Background: IL-6 signaling plays a role in immune and skeletal systems.
Results: sIL-6R mediated PTH-dependent hematopoietic cell expansions and blocking sIL-6R reduced PTH anabolic actions in mice.
Conclusion: sIL-6R is a mediator of PTH hematopoietic actions in marrow and anabolic actions in bone.
Significance: Novel orphan sIL-6R functions support PTH actions in bone and bone marrow.
Keywords: Bone, Bone Marrow, Interleukin, Myeloid Cell, Transforming Growth Factor Beta (TGFbeta), IL-6, Parathyroid Hormone, Soluble IL-6 Receptor
Abstract
Both PTH and IL-6 signaling play pivotal roles in hematopoiesis and skeletal biology, but their interdependence is unclear. The purpose of this study was to evaluate the effect of IL-6 and soluble IL-6 receptor (sIL-6R) on hematopoietic and skeletal actions of PTH. In the bone microenvironment, PTH stimulated sIL-6R protein levels in primary osteoblast cultures in vitro and bone marrow in vivo in both IL-6+/+ and IL-6−/− mice. PTH-mediated hematopoietic cell expansion was attenuated in IL-6−/− compared with IL-6+/+ bone marrow, whereas sIL-6R treatment amplified PTH actions in IL-6−/− earlier than IL-6+/+ marrow cultures. Blocking sIL-6R signaling with sgp130 (soluble glycoprotein 130 receptor) inhibited PTH-dependent hematopoietic cell expansion in IL-6−/− marrow. In the skeletal system, although intermittent PTH administration to IL-6+/+ and IL-6−/− mice resulted in similar anabolic actions, blocking sIL-6R significantly attenuated PTH anabolic actions. sIL-6R showed no direct effects on osteoblast proliferation or differentiation in vitro; however, it up-regulated myeloid cell expansion and production of the mesenchymal stem cell recruiting agent, TGF-β1 in the bone marrow microenvironment. Collectively, sIL-6R demonstrated orphan function and mediated PTH anabolic actions in bone in association with support of myeloid lineage cells in the hematopoietic system.
Introduction
Parathyroid hormone (PTH)2 is a well known modulator of calcium homeostasis with anabolic and catabolic actions in bone (1, 2). Based on its prominent anabolic actions, intermittent administration of PTH 1–34 is an effective therapeutic option for established osteoporosis and is emerging as a potential therapy for inflammatory mediated bone disease (3, 4). A growing body of evidence suggests an interaction between the skeletal and hematopoietic systems in the bone marrow microenvironment (9, 10). PTH positively supports the hematopoietic system by stimulating osteoblastic secretion of hematopoietic factors, such as IL-6 and MCP-1(CCL2) (5–8). Because PTH has notable actions in these systems, several investigators have suggested that PTH plays an essential role in the cross-talk between the skeletal and hematopoietic systems (5, 7, 11).
IL-6 is a well established downstream mediator of PTH signaling (12–14). PTH stimulates mRNA expression, protein synthesis, and secretion of IL-6 in vitro and in vivo (12–14). Similar to PTH, IL-6 signaling also plays dual actions in both the hematopoietic and skeletal system. IL-6 stimulates proliferation of early hematopoietic progenitor cells (15) and mediates PTH-dependent hematopoietic cell expansion (6). In the skeletal system, it has been reported that IL-6 stimulates osteoclast differentiation in vitro (16) and mediates PTH induced bone resorption in vivo (17). Relative to bone formation, IL-6 has been found to support osteoblast generation through the gp130-STAT1/3 pathway (18). As of yet, details linking PTH, IL-6, and their actions on bone and hematopoietic cells are ill defined.
In classic signaling, IL-6 acts through a receptor complex, composed of the membrane-bound IL-6 receptor (IL-6R) and gp130 (19). Alternatively, IL-6 also binds to its soluble receptor, sIL-6R. The IL-6·sIL-6R complex signals through the gp130 homodimer without membrane-associated IL-6R and is known as “IL-6 transsignaling” (20). Both IL-6 classic signaling and transsignaling similarly lead to the activation of STAT1, STAT3, and the MAPK pathway (21, 22). Recently, sIL-6R has been reported to play a central role in several inflammatory and autoimmune diseases, by regulating systemic or local actions of IL-6 signaling (23, 24). However, the role of sIL-6R in the bone marrow microenvironment and in the context of PTH actions remains elusive. The purpose of this study was to investigate the impact of sIL-6R in PTH-mediated hematopoietic cell expansion and to delineate the relationship between hematopoietic and skeletal actions of PTH through sIL-6R signaling.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies for flow cytometric analyses included anti-mouse Gr-1, B220, CD19, CD3, F4/80, and STAT3(pY705) which, along with the FITC annexin V staining kit for flow cytometry, were obtained from BD Biosciences (San Jose, CA). Anti-mouse CD11b antibody was purchased by eBioscience (San Diego, CA). Serum TRAP5b and P1NP immunoassays were obtained from Immunodiagnostic Systems Ltd. (Fountain Hills, AZ). The mouse sIL-6R DuoSet ELISA development system, TGF-β1 Quantikine ELISA system, recombinant mouse sIL-6R, mouse IL-6, and human sgp130 were purchased from R & D Systems (Minneapolis, MN).
Animal Care and Experiments
All of the animal experiments were performed in compliance with the institutional ethical requirements and approved by the University of Michigan Committee for the Use and Care of Animals. C57B6 IL-6+/− mice (25) were kindly provided by Evan Keller (University of Michigan, Ann Arbor, MI), and breeding was established to generate desired genotypes.
To evaluate anabolic actions of PTH, IL-6−/− and IL-6+/+ mice at 3–24 days (young) or 16–22 weeks (adult) of age were used. The mice received daily subcutaneous injections of vehicle (saline) or 50 μg/kg human PTH (hPTH 1–34; Bachem, Torrance, CA) for 3 (young) or 6 (adult) weeks. For fluorochrome labeling, calcein (20 mg/kg) (Sigma-Aldrich) dissolved in buffer (0.15 m NaCl, 2% NaHCO3) was injected intraperitoneally to adult mice at 5 and 2 days prior to sacrifice. For treatment with sgp130, the mice received daily subcutaneous injections of vehicle (PBS) or sgp130 (0.3 μg/mice) 1 h prior to vehicle (saline) or hPTH (50 μg/kg) injection. This regimen was followed for 2 weeks. All of the mice were sacrificed at least 24 h after the last injection in all of the above experiments.
Measurement of sIL-6R in Cell Cultures, Blood, and Bone Marrow Serums
For measurement of osteoblast production of sIL-6R, primary calvarial cells from IL-6−/− or IL-6+/+ mice were plated (3 × 106/well) in 12-well plates overnight following PTH (10 nm) treatment in α-MEM including 1% FBS. Culture media were harvested and used for sIL-6R measurement at 12 and 24 h after PTH treatment, and untreated medium at 0 h was used for control. For in vivo measurement of sIL-6R, young (3 weeks) and adult (16 weeks) IL-6+/+ and IL-6−/− mice received intermittent PTH or vehicle (saline) injection for 2 weeks and were sacrificed 5–6 h after the last injection. At sacrifice, whole blood was obtained by intracardiac blood draw, and serum was separated and kept frozen until biochemical assays were performed. Total bone marrow was centrifuged from hindlimb bones into PBS containing a proteinase inhibitor mixture (Sigma-Aldrich), and supernatant was transferred and frozen until assay. For measuring bone marrow sIL-6R levels, the cells were seeded at 6 × 106/well in 24-well plates and cultured for 8 days, and whole cells and conditioned media were harvested at days 1 and 8. In parallel experiments, the cells were seeded at 4 × 106/well in 24-well plates and treated once with or without fms-like tyrosine kinase-3 ligand (Flt-3L) (0.1 μg/ml) and/or PTH (10 nm), and whole cells and conditioned media were harvested at days 1, 6, and 8. Supernatants were used for measurement of sIL-6R. The remaining cells were lysed in buffer (CelLytic MT; Sigma-Aldrich), and the protein concentrations were measured by Lowry method using the DC protein assay kit (Bio-Rad). Levels of sIL-6R were adjusted by total protein concentrations of cell lysates. The sIL-6R levels were measured using ELISA according to the manufacturer's protocols.
