Abstract
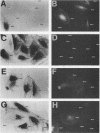
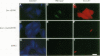
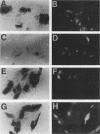
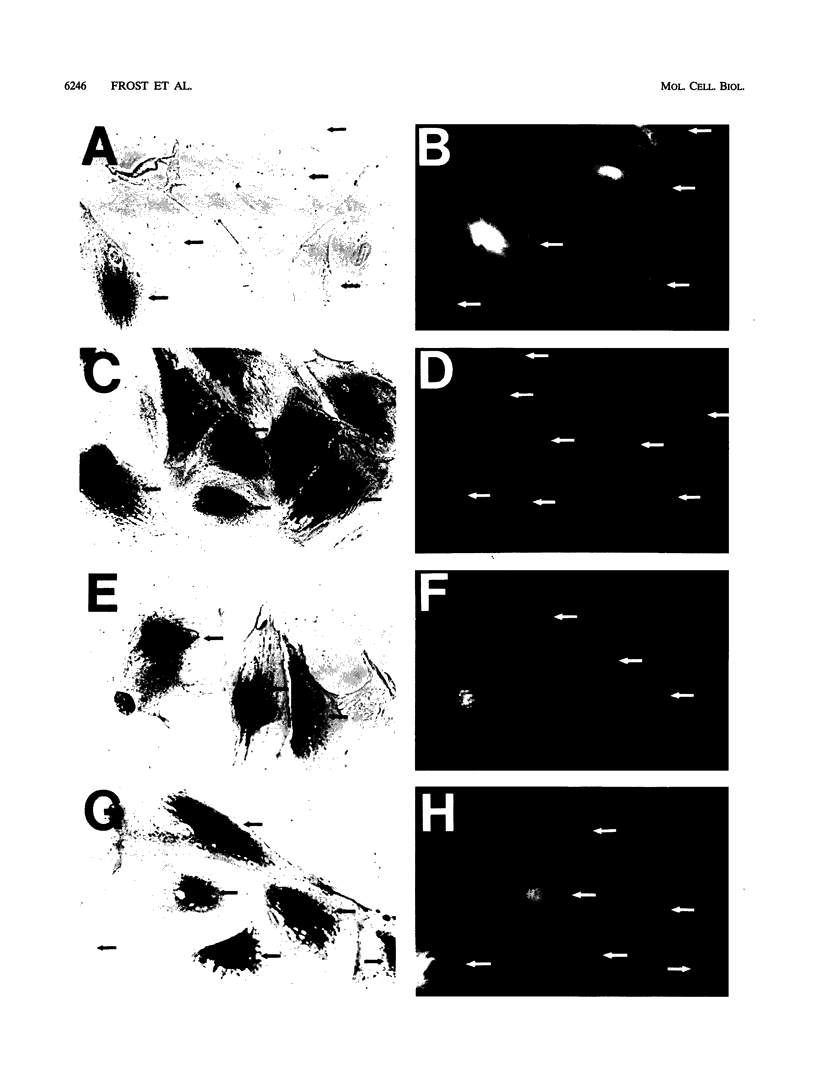
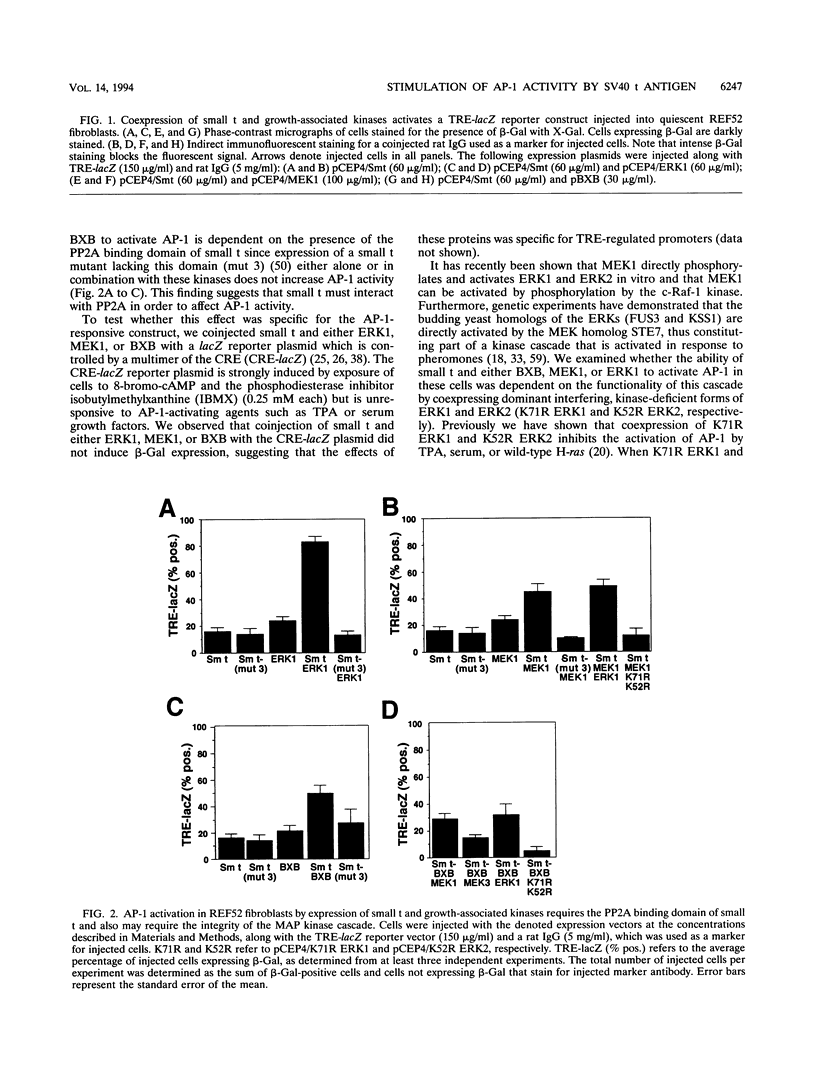
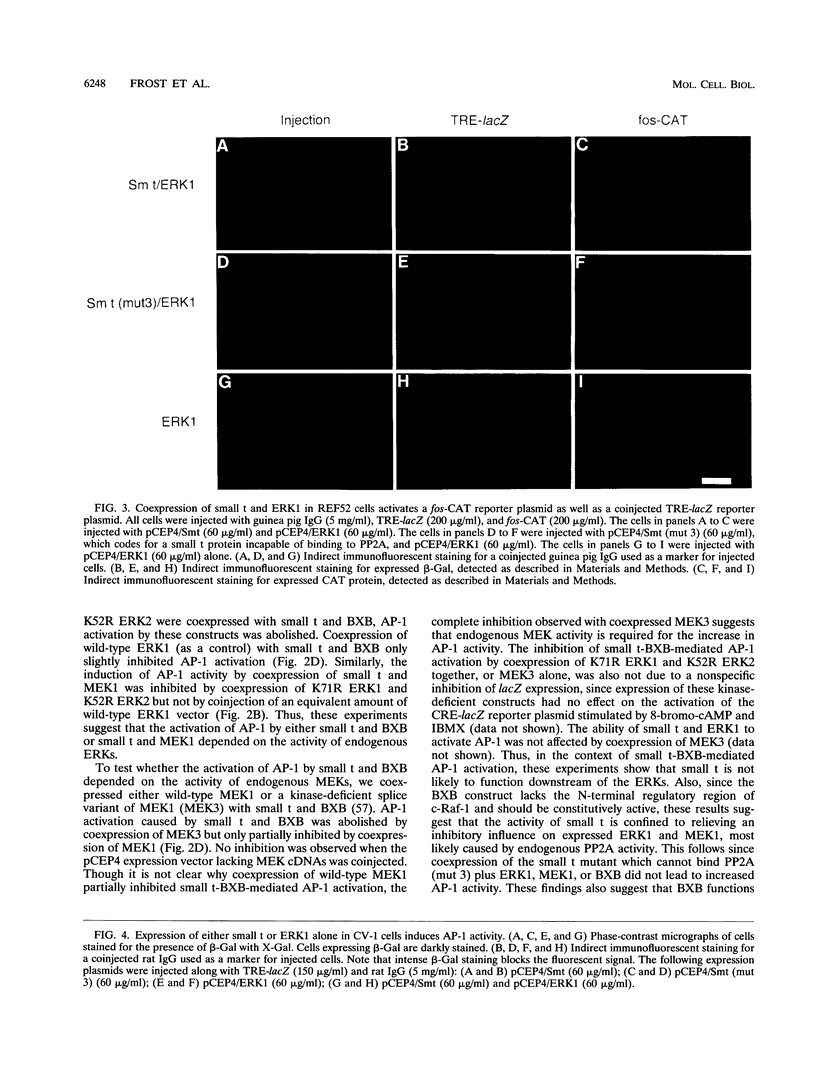
The simian virus 40 small tumor antigen (small t) specifically interacts with protein phosphatase type 2A (PP2A) in vivo and alters its catalytic activity in vitro. Among the substrates for PP2A in vitro are the activated forms of MEK and ERK kinases. Dephosphorylation of the activating phosphorylation sites on MEK and ERKs by PP2A in vitro results in a decrease in their respective kinase activities. Recently, it has been shown that overexpression of small t in CV-1 cells results in an inhibition of PP2A activity toward MEK and ERK2 and a constitutive upregulation of MEK and ERK2 activity. Previously, we have observed that overexpression of either ERK1, MEK1, or a constitutively active truncated form of c-Raf-1 (BXB) is insufficient to activate AP-1 in REF52 fibroblasts. We therefore examined whether overexpression of small t either alone or in conjunction with ERK1, MEK1, or BXB could activate AP-1. We found that coexpression of small t and either ERK1, MEK1, or BXB resulted in an increase in AP-1 activity, whereas expression of either small t or any of the kinases alone did not have any effect. Similarly, coexpression of small t and ERK1 activated serum response element-regulated promoters. Coexpression of kinase-deficient mutants of ERK1 and ERK2 inhibited the activation of AP-1 caused by expression of small t and either MEK1 or BXB. Coexpression of an interfering MEK, which inhibited AP-1 activation by small t and BXB, did not inhibit the activation of AP-1 caused by small t and ERK1. In contrast to REF52 cells, we observed that overexpression of either small or ERK1 alone in CV-1 cells was sufficient to stimulate AP-1 activity and that this stimulation was not enhanced by expression of small t and ERK1 together. These results show that the effects of small t on immediate-early gene expression depend on the cell type examined and suggest that the mitogen-activated protein kinase activation pathway is distinctly regulated in different cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alberts A. S., Deng T., Lin A., Meinkoth J. L., Schönthal A., Mumby M. C., Karin M., Feramisco J. R. Protein phosphatase 2A potentiates activity of promoters containing AP-1-binding elements. Mol Cell Biol. 1993 Apr;13(4):2104–2112. doi: 10.1128/mcb.13.4.