Abstract
Objectives
The aim of this study was to evaluate the association between physical activity and changes in levels of highly sensitive troponin T (cTnT) and N-terminal pro–B-type natriuretic peptide (NT-proBNP), and the subsequent risk of the development of heart failure (HF) in community-dwelling older adults.
Background
Higher baseline levels of cTnT and NT-proBNP and increases over time correlate with the risk of HF in older adults. Factors modifying these levels have not been identified.
Methods
NT-proBNP and cTnT were measured at baseline and 2 to 3 years later in adults 65 years of age and older free of HF participating in the Cardiovascular Health Study. Self-reported physical activity and walking pace were combined into a composite score. An increase was prespecified for NT-proBNP as a >25% increment from baseline to ≥190 pg/ml and for cTnT as a >50% increment from baseline in participants with detectable levels (≥3 pg/ml).
Results
A total of 2,933 participants free of HF had NT-proBNP and cTnT measured at both time points. The probability of an increase in biomarker concentrations between baseline and follow-up visits was inversely related to the physical activity score. Compared with participants with the lowest score, those with the highest score had an odds ratio of 0.50 (95% confidence interval: 0.33 to 0.77) for an increase in NT-proBNP and an odds ratio of 0.30 (95% confidence interval: 0.16 to 0.55) for an increase in cTnT, after adjusting for comorbidities and baseline levels. A higher activity score associated with a lower long-term incidence of HF. Moreover, at each level of activity, an increase in either biomarker still identified those at higher risk.
Conclusions
These findings suggest that moderate physical activity has protective effects on early heart failure phenotypes, preventing cardiac injury and neurohormonal activation.
Keywords: aging, exercise, heart failure, natriuretic peptides
With the introduction of highly sensitive (hs) assays for cardiac troponin T (cTnT), small amounts of cardiac injury can be detected in the majority of older adults (1,2). Very low concentrations of cTnT, detectable with the hs assay, demonstrate graded associations with risk of heart failure (HF). Furthermore, an increase or decrease in concentration >50% over 2 to 3 years is common among older adults and predicts a subsequent change in the risk of HF (1). Similarly, both baseline concentrations and change in levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP) are associated with new-onset HR in community-dwelling adults (3,4).
The finding that substantial changes in cTnT and NT-proBNP occur frequently and correlate with changes in a subsequent risk of HF suggests that the high-risk phenotypes characterized by these biomarkers (cardiac injury and neurohormonal activation) may be modifiable. However, the factors that determine changes in these biomarkers are uncertain. Specifically, little is known about the influence of lifestyle factors on the trajectory of these biomarker concentrations, particularly in older adults who may have had decades of exposure to traditional cardiovascular risk factors. The influence of moderate physical activity is of particular interest because change in activity level is associated with a relatively rapid change in the risk of cardiovascular events, including HF (5–7). However, the effect of a modifiable preventive strategy, such as physical activity, on the levels of cardiac-specific biomarkers has never been assessed.
We hypothesized that in an ambulatory cohort of older adults (65 years of age and older) free of HF, cTnT, and NT-proBNP would be less likely to increase and more likely to decrease at follow-up in individuals with a greater duration and intensity of habitual leisure-time physical activity.
Methods
Study population
The CHS (Cardiovascular Heart Study) is a multicenter, prospective, observational cohort study of cardiovascular disease in older adults. The present analyses include all individuals without a diagnosis of prevalent HF at study entry or incident HF between the first and second biomarker measurement, with availability of cTnT and NT-proBNP serum samples at both baseline (1989 to 1990 for the main cohort and 1992 to 1993 for the African-American cohort) and follow-up time points (2 and 3 years later for the main and African-American cohorts, respectively), and complete data on baseline physical activity (see “Measures of Physical Activity” section). Of the 5,888 CHS participants, 2,933 individuals were included in this current analysis for NT-proBNP and 1,757 for cTnT (a measurable baseline level [i.e., >3 pg/ml] was required for inclusion in the cTnT analysis) (Fig. 1). Details of data collection and definition of comorbidities were published previously (1). Differences between those with and without available sera for biomarker measurements were previously reported (1,3). The CHS was approved by the institutional review boards of the University of Washington, Seattle, and the participating centers. All participants gave written informed consent to participate at the time of study enrollment. Our analysis was approved by the institutional review board of the University of Maryland–Baltimore.
Figure 1. Study Participants Included in the Analysis of Physical Activity and Change in Biomarkers.
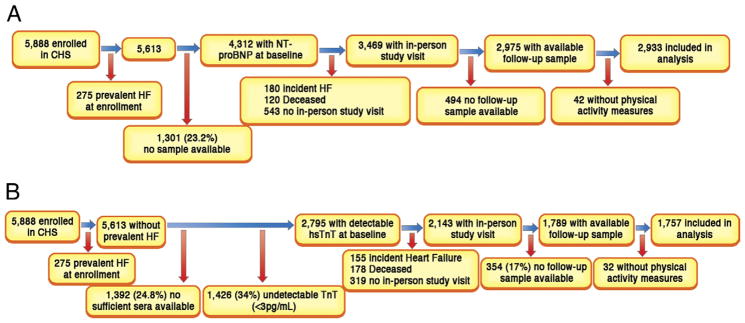
(A) Flow of study participants included in the N-terminal pro–B-type natriuretic peptide (NT-proBNP) analysis.
