Abstract
Background:
In elective open infrarenal aortic aneurysm repair the use of epidural anesthesia and analgesia may preserve splanchnic perfusion. The aim of this study was to investigate the effects of epidural anesthesia on gut perfusion with gastrointestinal tonometry in patients undergoing aortic reconstructive surgery.
Methods:
Thirty patients, scheduled to undergo an elective infrarenal abdominal aortic reconstructive procedure were randomized in two groups: the epidural anesthesia group (Group A, n=16) and the control group (Group B, n=14). After induction of anesthesia, a transanally inserted sigmoid tonometer was placed for the measurement of sigmoid and gastric intramucosal CO2 levels and the calculation of regional–arterial CO2 difference (ΔPCO2). Additional measurements included mean arterial pressure (MAP), cardiac output (CO), systemic vascular resistance (SVR), and arterial lactate levels.
Results:
There were no significant intra- and inter-group differences for MAP, CO, SVR, and arterial lactate levels. Sigmoid pH and PCO2 increased in both the groups, but this increase was significantly higher in Group B, 20 min after aortic clamping and 10 min after aortic declamping.
Conclusions:
Patients receiving epidural anesthesia during abdominal aortic reconstruction appear to have less severe disturbances of sigmoid perfusion compared with patients not receiving epidural anesthesia. Further studies are needed to verify these results.
Keywords: Anesthesia, aorta, epidural analgesia, intestinal tonometry, splanchnic perfusion
INTRODUCTION
Open surgical repair of abdominal aortic aneurysms is definitely associated with intraoperative hypovolemia, significant blood loss and thus impaired systemic oxygenation, even when the procedure is conducted on scheduled basis and not upon rupture of the aneurysm.[1] To safely detect and prevent systematic hypotension with the accompanying consequences of organ hypoperfusion and generalized metabolic de-arrangement, the intraoperative assessment of adequate global perfusion and oxygenation is of vital importance. The above-mentioned parameters are mainly evaluated by monitoring cardiac output, global oxygen delivery or consumption, arterial pH, and lactate blood concentration.[2]
Nevertheless, it should be taken into consideration that an apparently sufficient global perfusion does not always guarantee adequate regional perfusion of various organs, while, making a further step, deterioration in systemic and regional perfusion may increase the rate of postoperative complications, leading even to multiple organ dysfunction and failure.[3]
Being in accordance with these remarks, there is an increasing amount of data that highlight the existence of considerably high prevalence of ischemic colitis in patients undergoing open elective repair of abdominal aortic aneurysms (AAA), particularly in cases of prolonged cross-clamping of the aorta.[4,5] Although transient episodes of gut mucosal ischemia are inevitable in open AAA surgery, the maintenance of hemodynamic stability with adequate perfusion of the vital organs, as well as the diminishment of the period when aorta is clamped, are the key components in terms of preventing the catastrophic consequences of irreversible intestinal ischemia, which occur due to activation of coagulation cascade, release of inflammatory mediators, systemic acidosis, and de-arrangement of the intestinal barrier.[6,7]
In the absence of special biomarkers that could be used to assess the level of intestinal perfusion, sigmoid tonometry has been implemented in a variety of relevant studies, appearing both reliable and sensitive in detecting bowel ischemia.[1,4,8] Moreover, regarding the possible impact the implemented anesthetic techniques may have on intestinal perfusion in elective open AAA repair, solid data suggest that the use of epidural anesthesia has a definitively beneficial effect concerning intestinal perfusion, mainly due to sympathetic blockade.[9,10]
In order to investigate whether combined general–epidural anesthesia with perioperative continuous epidural analgesia alters splanchnic perfusion in patients undergoing infrarenal abdominal aortic surgery, we conducted a prospective control study in which, apart from the assessment of the conventional parameters of hemodynamic and metabolic monitoring, sigmoid tonometry was used, being a minimally invasive and highly reliable way to measure intestinal perfusion and oxygenation.
METHODS
Our initial study sample constituted of 44 male patients, admitted to undergo elective open repair of infrarenal aortic aneurysm. Setting as exclusion criteria the existence of positive history of previous operations in the gastrointestinal track and chronic intestinal inflammation, 14 patients were excluded from the study (n=30).
