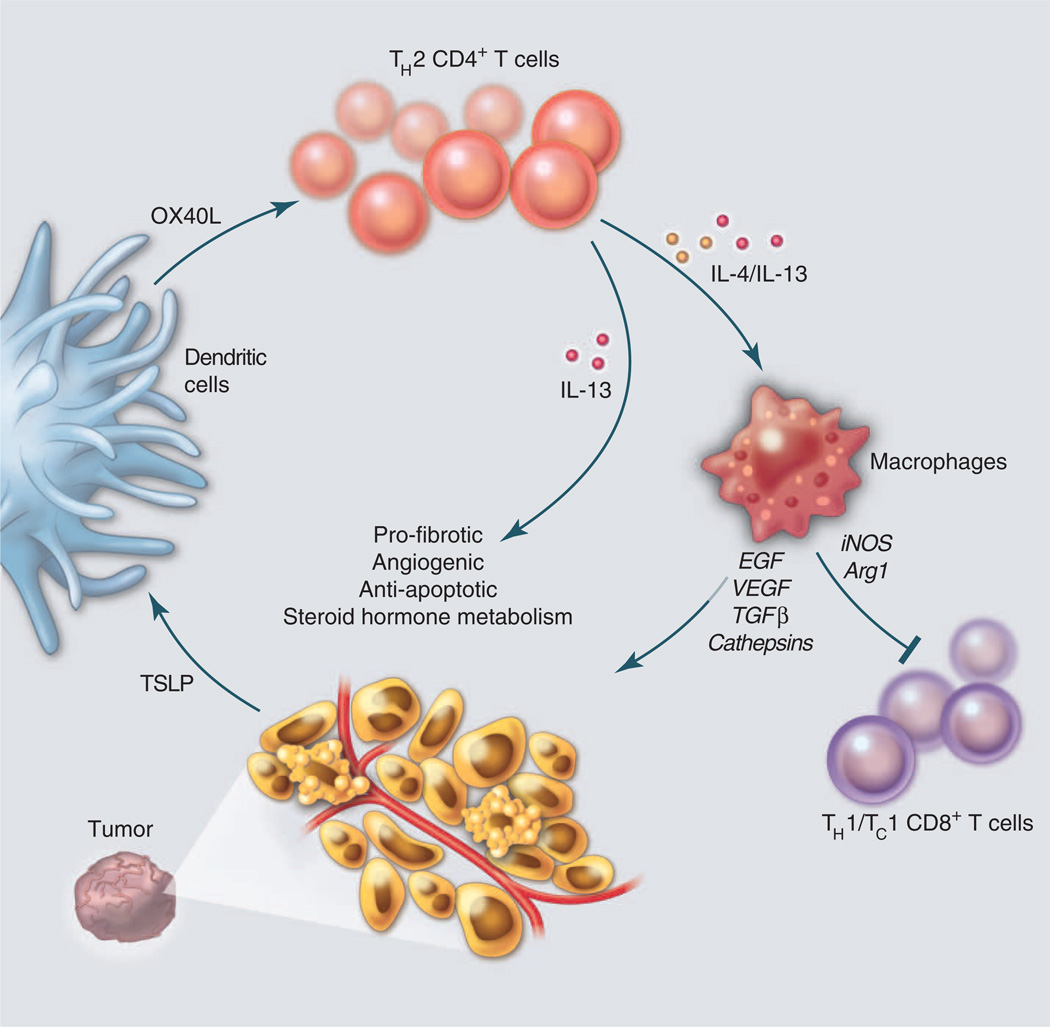
TH2 cells are well recognized for their tumor-promoting capabilities. Breast and pancreatic cancer, for example, are heavily infiltrated by TH2 cells (25) that coexpress IL-4/IL-13 and TNFα, but lack IL-10 secretion (26). These TH2 cells are “driven” by OX40 ligand (L)–expressing DCs in response to cancer-derived thymic stromal lymphopoietin (TSLP) (27) (Fig. 2). TH2 cells accelerate growth of breast carcinomas in humanized mouse models through production of IL-13 (25). In genetically engineered mouse models of mammary carcinogenesis, TH2 cells accelerate development of pulmonary metastasis via IL-4 activation of macrophages that thereby become type 2–polarized and provide survival signals to neoplastic epithelia and chemotherapy resistance (28, 29).