(B) Flow of study participants included in the cardiac troponin T (TnT) analysis. HF = heart failure; hs = highly sensitive.
Biomarker measures
cTnT and NT-proBNP were measured from serum collections at baseline and first follow-up time points (defined previously) in the main and supplemental CHS cohorts. All measurements were performed on serum samples stored at −70°C to −80°C and thawed just before testing by individuals blinded to participant data. NT-proBNP and cTnT were measured on the Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, Indiana), as previously reported (1). The analytical measurement range for the hs cTnT assay is 3 to 10,000 pg/ml with a 99th percentile of a healthy reference population defined at 13.5 pg/ml and a 10% coefficient of variation less than the 99th percentile (8). The coefficient of variation for the NT-proBNP assay was 2% to 5% during the testing period, and the analytical measurement range for NT-proBNP was 5 to 35,000 pg/ml.
Measures of physical activity
Leisure-time activity was a self-reported measure of weekly energy expenditure in kilocalories quantified by participant responses to a modified Minnesota Leisure-Time Activity Questionnaire that evaluated frequency and duration of 15 different activities during the previous 2 weeks (9). Average walking pace, a self-reported measure, was classified in ordinal categories < (2, 2 to 3, and >3 mph). Activity and walking pace information were collected at the time of the baseline biomarker measures. Using a composite scoring system previously developed in CHS to predict new-onset diabetes and validated to predict the risk of decline in renal function, we summed leisure time activity quartiles (range, 1 to 4) and walking pace (range, 1 to 3) to generate a combined physical activity score with a possible range of 2 to 7. To test for trends, we further categorized individuals with a score of 2 as low activity, those with a score of 3 to 6 as moderate activity, and those with a score of 7 as high activity (10,11).
Other covariates
Comorbidities, demographic data, and risk factors were measured at the baseline CHS visit as previously described (1). CHD was defined as a history of angina, coronary revascularization, or myocardial infarction. General health status was self-reported on a Likert scale of 1 to 5; for the present analysis, health status was categorized as fair or poor versus good, very good, or excellent.
Main outcomes
Main outcomes were a significant increase in cTnT or NT-proBNP and incident HF. A significant increase in the biomarkers was prespecified on the basis of previous studies from this cohort (1,3). The cut points used have been shown to strongly predict the risk of incident HF and cardiovascular death independently of baseline concentrations and cardiovascular risk factors (1,3). A significant increase in cTnT was defined as a >50% increase in cTnT level between measures among individuals with detectable (≥3 pg/ml) baseline levels (1). The rationale for excluding individuals with undetectable cTnT concentrations is that their relative change from baseline cannot be quantified. A sensitivity analysis was performed including those with undetectable baseline cTnT, imputing a baseline concentration of 2.99 pg/ml. A significant increase in NT-proBNP was defined as a >25% increase to a level ≥190 pg/ml (3). Incident HF was defined as the first adjudicated HF event occurring after the second biomarker measurement over the course of follow-up. The CHS Events Committee adjudicated incident HF by reviewing all pertinent data, including history, physical examination, chest radiography report, and medication use (12). NT-proBNP and cTnT data were not available to the adjudicators.
Statistical analysis
Baseline participant characteristics were compared across categories of the composite physical activity score using 1-way analysis of variance for Gaussian continuous measures, Cuzik’s nonparametric trend test for non-Gaussian continuous variable (13), or the score test for trend in proportions (14). The associations between baseline physical activity and the odds of a significant increase in NT-proBNP or cTnT were estimated using separate binary logistic regression models. Two sets of adjustment covariates were selected a priori on the basis of factors that might plausibly confound the relationship of activity with biomarker change: 1) demographic variables (age, sex, race [African American vs. other]), and education (more than a high school education vs. other); and 2) cardiovascular risk factors and comorbidity (diabetes, hypertension, and coronary heart disease [all characterized as absent, prevalent at baseline, or incident between the baseline and follow-up biomarker measurements], body mass index, stroke, emphysema, claudication, and self-reported health status). Because baseline NT-proBNP and cTnT could plausibly relate to physical activity level, all logistic regression models were additionally adjusted for these concentrations.
Incident rates of HF after the second biomarker measurement were estimated with the Kaplan-Meier method among subgroups defined by baseline physical activity and change in biomarker, and the difference in incidence rates was tested using unadjusted Cox proportional hazards models. Statistical analyses were performed using Stata version 11 (StataCorp, College Station, Texas).