The study was approved from our local Human Ethics Committee and informed written consent was obtained from all participating patients. Patients were randomly allocated in two groups: Group A (n=16) received combined anesthesia (general and epidural anesthesia), whereas Group B (n=14) received only general anesthesia. The day before surgery, the epidural catheter in patients of Group A was inserted at L1–L2 or L2–L3 segment using a standard loss of residence technique. On the day of surgery, when patients entered the operation room and before induction of general anesthesia, a monitor of online calculation of cardiac output by impedance cardiogaphy (Lifeguard ICG, Analogic, Boston, USA) was instituted in all patients. Under local anesthesia with lidocaine, a 20-gauge radical arterial catheter and a right internal jugular venous catheter, which was used for fluid infusions and central venous pressure monitoring were inserted percutaneously.
All patients received premedication with 0.02 mg/kg lorazepam 30–45 min before surgery. Before induction of 1–2.5 mg midazolam, a prophylactic antiemetic regimen with 0.5 mg droperidol and 3 mg granisetron was administered in all patients. Electrocardiogram (ECG), invasive blood pressure, and SpO2 monitoring was used in all patients from the time they entered the operating theater until 24 h postoperatively. Induction of general anesthesia was standardized and included intravenous infusion of remifentanil 1 μg/kg, propofol 2 mg/kg, and cis-atracurium 0.2 mg/kg. Anesthesia was maintained with sevoflurane 2–3%, air 50%, and oxygen 50%. Mechanical ventilation was used in all patients with a positive end-expiratory pressure (PEEP) of 3 cm H2O. Cis-atracurioum was infused to supplement general anesthesia and ventilation. Group E patients received before the beginning of the operation 10–15 mL levobupivacaine 0.25% (0.6–1 mL/segment depends on the age) with 100 μg fentanyl and then perioperatively at 4–6 mL/h. Anesthetic blockade was achieved until T4. The evaluation was performed with the cold/hot difference. Group B patients received a continuous intravenous infusion of remifentanil with a rate of 0.25–1.0 μg/kg/min. Perioperatively nitroglycerine was used for the management of hypertension, when systolic blood pressure exceeded 160 mmHg and phenylephrine was used when systolic blood pressure was less than 90 mmHg. For postoperative pain management, Group A patients had a patient controlled epidural analgesia (PCEA) pump, whereas Group B patients had a patient controlled intravenous analgesia (PCIA) pump. Postoperative pain management was achieved with continuous epidural infusion of levobupivacaine 0.125% and fentanyl 5 μg/mL, with an infusion rate of 3–5 mL/h in Group A. Group B patients had a PCIA pump and received fentanyl 10 μg/ mL with a rate of 1–2.5 mL/h intravenously.
To assess intestinal mucosal perfusion, we utilized a sigmoid tonometer (Tonocap, Datex Ohmeda, Helsinki, Finland). The sigmoid tonometer was inserted in the sigmoid colon by a sigmoidoscope and its correct position was verified intraoperatively by the surgeon. Sigmoid mucosal pH (pHi), PCO2 (PsCO2), and ΔPCO2 (difference between arterial PCO2 and PCO2 in sigmoid colon) were measured during the intra- and postoperative periods, while pHi was calculated using a modification of the Henderson–Hasselbach equation.
In all patients, the following parameters were recorded for our analysis: Cardiac output (CO), mean arterial blood pressure (MAP), and systemic vascular resistance (SVR) were measured at immediate preoperative period (T0 baseline), before aortic clamping (T1), 20 min after aortic clamping (T2), 10 min after aortic declamping (T3), before tracheal extubation (T4), as well as 2, 6, 12, and 24 h postoperatively (T5, T6, T7, and T8, respectively). Tonometric parameters (pHi, Ps CO2, ΔPCO2) in sigmoid colon were measured at T1–T8, following the guidelines of the manufacturer. Duration of surgery, duration of aortic clamping, intraoperative fluid–blood infusions, and arterial lactate were also recorded.
As far as statistical analysis is concerned, results for continuous variables were expressed as mean and standard deviation (SD). Categoric variables were presented as numbers. Continuous and categoric data were compared by the Mann–Whitney test. Probability (P) values less than 0.05 were considered statistically significant.
