Abstract
Claudin-2 is a unique member of the claudin family of transmembrane proteins as its expression is restricted to the leaky epithelium in vivo and correlates with epithelial leakiness in vitro. However, recent evidence suggests potential functions of claudin-2 that are relevant to neoplastic transformation and growth. In accordance here we report, based upon analysis of mRNA and protein expression using a total of 309 patient samples, that claudin-2 expression is significantly increased in colorectal cancer and correlates with cancer progression. We also report similar increases in claudin-2 expression in inflammatory bowel disease (IBD)-associated colorectal cancer. Most importantly, we demonstrate that the increased claudin-2 expression in colorectal cancer is causally associated with tumor growth as forced claudin-2 expression in colon cancer cells that do not express claudin-2 resulted in significant increases in cell proliferation, anchorage-independent growth, and tumor growth in vivo. We further show that the colonic microenvironment regulates claudin-2 expression in a manner dependent on signaling through the EGF receptor (EGFR), a key regulator of colon tumorigenesis. In addition, claudin-2 expression is specifically decreased in the colon of waved-2 mice, naturally deficient in EGFR activation. Furthermore, genetic silencing of claudin-2 expression in Caco-2 , a colon cancer cell line, prevents the EGF-induced increase in cell proliferation. Taken together, these results uncover a novel role for claudin-2 in promoting colon cancer, potentially via EGFR transactivation.
Keywords: Tight Junction, Claudin, EGFR, Colon Cancer, Proliferation
Introduction
Tight junctions, the most apical cell-cell adhesions, create a barrier for paracellular transport and help maintain epithelial cell polarity (Cereijido, Contreras et al. 2004). Pathological conditions, including cancers of epithelial origin, are associated with the loss of epithelial barrier function and polarity (Mullin 2004). The claudin family of proteins is integral to the tight junction and contains 24 members that are expressed in a tissue/cell specific manner (Furuse and Tsukita 2006). Various diseases have been associated with structural and functional defects in claudin family members (Lal-Nag and Morin 2009). However, precise role of claudins in colonic epithelial cell function and pathological conditions remain largely unknown.
Among claudins, claudin-2 is unique as its expression is restricted to the leaky epithelia (Reyes, Lamas et al. 2002; Escaffit, Boudreau et al. 2005). In colon, claudin-2 expression is highest among the undifferentiated colonocytes at the crypt base and decreases with colonocyte differentiation (Escaffit, Boudreau et al. 2005; Holmes, Van Itallie et al. 2006). Notably, initial studies using limited patient samples have suggested that claudin-2 expression increases in colorectal cancer samples (Kinugasa, Huo et al. 2007). However, a detailed analysis is warranted, especially in the light of recent reports that claudin-2 expression changes with the modulation of proliferation and migration (Guillemot and Citi 2006; Buchert, Papin et al. 2010; Takehara M 2009 ). Furthermore, it is important that we determine whether increased claudin-2 expression is causally associated with colorectal carcinogenesis, or is a simple bystander, and that we understand mechanism/s underlying regulation of colonic claudin-2 expression. In the current study, we demonstrate that claudin-2 expression is significantly increased in colorectal cancer compared to the normal colon and is causally associated with the colorectal cancer growth. We further show that EGFR tyrosine kinase activity helps maintain the endogenous colonic claudin-2 expression and colonic tissue microenvironment induces claudin-2 expression in an EGFR-dependent manner. Taken together, our current findings confirm a tumor-promoting role for claudin-2 in colon cancer, and implicate EGFR-mediated signaling in these events.
Results
Claudin-2 expression is significantly upregulated in colorectal cancer
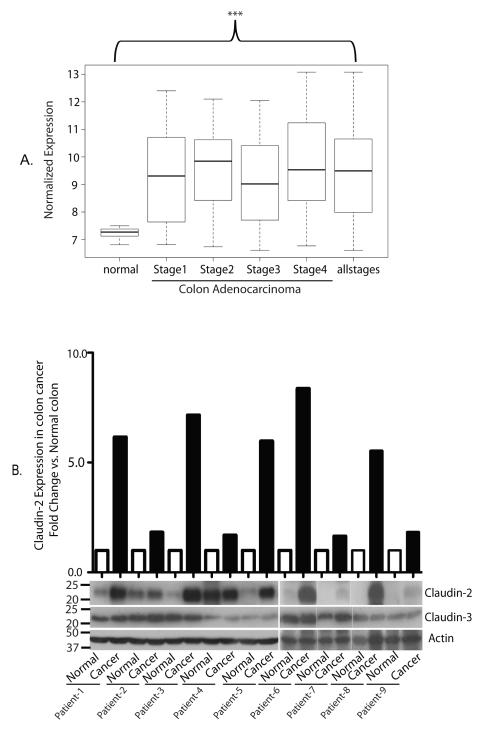
To determine claudin-2 expression in colorectal cancer, we investigated expression levels between normal adjacent colon specimens (n=10) and patients with colorectal adenocarcinoma (n=250). We found that claudin-2 expression was significantly upregulated in the adenocarcinoma group for each stage compared with normal adjacent tissue specimens (Fig. 1A, ***p<0.001 for all comparisons). These data suggest that claudin-2 is significantly upregulated in both early and late stage colorectal cancer tumors.
Fig. 1. Claudin-2 expression in colon cancer patients.
A. Normalized expression values for claudin-2 in normal adjacent colon specimens (n=10) compared with adenocarcinoma samples (n=250) from the Vanderbilt Medical Center and the Moffitt Cancer Center using microarray analysis. The demographics for this group are listed in Supplementary Table 1. Claudin-2 was significantly increased in the adenocarcinoma group compared with the normal specimens (***p<0.001 for all comparisons). B. Claudin-2 expression in colon cancer and adjacent normal colon samples. Total tissue lysates were prepared using frozen matched normal and cancer colon tissues from the same patient. β-Actin was used as loading control. The values on the left are molecular sizes in kilodaltons.
To determine a similar increase at the protein level, we examined claudin-2 expression in tissue lysates prepared from frozen, matched colon cancer and normal colon samples from nine patients. Immunoblot analysis showed an increase in claudin-2 expression in all tumor samples compared to their normal counterparts. Notably, expression of claudin-3, another claudin family member, remained largely unaltered in the same lysates (Fig. 1B).
We next performed immunohistochemical analysis using anti-claudin-2 antibody and sections from 40 colorectal cancer patients. Mouse kidney was used as positive control where claudin-2 immunoreactivity was found in tight junctions in the proximal tubules and thus validated the staining (data not shown). Intensity of claudin-2 staining was independently scored by a pathologist, and low and high scores were assigned (see figure legend). Claudin-2 expression was low in all normal colon cases, however increased in colorectal adenomas (72% low vs. 28% high) and further increased in adenocarcinomas [23% low vs. 77% high) [Table 1 & Supplementary fig. 1]. Chi square analysis of these data demonstrated a highly significant increase in claudin-2 levels in colorectal adenocarcinoma specimens compared to normal colon and adenomas (p<0.001). Taken together, our data show significantly increased claudin-2 expression in colorectal cancer and suggest the potential correlation of claudin-2 with cancer progression.
Table. 1. Claudin-2 immunolocalization in colonic mucosa and colon cancer.
Claudin-2 immunoreactivity in human colon samples was scored by a pathologist in a blinded fashion where lowest and highest immunoreactivity were scored as +1 and +5, respectively, and represented a sum of membrane and cytoplasmic staining.. Data has been presented as low (+1 to +2) or high (+3 to +5) expression for claudin-2. Claudin-2 staining was significantly higher in the adenocarcinoma group compared to the normal colon group (P<0.001, Chi square test).
| Claudin-2 Staining | Normal | Adenoma | Adenocarcinoma |
|---|---|---|---|
| Low claudin-2 (+1 to +2) | 13 | 10 | 0 |
| High claudin-2 (+3 to +5) | 0 | 3 | 10 |
Claudin-2 expression is markedly increased in inflammatory bowel disease (IBD)-associated cancer
A robust increase in claudin-2 expression has emerged as a consistent feature of IBD (Heller, Florian et al. 2005; Ridyard, Brown et al. 2007; Zeissig, Burgel et al. 2007). Increased epithelial permeability characterizes IBD patients (D’Inca, Di Leo et al. 1999). IBD patients also have increased risk of developing colon cancer (Eaden, Abrams et al. 2001). Therefore, we examined the potential correlation of claudin-2 expression with IBD-associated cancer. IHC analysis showed that in the non-cancerous colitis tissues, claudin-2 staining was present at a low level in the surface colonocytes in the apical cytoplasm and tight junctions, and in the crypt epithelium, claudin-2 staining was punctate along the lateral membranes and was also present in the cytoplasm (Supplementary fig. 2A(i-ii) and 2B(i-ii). Importantly, claudin-2 staining was highly increased in the cancers associated with Crohn’s disease [Supplementary fig. 2A(iii-iv)] as well as ulcerative colitis [Supplementary fig. 2B(iii-iv)]. This increased claudin-2 staining was present in lateral membranes, cytoplasmic granules, and/or was diffusely cytoplasmic.