Ex Vivo Cell Amplification Study and Flow Cytometric Analyses
The ex vivo amplification protocol is based on Servet-Delprat's model system and utilized to examine PTH actions as we have described previously (26). In brief, total bone marrow cells from IL-6−/− or IL-6+/+ mice (4–6 weeks old) were seeded at 2.5 × 105/cm2 in IMDM (RPMI or α-MEM was also used in the supplementary data set) supplemented with 20% fetal bovine serum, 100 units/ml penicillin, 50 μg/ml streptomycin, and 1% glutamine. At the time of plating, the cells were treated once with Flt-3L (0.1 μg/ml) and/or PTH (10 nm). For sIL-6R treatment, all groups received vehicle or sIL-6R (0.5 μg/ml) alone or in conjunction with designated treatments. For sgp130 treatment, the cells were pretreated with vehicle or sgp130 (1 μg/ml) for 1 h and then treated once with Flt-3L (0.1 μg/ml) and/or PTH (10 nm). The cells were enumerated using a hemocytometer on day 6 or 8, and cell viability was determined by trypan blue dye exclusion. Flow cytometric analyses (FACS) were performed for cell characterization at day 6 or 8. Nonadherent cells (1 × 106/sample) were pelleted, rinsed with PBS, resuspended in cold FACS buffer, and incubated for 30 min at 4 °C with appropriate antibodies while protected from light exposure. To analyze cell apoptosis, the FITC annexin V apoptosis assay system was used according to the manufacturer's protocol.
Phospho-flow Cytometric Analyses for Bone Marrow pSTAT3
For ex vivo studies, whole bone marrows from 4-week-old IL-6+/+ and IL-6−/− mice were treated once with vehicle (VEH), PTH (10 nm), or PTH with 1-h pretreatment of sgp130 and treated once with VEH, IL-6 (20 ng/ml), or sIL-6R (0.5 μg/ml) immediately after isolation. For in vivo studies, 3-week-old IL-6−/− mice received single injections of vehicle (PBS) or sgp130 (0.3 μg/mice) 1 h prior to PTH injection (50 μg/kg) and were sacrificed 2 h after PTH injection. Total bone marrow cells were flushed with fixation buffer (0.37% formaldehyde). To analyze STAT3 phosphorylation, the cells were treated with Lyse/Fix buffer (BD Biosciences) for 15 min at 37 °C followed by permeabilization with Perm buffer IV (BD Biosciences) for 20 min at room temperature. After washing with FACS buffer, the cells were stained with mouse anti-STAT3(pY705) antibody for 60 min at room temperature, and the frequency of pSTAT3+ cells was analyzed using the FACSCalibur system and Cell Quest Pro software (BD Biosciences). To determine the pSTAT3 status in CD11b+GR-1+ cells, bone marrow cells were flushed with fixation solution, washed twice in cold FACS buffer (PBS with 2% FBS and 2 mm EDTA), and stained with cell surface markers, mouse CD11b, and Gr-1 antibodies for 30 min at 37 °C followed by cell permeabilization and staining with mouse anti-STAT3(pY705) antibody. The cells were analyzed as described above.
Histologic Assays
Tibiae, femurs, or vertebrae were fixed in 10% phosphate-buffered formalin for 24 (young mice) or 48 h (adult mice) at 4 °C and decalcified in 10% EDTA for 10 (young mice) or 21 days (adult mice) at room temperature. Paraffin-embedded tibiae, femurs, and vertebrae were cut (5 μm) and stained with hematoxylin and eosin, and bone areas were measured using a computer-assisted histomorphometric analyzing system (Osteomeasure; OsteoMetrics Inc. Atlanta, GA). For tibiae and femurs, the region of interest began 200 μm proximal to the growth plate and 50 μm from the endocortical surface, including a 1200-μm (width) × 800-μm (length) area.
For dynamic histomorphometric analysis, tibiae from adult mice were fixed, dehydrated in xylene and graded ethanols without decalcification, and embedded in modified methylmethacrylate. Sections were cut (8 μm) with vertical bed microtomes (2065 and 2165; Leica/Jung, Bannockburn, IL) and affixed to gelatin-precoated (1%) slides. Calcein labels were visualized in unstained slides under Nikon Eclipse E800 light/epifluorescent microscopy to analyze bone formation rate, mineral apposition rate, and mineralizing surface.
MicroCT
Right femorae were scanned in water using cone beam microcomputed tomography (eXplore Locus SP; GE Healthcare Pre-Clinical Imaging, London, Canada). All of the images were reconstructed and calibrated at an 18-μm voxel size. A trabecular region of interest equal to 2 mm in length beginning at 200 μm proximal to the distal growth plate was defined with a spline algorithm encompassing the endocortical surface (MicroView v2.2 Advanced Bone Analysis Application; GE Healthcare Pre-Clinical Imaging).
Quantitative RT-PCR-For RT-PCR, TaqMan universal PCR Master Mix was used with the TGF-β1 (Mm03024053_m1), alkaline phosphatase (Mm00475831_m1), and osteocalcin (Mm03413826_mH) probe/primer sets from Applied Biosystems. Rodent GAPDH (VIC reporter dye) was used as an endogenous control.
Cell Proliferation and Differentiation Assays
For cell proliferation assays, calvarial cells were plated (50,000/well) in 24-well plates overnight followed by synchronization by serum starvation for 12 h before sIL-6R (0.1 or 0.5 μg/ml) or vehicle treatment in 2% serum. The media with sIL-6R or vehicle were changed every other day. The numbers of viable cells were determined at the indicated times (days 1, 3, 4, and 5) by trypan blue exclusion. For the osteogenic differentiation study, calvarial cells (700,000/well) were plated in 12-well plates, and at confluence, mineralization was initiated with osteogenic medium (α-MEM including 10% FBS, 50 μg/ml l-ascorbic acid, and 10 mm β-glycerophosphate) with or without PTH (10 nm) and/or sIL-6R (0.5 μg/ml). Osteogenic medium (± PTH ± sIL-6R) were refreshed every 2 days for 12 (for BMSC) or 14 days (calvarial cells), and Von Kossa staining was performed as described previously (27).
Statistical Analyses
Statistical analysis was performed by two-way analysis of variance or t test using the GraphPad Instat statistical program with significance at p < 0.05. For determination of PTH effects in each group, the values were expressed as treatment over control prior to statistical analysis. The data are presented as the means ± S.E.
RESULTS
Effect of PTH on sIL-6R Production in Bone
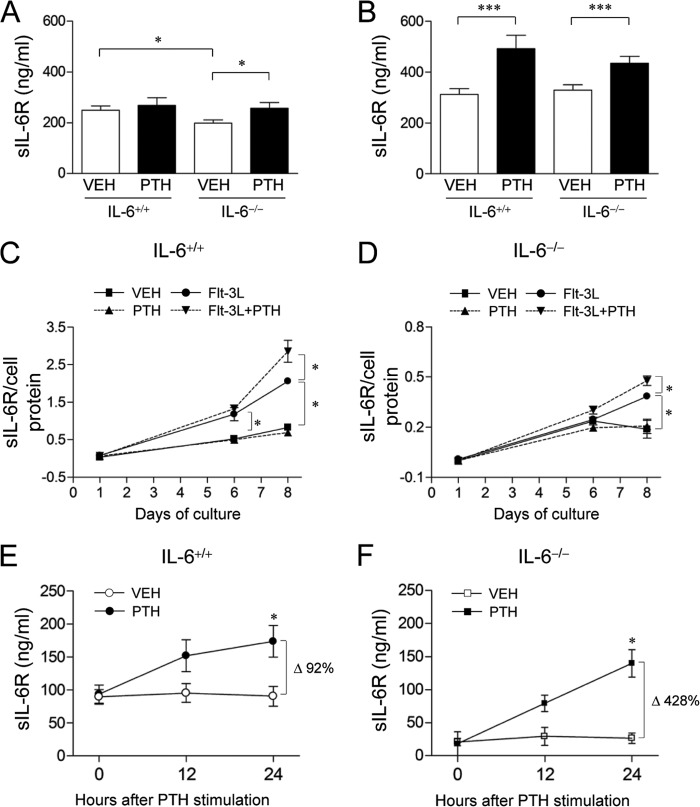
To evaluate the effect of PTH on sIL-6R production in bone marrow, sIL-6R protein levels were evaluated in young (3 weeks) and adult (16 weeks) IL-6−/− and IL-6+/+ mice after intermittent PTH administration for 2 weeks. Bone marrow sIL-6R levels were significantly lower in young IL-6−/− versus IL-6+/+ mice, and PTH administration elevated sIL-6R in IL-6−/− mice significantly to wild type levels (Fig. 1A). In adult mice, both genotypes showed similar bone marrow sIL-6R levels, as well as similar PTH increases (Fig. 1B). In contrast, serum sIL-6R levels showed no difference between young IL-6−/− and IL-6+/+ mice and were not altered in response to PTH (data not shown).
FIGURE 1.