Fig. 2.
Induction of TH2-type immune responses downstream of TSLP. DCs in tumor microenvironments are exposed to cancer-derived factors—for example, TSLP—that skew their maturation toward TH2-type inflammation, including their expression of OX40L. In this environment, responding TH2 cells (CD4+ T cells) secreting IL-4 and IL-13 promote tumor development either directly or indirectly via macrophages. Direct effects include triggering anti-apoptotic pathways and steroid metabolism in epithelial cancer cells, as well as promoting stromal fibroblast proliferation and differentiation. Indirect effects include triggering secretion of growth (EGF) and pro-angiogenic (VEGF) factors by tumor-infiltrating macrophages that also express inducible nitric oxide synthase (iNOS) and arginase (73) and thereby blunt CD8+ T cell proliferation.
In addition, IL-13 produced by NK T cells induces myeloid cells to make TGFβ, which ultimately fosters Treg cell development and inhibits cytotoxic T cells (30). Autocrine IL-13 is important in the pathophysiology of Hodgkin’s disease (31), where it stimulates Hodgkin and Reed-Sternberg cells growth. Similar to Hodgkin’s cells, breast cancer cells express phospho–signal transducer and activator of transcription 6 (STAT6) that is activated downstream of IL-13 receptor–dependent signaling (25), which can result in up-regulation of anti-apoptotic pathways in cancer cells that may be involved in resistance to cytotoxic CD8+ T cells and cytotoxic drugs (2, 32).
Clinically, the TH2 signature in breast cancer (33) and the expression of the TH 2 master regulator transcription factor GATA-3 is increased in metastatic sentinel lymph nodes in breast cancer, and it is associated with rapid disease progression and diminished overall survival in pancreatic cancer (34). Furthermore, the pathogenic TSLP/IL-13 pathway has also been detected in the context of Helicobacter pylori infection that leads to chronic gastritis, the causative factor in gastric cancer (35). Thus, interference with this inflammatory protumor TSLP-OX40L– IL-13 axis (Fig. 2) can be considered as a novel investigational therapeutic approach for several cancer types. Nevertheless, likely owing to tissue specificity, blockade of TSLP in squamous neoplasms instead accelerates malignancy by invoking protumorigenic activities of infiltrating monocytes that in turn blunt antitumor cytotoxic CD8+ T cells (36, 37).
Expression of immune checkpoint molecules such as PD-1 (a T cell receptor that mediates T cell inhibition) and its ligands, PD-L1 and PD-L2, forms a major receptor/ligand inhibitory pathway regulating T cell responses. Expression of PD-L1 on surfaces of tumor cells and tumor-infiltrating myeloid cells provides an off signal to PD-1–expressing T cells and thus enables tumor cells to escape immunosurveillance. Under persistent antigen exposure (such as in chronic infections or in tumor microenvironments), both CD4+ and CD8+ T cells up-regulate PD-1 expression, contributing to Tcell exhaustion (38). Blocking this pathway, for example, during chronic viral infection, reinvigorates virus-specific CD8+ T cell responses and results in enhanced T cell effector responses and viral clearance (39). However, other studies have revealed that conventional chemotherapy paradoxically increases the number of macrophages expressing PD-L1, thereby inhibiting CD8+ T cells and increasing the risk of treatment failure (40).