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. S., Frost J. A., Thorburn A. M. Rapid transcriptional assay for the expression of two distinct reporter genes by microinjection. DNA Cell Biol. 1993 Dec;12(10):935–943. doi: 10.1089/dna.1993.12.935. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Kilgour E., Sturgill T. W. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991 Jun 5;266(16):10131–10135. [PubMed] [Google Scholar]
- Bikel I., Mamon H., Brown E. L., Boltax J., Agha M., Livingston D. M. The t-unique coding domain is important to the transformation maintenance function of the simian virus 40 small t antigen. Mol Cell Biol. 1986 Apr;6(4):1172–1178. doi: 10.1128/mcb.6.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., McCormack M., Zinn K. G., Farrell M. P., Bikel I., Livingston D. M. A recombinant murine retrovirus for simian virus 40 large T cDNA transforms mouse fibroblasts to anchorage-independent growth. J Virol. 1986 Oct;60(1):290–293. doi: 10.1128/jvi.60.1.290-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Charles C. H., Sun H., Lau L. F., Tonks N. K. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5292–5296. doi: 10.1073/pnas.90.11.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Murphy D., Lovett M., Rigby P. W. A fragment of the SV40 large T-antigen gene transforms. Nature. 1982 Sep 2;299(5878):59–61. doi: 10.1038/299059a0. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992 Oct 16;258(5081):478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Erikson R. L. Purification of a murine protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product: relationship to the fission yeast byr1 gene product. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8205–8209. doi: 10.1073/pnas.89.17.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Errede B., Gartner A., Zhou Z., Nasmyth K., Ammerer G. MAP kinase-related FUS3 from S. cerevisiae is activated by STE7 in vitro. Nature. 1993 Mar 18;362(6417):261–264. doi: 10.1038/362261a0. [DOI] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K., Hakes D. J., Wang Y., Park H. D., Cooper T. G., Dixon J. E. A yeast protein phosphatase related to the vaccinia virus VH1 phosphatase is induced by nitrogen starvation. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12175–12179. doi: 10.1073/pnas.89.24.