Effect of PTH on sIL-6R production in bone. A and B, young (A, 3 weeks) and adult (B, 16 weeks) male and female IL-6+/+ and IL-6−/− mice were treated with PTH (50 μg/kg) or VEH for 2 weeks. sIL-6R levels were measured in bone marrow (n = 9–11/group). C and D, whole bone marrows from IL-6+/+ (C) or IL-6−/− (D) mice (4–6 weeks old, female) were seeded at 4 × 106 cells/well in 24-well plates and treated once with VEH or PTH and/or Flt-3L. sIL-6R protein levels were measured from culture medium at days 1, 6, and 8. The graphs show the ratio of sIL-6R/total cell protein. Note the different y axis scales (n = 4/group). E and F, primary neonatal calvarial cells from IL-6+/+ (E) or IL-6−/− (F) mice were plated overnight in 12-well dishes at 3 × 106/well followed by PTH (10 nm) stimulation in 1% serum. Culture medium was harvested 0, 12, or 24 h after PTH stimulation. Endogenous sIL-6R levels were measured by ELISA (n = 4/group). All of the numerical data are the means ± S.E. of two independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To investigate the endogenous sIL-6R levels in an ex vivo bone marrow culture system, sIL-6R levels were measured from plated bone marrow over 8 days with or without a single PTH and/or Flt-3L administration. At day 1, sIL-6R levels in untreated IL-6−/− cells were lower than those in IL-6+/+ cells (0.01 versus 0.02 pg/ml/106 cells, p < 0.01). PTH co-treatment with Flt-3L, not PTH alone, significantly increased sIL-6R production in both IL-6+/+ (Fig. 1C) and IL-6−/− (Fig. 1D) cells, whereas the extent was relatively less in IL-6−/− compared with IL-6+/+ cells (24.2% versus 38.7% increase, p < 0.05, note different y axis scale in IL-6+/+ versus IL-6−/−).
Because stromal cells and osteoblasts have PTH receptors in the bone marrow microenvironment (28), sIL-6R levels were measured in medium from primary calvarial cell cultures treated with PTH. Although sIL-6R levels were lower in IL-6−/− than IL-6+/+ calvarial cell supernatants under basal conditions, sIL-6R production was stimulated by PTH in cells from both genotypes with a greater fold increase in IL-6−/− cells at 24 h (Fig. 1, E and F). Collectively, PTH stimulated sIL-6R production not only in IL-6+/+ mice but also in IL-6−/− mice, suggesting that sIL-6R alone, without IL-6 (i.e. sIL-6R signaling), has orphan biological functions mediating PTH signaling.
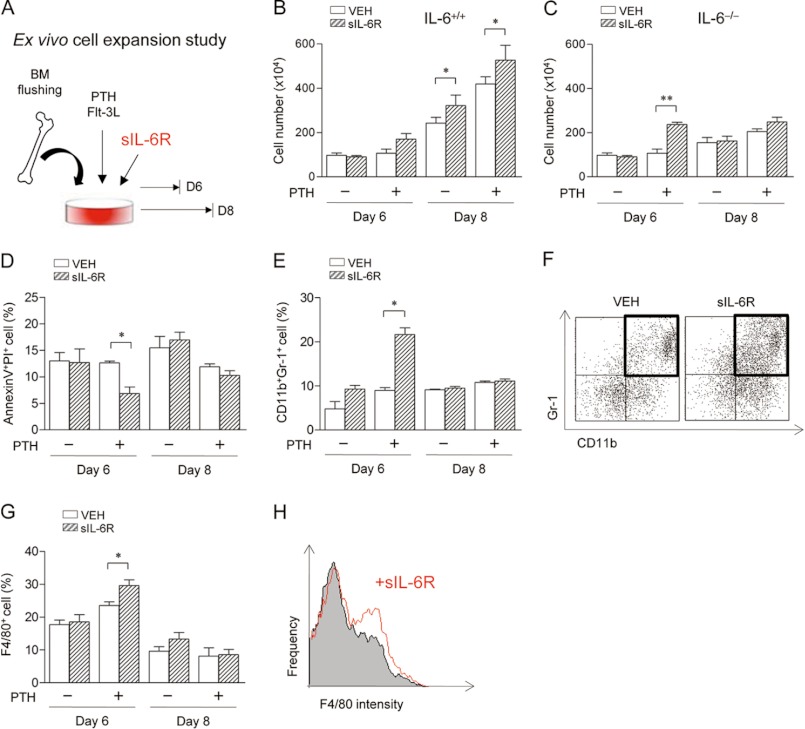
Effect of sIL-6R on ex Vivo Hematopoietic Cell Expansion
To verify sIL-6R orphan function, the ex vivo cell expansion study was performed with sIL-6R treatment. As described previously (6, 26), whole marrows were isolated and expanded ex vivo with Flt-3L, a stem cell factor that supports hematopoietic stem cell expansion especially CD45+ and CD11b+ cells. Exogenous sIL-6R was introduced in this system with or without PTH (Fig. 2A). Consistent with previous findings (6), PTH amplified Flt-3L-dependent cell expansion in IL-6+/+ cells at day 8. sIL-6R significantly increased cell expansion regardless of PTH treatment in IL-6+/+ cells at day 8 (Fig. 2B). In contrast, PTH had no effect on Flt-3L-dependent cell expansion in IL-6−/− cells (Fig. 2C). However, sIL-6R combined with PTH significantly increased cell expansion in IL-6−/− cells at an earlier time point (day 6) than in IL-6+/+ cells (Fig. 2, B versus C). Hence, cell characterizations were focused on day 6, which showed maximal effects of PTH on IL-6−/− cells.
FIGURE 2.
Effect of sIL-6R on ex vivo hematopoietic cell expansion. A, experimental design of ex vivo cell expansion study with sIL-6R treatment. Whole bone marrows from IL-6−/− or IL-6+/+ mice (6 weeks old, female) were seeded at 1.2 × 105 cells/cm2 with Flt-3L-containing medium and treated once with or without PTH. Additionally, VEH or sIL-6R (0.5 μg/ml) was co-treated in each group. Nonadherent cells were enumerated using trypan blue exclusion, and flow cytometric analysis was performed at days 6 and 8. B and C, numbers of total nonadherent cells from IL-6+/+ (B) and IL-6−/− (C) marrows. D, the percentages of annexin V+PI+ cells in IL-6−/− marrows. E, the percentages of annexin V−CD11b+Gr-1+ cells from IL-6−/− marrows. F, representative dot plots of annexin V−CD11b+Gr-1+ cells from PTH-treated IL-6−/− marrows at day 6. G, the percentages of annexin V−F4/80+ cells from IL-6−/− marrows. H, representative histograms of annexin V−F4/80+ cells from PTH-treated IL-6−/− marrows at day 6. All of the numerical data are the means ± S.E. of two independent experiments performed in triplicate. *, p < 0.05; **, p < 0.01.
Our previous study reported that the PTH-mediated hematopoietic cell expansion was due to reduced cell apoptosis and not to increased cell proliferation (6). Further analyses of sIL-6R showed significantly decreased apoptosis in PTH-treated cells at day 6 in IL-6−/− cells (Fig. 2D), suggesting that the effect of sIL-6R on cell expansion was at least in part due to PTH anti-apoptotic actions.
To further characterize the PTH-dependent cell expansions at day 6 in IL-6−/− cells, several cell subsets were analyzed via flow cytometry with exclusion of apoptotic (annexin V+) cells. sIL-6R significantly increased myeloid progenitor cells (CD11b+Gr-1+) (Fig. 2, E and F) and macrophages (F4/80+) (Fig. 2, G and H) under PTH-treated conditions at day 6. Other lymphoid cells analyzed including CD3 (T cell), CD19 (B cell), and B220 (B cell) showed no significant changes with sIL-6R treatment (data not shown).