B cells
As the sole producers of immunoglobulins (Igs), B cells are critical for humoral immunity and also influence other leukocyte subtypes. For example, B cell–derived paracrine factors can be causative and/or potentiate disease by sustaining chronic inflammation during autoimmunity (41). The role of B cells in cancer is under intense examination. In the skin, squamous carcinogenesis is limited in the absence of B cells (42–44). Two mechanisms appear to be involved in B cell–dependent skin carcinogenesis: (i) When autoantibody IgG is deposited into neoplastic parenchyma via leaky blood vessels, ligation of immune complex/Fcγ receptors on mast cells and macrophages fosters pro-angiogenic and immunosuppressive gene expression programs (42, 43); (ii) B cell secretion of IL-10 and TNFα activates protumorigenic myeloid cells that also foster cancer progression (44).Whether the IL-10– expressing B cells represent regulatory B cells (Bregs/B10) remains to be determined but is an important point to consider, because Bregs are resistant to aCD20 B cell–depleting therapy (45) and suppress the efficacy of CD20 immunotherapy (46). During prostate carcinogenesis, the Wnt family member wingless-type MMTV integration site family member 16B (WNT16B) is up-regulated by nuclear factor κ light polypeptide gene enhancer (NF-κB) in B cells after DNA damage and, via a paracrine mechanism, activates the canonical Wnt program in evolving tumor cells, the result of which is chemoresistance in combination with enhanced tumor cell survival and disease progression (47). In addition, B cell–derived lymphotoxin β promotes prostate metastasis in castration-resistant disease by stimulating inhibitor of NF-κB (IκB) kinase α (IKKα) and STAT3 activity in malignant cells, thus provoking androgen refractory regrowth and metastasis (48). Interestingly, B cells were found to be without functional significance during mammary carcinogenesis (49), further illustrating tissue specificity and perhaps oncogene specificity in the regulation of leukocyte protumorigenic activities. Taken together, immune cell functions vary by tissue and tumor type (Fig. 1), indicating that a one-size-fits-all approach will likely not be effective in immunebased therapeutic strategies.
Therapeutic Targets
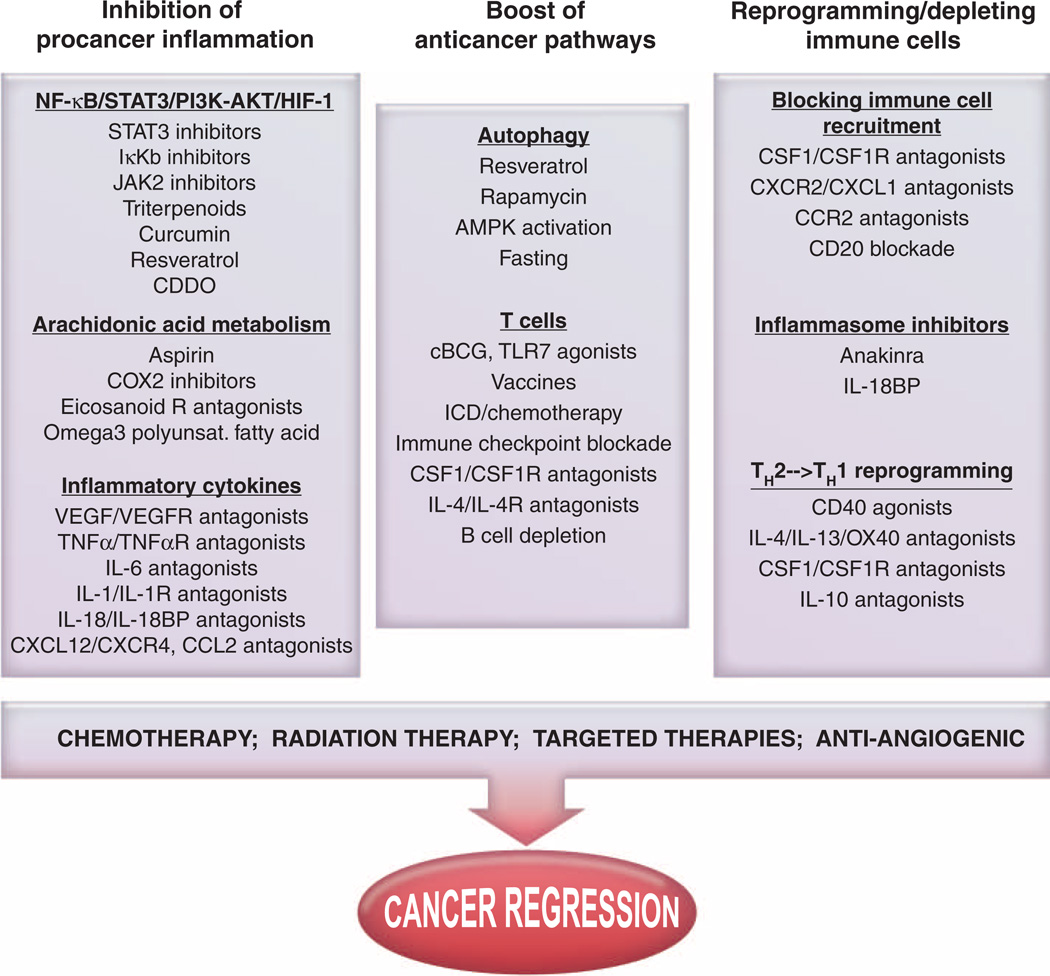