Results
Among the participants included in this analysis, the median weekly energy expenditure for leisure activity was 735 (interquartile range, 210 to 1,698) kcal with 768 (26.2%) reporting a walking pace of <2 mph, 1,251 (42.7%) between 2 and 3 mph, and 914 (31.2%) >3 mph. Kilocalories expended per week and walking pace were modestly correlated (ρ = 0.23). The baseline characteristics of the study participants on the basis of the 3 categories of physical activity are shown in Table 1. Higher physical activity score was associated with younger age, male sex, a lower body mass index, a lower prevalence of coronary heart disease and traditional cardiovascular risk factors, and better self-reported health status. Furthermore, greater activity was associated with lower baseline NT-proBNP, cTnT, and C-reactive protein levels.
Table 1.
Characteristics of Study Participants by Baseline Composite Physical Activity Score
| Composite Activity Score
|
||||
|---|---|---|---|---|
| Low Activity, (n = 310, 10.6%) | Moderate Activity (n = 2,331, 79.5%) | High Activity (n=292, 10%) | Test for Trend (p Value) | |
| Composite Activity Score | 2 | 3–6 | 7 | |
|
| ||||
| Leisure time activity, kcal/week | 0 (0–52.5) | 735 (310–1,462) | 2,240 (3,011–4,256) | <0.001 |
|
| ||||
| Age, yrs | 73.4 ± 5.5 | 71.9 ± 5.0 | 70.8 ± 4.0 | <0.001 |
|
| ||||
| % male | 24.8 | 38.3 | 52.7 | <0.001 |
|
| ||||
| % African American | 19.0 | 15.4 | 9.6 | 0.001 |
|
| ||||
| Baseline body mass index, kg/m2 | 28.1 ± 5.9 | 26.7 ± 4.4 | 25.3 ± 3.6 | <0.001 |
|
| ||||
| Change in body mass index, kg/m2 | −0.01 ± 1.9 | 0.08 ± 1.48 | 0.18 ± 1.18 | 0.12 |
|
| ||||
| Baseline SBP, mm Hg | 139.5 ± 21.8 | 135.3 ± 21.0 | 134.5 ± 20.5 | 0.003 |
|
| ||||
| Change in SBP from baseline to follow-up, mm Hg | −1 (−9 to 5) | 1 (−6 to 7) | 0 (−6 to 7) | 0.1 |
|
| ||||
| Baseline DBP, mm Hg | 70.7 ± 11.5 | 70.7 ± 10.9 | 71.7 ± 10.6 | 0.30 |
|
| ||||
| Change in DBP from baseline to follow-up, mm Hg | −1 (−9 to 5) | 1 (−6 to 7) | 0 (−6 to 7) | 0.07 |
|
| ||||
| Baseline heart rate, beats/min | 69.3 ± 11.3 | 67.4 ± 10.8 | 65.2 ± 10.5 | <.001 |
|
| ||||
| Change in heart rate from baseline to follow-up, beats/min | 0.3 ± 11.4 | −0.2 ± 10.4 | 0.2 ± 11.0 | 0.9 |
|
| ||||
| eGFR, ml/min/1.73 m2 | 77.6 ± 24.5 | 79.6 ± 22.4 | 80.3 ± 20.3 | 0.13 |
|
| ||||
| Coronary heart disease | ||||
| Prevalent at baseline | 72 (23.2%) | 351 (15.1%) | 47 (16.1%) | 0.006 |
| Prevalent at follow-up | 81 (26.1%) | 437 (18.8%) | 55 (18.8%) | 0.0008 |
|
| ||||
| Diabetes | ||||
| Prevalent at baseline | 70 (25.5%) | 359 (15.4%) | 33 (11.3%) | <0.001 |
| Prevalent at follow-up | 63 (20.3%) | 353 (15.1%) | 37 (12.7%) | 0.009 |
|
| ||||
| Hypertension | <0.001 | |||
| Prevalent at baseline | 203 (65.5%) | 1,333 (57.2%) | 150 (51.7%) | 0.01 |
| Prevalent at follow-up | 192 (62.1%) | 1,333 (57.2%) | 151 (51.9%) | 0.01 |
|
| ||||
| Previous stroke | 17 (5.5%) | 60 (2.6%) | 8 (2.7%) | 0.04 |
|
| ||||
| Major ECG abnormality | 87 (29.0%) | 579 (25.7%) | 57 (20.1%) | 0.01 |
|
| ||||
| Poor or fair self-reported health | 108 (34.5%) | 403 (17.3%) | 22 (7.5%) | <0.001 |
|
| ||||
| Beta-blocker use | ||||
| Baseline | 48 (15.5%) | 306 (13.1%) | 32 (11.0%) | 0.1 |
| Follow-up | 41 (13.2%) | 303 (13.0%) | 28 (9.6%) | 0.18 |
|
| ||||
| NT-proBNP, median, pg/ml | ||||
| Baseline | 112.9 (57.4–219.8) | 110.4 (53.1–190.0) | 86.1 (41.5–149.4) | <0.001 |
| Follow-up | 180.3 (85.3–370.8) | 135.1 (70.0–264.7) | 109.3 (61.3–216.3) | <0.001 |
|
| ||||
| cTnT, pg/ml | ||||
| Baseline* | 8.9 (5.4–14.0) | 7.5 (5.2–11.1) | 7.1 (4.6–10.4) | 0.001 |
| Below detection (<3 pg/ml) | 94 (31.1%) | 897 (39.3%) | 118 (41.1%) | <0.001 |
| Follow-up* | 10.6 (5.7–18.7) | 8.0 (4.3–13.6) | 6.4 (3.2–11.1) | 0.001 |
|
| ||||
| C-reactive protein, mg/dl | 3.1 (1.8–6.3) | 2.3 (1.2–4.0) | 1.7 (0.9–3.3) | 0.001 |
|
| ||||
| Walking distance, blocks/week | 4 (0–10) | 18 (6–48) | 60 (18–126) | <0.001 |
|
| ||||
| Alcohol use, >5 drinks/week | 24 (7.7%) | 320 (13.8%) | 57 (19.7%) | <0.001 |
|
| ||||
| Never smoked | 143 (46.1%) | 1,126 (48.4%) | 124 (42.5%) | 0.4 |
Values are median (interquartile range), %, mean ± SD, or n (%).