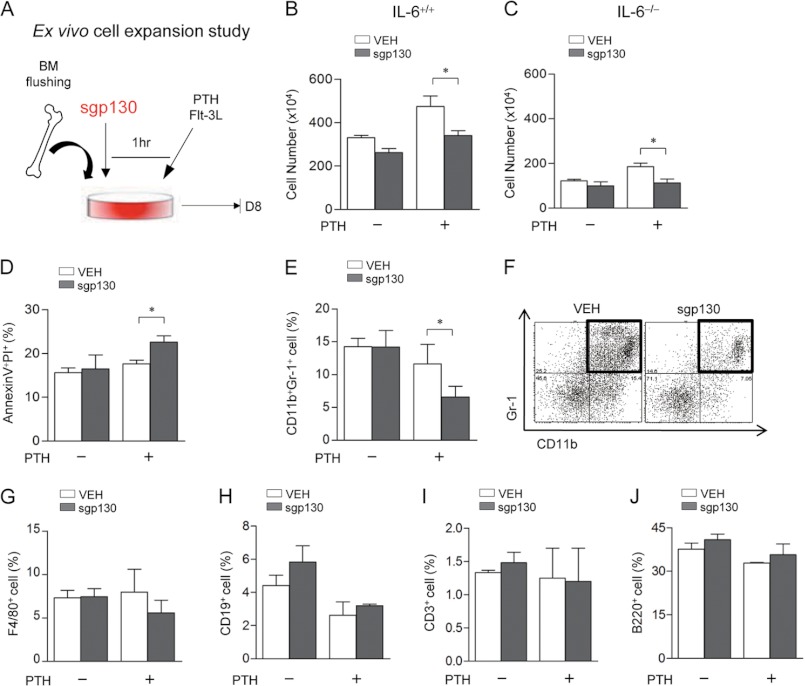
Effect of sgp130 on ex Vivo Hematopoietic Cell Expansion
To further analyze the effect of sIL-6R signaling on hematopoietic cell expansion, soluble gp130 receptor (sgp130), a specific inhibitor of sIL-6R, was introduced in the ex vivo cell expansion system (Fig. 3A). sgp130 significantly reduced the PTH-dependent increases in total cell numbers in both IL-6+/+ (Fig. 3B) and IL-6−/− (Fig. 3C) marrow cells, suggesting that sgp130 inhibited not only IL-6·sIL-6R transsignaling, actions from IL-6 and sIL-6R complex in IL-6+/+ cells, but also orphan sIL-6R signaling, actions from sIL-6R alone in IL-6−/− cells. Moreover, sgp130 increased apoptosis in PTH-treated IL-6−/− cells (Fig. 3D). Flow cytometric analyses of cell subtypes showed that sgp130 significantly decreased myeloid progenitor cells (Fig. 3, E and F), whereas macrophages (Fig. 3G) or lymphoid cells (Fig. 3, H–J) were not affected in IL-6−/− bone marrow populations. Collectively, sgp130 inhibited hematopoietic myeloid cell expansion by supporting anti-apoptotic actions of PTH on IL-6−/− cells.
FIGURE 3.
Effect of sgp130 on ex vivo hematopoietic cell expansion. A, experimental design of ex vivo cell expansion study with sgp130 treatment. Flushed whole marrows from IL-6−/− or IL-6+/+ mice (6 weeks old, female) were pretreated with VEH or sgp130 (1 μg/ml) for 1 h and seeded at 1.2 × 105 cells/cm2 with Flt-3L-containing medium. At the time of plating, each group was treated once with or without PTH. At day 8, nonadherent cells were harvested, enumerated using trypan blue exclusion, and used for flow cytometric analysis. B and C, numbers of total nonadherent cells from IL-6+/+ (B) and IL-6−/− (C) mice. D, the percentages of annexin V+PI+ cells in IL-6−/− marrows. E, the percentages of annexin V−CD11b+Gr-1+ cells from IL-6−/− marrows. F, representative dot plots of annexin V−CD11b+Gr-1+ cells from PTH-treated IL-6−/− marrows. G–J, plots of the percentages of annexin V−F4/80+ (G), annexin V−CD19+ (H), annexin V−CD3+ (I), and annexin V−B220+ (J) cells are shown from IL-6−/− marrows. All of the numerical data are the means ± S.E. of two independent experiments performed in triplicate. *, p < 0.05.
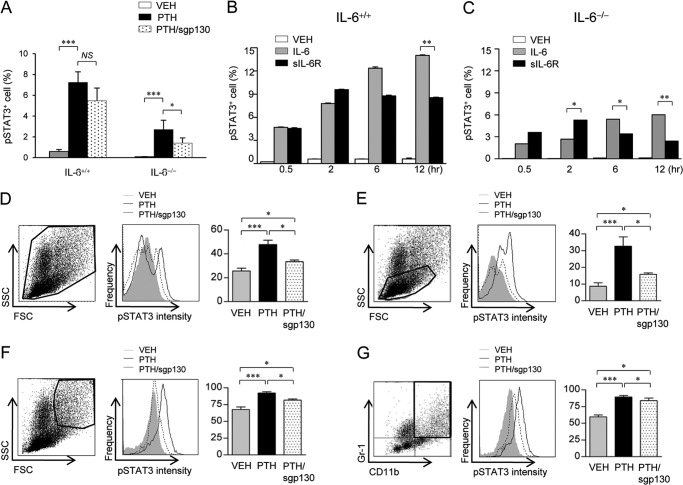
Effect of sIL-6R Signaling on STAT3 Phosphorylation in Hematopoietic Cells
To provide further mechanistic insight of sIL-6R signaling on PTH actions in hematopoietic cells, STAT3 phosphorylation, a major downstream transcriptional factor of IL-6 signaling, was evaluated. pSTAT3+ cells were measured by flow cytometric analysis in IL-6+/+ and IL-6−/− bone marrow 12 h after ex vivo PTH stimulation. The percentage of pSTAT3+ cells was significantly increased with PTH treatment in both IL-6+/+ and IL-6−/− marrow cells, and sgp130 inhibited PTH-mediated STAT3 phosphorylation only in IL-6−/− marrow cells (Fig. 4A and supplemental Fig. S1). Furthermore, both IL-6 and sIL-6R significantly up-regulated pSTAT3 in IL-6+/+ and IL-6−/− marrow cells from 0.5 to 12 h (Fig. 4, B and C, and supplemental Fig. S2). Interestingly, in IL-6−/− marrow cells, sIL-6R signaling resulted in earlier STAT3 phosphorylation than IL-6 (Fig. 4C and supplemental Fig. S2). At 2 h, a greater percentage of IL-6−/− marrow cells demonstrated STAT3 phosphorylation in marrow populations treated with sIL-6R as compared with those treated with IL-6. After 6 h, the earlier effects of sIL-6R signaling on STAT3 phosphorylation were decreased, whereas the effects of IL-6 were sustained at 12 h. Notably, the effect of sIL-6R on STAT3 phosphorylation in IL-6−/− marrows suggests that sIL-6R signaling has orphan biological function downstream of PTH signaling.
FIGURE 4.
Effect of sIL-6R signaling on STAT3 phosphorylation. A, whole bone marrow cells from 4-week-old female IL-6+/+ and IL-6−/− mice were treated once ex vivo with VEH, PTH (10 nm), or PTH with 1-h pretreatment of sgp130 (1 μg/ml) for 12 h, followed by flow cytometric analysis. The graph shows the percentages of pSTAT3+ cells. B and C, whole bone marrows from 4-week-old female IL-6+/+ and IL-6−/− mice were treated once ex vivo with VEH, IL-6 (20 ng/ml), or sIL-6R (0.5 μg/ml) for 0.5, 2, 6, and 12 h. The percentages of pSTAT3+ cells from IL-6+/+ (B) and IL-6−/− (C) mice are shown. D–G, young (3 weeks) female IL-6−/− mice were treated once with VEH or sgp130 (0.3 μg) in vivo 1 h prior to treatment with PTH (50 μg/kg) or VEH. Two hours later, whole bone marrows were isolated, and flow cytometric analysis of pSTAT3+ cells was performed. D–G, analyses of pSTAT3+ cells gated in whole marrows (D), lower side scattered cells (high end SSC threshold set at 440) representing lymphocytes (E), and higher forward and side scattered cells (F, low end FSC and SSC threshold set at 420 each) representing granulocytes and myeloid cells (G, CD11bhighGr-1high). Histograms show gray (VEH), black (PTH), and dotted (PTH+sgp130) bars. All numerical data are the means ± S.E. (n = 4/group). *, p < 0.05; **, p < 0.01; ***, p < 0.001. NS, not significant; SSC, side scattered cells; FSC, forward scattered cells.
Further, in vivo sgp130 treatment was performed with analyses of STAT3 phosphorylation. Bone marrow cells were harvested from IL-6−/− mice 2 h after a single treatment of PTH alone or PTH combined with sgp130. Flow cytometric analyses showed that PTH treatment induced a significant increase in the fraction of pSTAT3+ cells among whole bone marrow cells, and sgp130 treatment significantly attenuated the PTH-dependent increase in pSTAT3+ IL-6−/− cells (Fig. 4D). Similar findings were found when the analyses gated on small cells including lymphocytes (Fig. 4E), large cells including myeloid lineage cells (Fig. 4F), and CD11b+Gr-1+ cells, which are myeloid precursor cells (Fig. 4G). Collectively, sgp130 inhibited PTH-stimulated STAT3 phosphorylation in both lymphocytic and myeloid cell populations, suggesting that sIL-6R is critical for STAT3 activation in response to PTH.