Effectively counteracting or neutralizing tumor-promoting inflammation will necessitate simultaneous reprogramming or quelling of multiple immune-response programs activated in cancers. Alternatively, targeting the master regulators of adaptive immunity, DCs, and master effectors of tissue damage, macrophages, will allow a cascade of events favoring cancer rejection (Fig. 2). On the basis of available data, the pathways that present attractive targets today include (i) inhibition or sequestration of cytokines or chemokines, especially those that activate the STAT3/NF-κB pathway; (ii) depletion or reprogramming of procancer tumor-associated immune cells; and (iii) harnessing cytotoxic T cells by either neutralization of Treg cells, blockade of the PD-1/PD-L pathway, or inhibition of myeloid-based immunosuppressive molecules (Fig. 3). Combinations of these strategies to simultaneously favor (immunogenic) tumor cell death with conventional cytotoxic approaches may achieve a state akin to that present during acute inflammation during wound healing, thereby leading to activation of scavenging immune effectors and increased cancer cell death (Fig. 4). How these individual strategies, based on tissue, oncogene, or organ specificity and/or complexity of the immune infiltrate present, are being tailored is discussed below.
Fig. 3.
Therapeutic strategies against cancer-induced chronic inflammation. Inhibiting tumor cell–intrinsic proinflammatory functions [such as blunting NF-κB/STAT3/phosphatidylinositol 3-kinase (PI3K)–Akt pathways or downstream effectors]. Moreover, turning lymphocytes into effector TH1/TC1 cells necessitates effective reprogramming of type 2 macrophages or immunosuppressive DCs by a concerted action of pattern recognition receptors, the inflammasome platform, or CD40 costimulation, as well as neutralization of immune checkpoint ligand/receptor interaction. In parallel, reducing the accumulation or migration of suppressive myeloid cells in primary sites or distant niches while promoting cytoreduction/debulking with irradiation, cytotoxic compounds, or antiangiogenic molecules may synergistically gear the host/tumor imbalance toward durable tumor regression. HIF-1, hypoxia-inducible factor 1; AMPK, adenosine monophosphate–activated protein kinase; JAK2, Janus kinase 2; CDDO, 2-cyano- 3,12-dioxooleana-1,9(11)-dien-28-oic acid; TLR7, Toll-like receptor 7; COX2, cyclooxygenase; ICD, immunogenic cell death.