Forced claudin-2 expression increases tumorigenicity of colon cancer cells
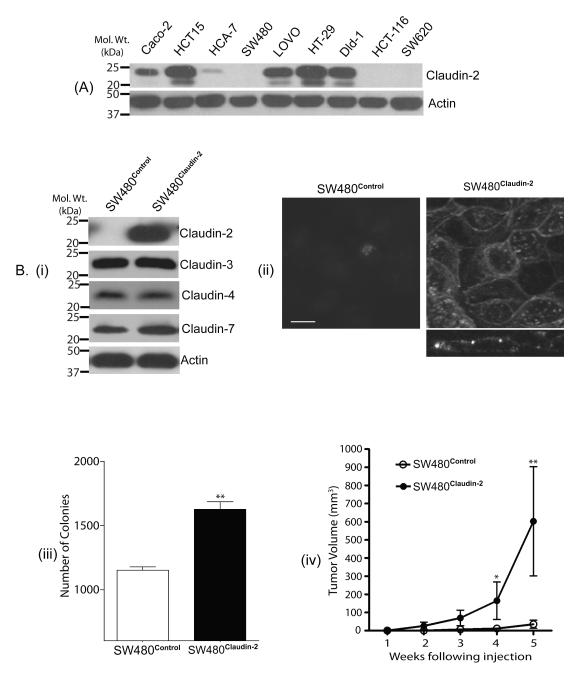
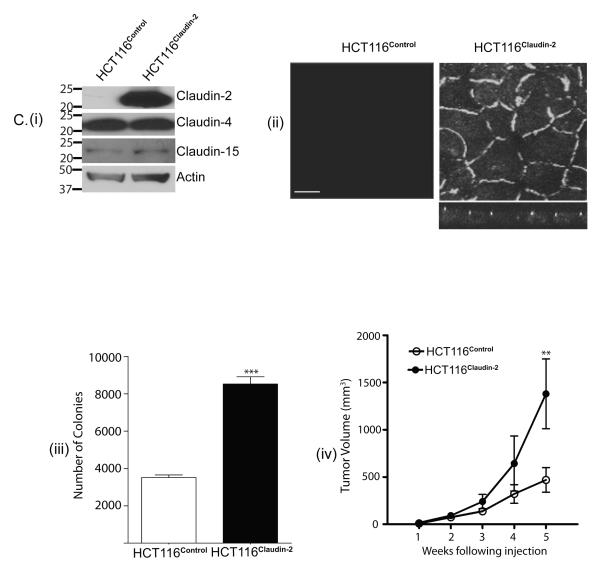
Based upon above data, we postulated a causal association of claudin-2 expression with colon cancer. In this regard, we first determined the expression profile of claudin-2 in a panel of colon cancer cell lines. As shown in Fig. 2A, claudin-2 was expressed in majority of the cell lines tested. To further test a causal association, we forced expressed claudin-2 cDNA in SW480 and HCT116 colon cancer cells that did not exhibit detectable claudin-2 expression. Claudin-2 overexpressing cells were named SW480Claudin-2 and HCT116Claudin-2. Immunoblot analysis confirmed robust claudin-2 overexpression in the selected polyclonal claudin-2 overexpressing population of both cell types, while endogenous claudin-3, claudin-4, claudin-7 or claudin-15 expression remained largely unaltered. [Fig. 2B(i), C(i) & Supplementary fig. 3]. Immunofluorescent analysis showed that the expressed claudin-2 was localized at the cell membrane and in the cytoplasm [Fig. 2B(ii) & C(ii)].
Figure 2. Forced claudin-2 expression increased tumorigenecity of colon cancer cells.
A. Expression profile of claudin-2 in colon cancer cells. B(i). Expression of claudin-2, claudin-3, claudin-4, claudin-7 and β-actin in SW480Control and SW480Claudin-2 cells. Control cells expressed the pcDNA4 plasmid vector. B(ii). Confocal image of the immunoflourescent staining using anti-claudin-2 antibody. Z-sectioning confirmed that the expressed claudin-2 is localized at the apical cell membrane along with minor cytoplasmic distribution. Scale bar, 10 μm. B(iii). Anchorage-independent growth. p<0.01 for SW480Claudin-2 vs. SW480Control. B(iv). Flank tumor xenografts after subcutaneous injection of SW480Control or SW480Claudin-2 cells (n=7 mice/group). (*p<0.05 and **p<0.01, SW480Claudin-2 group vs. SW480Control group, ANOVA). C(i) Claudin-2, claudin-4 or claudin-15 expression in HCT116control and HCT116Claudin-2 cells. β-actin was used for equal protein loading. The values on the left are molecular sizes in kilodaltons. C(ii). Confocal image analysis of immunoflourescent staining using anti-claudin-2 antibody. Z-sectioning demonstrated predominant membrane localization of the expressed claudin-2 Scale bar, 10 μm. C(iii). Anchorage-independent growth. p<0.001 for HCT116Claudin-2 vs. HCT116Control. C(iv). Flank tumor xenografts after subcutaneous injection of control HCT116 or HCT116Claudin-2 cells (n=7 mice/group). (**p<0.01, HCT116Claudin-2 group vs. HCT116Control group, ANOVA).
To determine effect of claudin-2 expression upon the tumorigenic ability of the respective cell lines we used anchorage-independent growth. As expected, SW480 and HCT116 control cells formed colonies in soft agarose. However, in both cell types forced claudin-2 expression resulted in significant increases in the number of colonies formed [Fig. 2B(iii) & C(iii)]. To further determine the effect of claudin-2 expression upon tumorigenesis in vivo, we injected SW480Claudin-2, HCT116Claudin-2, and respective control cells (1×106 cells) subcutaneously into the flanks of nude mice (n = 7/group). Irrespective of the cell type injected, majority of the mice had measurable tumors within 1 week post-inoculation [Fig. 2B(iv) & C(iv)]. However, tumor growth was faster in the mice injected with the claudin-2 overexpressing cells, and the average tumor volume was significantly higher in the mice injected with SW480Claudin-2 or HCT116Claudin-2 cells compared to those that received respective control cells (p<0.01 in both cases) after 5 weeks when the mice were sacrificed [Fig. 2B(iv) & C(iv)].
Claudin-2 overexpression increases proliferation and protects from the effects of 5-FU
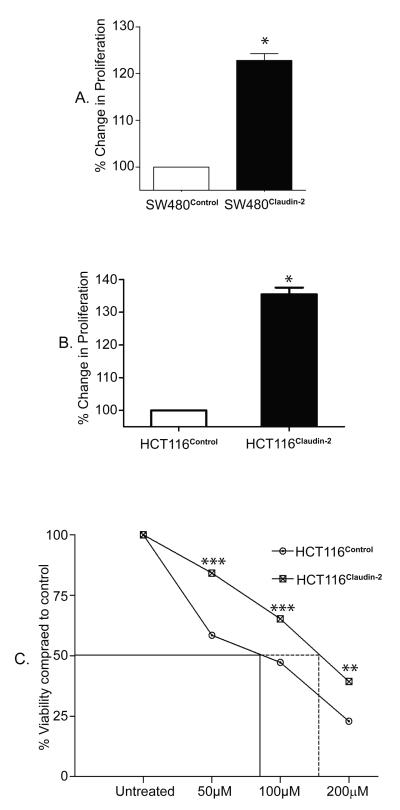
Forced claudin-2 expression significantly increased tumorigenicity of both cell types under study. Since, claudin-2 expression varies with changes in cell proliferation (Guillemot and Citi 2006; Buchert, Papin et al. 2010), we further determined effects of claudin-2 overexpression upon cell proliferation using MTT assay. As shown in Fig. 3, we observed significant increases in cell proliferation in both HCT116Claudin-2 and SW480Claudin-2 cells compared to respective controls [Fig. 3A & 3B; p<0.05]. Unchecked proliferation is central to tumorigenesis, including colon cancer, and is a key aspect of the resistance to the cancer treatment drugs. Therefore, we further examined effects of 5-FU, a colon cancer treatment drug (Ansfield, Klotz et al. 1977), upon HCT116Claudin-2 and control cells. Cells were exposed to progressively increasing concentrations of 5-FU and cell viability was determined at 24, 48, and 72 hours after exposure to 5-FU. As expected, 5-FU decreased cell viability in a time- and dose-dependent manner, however HCT116Claudin-2 cells were significantly (p<0.001 at 50 or 100 μM and p<0.01 at 200 μM) protected from the effects of 5-FU compared to the control cells (Fig. 3C). We further determined specific contribution of proliferation versus apoptosis to the 5-FU-dependent change in cell viability. As shown in supplementary fig. 4, 5-FU-treatment inhibited proliferation (p<0.001) while increased apoptosis (p<0.05) in HCT116control cells. However, HCT116claudin-2 cells were largely resistant to the effects of 5-FU upon proliferation as well as apoptosis.