Taken together, not only IL-6 but also IL-6R signaling alone mediated PTH actions in hematopoietic cell expansions through STAT3 phosphorylation. Notably, in IL-6 deficient conditions, actions of sIL-6R signaling on hematopoietic cells were rapid, suggesting possible compensation.
The Role of sIL-6R Signaling on PTH Skeletal Anabolic Actions
The second aim of this study was to delineate the relationship between hematopoietic and skeletal actions of PTH through IL-6 or sIL-6R signaling. To investigate the effect of IL-6 on PTH actions in bone, intermittent PTH was administered to male and female IL-6+/+ and IL-6−/− mice at two different ages, young (3–24 days) for 3 weeks and adult (16–22 weeks) for 6 weeks. Both static and dynamic histomorphometric analyses and microCT analysis demonstrated that PTH similarly increased bone mass and bone formation rates in IL-6+/+ and IL-6−/− mice (supplemental Fig. S3).
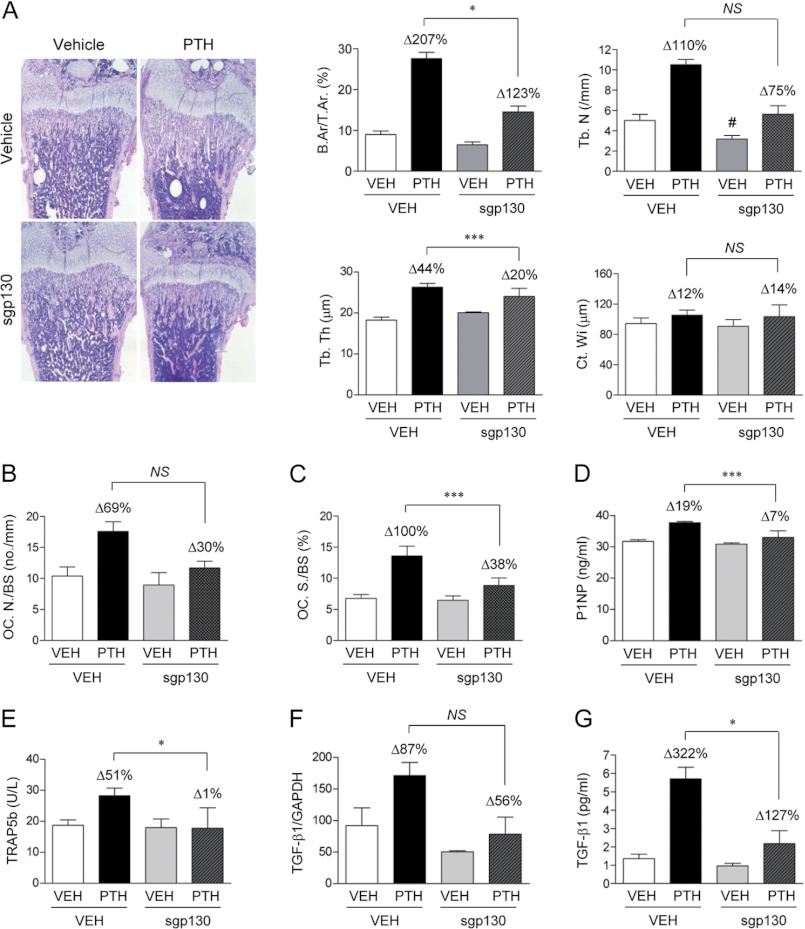
Because sIL-6R alone had biologic effects on hematopoietic cells in IL-6 deficient conditions, it is possible to deduce that sIL-6R signaling compensated for the absence of IL-6 in the skeletal system. To verify this hypothesis, young IL-6−/− mice were treated with vehicle, PTH, sgp130 alone, or combined with PTH for 2 weeks. Histomorphometric analyses showed that PTH significantly increased fractional bone area, trabecular number, and trabecular thickness in femurs (Fig. 5A) and vertebrae (supplemental Fig. S4). sIL-6R inhibition via sgp130 treatment attenuated these skeletal anabolic actions of PTH. When combined with PTH, sgp130 reduced PTH-dependent increase in fractional bone area by 26% (p = 0.016) and trabecular thickness by 18% (p < 0.001) in femurs (Fig. 5A). In vertebrae, sgp130 also showed a significant attenuation of the PTH-dependent increase in fractional bone area by 13% (p = 0.011) and trabecular number by 16% (p = 0.044) (supplemental Fig. S4). In addition, TRAP staining of bone sections showed that PTH increased osteoclast numbers (Fig. 5B, OC. N./BS) and surface (Fig. 5C, OC. S./BS), and sgp130 treatment significantly limited the PTH increase in osteoclast numbers. Consistent with histomorphometric data, comparisons of serum bone turnover markers also demonstrated that sgp130 reduced PTH-dependent increases in serum P1NP (Fig. 5D) and TRAP5b (Fig. 5E).
FIGURE 5.
The role of sIL-6R transsignaling on PTH skeletal actions. sgp130 (0.3 μg) or vehicle was administrated 1 h prior to PTH (50 μg/kg) or VEH to IL-6−/− mice (3 days old) daily for 2 weeks. A, representative hematoxylin and eosin images of femur (original magnification, ×100) and plots of fractional bone area (B.Ar/T.Ar), trabecular number (Tb.N), trabecular thickness (Tb.Th), and cortical width (Ct.Wi). B and C, histomorphometric analyses of TRAP-stained bone sections including osteoclast number (B, OC.N/BS), and osteoclast surface (C, OC.S/BS). D–F, serum levels of P1NP (D), TRAP 5b (E), and mRNA (F). G, protein levels of TGF-β1 in bone marrow (n = 10–13/group). All of the numerical data are the means ± S.E. *, p < 0.05; ***, p < 0.001 corresponding to comparisons between VEH (Δ) and sgp130 (Δ); #, p < 0.05 versus VEH (first bar). NS, not significant. A Student's t test or a two-way analysis of variance was used to calculate p values.
Because anabolic actions of PTH have recently been associated with increased TGF-β1 protein levels supporting mesenchymal stem cell recruitment (29, 30), TGF-β1 mRNA and active protein levels were measured. Mice treated with sgp130 had significantly reduced PTH-dependent increases in TGF-β1 mRNA (Fig. 5F) and active protein levels (Fig. 5G).
To discriminate direct versus indirect actions of sIL-6R in the skeletal system, the direct effect of sIL-6R on osteoblast proliferation and differentiation was investigated using primary calvarial cell cultures in vitro. Treatment with sIL-6R (0.1 or 0.5 μg/ml) did not alter the number of calvarial cells over time, suggesting that sIL-6R alone does not directly alter osteoblastic cell proliferation (supplemental Fig. S5A). Von Kossa staining and ostegenic marker gene studies such as alkaline phosphatase and osteocalcin showed that there was no difference in mineralized nodule formation in calvarial cells with and without sIL-6R treatment, whereas PTH (10 nm) inhibited mineralization in IL-6−/− calvarial cells (supplemental Fig. S5, B and C). Taken together, sIL-6R signaling, not IL-6, support PTH anabolic actions in bone without direct effects on IL-6−/− osteoblast/stromal cells.
The Role of sIL-6R Signaling on TGF-β1 Expressions in Myeloid Cells
Because sIL-6R signaling had no direct effects on osteoblast proliferation or differentiation, we then hypothesized that sIL-6R signaling supported anabolic actions of PTH in association with expansion of hematopoietic cells and TGF-β1 production. The direct effects of sIL-6R on TGF-β1 production in myeloid cells (significantly expanded by sIL-6R signaling in the ex vivo expansion study Fig. 2, E and F) were investigated.