Among those with detectable baseline
cTnT. cTnT = cardiac troponin T; DBP = diastolic blood pressure; ECG = electrocardiographic; eGFR = estimated glomerular filtration rate; NT-proBNP = N-terminal pro–B-type natriuretic peptide; SBP = systolic blood pressure.
Physical activity and increases in biomarker concentration
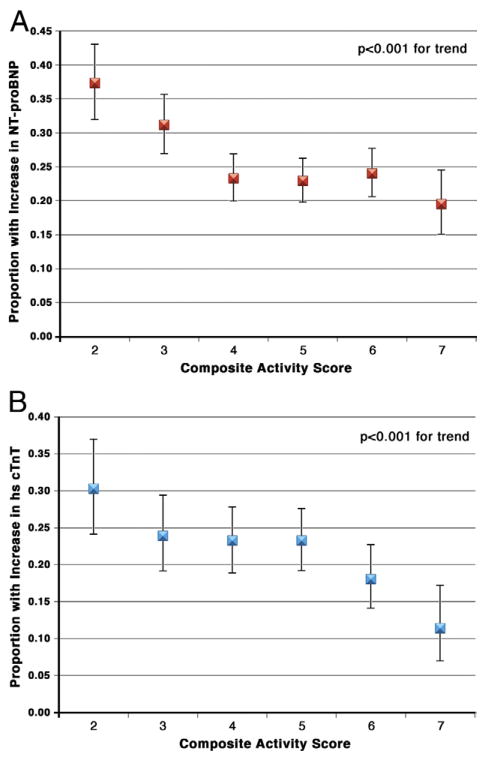
The probability of a significant increase in NT-proBNP concentration between baseline and follow-up visits decreased progressively across categories of greater baseline activity score, from 37.4% (95% confidence interval [CI]: 32.0% to 43.1%) in the lowest activity category to 19.5% (95% CI: 15.1% to 24.5%) in the highest activity category (p < 0.001) (Fig. 2A). Similarly, the likelihood of a significant increase in cTnT ranged from 30.2% (95% CI: 24.1% to 37.0%) for the low-activity group to 11.4% (95% CI: 7.0% to 17.2%) for the high-activity group (p < 0.001) (Fig. 2B).
Figure 2. Proportion of Participants With a Significant Increase in Biomarkers by Baseline Composite Physical Activity Score.
N-terminal pro–B-type natriuretic peptide (NT-proBNP) (A) or cardiac troponin T (cTnT) (B).
Adjustment for demographic variables and comorbidities did not significantly affect the associations of baseline physical activity with either NT-proBNP (Table 2) or cTnT (Table 3). In fully adjusted models, the odds ratio (OR) of a significant increase in cardiac biomarkers between visits, comparing the lowest with the highest baseline activity score category, was 0.50 (95% CI: 0.33 to 0.76) for NT-proBNP and 0.30 (95% CI: 0.16 to 0.55) for cTnT. To determine whether associations with significant increases in biomarker levels were similar for both components of the activity score, we separately tested quartiles of weekly physical activity and the 3 categories of walking pace. The unadjusted and adjusted ORs of a significant increase in NT-proBNP and cTnT across these categories of activity are shown in Online Tables 1 and 2, respectively. Progressively higher quartiles of leisure kilocalories per week and faster walking pace were both significantly associated with a lower adjusted OR for increase in each biomarker. There were no significant multiplicative interactions between quartiles of weekly leisure-time activity and usual walking pace with an increase in cTnT (p = 0.5 for test of interaction) or NT-proBNP (p = 0.14). We also tested whether a greater level of activity would be associated with an increased likelihood of a >50% decrease in cTnT. The frequency of >50% decrease was 16.2% among those with a high activity score and 8.2% among those with a low activity score (p = 0.15). This difference was not statistically significant after multivariable adjustment (OR: 1.48; 95% CI: 0.72 to 3.03).