Figure 3. Forced claudin-2 expression increased proliferation of colon cancer cells and protected from the effects of 5-FU.
A. Cell proliferation in SW480Claudin-2 and B. HCT116Claudin-2 cells and respective control cells using MTT assay (p<0.05, t-test). C. Effect of 5-FU upon cell viability in HCT116Control and HCT116Claudin-2 cells. Cell viability was determined at 24, 48, and 72 hours after exposure to 5-FU. Data representative of 24, 48 and 72 hours post 5-FU treatment, however shown only after 48 hours. The dose of 5-FU to kill 50% of cells (LD50) was 160 μM for HCT116Claudin-2 cells compared to 70 μM forHCT116Control cells when exposed for the same duration (**p<0.01 and ***p<0.001, ANOVA).
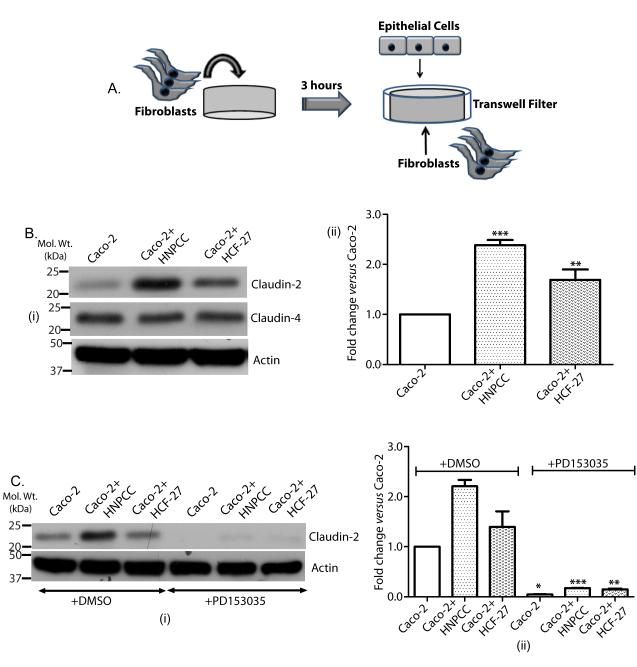
Co-culture of colon cancer cells with colonic fibroblasts increases claudin-2 expression in an EGFR-dependent manner
In colon, a close association exists between the crypt epithelial cells and pericryptal fibroblasts. Emerging evidence supports the importance of the stromal regulation of intestinal tumors (Cutler, Graves-Deal et al. 2003). As described, claudin-2 is uniquely, compared to other colonic claudins, expressed in the crypt base (Holmes, Van Itallie et al. 2006). Therefore, we further determined the potential role of the tissue microenvironment in the regulation of colonic claudin-2 expression.
In this regard, we performed co-culture of colonic fibroblasts (Fig. 4A) with Caco-2 colon cancer cells that express endogenous claudin-2 protein. Effect of co-culture upon claudin-2 and claudin-4 expression was examined. Co-culture with either HCF-27 (normal pericryptal fibroblasts) or HNPCC (fibroblasts from HNPCC individuals) (Cutler, Graves-Deal et al. 2003) cells had no appreciable effect upon claudin-4 expression, however claudin-2 expression was significantly increased (Fig. 4B). This fibroblast-dependent increase in claudin-2 expression was higher in cells co-cultured with the HNPCC (p<0.001) compared to the HCF-27 (p<0.01) fibroblasts (Fig. 4B). Importantly, this increase in claudin-2 expression was inhibited upon use of PD153035, an EGFR tyrosine kinase specific inhibitor [(Bos, Mendelsohn et al. 1997); Fig. 4C] or EGFR blocking antibody [Clone#528, 20 μg/ml; (Supplementary fig. 5)]. Together, our data supported the role of the colonic stroma in the regulation of claudin-2 expression in an EGFR-dependent manner.
Figure 4. Co-culture of colon cancer cells with colonic fibroblasts induced claudin-2 expression in an EGFR-dependent manner.
A. Cartoon summarizing the methodology of co-culture. Cells were grown in normal culture medium for 72 hours before collection to prepare lysate. B. (i) Effect of co-culture of Caco-2 cells with colonic fibroblast HNPCC (from the normal-appearing colonic mucosa of an individual with hereditary nonpolyposis colorectal cancer (Cutler, Graves-Deal et al. 2003) and normal colonic pericryptal fibroblasts HCF-27 on claudin-2 and claudin-4 expression; (ii) Representative densitometric analysis (**p<0.01 and ***p<0.001 co-culture vs. control, t-test). C. (i) Caco-2 cells were co-cultured with the colonic fibroblasts in the presence and/or absence of the EGFR inhibitor, PD 153035; (ii) Representative densitometric analysis (mean+SEM; (*p<0.05, **p<0.01 and p<0.001 co-culture+PD153035 vs. co-culture, t-test).
Exogenous EGF increases claudin-2 expression in colon cancer cells
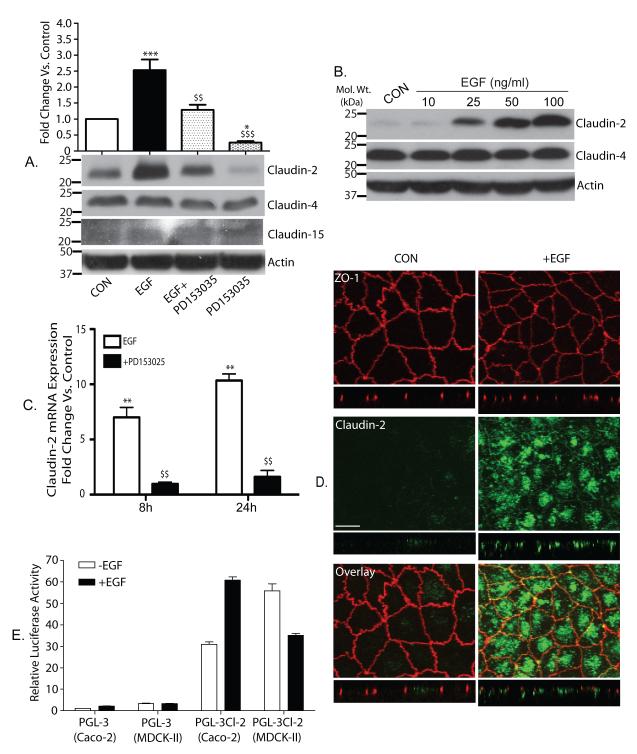
The data from the co-culture studies suggested a potential role of EGFR activation in the fibroblast-dependent induction of claudin-2 expression in Caco-2 cells. This finding was in sharp contrast to our earlier report that in renal epithelial (MDCK-II) cells, EGFR-activation decreases claudin-2 expression (Singh and Harris 2004). Therefore, we further determined effect of the exogenous EGF upon claudin-2 expression in the colon derived cells. Quiescent and polarized Caco-2 cells were exposed to EGF (100 ng/ml) and effect upon TER, paracellular permeability and claudins expression was determined. Notably, EGF treatment resulted in a time-dependent and significant decrease in TER (Supple. fig. 6A), while paracellular permeability for FITC-dextran increased (p<0,001; Supple. fig. 6B). Furthermore, EGF treatment resulted in a marked increase in claudin-2 expression (Fig. 5A; p<0.001), which was inhibited by PD153035. Notably, PD153035 alone inhibited the basal claudin-2 expression (p<0.05) in cells that did not receive EGF, suggesting that EGFR activation is required for the maintenance of baseline claudin-2 expression (Fig. 5A). This EGF-dependent increase in claudin-2 expression was dose-dependent (Fig. 5B) and was specific as expressions of claudin-3, claudin-4, claudin-8 and claudin-15 in EGF-treated Caco-2 cells was not different compared to the control Caco-2 cells (Fig. 5A & Supple. fig. 7) and involved regulation at the level of mRNA expression (Fig. 5C; p<0.01). We further performed immunofluorescent analysis to examine potential effects of EGFR activation upon cellular distribution of claudin-2 in co-localization with ZO-1. Confocal imaging showed predominant membrane distribution for ZO-1 in Caco-2 cells that remained largely unaltered in the EGF-treated cells (Fig. 5D). In contrast, claudin-2 staining was minimal in the untreated Caco-2 cells, but EGF stimulation markedly increased claudin-2 staining that was present along the cell membranes and cytoplasm in a granular pattern (Fig. 5D) and resembled claudin-2 staining in the colon cancer samples (Supplementary fig 1).