RESULTS
The patients of the two study arms did match concerning their demographic characteristics [Table 1]. The fluctuation of the main parameters of conventional intraoperative hemodynamic monitoring, CO, SVR, and MAP is demonstrated in Figure 1 and Table 2. More specifically, CO decreased at T1 and T2 in Group A and in T2 in Group B, but there was no statistically significant difference between groups at any time point of evaluation [Figure 1a]. Regarding SVR, it decreased in both groups at time points T3 and T4, but again no significant difference was noted comparing the two groups [Figure 1b]. At last, MAP decreased at T1 and T3 from baseline in Group A and at T1, T3, and T4 in Group B, with significant differences between groups occurring at T3–T4 [P=0.009 and P=0.036, respectively, [Figure 1c].
Table 1.
Demographic and perioperative data
Figure 1.
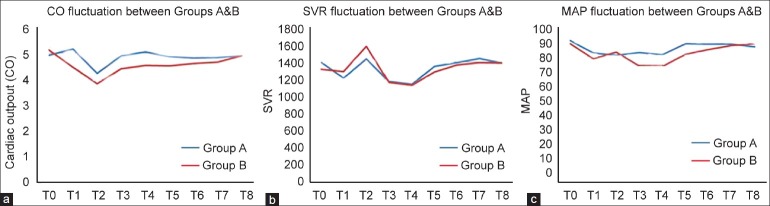
Monitoring and fluctuation of cardiac output (a) systematic vascular resistance (b) and mean arterial pressure (c) between groups A and B (T0– T8)
Table 2.
Hemodynamic parameters, tonometric variables, and arterial lactate during surgery and 24 h postoperatively
In contrast to the slight favorable effect the combined anesthesia appeared to have on basic hemodynamic parameters, sigmoid tonometry clearly revealed the supremacy of combined anesthesia concerning the preservation of intestinal perfusion and oxygenation [Table 2 and Figure 2]. As expected, intestinal pH (pHi) decreased in both groups; nevertheless, sigmoid pH values were significantly higher in the group of patients that received combined anesthesia (Group A), as measured at time points T2–T6 (P=0.005, P=0.011, P=0.004, P=0.017, and P=0.017 respectively) [Figure 2a]. In accordance with the fluctuation of intestinal pH values, sigmoid tonometry findings demonstrated that despite PsCO2 increased significantly at T2–T4 time points, compared with baseline levels in both Groups A and B, combined anesthesia resulted in maintaining lower PsCO2 levels from the time aorta was clamped up to the first 6 postoperative hours (T2–T6) [Figure 2b]. ΔPCO2 increased in Group A at T2 and decreased at T7–T8, while it increased in Group B at T2–T4. There was a significant difference between the groups at T2–T4 with higher values in Group B [Figure 2c]. Arterial lactate was significantly higher in Group B at T5–T6 (P=0.042 and P=0.019, respectively) [Figure 3].
Figure 2.
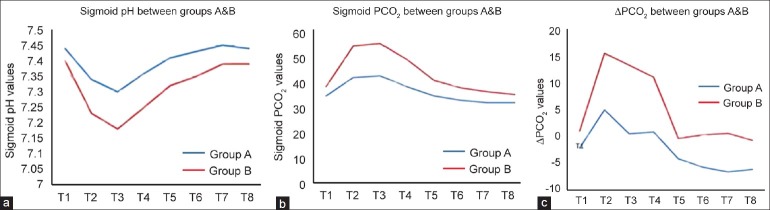
Sigmoid pH (a) PCO2 (b) and ΔPCO2 (c) values between groups A and B during the observation period T1–T8
Figure 3.