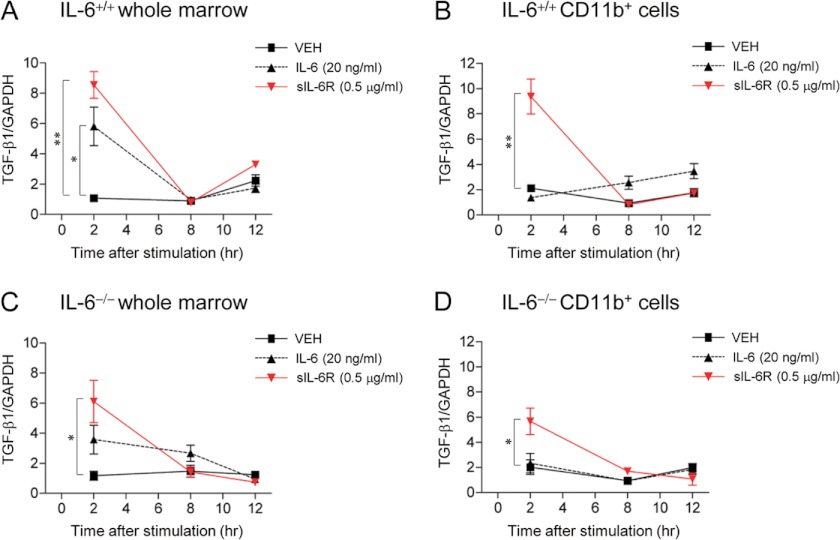
IL-6 (20 ng/ml) or sIL-6R (0.5 μg/ml) at the effective concentration reported for cell stimulation (31) was administered to whole marrow or myeloid (CD11b+) cells ex vivo and TGF-β1 gene expression was analyzed. At 2 h, sIL-6R significantly up-regulated TGF-β1 mRNA expression in whole marrow and myeloid cells in both IL-6+/+ (Fig. 6 A and B) and IL-6−/− (Fig. 6, C and D) cells, whereas IL-6 up-regulated TGF-β1 only in IL-6+/+ whole marrow cells (Fig. 6A). At 12 h, the effects of sIL-6R disappeared. Collectively, in the bone microenvironment sIL-6R rapidly stimulated myeloid TGF-β1 expression, whereas IL-6 stimulated TGF-β1 expression at a slower rate and to a lesser extent.
FIGURE 6.
Ex vivo TGF-β1 gene expression study. Whole bone marrows were harvested from 4-week-old female IL-6+/+ and IL-6−/− mice, and CD11b+ cells were sorted by magnetic-activated cell sorting. Whole marrows or CD11b+ cells were plated, and VEH, IL-6 (20 ng/ml), or sIL-6R (0.5 μg/ml) were administered for 2, 8, and 12 h ex vivo. TGF-β1 mRNA levels of were analyzed by real time PCR in IL-6+/+ whole marrow (A), IL-6+/+ myeloid (CD11b+) cells (B), IL-6−/− whole marrow (C), and IL-6−/− myeloid (D, CD11b+) cells (n = 4/group). All of the numerical data are the means ± S.E. *, p < 0.05; **, p < 0.01.
DISCUSSION
Recently, growing evidence suggests a regulatory relationship between bone and hematopoietic cells (9). Because PTH plays a dual function in both systems, it is reasonable to hypothesize that PTH is a bidirectional mediator of the skeletal and hematopoietic systems. Gp130, a common receptor of the IL-6 cytokine family, is an established regulator of bone modeling and remodeling (18, 32–34). In addition, IL-6 plays a role in hematopoietic cell expansion. Therefore, we hypothesized that IL-6 and sIL-6R are important mediators in both the skeletal and hematopoietic systems. To address this, the effect of IL-6 and sIL-6R on anabolic actions of PTH in bone was explored. Interestingly, sIL-6R signaling supported PTH anabolic actions in bone, whereas IL-6 did not.
The present study demonstrated that sIL-6R alone (orphan sIL-6R signaling) plays a role in PTH-mediated hematopoietic cell expansion. The effect of sIL-6R signaling on hematopoietic cell expansion was more rapid than classic IL-6 signaling, especially in myeloid cells. Furthermore, sIL-6R signaling, and not IL-6, supported anabolic actions of PTH in bone. sIL-6R signaling did not directly affect the osteoblast phenotype but instead up-regulated myeloid production of TGF-β1, a known mesenchymal stem cell inductive factor, suggesting that sIL-6R signaling mediated PTH anabolic actions in bone through its supportive actions on hematopoiesis, especially via cells in the myeloid linage.
This study provided new and direct evidence that PTH stimulated osteoblastic production of sIL-6R. Intriguingly, the lower basal level of sIL-6R in IL-6−/− osteoblasts was rescued by PTH stimulation, and this finding was consistently reproduced in PTH treatment of young IL-6−/− mice in vivo. A noteworthy finding was that the levels of sIL-6R in bone marrow (200–530 ng/ml) were much higher than in serum (70–200 ng/ml) and PTH up-regulated sIL-6R in marrow and not serum, substantiating an osteoblast role and suggesting the importance of local actions of sIL-6R in the bone microenvironment that may not be reflected in an endocrine manner in the serum. In comparison, the PTH-mediated amplification of endogenous sIL-6R levels, ex vivo, was restricted in IL-6−/− compared with IL-6+/+ cells. Because more than 90% of sIL-6R is produced from the cleavage of the membrane-bound sIL-6R through an ADAM (a disintegrin and metalloproteinase) family protein, different environmental conditions between in vivo and ex vivo systems could affect these protein cleavage processes and result in different PTH responses. Indeed, exogenous sIL-6R treatment effectively increased ex vivo hematopoietic cell expansion in IL-6−/− marrows.
Notably, this study found that sIL-6R itself, without IL-6, has orphan properties in the bone microenvironment. Production of sIL-6R in response to PTH in IL-6 deficient mice, showed that sIL-6R mediated downstream STAT3 phosphorylation independent of IL-6 and in response to PTH. Additionally, sIL-6R signaling mediated PTH-dependent hematopoietic cell expansion and anabolic actions in bone as revealed by its inhibition with sgp130. The sgp130 mediator quenches the IL-6·sIL-6R complex, resulting in interruption of its binding to gp130, without affecting IL-6 signaling through the classic IL-6 membrane-bound receptor (35). Future studies where PTH actions are evaluated when sgp130 is administered to adult mice and/or in osteoblast-targeted IL-6R deficient mice could provide further validation of the skeletal actions found here.
Several emerging lines of data support orphan properties of sIL-6R over an agonistic function to IL-6. Diamant et al. (36) first described an orphan effect of sIL-6R on proliferation of B9 cells, which was not neutralized by anti-IL-6 antibody, and Gabay et al. (37) reported a stimulatory effect of sIL-6R alone on the production of acute phase protein in hematoma cells or B9 cells. In HepG2 cells, which do not produce endogenous IL-6, sIL-6R alone up-regulated JunB mRNA expression via a gp130-dependent manner even in the presence of anti-IL-6 antibody (38). In addition, microarray analysis of HepG2 cells showed 11 genes regulated by sIL-6R alone and not by the IL-6·sIL-6R complex (39). To our knowledge, this is the first demonstration of orphan function of sIL-6R in the skeletal and hematopoietic system and the blocking effect of sgp130 on such sIL-6R orphan functions.
In this study, ex vivo cell expansions were performed in IMDM containing 4.5 g/liter glucose. Although high glucose-induced metabolic stress could affect hematopoiesis (40–42), cultures in other media containing lower glucose concentrations, such as RPMI (2 g/liter glucose) or α-MEM (1 g/liter glucose) showed similar cell expansions compared with IMDM (4.5 g/liter glucose) (supplemental Fig. S6), suggesting that the effects were independent of glucose concentration.
Because osteoblasts express low levels of the membrane IL-6R (16), the IL-6·sIL-6R complex and not IL-6 alone would have more potential to play a role in osteogenic differentiation (43, 44) and cell proliferation (43–45). However, in the present study, sIL-6R signaling had no direct effect on proliferation or differentiation in IL-6−/− osteoblasts, and sgp130 did not inhibit STAT3 phosphorylation of IL-6−/− osteoblasts. In contrast, sIL-6R signaling played a role in PTH-mediated hematopoietic cell expansion. Exogenous sIL-6R treatment resulted in hematopoietic cell expansion in a manner similar to PTH, and blocking endogenous sIL-6R with sgp130 markedly attenuated the PTH effect on hematopoietic cells in IL-6−/− cultures. That sgp130 inhibited hematopoietic cell expansion in vitro and skeletal actions of PTH in vivo, with no direct impact on osteoblasts, led us to conclude that PTH-mediated sIL-6R production and signaling in hematopoietic cells supports PTH anabolic actions in bone.
In the present study, the stimulatory effect of PTH on TGF-β1 in bone marrow was attenuated by blocking sIL-6R signaling with sgp130 in vivo. Furthermore, ex vivo treatment with sIL-6R, not IL-6, rapidly increased TGF-β1 gene expression in myeloid cells, indicating that up-regulation of TGF-β1 in the bone microenvironment is sIL-6R signaling-preferred. TGF-β1 is an osteotropic factor that is activated and secreted during osteoclastic bone resorption and recruits mesenchymal stem cells into remodeling sites (30, 46). Interestingly, treatment with alendronate, which induced osteoclast cell apoptosis, inhibited the release of TGF-β1 from the bone matrix, resulting in blunted anabolic actions of PTH (29). The data in the present study corroborated previous studies (29, 30, 46) and suggested a role for sIL-6R signaling in TGF-β1-related anabolic actions in bone. A previous report found that STAT3 positively regulated TGF-β1 production through binding to the TGF-β1 promoter region, suggesting that TGF-β1 is a direct downstream target of STAT3 (47), which supports the findings in the present study. Interestingly, the present study showed that sIL-6R signaling and IL-6 signaling have differing temporal actions on STAT3 phosphorylation with sIL-6R signaling, resulting in more rapid STAT3 phosphorylation. Collectively, we speculate that sIL-6R signaling up-regulated bone marrow TGF-β1 through its earlier action on STAT3 phosphorylation relative to the later action of IL-6.