Figure 5. Exogenous EGF increased claudin-2 expression in colon cancer cells.
A. Quiescent Caco-2 cells were exposed to EGF (100 ng/ml), PD153035 (0.5 μM) or both. The effect upon claudin-2, claudin-4 and claudin-15 expression was examined. β-Actin was used for normalization (***p<0.001 EGF treated vs. control, $$p<0.01 EGF treated vs. EGF+PD153035, $$$p<0.001 EGF treated vs. PD153035 alone and *p<0.05 PD153035 alone vs. control, ANOVA). The values on the left are molecular sizes in kilodaltons. B. EGF-induced increase in claudin-2 expression was dose-dependent. C. Real-time PCR to examine the effect of EGF-treatment on claudin-2 mRNA expression in Caco-2 cells. Data is presented as fold change compared to control (mean +SEM; **p<0.01, EGF treated vs. control and $$p<0.01, EGF treated vs. EGF+PD153035). D. Co-Immunoflourescent localization of claudin-2 (green) and ZO-1 (red) in control and EGF-treated Caco-2 cells. Confocal imaging showed that the intensity or membrane localization of ZO-1 was largely unaltered by EGF treatment while claudin-2 staining was markedly increased and was localized at the membrane and in the cytoplasm (see arrow). Scale bar, 10 μm. E. Luciferase reporter assay. Caco-2 and MDCK-II cells were transiently transfected with a luciferase reporter construct containing human (1. 2 Kb) claudin-2 promoter or empty PGL-3 vector. Twenty-four hours post-transfection cells were serum starved (overnight) and then exposed to EGF (100 ng/ml). Eighteen hours after EGF-treatment, samples were collected and processed. Firefly luciferase activity was normalized to R. reniformis luciferase activity (co-transfected reference plasmid) and plotted as mean ± SEM.
In further studies, we determined whether contrasting effect of EGFR-activation upon claudin-2 expression between MDCK-II and Caco-2 cells is due to differential transcriptional regulation. In this regard, we determined effect of EGF-stimulation upon human claudin-2 promoter activity in Caco-2 and MDCK-II cells as described in “Methods”. As shown in Fig. 5E, EGF-stimulation increased claudin-2 promoter activity in Caco-2 cells, however resulted in a sharp decrease in MDCK II cells and thus suggested differential effects of EGFR-activation upon claudin-2 transcriptional regulators between MDCK-II and Caco-2 cells.
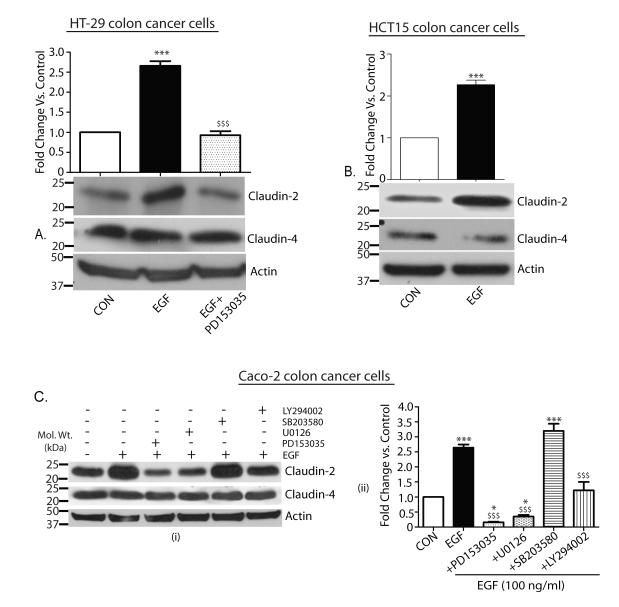
We further determined whether the EGF-dependent increase in claudin-2 expression was a phenomenon common among colon cancer cells or limited only to the Caco-2 cells. We used HCT15 and HT-29 colon cancer cells that also express claudin-2 for this purpose. Similar to the Caco-2 cells, EGF treatment increased claudin-2 expression in HT-29 cells (p<0.001) that was prevented upon use of PD153035 (Fig. 6A). Induction of claudin-2 by EGF was also demonstrated in HCT15 cells (Fig. 6B; p<0.001). As in Caco-2 cells, EGF treatment had no appreciable effects upon claudin-4 expression in either HT-29 or HCT15 cells (Fig. 6A-B).
Figure 6. EGF induced increase in claudin-2 expression was not limited to Caco-2 cells and was mediated through MAP ERK 1/2 and PI-3 Kinase.
A. Quiescent HT-29 cells were exposed to exogenous EGF (100 ng/ml), PD153035 (0.5 μM) or both. Effect on expression of claudin-2 and claudin-4 was determined. β-Actin was used as loading control (***p<0.001 EGF treated vs. control and $$$p<0.001 EGF treated vs. EGF+PD153035). B. Quiescent HCT-15 cells were exposed to EGF as above and effect upon claudin-2, claudin-4 and β-Actin was examined (***p<0.001 EGF treated vs. control). C. (i) Quiescent Caco-2 cells were exposed to EGF alone or in combination with PD153035 (0.5 μM), U0126 (10 μM), SB203580 (5 μM) or LY294002 (20 μm). The effect upon claudin-2 and claudin-4 expressions was determined. β-actin was used as loading control. (ii) Representative densitometric analysis (*p<0.05 vs. control; ***p<0.001 vs. control and $$$p<0.001 vs. EGF). The values on the left are molecular sizes in kilodaltons.
ERK1/2 and PI-3 kinase mediate the EGF-dependent increase in claudin-2 expression
We further determined signaling pathways downstream of EGFR activation that are important in the EGF-dependent increase in claudin-2 expression. The key role/s of ERK1/2 kinase, p-38 MAP kinase and/or PI-3 kinase, signaling pathways downstream of EGFR activation, in the regulation of EGFR-dependent cellular functions is established. We used pathway-specific pharmacological inhibitors, where cells were pretreated with either U0126 (10 μM; ERK1/2 kinase inhibitor), LY 294002 (20 μM; PI-3 kinase inhibitor) or SB202190 (5 μM; p-p38 kinase inhibitor) and control cells received vehicle (DMSO) for the same duration (Fig. 6C). The p-p38 MAP kinase inhibitor had no effect upon either baseline or EGF-induced claudin-2 expression while the PI-3 kinase inhibitor partially inhibited the EGFR-dependent increase in claudin-2 expression (p<0.001, EGF+LY294002 vs. EGF treated). In contrast, the ERK 1/2 kinase inhibitor inhibited the EGF-dependent increases in claudin-2 similar to and to the same extent as upon use of PD153035 (Fig. 6C; p<0.001, EGF+PD153035 or EGF+U0126 vs. EGF treated, and p<0.05 EGF+PD153035 or EGF+U0126 vs. control). Taken together, our data suggested the predominant role of the EGFR-ERK1/2 kinase axis in the regulation of colonic claudin-2 expression.
