Background: The molecular players regulating platelet life span are largely unexplored.
Results: Proteasome inhibition induced apoptotic changes in platelets associated with a rise in active Bax and significant drop in platelet life span.
Conclusion: Proteasome plays a crucial role in delimiting platelet life span through constitutive elimination of the conformationally active Bax.
Significance: The findings bear relevance in clinical settings where proteasome is therapeutically inhibited.
Keywords: Bax, Phagocytosis, Phosphatidylserine, Platelets, Thrombosis, Proteasome, Phagocytic Uptake, Platelet Life Span
Abstract
Limit of platelet life span (8–10 days) is determined by the activity of a putative “internal clock” composed of Bcl-2 family proteins, whereas the role of other molecular players in this process remains obscure. Here, we sought to establish a central role of proteasome in platelet life span regulation. Administration of mice with inhibitors of proteasome peptidase activity induced significant thrombocytopenia. This was associated with enhanced clearance of biotin-labeled platelets from circulation and reduction in average platelet half-life from 66 to 37 h. Cells pretreated in vitro with proteasome inhibitors exhibited augmented annexin V binding and a drop in mitochondrial transmembrane potential indicative of apoptotic cell death and decreased platelet life span. These cells were preferentially phagocytosed by monocyte-derived macrophages, thus linking proteasome activity with platelet survival. The decisive role of proteasome in this process was underscored from enhanced expression of conformationally active Bax in platelets with attenuated proteasome activity, which was consistent with pro-apoptotic phenotype of these cells. The present study establishes a critical role of proteasome in delimiting platelet life span ostensibly through constitutive elimination of the conformationally active Bax. These findings bear potential implications in clinical settings where proteasome peptidase activities are therapeutically targeted.
Introduction
Platelets are anucleate circulating cells that are major players in the process of hemostasis and thrombosis (1, 2). Platelets are produced from megakaryocytes and released into circulation, where they have a defined life span of 7–10 days in human (4–5 days in mouse), following which they are selectively cleared by the reticuloendothelial system (3). Thus, the steady-state platelet count in circulation is the balance between platelet biogenesis and clearance (4). Our knowledge of molecular mechanisms controlling platelet life span has so far been limited. The earlier hypothesis to explain platelet senescence at steady-state had been the “multiple-hit” model, which proposed restricted endurance of circulating platelets to “hits” inflicted from within environment, before platelets are recognized and cleared by macrophages (5, 6).
In a significant recent study platelet life span was demonstrated to be determined by putative internal clock based on opposing activities of anti- and pro-apoptotic Bcl-2 family proteins, particularly Bcl-XL, Bak, and Bax (7, 8). However, factors and processes regulating ticking of this clock are yet to be defined.
The 26 S proteasome is a multiprotein complex responsible for proteolysis of ubiquitinated peptides (9). Expressed ubiquitously, the ubiquitin-proteasome system is composed of a 20 S core cylinder endowed with peptidase activities and capped at each end with a 19 S regulatory particle (9). Proteasome activity is known to decline steadily upon aging, leading to accumulation of misfolded proteins, the hallmark of age-related disorders (10). Surprisingly, there have been few reports exploring the role of proteasome in platelets (11, 12). We have recently demonstrated calcium-mediated up-regulation of proteasomal activity in stimulated platelets (13). In the present study we demonstrate a critical role of proteasome in the regulation of platelet life span. We show that attenuation of proteasome activity significantly shortens life span of platelets associated with accumulation of conformationally active Bax and phagocytic clearance of the cells by macrophages.
EXPERIMENTAL PROCEDURES
ABT-737 was purchased from Selleck Chemicals. Anti-CD61-PE and annexin V-FITC were from BD Biosciences. Proteasome inhibitors PSI2 (Z-Ile-Glu(OtBu)-Ala-Leu-CHO), PSII (Z-Leu-Leu-Phe-CHO), MG132 (Z-Leu-Leu-Leu-CHO), and calpeptin were procured from Calbiochem. Whereas PSI and PSII specifically inhibit chymotryptic activity of 20 S proteasome, MG132 prevents degradation of ubiquitinated proteins by targeting 26 S proteasome. Bortezomib (Bortecad) was purchased from Cadila Pharmaceuticals. N-Hydroxysuccinimidobiotin (NHS-biotin), PE-streptavidin, 5,5′-6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolylcarbocyanine iodide (JC-1), thiazole orange, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), 6-carboxy-2′,7′-dichlorodihydrofluorescein (H2DCFDA), acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (AC-DEVD-AMC), apyrase, EGTA, EDTA, sodium orthovanadate, acetylsalicylic acid, bovine serum albumin (BSA) (fraction V), Triton X-100, protease inhibitors, dimethylsulfoxide (DMSO), oleuropein, mouse monoclonal anti-Bax (6A7), anti-Bax (2D2), and rabbit polyclonal anti-Bak were purchased from Sigma. Mouse monoclonal anti-ubiquitin was procured from Santa Cruz Biotechnology. Calcein-AM, Fura-2/AM and t-butoxycarbonyl-Leu-Metchloromethylcoumarin were from Invitrogen. RPMI 1640 medium was purchased from HiMedia. Reagents for electrophoresis were products of Merck. PVDF membranes and rabbit polyclonal active anti-caspase-3 were from Millipore. SuperSignal West Pico chemiluminescent substrate was from Pierce. Mouse monoclonal anti-cytochrome c and horseradish peroxidase (HRP)-labeled secondary antibodies were purchased from Transduction Laboratories and Bangalore Genei, respectively. All other reagents were of analytical grade. Milli-Q grade, type 1, deionized water (Millipore) was used for preparation of solutions.