IL-6 and sIL-6R are up-regulated in various human physiologic and pathologic conditions such as aging, cancer, fractures, and acute or chronic inflammatory diseases. Recently, beneficial effects of PTH on bone formation in fracture healing (48, 49) or osseous wound healing in periodontal disease (3) have been reported. Our findings provide evidence that potential therapeutic applications of PTH in such human conditions may be attributed to the integration of IL-6 signaling in the skeletal and hematopoietic compartments.
In summary (Fig. 7), sIL-6R, secreted in response to PTH stimulation of osteoblasts, plays an essential role in both PTH-mediated hematopoietic cell expansion and skeletal anabolism. In the skeletal system, sIL-6R mediates PTH anabolic actions through its supportive role in myeloid cell expansion and TGF-β1 production in the bone microenvironment in association with gp130/STAT3 signaling. In contrast, IL-6 contributes to PTH-mediated hematopoietic cell expansion but is dispensable for PTH anabolic actions in bone. This study provided evidence of the relationship between hematopoietic and skeletal systems through PTH and sIL-6R signaling.
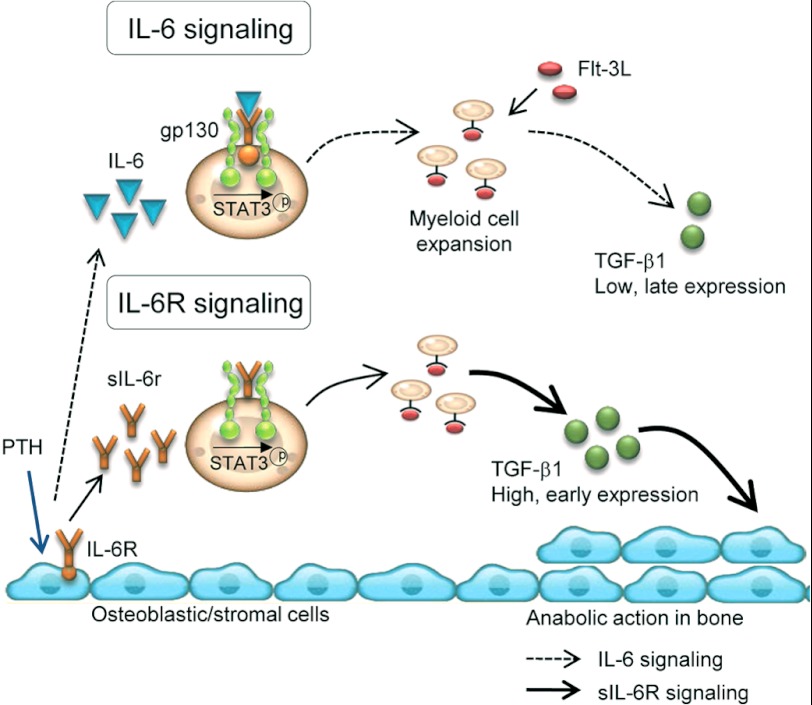
FIGURE 7.
Working model. sIL-6R signaling mediates PTH actions in both hematopoietic and skeletal systems. PTH binds to receptors on osteoblasts/stromal cells. In response, osteoblasts produce IL-6 and sIL-6R whose signaling phosphorylates STAT3 and results in myeloid cell expansion. sIL-6R/gp130/STAT3-mediated hematopoietic cell expansion positively impacts PTH anabolic actions in bone by stimulating TGF-β1 in myeloid cells. IL-6 contributes to PTH-mediated hematopoietic cell expansion but is not essential for PTH anabolic actions in bone. sIL-6R signaling in myeloid cells results in higher and more rapid TGF-β1 expression than IL-6/gp130/STAT3 signaling. TGF-β1 has been previously shown to recruit mesenchymal stem cells to bone surfaces and support bone formation (29, 30).
This work was supported, in whole or in part, by National Institutes of Health Grants DK53904 and PO1 CA093900 (to L. K. M.).

This article contains supplemental Figs. S1–S6.
- PTH
- parathyroid hormone
- sIL-6R
- soluble IL-6 receptor
- IMDM
- Iscove's modified Dulbecco's medium
- VEH
- vehicle
- α-MEM
- α-minimum Eagle's medium
- Flt-3L
- fms-like tyrosine kinase-3 ligand
- sgp130
- soluble glycoprotein 130 receptor.
REFERENCES
- 1. Demiralp B., Chen H. L., Koh A. J., Keller E. T., McCauley L. K. (2002) Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology 143, 4038–4047 [DOI] [PubMed] [Google Scholar]
- 2. Jilka R. L., Weinstein R. S., Bellido T., Roberson P., Parfitt A. M., Manolagas S. C. (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 104, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bashutski J. D., Eber R. M., Kinney J. S., Benavides E., Maitra S., Braun T. M., Giannobile W. V., McCauley L. K. (2010) Teriparatide and osseous regeneration in the oral cavity. N. Engl. J. Med. 363, 2396–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang J. K., Chang L. H., Hung S. H., Wu S. C., Lee H. Y., Lin Y. S., Chen C. H., Fu Y. C., Wang G. J., Ho M. L. (2009) Parathyroid hormone 1–34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 60, 3049–3060 [DOI] [PubMed] [Google Scholar]
- 5. Calvi L. M., Adams G. B., Weibrecht K. W., Weber J. M., Olson D. P., Knight M. C., Martin R. P., Schipani E., Divieti P., Bringhurst F. R., Milner L. A., Kronenberg H. M., Scadden D. T. (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 [DOI] [PubMed] [Google Scholar]
- 6. Pirih F. Q., Michalski M. N., Cho S. W., Koh A. J., Berry J. E., Ghaname E., Kamarajan P., Bonnelye E., Ross C. W., Kapila Y. L., Jurdic P., McCauley L. K. (2010) Parathyroid hormone mediates hematopoietic cell expansion through interleukin-6. PLoS One 5, e13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J. Y., Purton L. E., Rodda S. J., Chen M., Weinstein L. S., McMahon A. P., Scadden D. T., Kronenberg H. M. (2008) Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 105, 16976–16981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X., Qin L., Bergenstock M., Bevelock L. M., Novack D. V., Partridge N. C. (2007) Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J. Biol. Chem. 282, 33098–33106 [DOI] [PubMed] [Google Scholar]
- 9. Lorenzo J., Horowitz M., Choi Y. (2008) Osteoimmunology. Interactions of the bone and immune system. Endocr. Rev. 29, 403–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takayanagi H. (2009) Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 5, 667–676 [DOI] [PubMed] [Google Scholar]
- 11. Terauchi M., Li J. Y., Bedi B., Baek K. H., Tawfeek H., Galley S., Gilbert L., Nanes M. S., Zayzafoon M., Guldberg R., Lamar D. L., Singer M. A., Lane T. F., Kronenberg H. M., Weitzmann M. N., Pacifici R. (2009) T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 10, 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onyia J. E., Bidwell J., Herring J., Hulman J., Hock J. M. (1995) In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone 17, 479–484 [DOI] [PubMed] [Google Scholar]
- 13. Pollock J. H., Blaha M. J., Lavish S. A., Stevenson S., Greenfield E. M. (1996) In vivo demonstration that parathyroid hormone and parathyroid hormone-related protein stimulate expression by osteoblasts of interleukin-6 and leukemia inhibitory factor. J. Bone Miner. Res. 11, 754–759 [DOI] [PubMed] [Google Scholar]
- 14. Li X., Liu H., Qin L., Tamasi J., Bergenstock M., Shapses S., Feyen J. H., Notterman D. A., Partridge N. C. (2007) Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J. Biol. Chem. 282, 33086–33097 [DOI] [PubMed] [Google Scholar]
- 15. Michalevicz R., Lifshitz D., Revel M. (1989) Interferon β2/interleukin-6 and interleukin-3 synergize in stimulating proliferation of human early hematopoietic progenitor cells. Scanning Microsc. 3, 1143–1149; discussion 1149–1150 [PubMed] [Google Scholar]
- 16. Palmqvist P., Persson E., Conaway H. H., Lerner U. H. (2002) IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-κB ligand, osteoprotegerin, and receptor activator of NF-κB in mouse calvariae. J. Immunol. 169, 3353–3362 [DOI] [PubMed] [Google Scholar]
- 17. Grey A., Mitnick M. A., Masiukiewicz U., Sun B. H., Rudikoff S., Jilka R. L., Manolagas S. C., Insogna K. (1999) A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology 140, 4683–4690 [DOI] [PubMed] [Google Scholar]
- 18. Sims N. A., Jenkins B. J., Quinn J. M., Nakamura A., Glatt M., Gillespie M. T., Ernst M., Martin T. J. (2004) Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J. Clin. Invest. 113, 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami M., Hibi M., Nakagawa N., Nakagawa T., Yasukawa K., Yamanishi K., Taga T., Kishimoto T. (1993) IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260, 1808–1810 [DOI] [PubMed] [Google Scholar]
- 20. Narazaki M., Yasukawa K., Saito T., Ohsugi Y., Fukui H., Koishihara Y., Yancopoulos G. D., Taga T., Kishimoto T. (1993) Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood 82, 1120–1126 [PubMed] [Google Scholar]
- 21. Fukada T., Hibi M., Yamanaka Y., Takahashi-Tezuka M., Fujitani Y., Yamaguchi T., Nakajima K., Hirano T. (1996) Two signals are necessary for cell proliferation induced by a cytokine receptor gp130. Involvement of STAT3 in anti-apoptosis. Immunity 5, 449–460 [DOI] [PubMed] [Google Scholar]