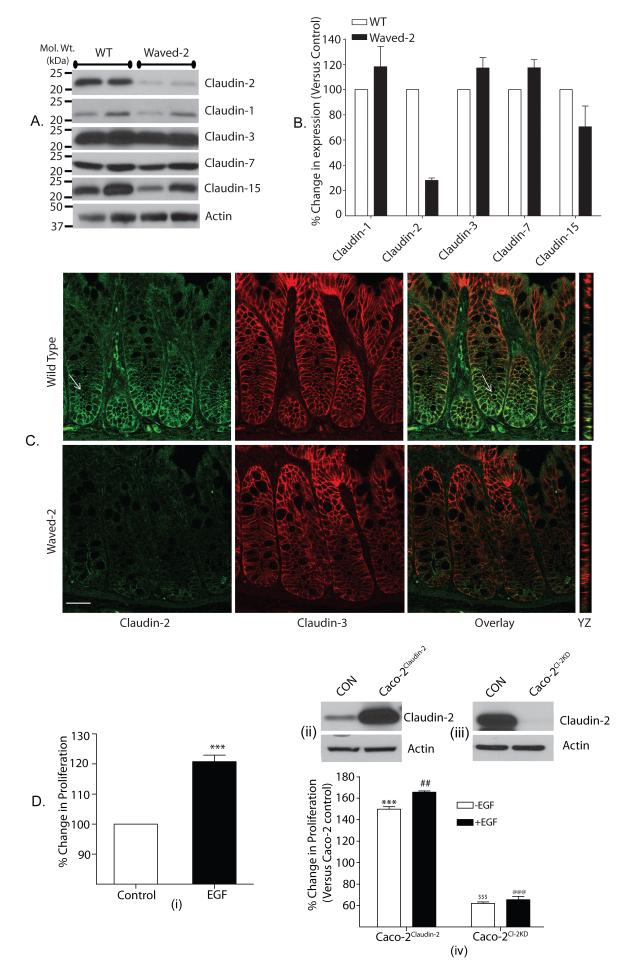
Colonic claudin-2 expression is decreased in waved-2 mice
Our above data highlighted the importance of EGFR activation in the regulation of claudin-2 expression in colon derived cell lines. Therefore, we examined whether similar correlation exists in vivo. We used waved-2 mice for this purpose where EGFR tyrosine kinase activity is naturally reduced by ~ 95% (Luetteke, Phillips et al. 1994). Colon was harvested from 8-10 week old waved-2 and littermate wild type (WT) mice. Immunoblot analysis using colon lysate showed robust claudin-3, claudin-7 and claudin-15 expressions while claudin-1 expression was minimal. These expression patterns were well in accordance with the published reports (Holmes, Van Itallie et al. 2006), however were not much different between the WT and waved-2 mice colon. In contrast, claudin-2 expression was sharply decreased in the waved-2 mice colon compared to the WT-mice colon (Fig. 7A & B). These findings were supported by further co-immunofluorescent analysis, which showed intense membrane localized claudin-3 expression in the WT and waved-2 mice colon that extended from the base to the tip of the colonic crypts. In contrast, claudin-2 expression was localized to the crypt base in the WT-mice colon, as previously described (Holmes, Van Itallie et al. 2006) and was sharply decreased in waved-2 mice colon (Fig. 7C). Taken together, our data showed that the EGFR-tyrosine kinase activity helps maintain colonic claudin-2 expression.
Figure 7. Colonic claudin-2 expression depends upon EGFR tyrosine kinase activity and modulates EGF-induced cell proliferation.
A. Expressions of claudin-1, claudin-2, claudin-3, claudin-7 and claudin-15 in the colon of waved-2 mice and WT littermates: A) Immunoblot analysis using total tissue lysates. Actin was used as loading control. B) Densitometric analysis of protein expression. Values are presented as mean+SEM (n#3). C) Representative confocal images from the co-immunofluorescent analysis of the colonic expression and cellular distribution of claudin-2 and claudin-3. Please note that claudin-2 expression in WT-colon is localized in the base of the crypt (see arrow) Scale bar, 10 μm, and D) Claudin-2 expression modulates EGF-induced increase in cell proliferation: D(i). Effect of EGF treatment upon cell proliferation (p<0.001), D(ii). Claudin-2 overexpression and D(iii). Knockdown of claudin-2 in Caco-2 cells, D(iv). Effect of EGF-treatment upon cell proliferation in Caco-2Claudin-2 and Caco-2Cl-2KD cells. ***p<0.001: compared to untreated Caco-2 control cells; ##p<0.01: compared to untreated Caco-2Claudin-2 cells; $$$ and @@@p<0.001: compared to untreated Caco-2 control cells. Values for the control cells were considered 100%. All values presented are mean +SEM.
Genetic silencing of claudin-2 expression prevents EGF-induced proliferation
We observed significant increase in cell proliferation upon expression of claudin-2 in colon cancer cells deficient in endogenous claudin-2 expression [Fig. 3A & 3B]. Therefore, in light of our findings that exogenous EGF increased claudin-2 expression in colon cells, we further examined effect of EGF-treatment upon cell proliferation in correlation with claudin-2 expression. Proliferation was determined in exponentially growing Caco-2 cells in the presence or absence of exogenous EGF (100 ng/ml), and significant increases in cell proliferation were observed with EGF addition [Fig. 7D(i), p<0.001]. To examine the association between EGF-induced claudin-2 expression and proliferation, we either silenced claudin-2 expression in Caco-2 cells (Caco-2Cl2KD cells) or stably overexpressed (Caco-2Claudin-2 cells) [Fig. 7D(iii) & (ii) respectively]. Further studies showed significant increase in proliferation in exponentially growing Caco-2Claudin-2 cells (p<0.01) and a decrease in Caco-2Cl2KD cells (p<0.001) compared to the Caco-2Control cells [Fig. 7D(iv)], which supported our findings from SW480 and HCT116 cells (Fig. 3). Importantly, further studies where Caco-2Claudin-2 and Caco-2Cl2KD cells were exposed to exogenous EGF (100 ng/ml) showed that claudin-2 overexpression did not affect the EGF-induced proliferation, however silencing of claudin-2 expression prevented the EGF-induced proliferation in Caco-2 cells [Fig. 7D(iv)].
Discussion
In this study, we have confirmed and conclusively demonstrated, using an extensive database of human colon cancer samples and performing mRNA and protein expression quantification, that claudin-2 expression is significantly increased in colorectal cancer and correlates with tumor progression. Most importantly, we have demonstrated that the increased claudin-2 expression is causally associated with colorectal cancer as forced claudin-2 expression increased the tumorigenic ability of colon cancer cells. We have further demonstrated that the colonic tissue microenvironment potentiates the induction of claudin-2 expression in an EGFR-dependent manner and colonic claudin-2 expression is specifically decreased in waved-2 mice. Importantly, EGFR signaling is a key regulator of colorectal carcinogenesis. Furthermore, in waved-2 mice, normal proliferative response is blunted following partial small bowel resection (Helmrath, Shin et al. 1998) and presence of waved-2 allele reduces intestinal polyps by ~90% in the APCmin mouse model (Roberts, Min et al. 2002). In addition, symplekin, a transcription cofactor and colon tumor promoter, increases claudin-2 expression in colon cancer cells (Buchert, Papin et al. 2010), while matriptase, a putative colon tumor suppressor, decreases claudin-2 expression (Buzza, Netzel-Arnett et al. 2010). Furthermore, non-steroidal anti-inflammatory drug (NSAID)-dependent inhibition of cell invasion in colon cancer cells can be reversed by claudin-2 expression (Mima, Takehara et al. 2008). In accordance, in our studies, claudin-2 overexpression protected HCT116 cells from the cytotoxic effects of 5-FU.
Claudin-2 is a tight junction (TJ) integral protein that converts TJ from a tight to a leaky strand phenotype (Furuse, Furuse et al. 2001; Singh, Sugimoto et al. 2007). In transformed epithelia, evidence of increased TJ permeability has been demonstrated and in colon adenocarcinomas, epithelial permeability was higher compared to the adjacent normal colon (Soler, Miller et al. 1999). Further, transepithelial impedance decreases across the colons of mice treated with chemical carcinogens (Davies, Joseph et al. 1989). These findings lead to the speculation that claudin-2 expression may underlie the increased permeability in colon cancer. This concept gains support from the documented increase in claudin-2 expression as well as colonic epithelial permeability in IBD patients (Heller, Florian et al. 2005; Prasad, Mingrino et al. 2005; Amasheh, Grotjohann et al. 2009). Notably, IBD patients are at increased risk of developing colon cancer (Eaden, Abrams et al. 2001). In the current report, we have observed increased claudin-2 expression in IBD-associated cancer. Our findings are in accordance with the finding of a recent study that reported a potential correlation of claudin-2 expression with IBD-associated neoplastic transformation (Weber, Nalle et al. 2008). In this regard, we found that EGF-induced claudin-2 expression was paralleled by an increase in paracellular permeability and a decrease in TER. An association between the tumor promoter-induced TJ permeability (Mullin and O’Brien 1986), transepithelial flux of growth factors (Mullin and McGinn 1987), and development of epithelial tumors (Mullin, Snock et al. 1992) has been shown by studies utilizing in vitro models of epithelial neoplasia. Thus, an increase in claudin-2 expression would render the colonic epithelium permeable and in turn luminal carcinogenic agents, including luminal growth factors such as EGF, would gain access to their receptor located basolaterally, and thus induce unregulated proliferation and potentially neoplastic transformation/growth. A similar hypothesis can be postulated for IBD-associated cancer where increased colonic epithelial permeability due to increased claudin-2 expression results in increased access of immunogenic substances, normally present in the lumen, to the mucosa and thereby initiate immune responses. In turn, cytokines (such as, TNF-α) further increase claudin-2 expression (Mankertz, Amasheh et al. 2009) and thus initiate a vicious cycle leading to chronic inflammation and thus increased susceptibility to colon cancer. Further investigations are needed to corroborate such postulations and are currently underway in our laboratory.