Platelet Preparation
Platelets were isolated from fresh human blood by differential centrifugation, as described (14). Briefly, blood from healthy volunteers was collected in citrate phosphate-dextrose adenine and centrifuged at 180 × g for 10 min. Platelet-rich plasma thus obtained was incubated with 1 mm acetylsalicylic acid for 15 min at 37 °C. After addition of EDTA (5 mm), platelets were sedimented by centrifugation at 800 × g for 15 min. Cells were washed in buffer A (20 mm HEPES, 138 mm NaCl, 2.9 mm KCl, 1 mm MgCl2, 0.36 mm NaH2PO4, 1 mm EGTA, supplemented with 5 mm glucose and 0.6 ADPase unit of apyrase/ml, pH 6.2) and were finally resuspended in buffer B (20 mm HEPES, 138 mm NaCl, 2.9 mm KCl, 1 mm MgCl2, 0.36 mm NaH2PO4, 5 mm glucose, pH 7.4). The final cell count was adjusted to 0.5–0.8 × 109/ml. All steps were carried out under sterile conditions, and precautions were taken to maintain the cells in resting condition.
Platelet Clearance Analysis
Mice were injected in tail vein with 600 mg of NHS-biotin and either DMSO (control) or PSI (0.3 mg/kg) (treated) (15). At various time points 50 μl of retro-orbital blood was drawn from both control as well as treated mice, mixed with 200 μl of buffered saline-glucose-citrate buffer (116 mm NaCl, 13.6 mm trisodium citrate, 8.6 mm Na2HPO4, 1.6 mm KH2PO4, 0.9 mm EDTA, 11.1 mm glucose), and followed by 1 ml of balanced salt solution (149 mm NaCl, 3.7 mm KCl, 2.5 mm CaCl2, 1.2 mm MgSO4, 7.4 mm HEPES, 1.2 mm KH2PO4, 0.8 mm K2HPO4, 3% bovine calf serum). Cells were pelleted at 1400 × g for 10 min, and resuspended in 300 μl of sheath fluid. They were stained with FITC-conjugated rat anti-CD41, which label only platelets, followed by PE-streptavidin for 1 h on ice, washed in balanced salt solution, and analyzed by flow cytometry to determine the fraction of platelet population labeled with PE (7).
Labeling of Reticulated Platelets
Mice were injected intravenously with either DMSO or PSI as described above. Blood was collected from retro-orbital plexus of mice at different time points (0, 24, 48, 72, and 96 h). Staining for reticulated platelets was carried out by incubation of 5 μl of blood with 50 μl of thiazole orange (0.1 mg/ml in phosphate-buffered saline (PBS)) and 1 μl of PE-conjugated CD41 antibody in the dark for 15 min at room temperature, followed by fixation with 1 ml of paraformaldehyde (1%) in PBS (7). Cells were washed with PBS, resuspended in 300 μl of sheath fluid, and analyzed by flow cytometry. After appropriate compensation, fluorescence data were collected using four-quadrant logarithmic amplification.
Cytofluorometric Analysis of Mitochondrial Transmembrane Potential
Mitochondrial transmembrane potential (Δψm) was measured using the potential-sensitive fluorochrome JC-1, which selectively moves across polarized mitochondrial membrane and forms aggregates (red). As membrane potential collapses, the fluorescence changes from red to green due to release of monomeric dye (16). To study Δψm, platelets were pretreated with PSI (20 μm), PSII (20 μm), MG132 (10 μm), CCCP (30 μm), or DMSO (vehicle) for 30 min, followed by incubation with 2 μm JC-1 for 15 min at 37 °C in the dark. Cells were washed in PBS, and JC-1 fluorescence was analyzed in FL1 and FL2 channels of flow cytometer (FACSCalibur; BD Biosciences) for detection of dye monomer and aggregates, respectively. The ratio of red to green (FL2/FL1) fluorescence reflected Δψm.
Flow Cytometric Measurement of Reactive Oxygen Species (ROS)
Platelets were treated with proteasome inhibitors or vehicle as above, washed with PBS, and incubated with H2DCFDA (1 μm) for 30 min at 37 °C in the dark. Cells were next washed twice with PBS and analyzed by flow cytometry as described previously (16).