Table 2.
Association of Composite Physical Activity Score With Clinically Significant Increases in NT-proBNP* Among All Patients With Baseline and Follow-up NT-proBNP
| Unadjusted (n = 2,933) | Demographic Variable† Adjusted (n = 2,933) | Comorbidity‡ Adjusted (n = 2,873) | |
|---|---|---|---|
| Composite Activity Score | |||
|
| |||
| 2 (n = 310, 10.6%) | Reference | Reference | Reference |
| 3 (n = 461, 15.7%) | 0.74 (0.54–1.02) | 0.79 (0.58–1.09) | 0.75 (0.53–1.04) |
| 4 (n = 600, 20.5%) | 0.52 (0.38–0.71) | 0.56 (0.41–0.76) | 0.56 (0.40–0.78) |
| 5 (n = 688, 23.5%) | 0.52 (0.38–0.70) | 0.52 (0.38–0.70) | 0.53 (0.39–0.74) |
| 6 (n = 582, 19.8%) | 0.58 (0.42–0.78) | 0.60 (0.44–0.82) | 0.60 (0.43–0.84) |
| 7 (n = 292, 10.0%) | 0.48 (0.33–0.71) | 0.46 (0.31–0.69) | 0.50 (0.33–0.76) |
|
| |||
| Test for trend | p < 0.001 | p < 0.001 | p = 0.001 |
Values are odds ratio (95% confidence interval).
Increase from baseline >25% and a follow-up NT-proBNP of >190 pg/ml. All models additionally adjusted for baseline NT-proBNP.
Demographic variables: age, sex, race, and education.
Comorbidities: body mass index, diabetes, hypertension, coronary heart disease, claudication, emphysema, stroke, major electrocardiographic abnormality, and self-reported health.
NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Table 3.
Association of Composite Physical Activity Score With Clinically Significant Increase* in cTnT Among Those With Detectable cTnT at Baseline (n = 1,757)
| Unadjusted (n = 1,757) | Demographic Variable† Adjusted (n = 1,757) | Comorbidity Adjusted‡ (n = 1,718) | ||
|---|---|---|---|---|
| Composite Activity Score | ||||
|
| ||||
| 2 (n = 208, 11.8%) | Reference | Reference | Reference | |
| 3 (n = 283, 16.1%) | 0.70 (0.37–1.05) | 0.73 (0.48–1.09) | 0.69 (0.45–1.05) | |
| 4 (n = 363, 20.7%) | 0.66 (0.45–0.97) | 0.70 (0.47–1.03) | 0.72 (0.48–1.08) | |
| 5 (n = 363, 23.1%) | 0.66 (0.45–0.96) | 0.65 (0.44–0.96) | 0.69 (0.46–1.04) | |
| 6 (n = 405, 23.1%) | 0.48 (0.32–0.72) | 0.49 (0.32–0.75) | 0.52 (0.33–0.81) | |
| 7 (n = 167, 9.5%) | 0.27 (0.15–0.47) | 0.27 (0.15–0.49) | 0.30 (0.16–0.55) | |
|
| ||||
| Test for trend | p < 0.001 | p < 0.001 | p < 0.001 | |
Values are odds ratio (95% confidence interval).
Increase >50% from baseline. Participants with a >50% decrease were combined with those with <50% change. All models additionally adjusted for baseline cTnT concentration.
Demographic variables: age, sex, race (African American or other), and education (more than high school).
Comorbidities: body mass index, diabetes, hypertension, coronary heart disease, claudication, emphysema, stroke, major electrocardiographic abnormality, and self-reported health.
cTnT = cardiac troponin T; other abbreviations as in Table 2.
Several sensitivity analyses were performed. First, when those participants with initially undetectable cTnT concentrations were included (imputing a value of 2.99 pg/ml), results were not materially changed (test for trend across activity score, p = 0.004). The association of physical activity with a risk of longitudinal increase in cTnT did not differ by baseline concentration of cTnT (test for interaction, p = 0.7). Second, when participants with any significant comorbidity (diabetes, coronary heart disease, stroke, claudication, emphysema, or obesity [body mass index >30 kg/m2]) were excluded, associations of increasing baseline physical activity with a reduced probability of increasing biomarker levels were similar to the overall study population (Online Tables 3a and 3b). Third, when we included only participants who self-reported their health as good, very good, or excellent, the adjusted odds of a significant bio-marker increase diminished across activity levels in a similar trend as in the entire study population (Online Tables 4a and 4b). Fourth, among all subjects, additional adjustment for serum creatinine did not change the associations of the composite activity score with a change in either biomarker (data not shown). Fifth, NT-proBNP levels were evaluated only in the cohort of subjects with baseline detectable cTnT levels (n = 1,757). Results were nearly identical to the results for the full NT-proBNP cohort (data not shown). Last, in the main CHS cohort (excluding the supplemental African-American cohort), a second assessment of physical activity and walking pace were available at the time of the follow-up biomarker measures. After adjusting for follow-up physical activity and walking pace, the results are not materially different from the primary analyses, and there remain highly significant associations of greater activity with lower risk of increasing biomarker levels (Online Table 5).