Colonic claudin-2 expression is uniquely restricted to the crypt base, which is the proliferative zone (Escaffit, Boudreau et al. 2005; Holmes, Van Itallie et al. 2006). Claudin-2 has also been identified as a target of Wnt/β-catenin signaling (Mankertz, Hillenbrand et al. 2004). Thus, potential role/s of claudin-2 in cellular functions other than the known effects on tight junction function can be suggested. In our studies, forced claudin-2 expression in colon cancer cells increased proliferation and anchorage-independent growth. Our findings gain support from the report that silencing of claudin-2 expression in HT-29 colon cells decreased cyclin-D expression (Buchert, Papin et al. 2010). Also, increased proliferation upon knockdown of cingulin was associated with an increase in claudin-2 expression (Guillemot and Citi 2006). Taken together, a direct/indirect role of claudin-2 in the regulation of colonocyte proliferation is postulated.
This hypothesis gains strength from our finding that EGFR activation not only induced colonic claudin-2 expression, but also appeared necessary to maintain the endogenous colonic claudin-2 expression. This EGF-dependent induction of claudin-2 in colon cancer cells is in contrast to our previously published report using renal epithelial cells, MDCK-II, where EGFR activation resulted in a sharp decrease in claudin-2 expression (Singh and Harris 2004). Other groups, since then, have reported similar effects of EGFR activation upon claudin-2 expression in MDCK-II cells (Angelow, Schneeberger et al. 2007; Flores-Benitez, Ruiz-Cabrera et al. 2007). Our current studies using human claudin-2 promoter suggest that the differential effects of EGFR activation upon claudin-2 transcriptional regulators may underlie the contrasting effect of EGF-stimulation on claudin-2 expression among renal and colonic epithelial cells. Currently, we are in the process of identifying putative transcription factors involved in this differential regulation. Notably, EGFR activation also increases claudin-2 expression in lung cancer cells (Peter, Comellas et al. 2009) and thus supports tissue specificity of such regulation and highlights the importance of the tissue microenvironment. Similar tissue specificity for claudin-2 regulation is reported for HNF-1α as HNF-1α is required for claudin-2 expression in liver but not in the kidney (Sakaguchi, Gu et al. 2002).
Further, the EGF-induced increase in claudin-2 expression in Caco-2 cells was paralleled by a significant increase in cell proliferation and silencing of claudin-2 expression abolished this EGF-induced increase in proliferation. EGFR kinase activation promotes intestinal epithelial cell proliferation and crypt size (Dahlhoff, Horst et al. 2008). EGFR plays an important role in the regulation of intestinal tumorigenesis (Roberts, Min et al. 2002). In colon cancer patients, EGFR activation is correlated with poor prognosis and increased mortality (Rego, Foster et al. 2009). The proliferative and survival roles of EGFR are commonly mediated through the activation of ERK1/2 and/or PI-3 kinase signaling pathways. Also, activation of these pathways has been associated with the growth/progression of colon cancer. In our studies, the EGF-dependent increase in claudin-2 was abolished completely upon inhibition of ERK1/2 signaling and partially upon inhibition of PI-3 kinase. Taken together, our data strongly supports a correlation between claudin-2 expression and colonocyte proliferation and/or survival. Our findings gain support from other published findings that have shown similar dependence of claudin-2 expression upon ERK1/2 or PI-3 kinase signaling in colon-derived cell lines (Kinugasa, Sakaguchi et al. 2000; Prasad, Mingrino et al. 2005).
Our finding that co-culture of colon cancer cells with colonic fibroblasts increases claudin-2 expression in an EGFR-dependent manner further supports the role of claudin-2 in the regulation of colon cancer, potentially via EGFR activation. Notably, a DSS colitis-induced decrease in colonic claudin-2 expression was reverted in response to mesenchymal stem cells (Yabana, Arimura et al. 2009). The role of the tissue microenvironment, including fibroblasts, has emerged as an important aspect of the regulation of tumorigenesis. However, the detailed mechanism/s underlying the fibroblast-dependent activation of EGFR remains unclear at present though our data does exclude the direct role of EGF (data not shown). In this regard, a potential candidate is prostaglandin E2 (PGE2), which is abundantly synthesized by colonic fibroblasts (Zhu, Hua et al. 2003) and activates EGFR in colon cancer cells (Pai, Soreghan et al. 2002). TNF-α, a known inducer of colonic claudin-2 expression (Mankertz, Amasheh et al. 2009), stimulates (PGE2) synthesis (Kim, Zhu et al. 1998), and activates EGFR in colon cancer cells (Yamaoka, Yan et al. 2008). However, EGFR can also be activated by other mechanism/s including the activation of G-protein coupled receptors (Singh and Harris 2005). Currently, we are in the process of examining the details of fibroblast-dependent activation of EGFR activation and claudin-2 expression.
Overall, in the current study, we have demonstrated a consistent and significant increase in claudin-2 expression in colon cancer and a causal association between claudin-2 and colon carcinogenesis. Importantly, we show that colonic claudin-2 expression is upregulated by the tissue microenvironment in an EGFR-dependent manner. Our findings will provide a new understanding of the molecular details of colon cancer regulation, specifically in the context of EGFR signaling. Our findings may also help understand the correlation between epithelial permeability, neoplastic transformation, and colon cancer. Additionally, we provide new data regarding the potential role of claudin-2 in the regulation of colonocyte proliferation and survival. Our findings may prove to have important clinical implications considering the known roles of EGFR and epithelial permeability in the regulation of colon cancer, since our identification of claudin-2 as an important link between EGFR and alterations in colonocyte function suggest that it could be a target for therapeutic intervention.
Materials and Methods
Plasmids and reagents
A pcDNA-4 expression plasmid (Invitrogen Inc.) containing the claudin-2 coding sequence was used for overexpression. Human claudin-2 specific and control shRNA-constructs were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies against claudin-1, -2, -3, -4, -7, -15 and ZO-1 were obtained from Invitrogen (Carlsbad, CA). EGF, PD 153035, U0126, SB-239063 and LY-294002 were from EMD Biosciences (Gibbstown, NJ).
Cell culture and transfection
The source and culture conditions of the cell lines used have been previously described (Dhawan, Singh et al. 2005). Claudin-2-overexpressing cell population was selected using Zeocin (0.4 mg/ml). For co-culture, fibroblasts were plated on the bottom surface of transwell filters (0.4 μm pore size). Caco-2 cells were plated on the filter top once fibroblasts were attached (Fig. 4A).
Human tissue, microarray platforms and statistical analysis
The protocols and procedures for the procurement of human tissue samples, details of the microarray platforms and statistical analysis has been described previously (Krishnan, Singh et al. 2009).
Immunoblot, immunohistochemistry (IHC) and immunofluorescence (IF) analyses
were performed as previously described (Dhawan, Singh et al. 2005).
MTT assay
The CellTiter assay (MTT assay; Promega Inc.) was conducted as described previously (Dhawan, Singh et al. 2005).
Anchorage-independent growth assay
was conducted as described previously (Dhawan, Singh et al. 2005). Colonies were counted using Gel Count (Bio Rad).
In vivo studies
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University. Subcutaneous flank inoculation of claudin-2 overexpressing or control cells was performed in 6-week-old athymic mice. Tumor incidence, tumor growth, and animal conditions were assessed on a continuous basis for 5 weeks after inoculation, at which time animals were sacrificed.
Luciferase reporter assay
was done using a human claudin-2 (1.2 kb) promoter (Sakaguchi, Gu et al. 2002) construct containing firefly luciferase reporter as described previously (Shiou, Singh et al. 2007).
Statistical analysis
Student’s t-test, Chi-square test and ANOVA were used to determine statistical significance as applicable, and differences were considered statistically significant at p< 0.05.
Supplementary Material
Supplementary Fig. 1: Representative images from the IHC analysis of claudin-2 expression using paraffin-embedded normal and colon cancer samples. A colon cancer progression tissue array (normal adjacent colon, n=13; adenomas n=14; and adenocarcinomas n=13) from the Cooperative Human Tissue Network (CHTN, NCI), and IHC-specific mouse anti-claudin-2 antibody (Invitrogen Corp; 32-5600) were used. (i & ii) Representative normal colon, (iii-iv) Representative colon adenocarcinoma. [Magnifications: 100x (i & iii) and 400x (ii & iv)].