Measurement of Annexin V Binding by Flow Cytometry
Platelets (1 × 108 cells in 100 μl) were incubated at 37 °C for 30 min in the presence of proteasome inhibitors or vehicle as stated above. For positive control the washed platelets were treated with thrombin (1 unit/ml) for 10 min without stirring. Then an equal amount of 4% paraformaldehyde was added to the cells and incubated for 30 min, which was followed by washing. Postfixed resuspended platelets were labeled with 5 μl of PE-labeled anti-CD61 antibody and 10 μl of FITC-labeled annexin V in the presence of 5 mm CaCl2 to promote binding. Samples were incubated for 30 min at room temperature in the dark and analyzed on the flow cytometer (16). After compensation between FITC and PE, all fluorescence data were collected using four-quadrant logarithmic amplification. Data from 10,000 CD61-positive events were collected for each sample.
Isolation of Mitochondria-rich and Cytosolic Fractions
Platelets suspended in mitochondria extraction buffer (225 mm mannitol, 75 mm sucrose, 0.1 mm EGTA, 1 mg/ml fatty acid-free BSA, 10 mm HEPES-KOH, 2 mm sodium orthovanadate, 21 μm leupeptin, 2 mm phenylmethylsulfonyl fluoride, 20 μm pepstatin A, and 0.56 unit of trypsin inhibitor/ml of aprotinin, pH 7.4) were lysed by freezing in liquid nitrogen for 1 min followed by thawing at 37 °C for 3 min (17). The freeze-thaw sequence was repeated for two more cycles. The lysate was centrifuged at 700 × g for 10 min at 4 °C, and pellet was discarded. The supernatant was further centrifuged at 15,000 × g for 10 min at 4 °C. The pellet was regarded as the mitochondria-rich fraction, and the supernatant was the mitochondria-free cytosolic fraction.
Caspase-3 Activity Assay
To determine cytosolic caspase-3 activity, samples were pretreated with 10, 25, and 50 μm of PSI or vehicle (DMSO) and lysed with equal amount of 2× radioimmune precipitation assay (RIPA) buffer. After a 10-min incubation in ice, an equal volume of 2× substrate buffer (20 mm HEPES, pH 7.4, 2 mm EDTA, 0.1% CHAPS, 5 mm DTT, and 10 μm caspase substrate AC-DEVD-AMC) was added to each lysate and further incubated for 30 min at 37 °C (16). Caspase-3 activity was determined from the extent of cleavage of fluorogenic substrate measured at 460 nm emission (excitation, 360 nm). In other experiments active caspase-3 was detected by Western blot analysis in cell lysate using specific antibody.
Calpain Activity Assay
Intracellular calpain activity was measured as described previously (18). Washed human platelets in a 96-well plate were exposed to DMSO or PSI for 30 min and then loaded with t-butoxycarbonyl-Leu-Metchloromethylcoumarin (10 μm). After a 30-min incubation, cellular fluorescence was quantified with a fluorescence microplate reader (BioTek model FLx800) at 37 °C using 351-nm excitation and 430-nm emission filters.
Measurement of Intracellular Free Calcium
Intracellular calcium measurements were carried out in platelets pretreated with either DMSO (vehicle) or different concentrations of PSI using Fura-2/AM dye as described previously (14). Fluorescence was recorded using Hitachi fluorescence spectrophotometer (model F-2500) and intracellular calcium levels were obtained using FL Solutions software.
Inmunoprecipitation
Ubiquitinated platelet proteins were immunoprecipitated as described earlier (16). Briefly, 500 μl of platelet suspension was lysed and immunoprecipitated by incubation with 2 μg of anti-ubiquitin antibody and 25 μl of protein A-agarose overnight at 4 °C on a rocking platform. It was washed three times with 500 μl of 2× RIPA buffer, and the pellet was subjected to polyacrylamide gel electrophoresis (PAGE).
Western Blotting
Proteins were separated by 13% SDS- or native-PAGE and transferred electrophoretically onto PVDF membrane (0.8 mA/cm2, 2 h) in a semidry blotter (TE 77 PWR; GE Healthcare) for subsequent probing as described previously (14). Blots were incubated for 1 h with 5% (w/v) BSA in Tris-buffered saline containing 0.05% Tween 20 (TBST) to block residual protein binding sites. Membranes were incubated overnight at 4 °C with the primary antibodies (anti-Bax (6A7), 1:500; anti-Bax (2D2), 1:1000; anti-Bak, 1:1000; anti-caspase-3, 1:200; and anti-cytochrome c, 1:500). Blots were incubated with the appropriate HRP-conjugated secondary antibody (diluted 1:10,000) and exposed to enhanced chemiluminescence reagents for 5 min. Blots were exposed to photographic films and densitometrically scanned. For protein loading control, membranes containing whole cell lysates were reprobed with the anti-actin antibody (1:1000).