Association of physical activity score and longitudinal increase in cardiac-specific biomarkers with risk of new-onset HF
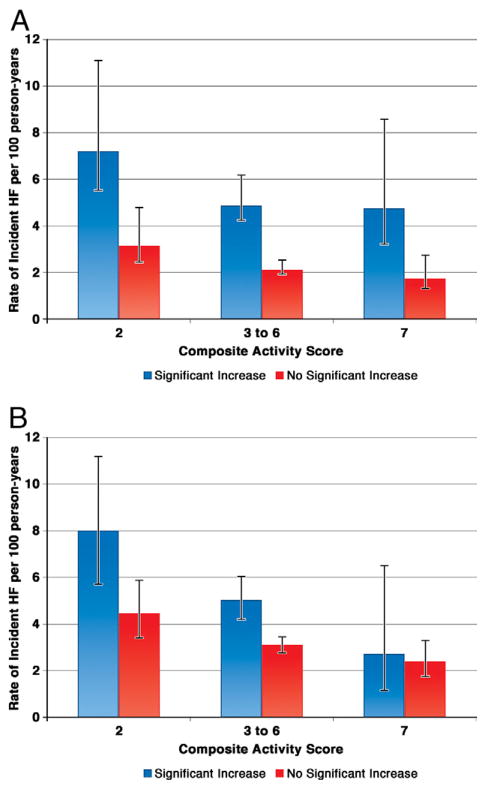
A total of 822 cases of incident HF occurred over a median follow-up after the second biomarker measurement of 10.8 years (range, 0.02 to 16.1 years). The incidence of HF diminished with progressively higher levels of fitness in both subjects with and without an increase in either biomarker level (Figs. 3A and 3B). To examine the joint effects of physical activity and longitudinal change in the cardiac-specific biomarkers on the risk of new-onset HF, we estimated HF incidence rate across 3 categories of physical activity representing low, moderate, or high. In each category of activity, we evaluated the incidence of new-onset HF on the basis of the presence or absence of a significant increase in NT-proBNP and cTnT. In each category of activity, individuals with an increase in NT-proBNP (Fig. 3A) or cTnT (Fig. 3B) level had a greater incidence rate of HF compared with those without an increase in level, with the exception of those in the highest activity category and an increase in cTnT. There was no significant interaction between the baseline activity group and an increase in NT-proBNP (p = 0.8) or cTnT (p = 0.6). To evaluate for the competing risk of cardiovascular death, we repeated the analysis shown in Figures 3A and 3B for the incidence of the combined endpoint of new-onset HF or cardiovascular death. The overall findings are similar for HF incidence alone, and the results are shown in Online Figures 1a and 1b for NT-proBNP and cTnT, respectively. When survival analyses were performed, accounting for the competing risk of all-cause mortality, associations of exercise with HF were similar to the standard survival analysis (data not shown).
Figure 3. Rate of Incident HF.
(A) Examined by baseline composite physical activity score and subsequent increase in NT-proBNP. Error bars represent 95% confidence intervals. Tests for trend across activity groups: p = 0.02 for a significant increase in the NT-proBNP group and p = 0.001 for no significant increase in the NT-proBNP group. (B) Examined by baseline composite activity score and subsequent increase in cTnT. Error bars represent 95% confidence intervals. Tests for trend across activity groups: p = 0.002 for a significant increase in the cTnT group and p = 0.005 for no significant increase in the cTnT group. HF = heart failure; other abbreviations as in Figure 2.
Discussion
The findings of this study demonstrate a strong inverse association between progressively higher levels of physical activity in ambulatory older adults and a lower probability of a longitudinal increase in cTnT (measured by an hs assay) and NT-proBNP. Our findings also show that the incidence of new-onset HF is inversely associated with the extent of self-reported physical activity. Importantly, a significant increase in biomarkers remained associated with an increased incidence of HF at any level of physical activity. The association of greater self-reported physical activity with a lower probability of an increase in cardiac biomarkers of injury and hemodynamic stress also remained present when analyzing only subjects free of significant comorbidity or those participants who perceived their health to be good, very good, or excellent. These findings provide support for the hypothesis that regular moderate physical activity can mitigate or delay progressive pathophysiological processes that ultimately result in symptomatic HF.