Supplementary Fig. 2: Claudin-2 expression in IBD-associated cancer. IHC staining for claudin-2 was performed using archived paraffin sections of Crohn’s disease- and ulcerative colitis (UC)-associated colon cancers (n=19). A. Representative claudin-2 expression in colon with colitis from Crohn’s disease patients [A(i) & A(ii)] and associated cancer [A(iii) & A(iv)]. B. Similar increase in claudin-2 expression was observed in ulcerative colitis (UC) associated cancer [B(iii) & B(iv)] compared to the colon with colitis only [B(i) & B(ii)]. [Magnifications: 100x [A(i), A(iii), B(i) & B(iii) and 200x [A(ii), A(iv), B(ii) & B(iv)].
Supplementary Fig. 3: Claudin-2 overexpression does not modulate expression of other claudin proteins. Total RNA was isolated from ~90 % confluent HCT116Control and HCTClaudin-2 cells and was subjected to real time RT-PCR using gene specific real-time PCR primers. No major change in the expression of claudin-3, claudin-4 or claudin-7 was observed due to the forced expression of claudin-2. Values presented are mean+SEM.
Supplementary Fig. 4: 5-FU treatment affected cell proliferation and apoptosis. Exponentially growing HCT116Control and HCTClaudin-2 cells were exposed to 5-FU (100 μM) for 48 hours. Cell proliferation and apoptosis were determined using MTT-assay and cell death ELISA assay, as described previously (Dhawan, Singh et al. 2005; Singh, Sugimoto et al. 2007). Values presented are mean+SEM and are presented as % change compared to the respective untreated control cells.
Supplementary Fig. 5: EGFR-blocking antibody prevented the co-culture dependent increase in claudin-2 expression. Caco-2 cells were subjected to co-culture in the presence or absence of EGFR blocking antibody (clone#528, 20 μg/ml) and effect on claudin-2 expression was determined.
Supplementary Fig. 6: EGF stimulation decreased trans-epithelial resistance (TER) and increased paracellular permeability. A. TER in Caco-2 cells (***p<0.001). Confluent cells plated on transwell filters (0.4-μm pore size) were serum starved and then exposed to EGF (100 ng/ml). TER was measured before treatment and 8 & 24 hours after EGF treatment. Results are expressed in Ω x cm2, and B. paracellular permeability for FITC-dextran (4 kDa) $$$p<0.001. Medium containing FITC-dextran (4 kDa) was added to the top (inner) chamber of the transwell. Samples were collected from the bottom (outer) chamber after 1, 4 and 24 hours after EGF-treatment. Data are presented as the total amount of FITC-dextran (4 kDa, Sigma-Aldrich Inc.) collected in the bottom chamber at the indicated time points.
Supplementary Fig. 7: EGF-dependent increase in claudin-2 expression in Caco-2 cells was specific. Total RNA was isolated from control and EGF-treated Caco-2 cells and was subjected to real time RT-PCR using gene specific primers. Among claudin-2, 3, 4 and 8, only claudin-2 expression was significantly increased in the EGF-treated cells compared to the control cells. Values presented are mean+SEM. **P<0.01 versus control cells.
Supplementary Table. 1: Demographics and case information for the VMC and MCC patients represented by 10 normal adjacent colon samples, 55 adenocarcinomas and 195 adenocarcinoma patients are presented in table format. The Vanderbilt test set includes 14 patients from the University of Alabama-Birmingham Medical Center (14 tumors provided by Martin J. Heslin, M.D.). All patients were diagnosed with colorectal adenocarcinoma (stages I-IV) according to current American Joint Commission on Cancer (AJCC) guidelines. Other in the VMC medical record implies not otherwise specified or Asian and Hispanic or Hispanic NOS.
Acknowledgement
This work was supported by 5P50DK044757 and P30DK058404 Pilot projects (A.B.S.) & NIH grants CA119005, CA124977 (P.D.). We would like to thank Drs. Ambra Pozzi and Mingjian Shi for waved-2 mice and Dr. Wael El-Rifai for 5-FU.
Footnotes
Conflict of Interest: None.
References
- Amasheh M, Grotjohann I, et al. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009:1–10. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- Angelow S, Schneeberger E, et al. Claudin-8 Expression in Renal Epithelial Cells Augments the Paracellular Barrier by Replacing Endogenous Claudin-2. Journal of Membrane Biology. 2007;215(2):147–159. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- Ansfield F, Klotz J, et al. A phase III study comparing the clinical utility of four regimens of 5-fluorouracil: a preliminary report. Cancer. 1977;39(1):34–40. doi: 10.1002/1097-0142(197701)39:1<34::aid-cncr2820390107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bos M, Mendelsohn J, et al. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clinical Cancer Research. 1997;3(11):2099–2106. [PubMed] [Google Scholar]
- Buchert M, Papin M, et al. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc Natl Acad Sci U S A. 2010;107(6):2628–2633. doi: 10.1073/pnas.0903747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza MS, Netzel-Arnett S, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A. 2010;107(9):4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, et al. Cell Adhesion, Polarity, and Epithelia in the Dawn of Metazoans. Physiol. Rev. 2004;84(4):1229–1262. doi: 10.1152/physrev.00001.2004. [DOI] [PubMed] [Google Scholar]
- Cutler NS, Graves-Deal R, et al. Stromal Production of Prostacyclin Confers an Antiapoptotic Effect to Colonic Epithelial Cells. Cancer Res. 2003;63(8):1748–1751. [PubMed] [Google Scholar]
- D’Inca R, Di Leo V, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94(10):2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M, Horst D, et al. Betacellulin stimulates growth of the mouse intestinal epithelium and increases adenoma multiplicity in Apc+/Min mice. FEBS Letters. 2008;582(19):2911–2915. doi: 10.1016/j.febslet.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Davies RJ, Joseph R, et al. Detection of the cancer-prone colon, using transepithelial impedance analysis. Arch Surg. 1989;124(4):480–484. doi: 10.1001/archsurg.1989.01410040090021. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. The Journal of Clinical Investigation. 2005;115(7):1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaden JA, Abrams KR, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escaffit F, Boudreau F, et al. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. Journal of Cellular Physiology. 2005;203(1):15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- Flores-Benitez D, Ruiz-Cabrera A, et al. Control of tight junctional sealing: role of epidermal growth factor. Am J Physiol Renal Physiol. 2007;292(2):F828–836. doi: 10.1152/ajprenal.00369.2006. [DOI] [PubMed] [Google Scholar]
- Furuse M, Furuse K, et al. Conversion of Zonulae Occludentes from Tight to Leaky Strand Type by Introducing Claudin-2 into Madin-Darby Canine Kidney I Cells. J. Cell Biol. 2001;153(2):263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends in Cell Biology. 2006;16(4):181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Citi S. Cingulin Regulates Claudin-2 Expression and Cell Proliferation through the Small GTPase RhoA. Mol. Biol. Cell. 2006;17(8):3569–3577. doi: 10.1091/mbc.E06-02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Florian P, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Helmrath MA, Shin CE, et al. The EGF\EGF-receptor axis modulates enterocyte apoptosis during intestinal adaptation. J Surg Res. 1998;77(1):17–22. doi: 10.1006/jsre.1998.5362. [DOI] [PubMed] [Google Scholar]
- Holmes JL, Van Itallie CM, et al. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expression Patterns. 2006;6(6):581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kim EC, Zhu Y, et al. Cytokine-mediated PGE2 expression in human colonic fibroblasts. Am J Physiol Cell Physiol. 1998;275(4):C988–994. doi: 10.1152/ajpcell.1998.275.4.C988. [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Huo Q, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A):3729–3734. [PubMed] [Google Scholar]
- Kinugasa T, Sakaguchi T, et al. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118(6):1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Krishnan M, Singh AB, et al. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2009;29(2):305–312. doi: 10.1038/onc.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M, Singh AB, et al. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2010;29(2):305–312. doi: 10.1038/onc.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal-Nag M, Morin P. The claudins. Genome Biology. 2009;10(8):235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke NC, Phillips HK, et al. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8(4):399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, et al. TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell and Tissue Research. 2009;336(1):67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336(1):67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Hillenbrand B, et al. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochemical and Biophysical Research Communications. 2004;314(4):1001–1007. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- Mima S, Takehara M, et al. NSAIDs suppress the expression of claudin-2 to promote invasion activity of cancer cells. Carcinogenesis. 2008;29(10):1994–2000. doi: 10.1093/carcin/bgn134. [DOI] [PubMed] [Google Scholar]