Monocyte Isolation, Culture, and Phagocytic Recognition of Platelets
Human monocytes were isolated and cultured as described (17, 19). Briefly, blood from healthy donors was collected in citrate, and peripheral blood mononuclear cells (PBMCs) were isolated using Hysep, according to the manufacturer's instructions. Monocytes were further isolated by plating the PBMCs on polystyrene-coated tissue culture flasks for 4 h at 37 °C, followed by three washes with PBS to remove nonadherent lymphocytes. Monocytes (250,000 in 500-μl volume) were then plated on 6-well plates in RPMI 1640 medium supplemented with 10% fetal bovine serum and cultured for 7 days to obtain the monocyte-derived macrophages (MDMs). Platelets labeled with calcein-AM were incubated with a monolayer of autologous MDMs for 45 min. Following the incubation period, the phagocyte monolayer was washed free of noninteracting platelets, and any adherent platelets were removed by treatment with trypsin at 37 °C for 5 min followed by 5 mm EDTA at 4 °C. MDMs were recovered by trypsin/EDTA treatment for 15 min at 37 °C and subjected to flow cytometric and epifluorescent microscopic analysis.
Statistical Methods
Standard statistical methods were used. Parametric methods (t test) were used for evaluation, and tests were considered significant at p < 0.05 (two-tailed tests). Data are presented as means ± S.D. of at least five individual experiments from different blood donors.
RESULTS
Proteasome Inhibition Leads to Decreased Platelet Life Span
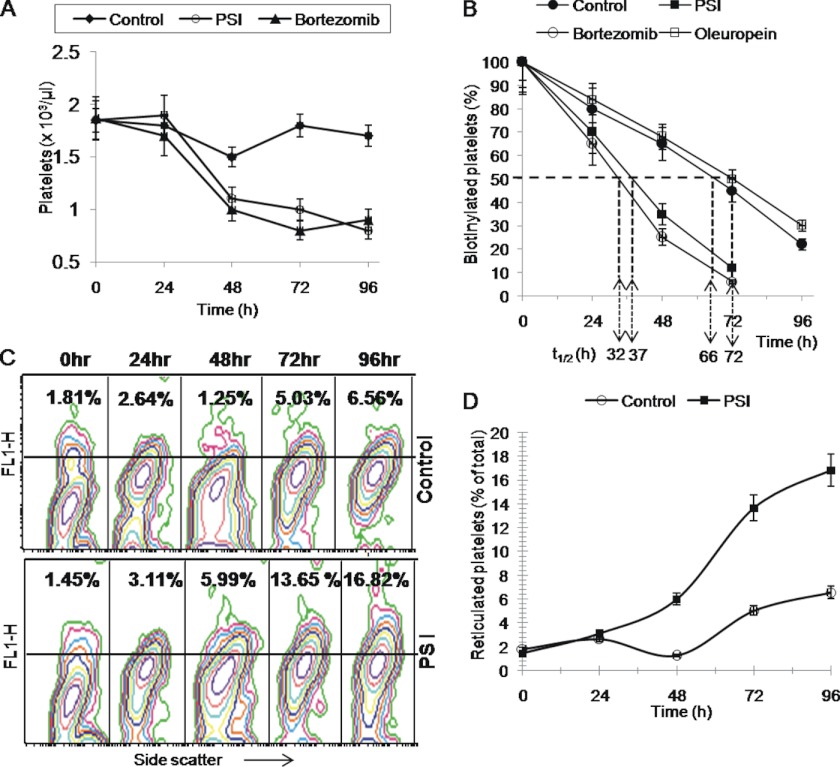
Platelet life span is determined by opposing activities of anti- and pro-apoptotic Bcl-2 family proteins (7, 8). As proteasomal activity in tumor cells influences cellular level of these proteins (20–22), we investigated whether proteasome has a role in regulating platelet lifespan in vivo. Intravenous administration of PSI (0.3 mg/kg) and bortezomib (0.1 mg/kg) in mice led to significant lowering of platelet count from 1.8 × 103/μl to 0.8 × 103/μl and 0.9 × 103/μl, respectively (n = 5), suggestive of close relationship between proteasome inhibition and induction of thrombocytopenia (Fig. 1A).
FIGURE 1.
A, platelet count in control, PSI-, and bortezomib-treated mice at different time points. B, proportion of biotinylated platelets (percentage) in peripheral blood sample drawn from DMSO (vehicle), PSI (0.3 mg/kg), bortezomib (0.1 mg/kg), or oleuropein (1.5 mg/kg) pretreated mice 0, 24, 48, 72, and 96 h after administration of NHS-biotin. t½ represents platelet half-life in hours. C, representative flow cytometric profiles of thiazole orange-stained platelets 0, 24, 48, 72, and 96 h after a single dose of DMSO (upper row) or PSI (0.3 mg/kg) (lower row). Percentage values indicated in upper panels correspond to younger (reticulated) platelets among entire platelet population. D, quantitative representation of results shown in C. Data are representative of five different experiments and are expressed as mean ± S.D. (error bars).
Reduction in platelet count could either be due to an increase in platelet clearance or decreased platelet production. To study possible changes in platelet clearance we conjugated mice platelets with biotin by intravenous administration of NHS-biotin and tracked the labeled platelets ex vivo by incubating cells with PE-streptavidin (7). Consistent with an earlier observation by Berger et al. (23), platelet half-life (t½-clearance of 50% biotin-conjugated platelets) was found to be 66 h in control mice. Platelet half-life dropped significantly to 37 h and 32 h in mice administered with PSI and bortezomib, respectively (Fig. 1B). Oleuropein, which has been recently demonstrated to be a potential proteasome activator, caused a small but consistent increment in platelet half-life to 72 h (Fig. 1B).