- 22. Nakajima K., Yamanaka Y., Nakae K., Kojima H., Ichiba M., Kiuchi N., Kitaoka T., Fukada T., Hibi M., Hirano T. (1996) A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 15, 3651–3658 [PMC free article] [PubMed] [Google Scholar]
- 23. Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M., Bartsch B., Holtmann M., Becker C., Strand D., Czaja J., Schlaak J. F., Lehr H. A., Autschbach F., Schürmann G., Nishimoto N., Yoshizaki K., Ito H., Kishimoto T., Galle P. R., Rose-John S., Neurath M. F. (2000) Blockade of interleukin 6 trans-signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation. Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 [DOI] [PubMed] [Google Scholar]
- 24. Matsumoto S., Hara T., Mitsuyama K., Yamamoto M., Tsuruta O., Sata M., Scheller J., Rose-John S., Kado S., Takada T. (2010) Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J. Immunol. 184, 1543–1551 [DOI] [PubMed] [Google Scholar]
- 25. Kopf M., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Köhler G. (1994) Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 [DOI] [PubMed] [Google Scholar]
- 26. Servet-Delprat C., Arnaud S., Jurdic P., Nataf S., Grasset M. F., Soulas C., Domenget C., Destaing O., Rivollier A., Perret M., Dumontel C., Hanau D., Gilmore G. L., Belin M. F., Rabourdin-Combe C., Mouchiroud G. (2002) Flt3+ macrophage precursors commit sequentially to osteoclasts, dendritic cells and microglia. BMC Immunol. 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh A. J., Beecher C. A., Rosol T. J., McCauley L. K. (1999) 3′,5′-Cyclic adenosine monophosphate activation in osteoblastic cells. Effects on parathyroid hormone-1 receptors and osteoblastic differentiation in vitro. Endocrinology 140, 3154–3162 [DOI] [PubMed] [Google Scholar]
- 28. Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr. (1992) Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells. A single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. U.S.A. 89, 2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu X., Pang L., Lei W., Lu W., Li J., Li Z., Frassica F. J., Chen X., Wan M., Cao X. (2010) Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 7, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koh A. J., Novince C. M., Li X., Wang T., Taichman R. S., McCauley L. K. (2011) An irradiation-altered bone marrow microenvironment impacts anabolic actions of PTH. Endocrinology 152, 4525–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sui X., Tsuji K., Tajima S., Tanaka R., Muraoka K., Ebihara Y., Ikebuchi K., Yasukawa K., Taga T., Kishimoto T., Nakahata T. (1996) Erythropoietin-independent erythrocyte production. Signals through gp130 and c-kit dramatically promote erythropoiesis from human CD34+ cells. J. Exp. Med. 183, 837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGregor N. E., Poulton I. J., Walker E. C., Pompolo S., Quinn J. M., Martin T. J., Sims N. A. (2010) Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodeling. Calcif. Tissue Int. 86, 261–270 [DOI] [PubMed] [Google Scholar]
- 33. Sims N. A., Jenkins B. J., Nakamura A., Quinn J. M., Li R., Gillespie M. T., Ernst M., Robb L., Martin T. J. (2005) Interleukin-11 receptor signaling is required for normal bone remodeling. J. Bone Miner. Res. 20, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 34. Walker E. C., McGregor N. E., Poulton I. J., Solano M., Pompolo S., Fernandes T. J., Constable M. J., Nicholson G. C., Zhang J. G., Nicola N. A., Gillespie M. T., Martin T. J., Sims N. A. (2010) Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J. Clin. Invest. 120, 582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jostock T., Müllberg J., Ozbek S., Atreya R., Blinn G., Voltz N., Fischer M., Neurath M. F., Rose-John S. (2001) Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 268, 160–167 [DOI] [PubMed] [Google Scholar]
- 36. Diamant M., Hansen M. B., Rieneck K., Svenson M., Yasukawa K., Bendtzen K. (1994) Stimulation of the B9 hybridoma cell line by soluble interleukin-6 receptors. J. Immunol. Methods 173, 229–235 [DOI] [PubMed] [Google Scholar]
- 37. Gabay C., Silacci P., Genin B., Mentha G., Le Coultre C., Guerne P. A. (1995) Soluble interleukin-6 receptor strongly increases the production of acute-phase protein by hepatoma cells but exerts minimal changes on human primary hepatocytes. Eur. J. Immunol. 25, 2378–2383 [DOI] [PubMed] [Google Scholar]
- 38. Igaz P., Tóth S., Rose-John S., Madurka I., Fejér G., Szalai C., Falus A. (1998) Soluble interleukin 6 (IL-6) receptor influences the expression of the protooncogene junB and the production of fibrinogen in the HepG2 human hepatoma cell line and primary rat hepatocytes. Cytokine 10, 620–626 [DOI] [PubMed] [Google Scholar]
- 39. Holub M. C., Hegyesi H., Igaz P., Polgár A., Toth S., Falus A. (2002) Soluble interleukin-6 receptor enhanced by oncostatin M induces major changes in gene expression profile of human hepatoma cells. Immunol. Lett. 82, 79–84 [DOI] [PubMed] [Google Scholar]
- 40. Kobayashi S., Xu X., Chen K., Liang Q. (2012) Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 8, 577–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han D., Yang B., Olson L. K., Greenstein A., Baek S. H., Claycombe K. J., Goudreau J. L., Yu S. W., Kim E. K. (2010) Activation of autophagy through modulation of 5′-AMP-activated protein kinase protects pancreatic beta-cells from high glucose. Biochem. J. 425, 541–551 [DOI] [PubMed] [Google Scholar]
- 42. Younce C. W., Wang K., Kolattukudy P. E. (2010) Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc. Res. 87, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishimura R., Moriyama K., Yasukawa K., Mundy G. R., Yoneda T. (1998) Combination of interleukin-6 and soluble interleukin-6 receptors induces differentiation and activation of JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells. J. Bone Miner. Res. 13, 777–785 [DOI] [PubMed] [Google Scholar]
- 44. Erices A., Conget P., Rojas C., Minguell J. J. (2002) Gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 280, 24–32 [DOI] [PubMed] [Google Scholar]
- 45. Taguchi Y., Yamamoto M., Yamate T., Lin S. C., Mocharla H., DeTogni P., Nakayama N., Boyce B. F., Abe E., Manolagas S. C. (1998) Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc. Assoc. Am. Physicians 110, 559–574 [PubMed] [Google Scholar]
- 46. Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T. R., Peng X., Hu J., Feng X., Van Hul W., Wan M., Cao X. (2009) TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15, 757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kinjyo I., Inoue H., Hamano S., Fukuyama S., Yoshimura T., Koga K., Takaki H., Himeno K., Takaesu G., Kobayashi T., Yoshimura A. (2006) Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J. Exp. Med. 203, 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ellegaard M., Jørgensen N. R., Schwarz P. (2010) Parathyroid hormone and bone healing. Calcif. Tissue Int. 87, 1–13 [DOI] [PubMed] [Google Scholar]
- 49. Aspenberg P., Genant H. K., Johansson T., Nino A. J., See K., Krohn K., García-Hernández P. A., Recknor C. P., Einhorn T. A., Dalsky G. P., Mitlak B. H., Fierlinger A., Lakshmanan M. C. (2010) Teriparatide for acceleration of fracture repair in humans. A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 25, 404–414 [DOI] [PubMed] [Google Scholar]