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Bottaro D. P., Chan A., Miki T., Aaronson S. A. Expression cloning of a human dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12170–12174. doi: 10.1073/pnas.89.24.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992 Oct;17(10):418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lin A., Frost J., Deng T., Smeal T., al-Alawi N., Kikkawa U., Hunter T., Brenner D., Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992 Sep 4;70(5):777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A. Roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells: studies with simian virus 40 double mutants. J Virol. 1979 Sep;31(3):596–607. doi: 10.1128/jvi.31.3.596-607.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Meinkoth J. L., Montminy M. R., Fink J. S., Feramisco J. R. Induction of a cyclic AMP-responsive gene in living cells requires the nuclear factor CREB. Mol Cell Biol. 1991 Mar;11(3):1759–1764. doi: 10.1128/mcb.11.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Rubin H., Figge J., Bladon M. T., Chen L. B., Ellman M., Bikel I., Farrell M., Livingston D. M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982 Sep;30(2):469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Ruediger R., Roeckel D., Fait J., Bergqvist A., Magnusson G., Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992 Nov;12(11):4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne P. H., Viciana P. R., Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993 Jul 22;364(6435):352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Dent P., Lynch K. R., Weber M. J., Sturgill T. W. Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol. 1993 Aug;13(8):4539–4548. doi: 10.1128/mcb.13.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Vincent L. A., Haystead C., Haystead T. A., Michel H., Hunt D. F., Lynch K. R., Sturgill T. W. Molecular structure of a protein-tyrosine/threonine kinase activating p42 mitogen-activated protein (MAP) kinase: MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):173–177. doi: 10.1073/pnas.90.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994 Mar 1;13(5):1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem. 1993 May 25;268(15):11435–11439. [PubMed] [Google Scholar]
- Zhou Z., Gartner A., Cade R., Ammerer G., Errede B. Pheromone-induced signal transduction in Saccharomyces cerevisiae requires the sequential function of three protein kinases. Mol Cell Biol. 1993 Apr;13(4):2069–2080. doi: 10.1128/mcb.13.4.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]