Thiazole orange stains young reticulated platelets and hence reflects adequacy of platelet generation by bone marrow (7). Platelets exhibited a steady time-dependent increase in thiazole orange staining (11-fold rise in 96 h) in mice administered with PSI, whereas those in control mice remained significantly less stained with minor increment observed at 96 h (Fig. 1, C and D). This was consistent with progressive expansion in young platelet population and increased platelet generation as a consequence of enhanced platelet clearance in PSI-administered mice. Thus, proteasome inhibition in vivo increases platelet clearance and limits its life span in circulation.
Proteasome Inhibition Induces Apoptosis-like Changes in Human Platelets Associated with Up-regulation of Bax
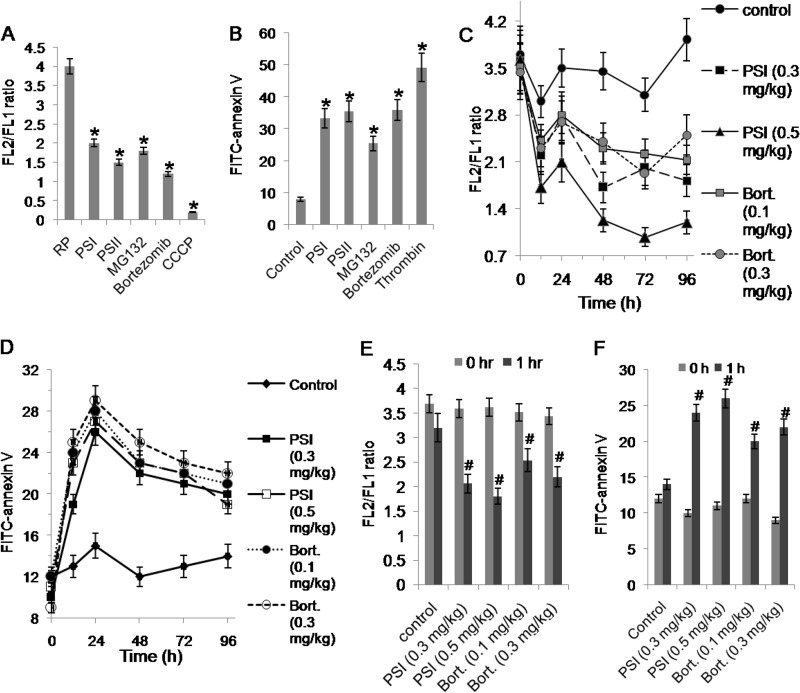
Bortezomib, a highly selective inhibitor of proteasomal peptidase activity, has been employed in cancer therapy for its ability to induce apoptosis in tumor cells (24). To determine whether proteasome inhibition regulates platelet life span through induction of apoptosis, we investigated the effect of different inhibitors of proteasome activity on Δψm and surface exposure of phosphatidylserine (PS) in mice platelets in vivo and human platelets in vitro. JC-1, a lipophilic cation, was employed to determine alterations in Δψm. As expected, a high aggregate to monomer fluorescence ratio (FL2/FL1) of the fluor was observed in untreated (control) washed human platelets indicative of stabilized Δψm, which dropped drastically in CCCP (protonophore)-treated platelets (Fig. 2A). Incubation of platelets with different proteasome inhibitors (PSI, 20 μm; PSII, 20 μm; MG132, 10 μm; and bortezomib, 25 μm) was associated with significant decrements in Δψm (by 50, 62.5, 55, and 70%, respectively) compared with control (Fig. 2A). Consistent with above results, PSI or bortezomib, when administered intravenously to mice, evoked a dose-dependent drop in Δψm starting 1 h after treatment ex vivo, whereas those from vehicle-treated mice exhibited stable Δψm (Fig. 2, C and E).
FIGURE 2.
Study of apoptotic features in platelets following proteasomal inhibition. A and B, mitochondrial transmembrane potential (FL2/FL1 ratio) (A), and PS exposure (FITC-annexin V binding) (B) in platelets pretreated with proteasome inhibitors (PSI, 20 μm; PSII, 20 μm; MG132, 10 μm; and bortezomib, 25 μm), CCCP (30 μm), thrombin (1 unit/ml) or DMSO (RP or control), as indicated. C and D, changes in mitochondrial transmembrane potential (C) and FITC-annexin V binding (D) with time observed in platelets from mice 0, 24, 48, 72, and 96 h after injection of DMSO (control), PSI, or bortezomib (bort.). E and F, mitochondrial transmembrane potential (E) and FITC-annexin V binding (F) in mice platelets 1 h after intravenous treatment with DMSO (control), PSI, or bortezomib. Data are representative of five different experiments and are expressed as mean ± S.D. (error bars). *, p < 0.05 compared with DMSO-pretreated platelets; #, p < 0.05 compared with 0 h platelets.