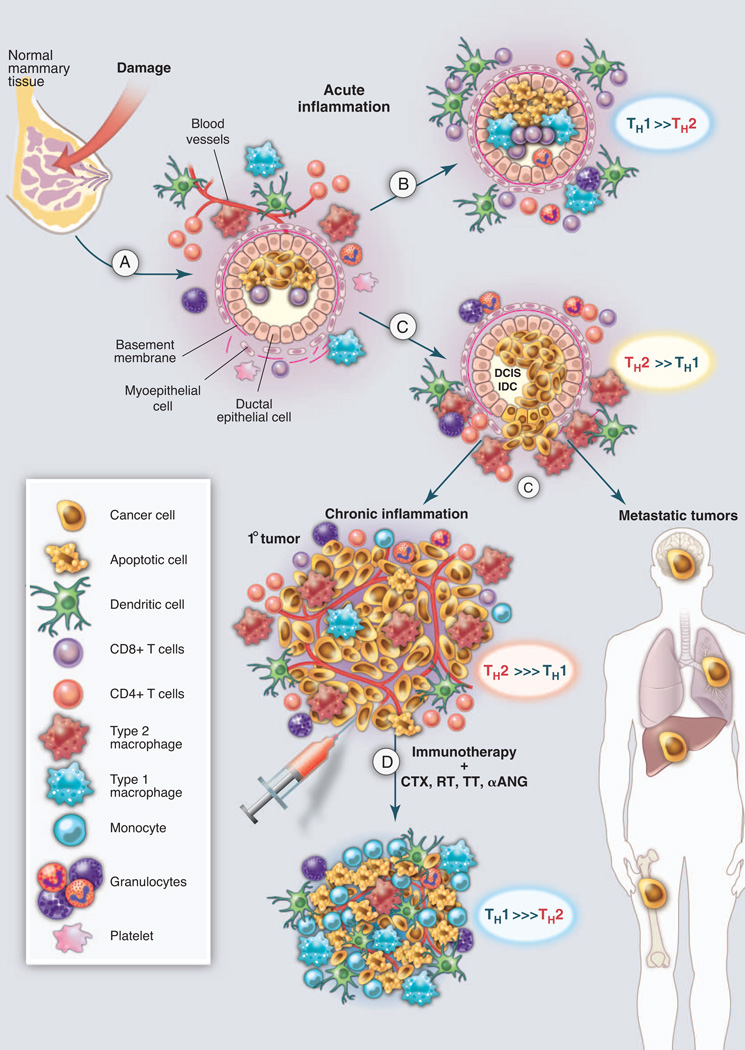
Fig. 4.
Targeting tumor-promoting chronic inflammation as a therapeutic strategy. (A) Tissue damage results in activation of hard-wired pathways (angiogenic and immune) embedded in all tissues to facilitate healing and homeostasis. (B) Type 1 immune responses, aided by TH1 cells, eradicate damaged cells to aid the healing prcess. (C) In tissues harboring initiated cells, neoplastic epithelial cells secrete factors such as TSLP, GM-CSF, CSF-1, and TNFα, thereby inducing recruitment of leukocytes that become TH2-polarized and resulting in chronic activation of angiogenic and tissue remodeling programs, enhanced survival signaling to aid proliferation and blunt cell death, and generation of an immunosuppressive environment that fosters primary tumor development and aids in metastatic disseminations. (D) Effectively counter acting or neutralizing tumor-promoting chronic inflammation may be achieved by resetting or reprogramming the prominent TH2-based programs activated in cancer; this may result in simultaneously favoring (immunogenic) tumor cell death, where TH1-based immunity emerges akin to that present during acute inflammation during wound healing, thus enabling a cascade of events favoring cancer rejection, perhaps as monotherapy but more likely in combination with chemotherapy (CTX), radiotherapy (RT), targeted therapy (TT), or antiangiogenic modalities (αANG). DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
Selective inhibition or sequestration of cancer inflammation–induced cytokines and chemokines
High serum concentrations of proinflammatory TNFα, IL-6, or inflammasome-related IL-1β/IL-18 correlate with advanced malignancies and are associated with reduced survival (50, 51). Several anticytokine agents are already in use for treatment of cancer (51). For example, in a phase II trial of a chimeric antibody against IL-6 in ovarian cancer, those patients exhibiting a prolonged stabilization of disease showed significant declines in plasma levels of the chemokines promoting immune cell recruitment (CCL2 and CXCL12), as well as angiogenesis (VEGF) (52). Blockade of TNFα represents another pathway; however, chronic administration of TNF inhibitors in patients suffering from rheumatoid arthritis may increase the risk of developing lymphoma (53, 54). Whether inhibiting the membrane-bound or the soluble form of TNFα makes a difference is currently under investigation.