- Mullin JM. Epithelial barriers, compartmentation, and cancer. Sci STKE. 2004;2004(216):pe2. doi: 10.1126/stke.2162004pe2. [DOI] [PubMed] [Google Scholar]
- Mullin JM, McGinn MT. The phorbol ester, TPA, increases transepithelial epidermal growth factor flux. FEBS Letters. 1987;221(2):359–364. doi: 10.1016/0014-5793(87)80956-0. [DOI] [PubMed] [Google Scholar]
- Mullin JM, O’Brien TG. Effects of tumor promoters on LLC-PK1 renal epithelial tight junctions and transepithelial fluxes. Am J Physiol Cell Physiol. 1986;251(4):C597–602. doi: 10.1152/ajpcell.1986.251.4.C597. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Snock KV, et al. Effects of acute vs. chronic phorbol ester exposure on transepithelial permeability and epithelial morphology. J Cell Physiol. 1992;152(1):35–47. doi: 10.1002/jcp.1041520106. [DOI] [PubMed] [Google Scholar]
- Pai R, Soreghan B, et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- Peter Y, Comellas A, et al. Epidermal growth factor receptor and claudin-2 participate in A549 permeability and remodeling: implications for non-small cell lung cancer tumor colonization. Mol Carcinog. 2009;48(6):488–497. doi: 10.1002/mc.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Mingrino R, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85(9):1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- Rego RL, Foster NR, et al. Prognostic effect of activated EGFR expression in human colon carcinomas: comparison with EGFR status. Br J Cancer. 2009;102(1):165–172. doi: 10.1038/sj.bjc.6605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JL, Lamas M, et al. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62(2):476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- Ridyard AE, Brown JK, et al. Apical junction complex protein expression in the canine colon: differential expression of claudin-2 in the colonic mucosa in dogs with idiopathic colitis. J Histochem Cytochem. 2007;55(10):1049–1058. doi: 10.1369/jhc.7A7211.2007. [DOI] [PubMed] [Google Scholar]
- Roberts RB, Min L, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2002;99(3):1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RB, Min L, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Gu X, et al. Cloning of the Human Claudin-2 5â€2-Flanking Region Revealed a TATA-less Promoter with Conserved Binding Sites in Mouse and Human for Caudal-related Homeodomain Proteins and Hepatocyte Nuclear Factor-1α. Journal of Biological Chemistry. 2002;277(24):21361–21370. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- Shiou SR, Singh AB, et al. Smad4 regulates claudin-1 expression in a transforming growth factor-beta-independent manner in colon cancer cells. Cancer Res. 2007;67(4):1571–1579. doi: 10.1158/0008-5472.CAN-06-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, Harris RC. Epidermal Growth Factor Receptor Activation Differentially Regulates Claudin Expression and Enhances Transepithelial Resistance in Madin-Darby Canine Kidney Cells. J. Biol. Chem. 2004;279(5):3543–3552. doi: 10.1074/jbc.M308682200. [DOI] [PubMed] [Google Scholar]
- Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular Signalling. 2005;17(10):1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Singh AB, Sugimoto K, et al. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol. 2007;293(5):C1660–1668. doi: 10.1152/ajpcell.00274.2007. [DOI] [PubMed] [Google Scholar]
- Singh AB, Sugimoto K, et al. Juxtacrine activation of epidermal growth factor (EGF) receptor by membrane-anchored heparin-binding EGF-like growth factor protects epithelial cells from anoikis while maintaining an epithelial phenotype. J Biol Chem. 2007;282(45):32890–32901. doi: 10.1074/jbc.M702677200. [DOI] [PubMed] [Google Scholar]
- Soler AP, Miller RD, et al. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20(8):1425–1432. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Takehara M, N. T, Mima S, Hoshino T, Mizushima T. Effect of Claudin Expression on Paracellular Permeability, Migration and Invasion of Colonic Cancer Cells. Biol Pharm Bull. 2009;32(5):825–831. doi: 10.1248/bpb.32.825. [DOI] [PubMed] [Google Scholar]
- Weber CR, Nalle SC, et al. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88(10):1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabana T, Arimura Y, et al. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. The Journal of Pathology. 2009;218(3):350–359. doi: 10.1002/path.2535. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Yan F, et al. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proceedings of the National Academy of Sciences. 2008;105(33):11772–11777. doi: 10.1073/pnas.0801463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Burgel N, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hua P, et al. Cyclooxygenase-2 Expression and Prostanoid Biogenesis Reflect Clinical Phenotype in Human Colorectal Fibroblast Strains. Cancer Res. 2003;63(2):522–526. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Representative images from the IHC analysis of claudin-2 expression using paraffin-embedded normal and colon cancer samples. A colon cancer progression tissue array (normal adjacent colon, n=13; adenomas n=14; and adenocarcinomas n=13) from the Cooperative Human Tissue Network (CHTN, NCI), and IHC-specific mouse anti-claudin-2 antibody (Invitrogen Corp; 32-5600) were used. (i & ii) Representative normal colon, (iii-iv) Representative colon adenocarcinoma. [Magnifications: 100x (i & iii) and 400x (ii & iv)].
Supplementary Fig. 2: Claudin-2 expression in IBD-associated cancer. IHC staining for claudin-2 was performed using archived paraffin sections of Crohn’s disease- and ulcerative colitis (UC)-associated colon cancers (n=19). A. Representative claudin-2 expression in colon with colitis from Crohn’s disease patients [A(i) & A(ii)] and associated cancer [A(iii) & A(iv)]. B. Similar increase in claudin-2 expression was observed in ulcerative colitis (UC) associated cancer [B(iii) & B(iv)] compared to the colon with colitis only [B(i) & B(ii)]. [Magnifications: 100x [A(i), A(iii), B(i) & B(iii) and 200x [A(ii), A(iv), B(ii) & B(iv)].
Supplementary Fig. 3: Claudin-2 overexpression does not modulate expression of other claudin proteins. Total RNA was isolated from ~90 % confluent HCT116Control and HCTClaudin-2 cells and was subjected to real time RT-PCR using gene specific real-time PCR primers. No major change in the expression of claudin-3, claudin-4 or claudin-7 was observed due to the forced expression of claudin-2. Values presented are mean+SEM.
Supplementary Fig. 4: 5-FU treatment affected cell proliferation and apoptosis. Exponentially growing HCT116Control and HCTClaudin-2 cells were exposed to 5-FU (100 μM) for 48 hours. Cell proliferation and apoptosis were determined using MTT-assay and cell death ELISA assay, as described previously (Dhawan, Singh et al. 2005; Singh, Sugimoto et al. 2007). Values presented are mean+SEM and are presented as % change compared to the respective untreated control cells.
Supplementary Fig. 5: EGFR-blocking antibody prevented the co-culture dependent increase in claudin-2 expression. Caco-2 cells were subjected to co-culture in the presence or absence of EGFR blocking antibody (clone#528, 20 μg/ml) and effect on claudin-2 expression was determined.
Supplementary Fig. 6: EGF stimulation decreased trans-epithelial resistance (TER) and increased paracellular permeability. A. TER in Caco-2 cells (***p<0.001). Confluent cells plated on transwell filters (0.4-μm pore size) were serum starved and then exposed to EGF (100 ng/ml). TER was measured before treatment and 8 & 24 hours after EGF treatment. Results are expressed in Ω x cm2, and B. paracellular permeability for FITC-dextran (4 kDa) $$$p<0.001. Medium containing FITC-dextran (4 kDa) was added to the top (inner) chamber of the transwell. Samples were collected from the bottom (outer) chamber after 1, 4 and 24 hours after EGF-treatment. Data are presented as the total amount of FITC-dextran (4 kDa, Sigma-Aldrich Inc.) collected in the bottom chamber at the indicated time points.
Supplementary Fig. 7: EGF-dependent increase in claudin-2 expression in Caco-2 cells was specific. Total RNA was isolated from control and EGF-treated Caco-2 cells and was subjected to real time RT-PCR using gene specific primers. Among claudin-2, 3, 4 and 8, only claudin-2 expression was significantly increased in the EGF-treated cells compared to the control cells. Values presented are mean+SEM. **P<0.01 versus control cells.
Supplementary Table. 1: Demographics and case information for the VMC and MCC patients represented by 10 normal adjacent colon samples, 55 adenocarcinomas and 195 adenocarcinoma patients are presented in table format. The Vanderbilt test set includes 14 patients from the University of Alabama-Birmingham Medical Center (14 tumors provided by Martin J. Heslin, M.D.). All patients were diagnosed with colorectal adenocarcinoma (stages I-IV) according to current American Joint Commission on Cancer (AJCC) guidelines. Other in the VMC medical record implies not otherwise specified or Asian and Hispanic or Hispanic NOS.