A consistent finding across multiple community-based populations is that more physically active individuals are at lower risk of multiple adverse cardiovascular endpoints including new-onset HF (15–20). A recent meta-analysis confirmed that greater self-reported physical activity, whether measured by the weekly energy expenditure in leisure activities or by walking pace, is inversely associated with adverse cardiovascular endpoints including death (21). However, physical activity, unlike many traditional risk factors, is a dynamic factor in older adults. Adaptation to a more physically active or sedentary lifestyle appears to be associated with a change in cardiovascular risk over a period of only 5 to 7 years (5–7). In sedentary older adults, exercise training over 1 year is associated with increased arterial compliance, physiological left ventricular remodeling, and increased aerobic exercise capacity (22). These findings suggest that physical activity may favorably modify the cardiovascular and arterial substrate leading to HF over a relatively short period of time.
We previously showed in this cohort that increases in cTnT or NT-proBNP levels over 2 to 3 years are associated with approximately a 60% and 200% higher long-term risk, respectively, for the development of new-onset HF, independent of baseline levels, demographic variables, and traditional cardiovascular risk factors (1,3). Using these same definitions for a significant increase in biomarkers, we found that the most physically active individuals in the CHS cohort were 70% and 50% less likely to have a significant increase in cTnT and NT-proBNP, respectively, compared with the most sedentary participants. The associations of physical activity with biomarker increases were dose dependent, with significant effects seen at even low levels of activity and more robust effects at high levels. Similar dose-dependent effects can be seen on outcomes starting with low levels of physical activity (20). Potentially, the absence of longitudinal increases in cTnT and NT-proBNP could reflect the favorable effects of physical activity on pathological left ventricular remodeling and arterial elastance, resulting in less left ventricular wall stress and cardiac injury. Higher levels of both NT-proBNP and cTnT are associated with left ventricular hypertrophy and systolic dysfunction in community-based cohort studies (23–25). An increase in concentrations of these 2 cardiac biomarkers likely reflects ongoing subclinical progression of cardiac pathophysiology. In support of this concept, we recently showed that participants with increasing NT-proBNP levels were nearly twice as likely to progress from a normal to abnormal (<55%) left ventricular ejection fraction on follow-up echocardiography compared with participants without increasing levels (25).
Several additional mechanisms may contribute to the inverse association between physical activity and increase in markers of cellular injury and hemodynamic stress. For example, telomere shortening occurs with aging and is associated with cellular apoptosis and increased risk of cardiovascular events (26–28). Habitual physical activity has been shown to prevent myocyte, endothelial, and leukocyte apoptosis via up-regulation of telomere-stabilizing proteins and telomerase in both animal models and humans (26,27). In another animal model of aging, long-term exercise was associated with improved neo-revascularization of the myocardium, with increased capillary density in response to muscle ischemia and enhanced endothelial progenitor cell homing to the ischemic territory (29). Such a mechanism could result in mitigation of myocyte cell death after pathological insult. Furthermore, in a mouse model of mitochondrial DNA mutagenesis resulting in rapid aging, long-term exercise induced systemic mitochondrial biogenesis and improved function, resulting in the prevention of left ventricular hypertrophy compared with sedentary mice (30). Increasing levels of NT-proBNP and hs cTnT may reflect preclinical pathological precursors of hemodynamic stress and injury that are prevented with physical activity.
Despite a large volume of epidemiological evidence and guidelines recommending at least moderate physical activity 5 days a week, only 3.8% of Americans achieve this recommendation (31). Given the long latency period between the onset of HF risk factors and the development of symptoms, it can be challenging to motivate patients to adopt or maintain lifestyles in which there is no near-term clinical outcome or objective surrogate evidence of successful implementation of a preventive strategy (32). The findings of our study raise the possibility that hs markers of myocardial injury and cardiac stretch/dysfunction could be used to evaluate the efficacy and adherence to a prescription of moderate to more intense physical activity by following serial levels of these biomarkers over time. However, such a strategy would need to be evaluated prospectively in a controlled trial.
We did not find that those participants in the highest physical activity category were more likely to have a significant longitudinal reduction in either cTnT or NT-proBNP. Several factors may have contributed to our inability to demonstrate a reduction in biomarker levels with higher activity. It is possible that although physical activity, at the amounts typically performed by older adults, can prevent further injury, it cannot reverse the process. Also, the statistical power for detecting associations with decreasing biomarker levels was modest due to the smaller number of participants who have a decrease compared with an increase in their cTnT level. Our findings are in contrast to the beneficial effects of physical exercise that have been documented to lead to a marked decrease in NT-proBNP and hs cTnT levels after acute myocardial infarction or cardiac surgery hospitalization, respectively (33,34). However, in these patients, the baseline biomarkers were generally 9- to 10-fold higher at baseline than in our community-dwelling subjects and represent a more acute change in pathophysiology likely involving favorable left ventricular remodeling. Last, acute increases in both hs cTnT and NT-proBNP can be seen immediately after strenuous exercise such as a marathon race, but typically return to baseline within 72 h (35). Of course, the intensity of the estimated physical activity in the older CHS participants is much less rigorous. Therefore, the biomarker levels measured at the study visits are unlikely to be influenced by the amount of mild to moderate exercise within the previous days.