Blockade of CCL2 may also represent a viable therapeutic strategy. In mammary cancer models, depletion of tumor cell–derived CCL2 inhibits metastatic seeding (55). In prostate carcinogenesis, CCL2 protects malignant cells from chemotherapy-induced cytotoxicity, and suppression of CCL2 leads to enhanced responses to taxane-based chemotherapy (56). Similarly, interrupting the CXCR4/CXCL12 chemokine axis can be used to sensitize resistant tumor cells to chemotherapy or radiotherapy and potentially inhibit vascularization and tumor cell spreading. This response is in part related to bone marrow–derived TIE-2–positive macrophages that are pro-angiogenic and specifically attracted to irradiated tumors in a CXCL12-dependent fashion and thereby contribute to tumor regrowth post-therapy (57). AMD3100 (plerixafor), approved by the Food and Drug Administration (FDA) for hematopoietic progenitor cell mobilization, reduces TIE-2–positive macrophage recruitment (58); the CXCL12 peptide analog was assigned an orphan drug status by the FDA for treatment of osteosarcoma.
Depletion or reprogramming of tumor-associated immune cells
We have already discussed the master regulatory role of macrophages in tumor initiation and maintenance. Consequently, blockade of macrophage colony-stimulating factor 1 or its receptor (CSF1/CSF1R) rapidly diminishes macrophage presence and promotes TH1 responses in late-stage mammary adenocarcinomas (59). CSF-1–related gene signatures (60) and the presence of proliferating macrophages predict risk of recurrence (61), as well as response to chemotherapy in breast cancer (59). Antagonist αIL-4 therapeutic antibodies reprogram tumor-associated type 2 macrophages, monocytes, and other TH2 cells toward TH1 phenotypes in mammary cancer (49). Reprogramming macrophages can also be achieved by administration of agonistic αCD40 therapeutic antibodies as already discussed. Lastly, as another example of therapeutic interference with myeloid cells, treatment of pancreatic cancers in mice with granulocyte-macrophage colony-stimulating factor (GM-CSF) antagonists blocks monocyte recruitment and thereby favors CD8+ T cell infiltrates that slow tumor development (62, 63).
Rituximab, a chimeric monoclonal antibody against CD20 that is predominantly expressed on the surface of B cells, leads to B cell depletion (64) and thus could be considered in solid tumors. Indeed, a pilot clinical study in advanced colon cancer patients treated with rituximab reported encouraging tumor regressions [reviewed in (65)].
Immune cells can also be targeted and manipulated by using innate receptors involved in pathogen responses or pathogens themselves. For example, intravesical instillation of bacillus Calmette-Guérin (BCG) is effective at eliciting actute inflammation and successful tumor immunity in patients with nonmuscle invasive bladder cancer, leading to 50 to 70% clinical response (66), and was FDA-approved in 1990. Other TLR agonists (synthetic imidazoquinoline, imiquimod, or resiquimod) approved for treatment of genital warts and superficial basal cell carcinoma could also be envisioned to induce immune-mediated rejection of skin metastases in breast and melanoma patients (67, 68)
Harnessing cytotoxic T cells
Mobilizing effector/memory antitumor-specific CD4+ and CD8+ T cells producing high levels of IFN-γ (called TH1 and TC1, respectively) may, at least in part, reverse immunosuppression mediated by the tumor microenvironment. IFN-γ has pleiotropic effects on the tumor microenvironment, such as antiangiogenic activities, quelling protumorigenic properties of macrophages while also enhancing their tumoricidal properties, and enhanced processing and presentation of tumor antigens to T lymphocytes. Hence, therapeutics bolstering TH1 programming may provide a survival advantage (Fig. 4). Vaccination—that is, the provision of an antigen together with an adjuvant to elicit therapeutic T cells in vivo—combined with modulation of the tumor microenvironment represents a very promising and powerful therapeutic strategy to boost antitumor T cell immunity as well. However achieved, the T cells elicited by a vaccine, adoptively transferred, or unleashed by modulation of the tumor microenvironment will likely require additional help provided by interference with off signals able to block their anti-tumor function. In particular, phase I clinical trials in patients indicate that blocking the PD-1 pathway is a promising strategy for achieving immunological control of human cancers, including lung cancer (40, 69). This is somewhat analogous to the improved survival now documented in metastatic melanoma patients treated with an antibody against the immunoregulatory molecule CTLA-4 (70) (e.g., ipiluminab), recently approved by the FDA. Given that PD-1 ligands are expressed in many tumor microenvironments, targeting the ligands, as opposed to their receptors, has the potential to be more effective and less toxic than current therapies targeting PD-1 and/or CTLA-4.