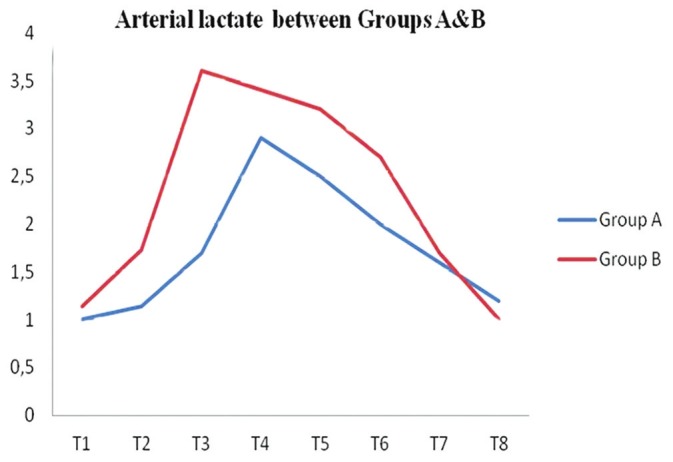
Comparison of arterial lactate levels between groups A and B (T0–T8)
DISCUSSION
In patients undergoing abdominal aortic surgery, hemodynamic stability appears to be a prerequisite to avoid postoperative organ dysfunction.[11] Particularly when it comes to open AAA repair, the occurring hypovolemia, the increase of the sympathetic tone, the significant blood loss, mesentery traction, and prolonged time of aortic clamping stand for the major factors that predispose to intestinal hypoperfusion.[12–14] In addition, the systemic hemodynamic effects of infrarenal aortic cross-clamping are unfavorable, as clamping increases myocardial work, reduces cardiac output, causing progressive tissue hypoxia, while aortic declamping results in reperfusion of the ischemic tissue, causing further de-arrangement of physiologic metabolic homeostasis and adequate compensation of the oxygen debt.[15] As a result, all the above-mentioned pathophysiologic changes that occur during open AAA repair, pose justified concerns regarding the feasibility of maintaining sufficient splachnic flow. Thus, ischemic colitis is a not uncommon complication of elective open surgery for abdominal aortic aneurysms, being observed in approximately 5%–25% of patients and considered to be a major factor for development of multiple organs dysfunction syndrome, mainly due to increased mucosal permeability of the intestine and consequent endotoxinemia.[5,16,17]
Taking all these considerations into account, the maintenance of an adequate intestinal perfusion appears to be a crucial factor for reducing the postoperative complication rates, mortality, and morbidity, in patients that undergo open AAA reconstruction.
As expected, and in accordance to these remarks, evaluating the main hemodynamic parameters recorded in our study, CO reduced in both groups after aorta was clamped (T2), compared with baseline values. MAP decreased at T1, T2, and T3 from baseline in Group A and at T1, T3, and T4 in Group B, with significant difference between the groups at T3–T4, with lower values in Group B. Vesainen et al.[12] similarly observed a reduction in MAP before aortic clamp and during clamping compared with preoperative value. On the other hand, Piper et al.[18] reported significant difference in MAP before clamping and after release of clamp, with lower values in patients under combined general–epidural anesthesia. Finally, SVR was significantly lower in Group A at T3–T4 compared with T0, whereas in Group B SVR was significantly higher during clamping (T2) compared with T0, highlighting the beneficial effect of epidural anesthesia on sympathetic blockage. Our results indicate that immediately after aortic clamping, both epidural and general anesthesia groups suffered a significant drop of sigmoid intramucosal pH, as calculated from mucosal CO2, as well as regional–systemic CO2 level difference increase in comparison with their respective preclamping values, indicative of the adverse outcomes of aortic clamping in sigmoid mucosal perfusion. However, these perfusion abnormalities were significantly less severe in the epidural anesthesia group in comparison with the general anesthesia group. Interpreting these results, epidural anesthesia seems to have a positive effect on intestinal blood flow, in the colonic portion perfused by the inferior mesenteric artery, after its inflow has been interrupted. These effects on intestinal blood flow may be due to vasodilatation and decreased mesenteric vascular resistance secondary to sympathetic block, resulting in an adequacy of intramucosal oxygenation.
Epidural anesthesia has proven to be extremely useful in terms of preventing intestinal hypoperfusion during open AAA reconstruction surgery. Specifically, the consequent sympathetic blockage appears to reduce the metabolic demands of the intestine and improve oxygen delivery, due to favorable re-distribution of gut mucosal blood flow.[19] Moreover, sympathetic blocking inhibits a potential increase of the intestinal arteriole or precapillary sphincters, preventing tissue hypoxia and acidosis.[20] Additionally, epidural anesthesia exhibits both a favorable effect upon intestinal inflammation status, modifying local entrapment of leukocytes,[21] as well as upon rapid restoration of bowel motility, preventing the complications of prolonged intestinal paralysis.[22]
Previous studies have examined the clinical benefit of application of epidural anesthesia. Kapral et al.[23] and Suttcliffe et al.[24] demonstrated that thoracic epidural analgesia prevented a decrease in gastric intramucosal pH in patients undergoing major abdominal surgery. Also, in patients undergoing aortic surgery, Seeger et al.[15] reported lower blood pH levels after declamping in patients with general anesthesia compared with patients with epidural plus general anesthesia.