Study limitations
There are several limitations that need to be considered when interpreting the results of this study. Leisure-time physical activity and walking pace were measured by self-reported questionnaires. Although these self-reported measures and their combination have been strongly associated with multiple long-term cardiovascular and non-cardiovascular outcomes within the CHS and in other cohort studies (10,11,18,21), they lack the objectivity of direct measurement of activity levels. Therefore, misclassification of physical activity may be present, which could have biased the association with an increase in cardiac-specific biomarkers toward the null. However, the finding that self-reported physical activity was inversely associated with incident HF over a median of 10 years suggests that, as in other large cohort studies, this method of assessment is valid (21). Furthermore, despite adjustment for multiple comorbidities associated with the risk of HF and impeding regular exercise, physical activity may simply be a marker rather than a mediator of cardiac health in this cohort. However, the finding of a similar association in individuals free of major comorbidities and after excluding participants who perceived their health as fair or poor suggests that the relationship between physical activity level and biomarker evidence of progressive cardiac pathophysiology may be causal.
Conclusions
The proportion of older adults who experienced an increase in concentrations of cardiac-specific biomarkers of injury and hemodynamic stress are inversely associated with self-reported levels of physical activity in this cohort of community-dwelling adults free of HF. Physical activity was also inversely associated with the risk of the development of HF. Serial monitoring of these biomarkers may provide an early window into the pathophysiology associated with sedentary lifestyle and the risk of HF. Our findings raise the possibility that the trajectory of biomarker change and the subsequent HF risks associated with increasing levels may be modifiable by changes in lifestyle even at an advanced age.
Supplementary Material
Acknowledgments
This research was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and NHLBI grant HL080295, with additional support from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. For a list of the principal investigators and study sites of the Cardiovascular Health Study, see http://www.chs-nhlbi.org/pi.htm. Additional funding for this analysis was provided by an investigator-initiated grant from Roche Diagnostics Corporation. Drs. Seliger, deFilippi, deLemos, and Christenson have received grant funding from Roche Diagnostics Corporation. Dr. Seliger has received consulting fees from Roche Diagnostics. Dr. deFilippi has received honoraria and consulting fees from Roche Diagnostics. Dr. deLemos has received research support from Abbott.
Abbreviations and Acronyms
- CI
confidence interval
- cTnT
cardiac troponin T
- HF
heart failure
- hs
highly sensitive
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- OR
odds ratio
APPENDIX
For supplemental tables and figure, please see the online version of this article.
Footnotes
All other authors report that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–62. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Cauley JA, Stone K, et al. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289:2379–86. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 7.Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men/clinical perspective. Circulation. 2011;124:2483–90. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 9.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169:2116–23. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schellenbaum GD. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–35. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 14.Clayton DHM. Statistical Models in Epidemiology. Oxford, England: Oxford University Press; 1993. [Google Scholar]
- 15.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122:790–7. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–7. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Tuomilehto J, Jousilahti P, et al. Occupational, commuting, and leisure-time physical activity in relation to heart failure among Finnish men and women. J Am Coll Cardiol. 2010;56:1140–8. doi: 10.1016/j.jacc.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 21.Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee I-M. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–95. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797– 805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lemos JA, McGuire DK, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53.e2. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 25.deFilippi CR, Christenson RH, Kop WJ, Gottdiener JS, Zhan M, Seliger SL. Left ventricular ejection fraction assessment in older adults an adjunct to natriuretic peptide testing to identify risk of new-onset heart failure and cardiovascular death? J Am Coll Cardiol. 2011;58:1497–506. doi: 10.1016/j.jacc.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner C, Fürster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–47. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 27.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–82. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 28.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 29.Cheng XW, Kuzuya M, Kim W, et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1α reactivation in mice of advanced age. Circulation. 2010;122:707–16. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safdar A, Bourgeois JM, Ogborn DI, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–40. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troiano Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease. Circulation. 2011;124:967–90. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 33.Ferratini M, Ripamonti V, Masson S, et al. Pentraxin-3 predicts functional recovery and 1-year major adverse cardiovascular events after rehabilitation of cardiac surgery patients. J Cardiopulm Rehabil Prev. 2012;32:17–24. doi: 10.1097/HCR.0b013e31823be0f4. [DOI] [PubMed] [Google Scholar]
- 34.Giallauria F, Lorenzo AD, Pilerci F, et al. Reduction of N terminal-pro-brain (B-type) natriuretic peptide levels with exercise-based cardiac rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Eur J Cardiov Prev Rehabil. 2006;13:625–32. doi: 10.1097/01.hjr.0000209810.59831.f4. [DOI] [PubMed] [Google Scholar]
- 35.Scherr J, Braun S, Schuster T, et al. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc. 2011;43:1819–27. doi: 10.1249/MSS.0b013e31821b12eb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.