Concluding Remarks
Inflammation represents a link between intrinsic (oncogenes, tumor suppressors, and genome stability genes) and extrinsic (immune and stromal components) factors contributing to tumor development. This knowledge offers new and novel candidate targets for therapeutic intervention, in combination with more conventional therapeutic approaches such as chemotherapy, radiotherapy, and targeted therapy. Therapeutic manipulation of chronic inflammation in tumors is likely to enhance the clinical efficacy of therapeutic vaccination as well as adoptive T cell transfer, thus turning the chronic procancer inflammatory microenvironment into an anticancer microenvironment that is more likely to also liberate and activate existing anticancer effector T cells. Given the functional relevance of immune networking in tumors, it is imperative to incorporate immunometrics such as “the immunoscore” into traditional classification schemes to provide new prognostic and/or predictive tools to clinical practice (71, 72). A better identification of tissue and/or tumor-specific inflammatory mechanisms (obtained through next-generation sequencing, metabolomics, and epigenetics) will allow us to direct the clinical management of cancer toward a more personalized medicine. A magic bullet? Yes, but not as stand-alone monotherapy. Rather, inflammation is another piece of the puzzle constituting hallmarks of cancer, the targeting of which can bring us closer to successful therapy for this dreaded and deadly disease.
References and Notes
- 1.Hanahan D, Weinberg RA. Cell. 2011;144:646. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Cancer Cell. 2012;21:309. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Cell. 2010;140:883. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Bickels J, Kollender Y, Merinsky O, Meller I. Isr. Med. Assoc. J. 2002;4:471. [PubMed] [Google Scholar]
- 6.Ruffell B, Affara NI, Coussens LM. Trends Immunol. 2012;33:119. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty GL, et al. Science. 2011;331:1612. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wooster R, Bachman KE. Curr. Opin. Genet. Dev. 2010;20:336. doi: 10.1016/j.gde.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, Ward E. CA Cancer J. Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 10.Wei EK, Wolin KY, Colditz GA. J. Clin. Oncol. 2010;28:4052. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thun MJ, Henley SJ, Gansler T. Novartis Found. Symp. 2004;256:6. [PubMed] [Google Scholar]
- 12.Balkwill F, Charles KA, Mantovani A. Cancer Cell. 2005;7:211. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Nature. 2002;420:860. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freund A, Orjalo AV, Desprez PY, Campisi J. Trends Mol. Med. 2010;16:238. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tlsty TD, Coussens LM. Annu. Rev. Pathol. 2006;1:119. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 16.Steinman RM, Banchereau J. Nature. 2007;449:419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 17.Lu P, Takai K, Weaver VM, Werb Z. Cold Spring Harb. Perspect. Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvorak HF. N. Engl. J. Med. 1986;315:1650. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 19.Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. Nat. Rev. Immunol. 2009;9:811. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanchot C, et al. Cancer Microenviron. published online 27 October 2012. [Google Scholar]
- 21.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. Nat. Immunol. 2007;8:239. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita H, et al. Nature. 2012;482:400. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong C. Nat. Rev. Immunol. 2008;8:337. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 24.Wei S, Zhao E, Kryczek I, Zou W. Oncoimmunology. 2012;1:516. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspord C, et al. J. Exp. Med. 2007;204:1037. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YJ, et al. Annu. Rev. Immunol. 2007;25:193. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 27.Pedroza-Gonzalez A, et al. J. Exp. Med. 2011;208:479. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gocheva V, et al. Genes Dev. 2010;24:241. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shree T, et al. Genes Dev. 2011;25:2465. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terabe M, Park JM, Berzofsky JA. Cancer Immunol. Immunother. 2004;53:79. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinnider BF, Mak TW. Blood. 2002;99:4283. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WJ, et al. Cytokine. 2008;42:39. doi: 10.1016/j.cyto.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen VN, et al. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2802. [Google Scholar]