In our study, we attempted to investigate the effect of lumbar perioperative continuous epidural analgesia on intestinal perfusion. We chose to insert an epidural catheter in lumbar region because there is less possibility for complications. We administrated an appropriate initial dose epidurally, in order to produce and maintain a blockade up to T4 level. Postoperative doses were sufficient enough to maintain the analgesic effect. We examined the impact of continuous epidural infusion, since no previous study has demonstrated similar results in humans under combined general–epidural anesthesia with continuous perioperative epidural analgesia. Piper et al.[18] concluded that perioperatively administration of bupivacaine epidurally has no beneficial effects on hemodynamics or intestinal pH, but in this particular study local anesthetic was administered at bolus doses in constant time intervals.
In order to monitor intraoperatively and postoperatively the status of intestinal perfusion, we implemented gastrointestinal tonometry. Although various techniques have been proposed for this reason, tonometric monitoring of regional CO2 and subsequent calculation of intramucosal pH appear to be the most accurate and reliable predictors of intestinal ischemia and other complications after abdominal aortic surgery, with a reported sensitivity of 100% and specificity of 92% for major morbidity.[7,8] Furthermore, the regional–systemic difference of CO2 levels stands for a sensitive marker in identifying regional splachnic hypoperfusion, as well as an independent predictor of postoperative organ failure.[25,26]
CONCLUSIONS
These data indicate that the severe consequences of aortic cross-clamping during abdominal aortic surgical reconstruction can be mitigated by the use of epidural anesthesia. Our study suggests that epidural anesthesia addresses effectively the distal colonic reduced perfusion by positively affecting indirect indices of intestinal mucosal blood flow and oxygenation. It could serve as a valuable method for improving intestinal blood perfusion, as long as anesthetic manipulation handles hypotension effectively, which consists one of its major adverse effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Nakatsuka M. Assessment of gut mucosal perfusion and colonic tissue blood flow during abdominal aortic surgery with gastric tonometry and laser Doppler flowmetry. Vasc Endovasc Surg. 2002;36:193–8. doi: 10.1177/153857440203600306. [DOI] [PubMed] [Google Scholar]
- 2.Shoemaker WC, Appel PL, Kramm HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–86. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 3.Muehling BM, Ortlieb L, Oberhuber A, Orend KH. Fast track management reduces the systemic inflammatory response and organ failure following elective infrarenal aortic aneurysm repair. Interact Cardiovasc Thorac Surg. 2011;12:784–8. doi: 10.1510/icvts.2010.262337. [DOI] [PubMed] [Google Scholar]
- 4.Redaelli C, Schilling M, Carrel T. Intraoperative assessment of intestinal viability by laser flowmetry for surgery of ruptured abdominal aortic aneurysms. World J Surg. 1998;22:283–9. doi: 10.1007/s002689900383. [DOI] [PubMed] [Google Scholar]
- 5.Soong CV, Halliday MI, Hood JH, McCaigue MD, Hood JM, Rowlands BJ, et al. The relationship between bowel ischemia and organ impairment in elective abdominal aortic surgery repair. Br J Surg. 1992;80:517–32. [Google Scholar]
- 6.Mythen MG, Webb AR. Intraoperative gut mucosal hypoperfusion is associated with increased postoperative complications and cost. Intensive Care Med. 1994;20:99–104. doi: 10.1007/BF01707662. [DOI] [PubMed] [Google Scholar]
- 7.Fiddian-Green RG, Gantz NM. Transient episodes of sigmoid ischemia and their relation to infection from intestinal organisms after abdominal aortic operations. Crit Care Med. 1987;15:335–9. doi: 10.1097/00003246-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fiddian-Green RG. Should measurements of tissue pH and PO2 be included in the routine monitoring of intensive care unit patients? Crit Care Med. 1991;19:141–3. doi: 10.1097/00003246-199102000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Schwarte LA, Picker O, Höhne C, Fournell A, Scheeren T. Effects of thoracic epidural anaesthesia on microvascular gastric mucosal oxygenation in physiological and compromised circulatory conditions in dogs. Br J Anaesth. 2004;93:552–9. doi: 10.1093/bja/aeh235. [DOI] [PubMed] [Google Scholar]