Externalization of PS, an anionic phospholipid, from the inner leaflet to outer layer of cell membrane is an invariable early feature in the apoptotic process (16). FITC-annexin V binding to cell surface was studied next to detect platelets undergoing apoptosis. As expected, surface membrane was found to be significantly enriched with PS in thrombin-stimulated platelets compared with the resting cells. Remarkably, pretreatment of platelets with different proteasome inhibitors, PSI, PSII, MG132, and bortezomib resulted in dramatic increase (by 4-, 5-, 3-, 4 and 5-fold, respectively) in annexin V binding compared with the untreated (control) cells, indicative of induction of apoptosis-like changes upon proteasome inhibition (Fig. 2B). A comparable dose-dependent increase in annexin V binding was also observed in mouse platelets after administering mice with PSI and bortezomib (Fig. 2, D and F). Because apoptosis is known to be associated with cellular ROS generation (16, 25, 26), we sought to determine whether proteasome inhibition induced ROS production in platelets. Using the cell-permeable dye H2DCFDA, ROS was found to be significantly enhanced (by 3.8-, 3.6-, and 6.1-fold) in platelets treated with PSI, MG132, and PSII, respectively (data not shown), which further validated the reciprocal relationship between proteasome activity and induction of platelet apoptosis.
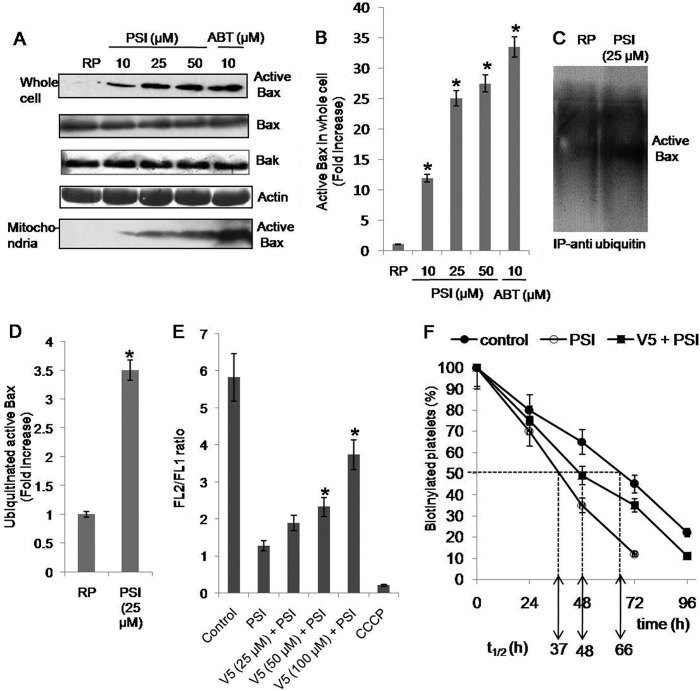
Next, we searched for putative molecular mediators of apoptosis in platelets treated with proteasome inhibitors. Early in the process of apoptosis Bax, the pro-apoptotic member of Bcl-2 family, is known to undergo conformational change and translocate to the mitochondrial membrane (27). We evaluated changes in levels of both total Bax and conformationally changed active Bax in platelets with or without proteasomal inhibition. Active Bax was detected by immunoblotting with an antibody (clone 6A7), which specifically detects Bax in its conformationally altered state. As expected BH3-mimetic ABT737 induced significant Bax activation in platelets (Fig. 3A). We found progressive increments in conformationally active Bax (by 11-, 25-, and 27-fold) but not total Bax in platelets pretreated with increasing concentrations of PSI (10, 25, and 50 μm, respectively) (Fig. 3, A and B), indicative of its accumulation upon proteasome inhibition. Active Bax was also found to translocate to platelet mitochondrial fraction under identical experimental condition when proteasome was inhibited by PSI in a concentration-dependent manner (Fig. 3A). No change in the level of other pro-apoptotic Bcl-2 family protein Bak was observed upon proteasomal inhibition (Fig. 3A).
FIGURE 3.
A, Western blots showing expression levels of active Bax, total Bax, and Bak in whole cell lysate or mitochondrial fraction prepared from platelets pretreated with DMSO (RP), PSI, or ABT737. B, quantitative representation of active Bax levels in platelet whole cell lysates as determined by densitometry of Western blots. C, Western blots showing expression levels of ubiquitinated active Bax immunoprecipitated from platelets pretreated with DMSO (RP) or PSI for 30 min. D, quantitative representation of expression levels in Western blots shown in C as determined by densitometry. E, mitochondrial transmembrane potential (FL2/FL1 ratio) in PSI-treated platelets with or without preincubation with V5 peptide as indicated. F, proportion of biotinylated platelets (percentage) in peripheral blood samples drawn from mice treated with DMSO alone (control) PSI (0.3 mg/kg) alone, or a combination of V5 peptide (2 mg/kg) and PSI (0.3 mg/kg) 0, 24, 48, 72, and 96 h after administration of NHS-biotin. t½ represents platelet half-life in hours. *, p < 0.05 compared with DMSO-pretreated platelets. Data are representative of five different experiments and are expressed as mean ± S.D. (error bars).