- 34.De Monte L, et al. J. Exp. Med. 2011;208:469. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kido M, et al. Infect. Immun. 2010;78:108. doi: 10.1128/IAI.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Cancer Cell. 2012;22:479. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Demehri S, et al. Cancer Cell. 2012;22:494. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Chen L. Curr. Top. Microbiol. Immunol. 2011;344:245. doi: 10.1007/82_2010_81. [DOI] [PubMed] [Google Scholar]
- 39.Sakthivel P, Gereke M, Bruder D. Rev. Recent Clin. Trials. 2012;7:10. doi: 10.2174/157488712799363262. [DOI] [PubMed] [Google Scholar]
- 40.Hasan A, Ghebeh H, Lehe C, Ahmad R, Dermime S. Expert Opin. Ther. Targets. 2011;15:1211. doi: 10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- 41.Pillai S, Mattoo H, Cariappa A. Curr. Opin. Immunol. 2011;23:721. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Visser KE, Korets LV, Coussens LM. Cancer Cell. 2005;7:411. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Andreu P, et al. Cancer Cell. 2010;17:121. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schioppa T, et al. Proc. Natl. Acad. Sci. U.S.A. 2011;108:10662. [Google Scholar]
- 45.Haas KM, Poe JC, Steeber DA, Tedder TF. Immunity. 2005;23:7. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. J. Clin. Invest. 2011;121:4268. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Y, et al. Nat. Med. 2012;18:1359. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. Nature. 2010;464:302. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeNardo DG, et al. Cancer Cell. 2009;16:91. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinarello CA. Cancer Metastasis Rev. 2010;29:317. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkwill FR, Mantovani A. Semin. Cancer Biol. 2012;22:33. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Anglesio MS, et al. Clin. Cancer Res. 2011;17:2538. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 53.Geborek P, et al. Ann. Rheum. Dis. 2005;64:699. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bongartz T, et al. JAMA. 2006;295:2275. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 55.Qian BZ, et al. Nature. 2011;475:222. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian DZ, et al. Prostate. 2010;70:433. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozin SV, et al. Cancer Res. 2010;70:5679. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welford AF, et al. J. Clin. Invest. 2011;121:1969. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeNardo DG, et al. Cancer Discov. 2011;1:54. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck AH, et al. Clin. Cancer Res. 2009;15:778. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell MJ, et al. Breast Cancer Res. Treat. 2011;128:703. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Cancer Cell. 2012;21:836. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayne LJ, et al. Cancer Cell. 2012;21:822. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kessel A, Rosner I, Toubi E. Clin. Rev. Allergy Immunol. 2008;34:74. doi: 10.1007/s12016-008-8074-1. [DOI] [PubMed] [Google Scholar]
- 65.Tan TT, Coussens LM. Curr. Opin. Immunol. 2007;9:209. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Zbar B, Tanaka T. Science. 1971;172:271. doi: 10.1126/science.172.3980.271. [DOI] [PubMed] [Google Scholar]
- 67.Adams S, et al. Clin. Cancer Res. 2012;18:6748. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heber G, et al. J. Dtsch. Dermatol. Ges. 2009;7:534. doi: 10.1111/j.1610-0387.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- 69.Brahmer JR, et al. J. Clin. Oncol. 2010;28:3167. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hodi FS, et al. N. Engl. J. Med. 2010;363:711. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galon J, et al. J.Transl. Med. 2012;10:205. [Google Scholar]
- 72.Galon J. J. Transl. Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doedens AL, et al. Cancer Res. 2010;70:7465. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]