- 10.Gould TH, Grace K, Thorne G, Thomas M. Effect of epidural anaesthesia on colonic blood flow. Br J Anaesth. 2002;89:446–51. [PubMed] [Google Scholar]
- 11.Schlichtig R, Mehta N, Gayowski TJ. Tissue-arterial PCO2 difference is a better marker of ischemia than intramural pH (pHi) or arterial pH-Phi difference. J Crit Care. 1996;11:51–6. doi: 10.1016/s0883-9441(96)90020-9. [DOI] [PubMed] [Google Scholar]
- 12.Väisänen O, Parviainen I, Ruokonen E, Hippeläinen M, Berg E, Hendolin H, et al. Epidural analgesia with bupivacaine does not improve splanchnic tissue perfusion after aortic reconstruction surgery. Br J Anaesth. 1998;81:893–8. doi: 10.1093/bja/81.6.893. [DOI] [PubMed] [Google Scholar]
- 13.Davidson D, Stalcup SA. Systemic circulatory adjustments to acute hypoxia and reoxygenation in unanesthetized sheep. J Clin Invest. 1984;73:317–20. doi: 10.1172/JCI111216. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bjorck M, Hedberg B. Early detection of major complications after abdominal aortic surgery: Predictive value of sigmoid colon and gastric intramucosal pH monitoring. Br J Surg. 1994;81:25–30. doi: 10.1002/bjs.1800810108. [DOI] [PubMed] [Google Scholar]
- 15.Seeger JM, Coe DA, Kaelin LD. Routine reimplantation of patent inferior mesenteric arteries limits colon infarction after aortic reconstructions. J Vasc Surg. 1992;15:635–41. [PubMed] [Google Scholar]
- 16.Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74:549–63. [PubMed] [Google Scholar]
- 17.Tollefson DJ, Ernst CB. Colon ischemia following aortic aneurysm reconstruction. Ann Vasc Surg. 1991;5:485–9. doi: 10.1007/BF02133058. [DOI] [PubMed] [Google Scholar]
- 18.Piper SN, Boldt J, Schmidt CC, Maleck WH, Brosch C, Kumle B. Hemodynamics, intramucosal pH and regulators of circulation during perioperative epidural analgesia. Can J Anaesth. 2000;47:631–7. doi: 10.1007/BF03018995. [DOI] [PubMed] [Google Scholar]
- 19.Sielenkämper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000;93:844–51. doi: 10.1097/00000542-200009000-00036. [DOI] [PubMed] [Google Scholar]
- 20.Bohlen HG. Intestinal tissue PO2 and microvascular responses during glucose exposure. Am J Physiol. 1980;238:H164–71. doi: 10.1152/ajpheart.1980.238.2.H164. [DOI] [PubMed] [Google Scholar]
- 21.Rem J, Brandt MR, Kehlet H. Prevention of postoperative lymphopenia and granulocytosis by epidural analgesia. Lancet. 1980;1:283–4. doi: 10.1016/s0140-6736(80)90780-1. [DOI] [PubMed] [Google Scholar]
- 22.Ai K, Kotake Y, Satoh T, Serita R, Takeda J, Morisaki H. Epidural anesthesia retards intestinal acidosis and reduces portal vein endotoxin concentrations during progressive hypoxia in rabbits. Anesthesiology. 2001;94:263–9. doi: 10.1097/00000542-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Kapral S, Gollmann G, Lehofer F. Gastric tonometry as a visceral perfusion monitoring during thoracic epidural anaesthesia. Acta Anaesthesiol Scand. 1996;109:178–80. [PubMed] [Google Scholar]
- 24.Sutcliffe NP, Mostafa SM, Gannon J, Harper SJ. The effect of epidural blockade on gastric intramucosal pH in the peri-operative period. Anaesthesia. 1996;51:37–40. doi: 10.1111/j.1365-2044.1996.tb07651.x. [DOI] [PubMed] [Google Scholar]
- 25.Schlichtig R, Bowles SA. Distinguishing between aerobic and anaerobic appearance of dissolved CO2 in intestine during low flow. J Appl Physiol. 1994;76:2443–51. doi: 10.1152/jappl.1994.76.6.2443. [DOI] [PubMed] [Google Scholar]
- 26.Donati A, Cornacchini O, Loggi S, Caporelli S, Conti G, Falcetta S, et al. A comparison among portal lactate, intramucosal sigmoid pH, and deltaCO2 (PaCO2 - regional PCO2) as indices of complications in patients undergoing abdominal aortic aneurysm surgery. Anesth Analg. 2004;99:1024–31. doi: 10.1213/01.ANE.0000132543.65095.2C. [DOI] [PubMed] [Google Scholar]