We then looked for ubiquitination of conformationally active Bax in unstimulated platelets. Ubiquitinated proteins were immunoprecipitated from platelet cell lysate and probed using anti-Bax (6A7) antibody. We observed multiple bands of high molecular weight typical of polyubiquitinated proteins. Further, significantly greater levels of polyubiquitinated active Bax were observed in PSI-treated platelets (Fig. 3, C and D). Thus, active Bax was found to be constitutively targeted to proteasomal degradation by polyubiquitination.
To establish the causal role of active Bax in regulating platelet life span, the effect of proteasome inhibitor was observed in platelets preincubated with V5 peptide, a Bax inhibitor. PSI-induced drop in Δψm was reversed by 14, 23, and 54% with 25, 50, and 100 μm V5 peptide, respectively (Fig. 3E). In agreement with this, preadministration of mice with V5 peptide (2 mg/kg) by an intravenous route also caused 38% amelioration of the drop in platelet life span induced by PSI (Fig. 3F).
Platelet Cell Death upon Proteasome Inhibition Is Caspase-independent
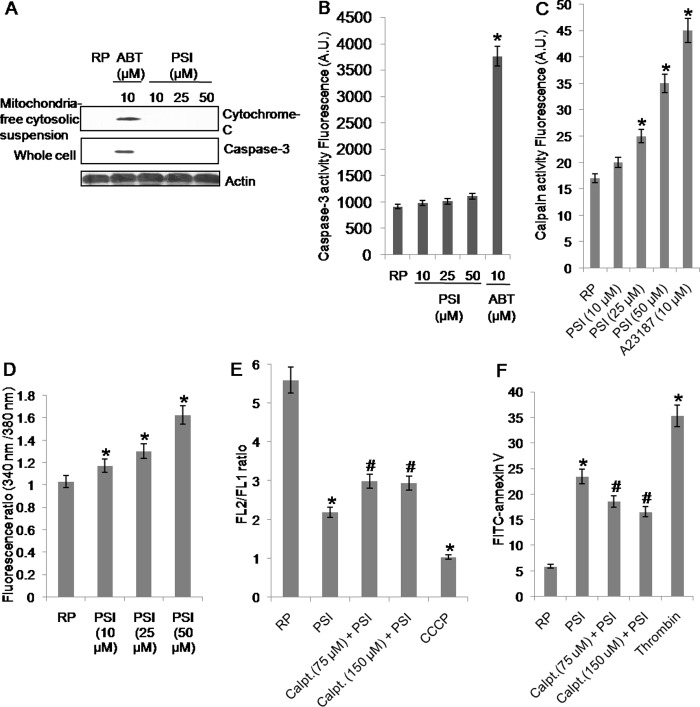
The pro-apoptotic members of Bcl-2 family induce release of mitochondrial cytochrome c into cytosol, which eventually leads to caspase-3 activation through constitution of apoptosome complex with Apaf-1 and caspase-9 (28). Because proteasome inhibition was associated with apoptosis-like features such as PS exposure and accumulation of active Bax in platelets, we sought to determine caspase-3 activity under identical experimental condition. As reported earlier (16), ABT737 induced significant release of mitochondrial cytochrome c as well as caspase-3 activation in platelets (Fig. 4A). However, we neither detected cytochrome c in extramitochondrial cytosolic fraction (Fig. 4A, top), nor could we record up-regulation in caspase-3 activity (Fig. 4, A, bottom, and B) in platelets exposed to increasing doses of PSI (10, 25, and 50 μm). These observations underscore the possibility of caspase-independent cell death pathway operational in platelets (19) as well as monocytes (29, 30), as described earlier. Studies including our own have suggested the role of calpain, a calcium-dependent thiol protease, in mediating cell death in platelets especially upon storage (19, 31). Hence, we looked for the relationship between proteasome inhibition and calpain activity in platelets. Platelets pretreated with PSI were found to exhibit higher calpain activity than their vehicle-treated counterparts (Fig. 4C). Pretreatment of cells with PSI also induced release of cytosolic free calcium from platelet intracellular stores in a dose-dependent manner (Fig. 4D), which is consistent with previous reports of Bax regulating release of calcium from the stores in endoplasmic reticulum (32). The effect of proteasome inhibition on platelet Δψm and PS exposure was examined in the presence of calpeptin, a specific calpain inhibitor, to establish the role of calpain in mediating cell death. Calpeptin brought about 39 and 24% reversal of PSI-induced PS externalization and drop in Δψm, respectively (Fig. 4, E and F). Thus, platelet cell death upon proteasome inhibition is Bax-dependent and attributable to calpain activation.
FIGURE 4.
A, Western blot analysis showing levels of cytochrome c and active caspase-3 in platelet whole cell lysate or mitochondria-free cytosolic suspension, as indicated, 30 min after treatment of platelets with DMSO (RP), PSI, or ABT737. B and C, caspase-3 activity (B) and calpain activity (C) as determined from the extent of cleavage of fluorogenic substrate AC-DEVD-AMC or t-butoxycarbonyl-Leu-Metchloromethylcoumarin, respectively, in platelets pretreated with DMSO (RP), PSI, or ABT737. D, intracellular calcium levels represented as ratio of fluorescence intensity at 340 nm to that at 380 nm measured in washed human platelets labeled with Fura-2/AM dye, 1 min after treatment with DMSO or PSI. E and F, mitochondrial transmembrane potential (FL2/FL1 ratio) (E), and PS exposure (FITC-annexin V binding) (F) in platelets pretreated with calpeptin (Calpt. 75 μm and 150 μm) and PSI (25 μm), PSI (25 μm) alone, CCCP (30 μm), thrombin (1 unit/ml), or DMSO (RP), as indicated. *, p < 0.05 compared with DMSO-pretreated platelets; #, p < 0.05 compared with PSI-pretreated platelets. Data are representative of five different experiments and are expressed as mean ± S.D. (error bars).
Proteasome Inhibition Is Associated with Enhanced Uptake of Platelets by Macrophages
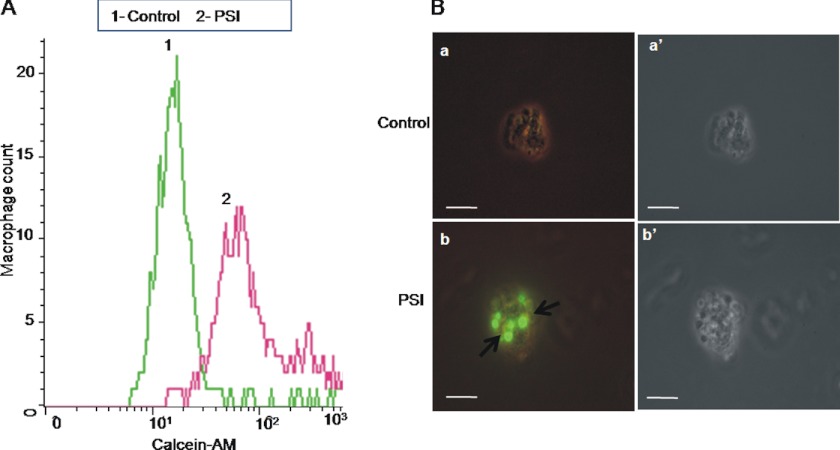
Platelets undergoing apoptosis-like changes are known to be removed by phagocytes in the reticuloendothelial system (33). Hence, we evaluated macrophage-assisted clearance of platelets following proteasomal inhibition. Calcein-stained platelets, either pretreated with PSI (20 μm) or DMSO, were incubated with a monolayer of autologous monocyte-derived adherent human macrophages for 45 min. The phagocyte monolayer was next washed free of noninteracting platelets and subjected to flow cytometry as well as epifluorescence microscopy to examine phagocytic uptake of platelets by macrophages.
Macrophages were gated and analyzed by flow cytometry for associated fluorescence (FL1). They exhibited significantly higher calcein fluorescence following incubation with PSI-treated fluorescent platelets, which was reflective of enhanced phagocytic uptake of treated platelets (Fig. 5A). This finding was further corroborated from epifluorescence microscopy of macrophages. Significantly higher mean fluorescence intensity (per high power field) was found to be associated with macrophages co-incubated with PSI-treated platelets than the control cells, which reflects facilitated phagocytic uptake of platelets upon proteasomal inhibition (Fig. 5B).
FIGURE 5.
Phagocytic uptake of platelets by autologous macrophages. A, flow cytometry of macrophages co-incubated with calcein-labeled platelets pretreated either with PSI (20 μm) or DMSO (control). B, epifluorescence microscopy of macrophages co-incubated with platelets pretreated either with DMSO (a) or PSI (20 μm) (b). Panels a′ and b′ represent corresponding phase-contrast micrographs. Scale bars, 10 μm. Data are representative of five different experiments.
DISCUSSION
The results presented so far identify the proteasome peptidase complex as a novel regulator of platelet life span. A putative internal “timer” comprising anti- and pro-apoptotic factors of Bcl-2 family has been suggested as a determinant of platelet longevity through apoptosis induction (7, 8). The present study, while contributing further evidence in support of control of platelet life span by Bcl-2 family proteins, introduces proteasome system as an essential and additional component of this timer.
The role of proteasome in regulation of cellular apoptosis has been well documented (34–38). Cellular levels of Bcl-2, Bcl-XL, and Bax are regulated by proteasomal activity in endothelial cells and cancer cell lines (20–22). Here, we show that platelets exhibited features of apoptosis following inhibition of proteasomal peptidase activity, which included drop in Δψm, enhanced surface exposure of PS, and rise in cytosolic ROS. Platelets abundantly express Bax (39) as well as the Bax-specific mRNA (40). Because Bax is a known substrate of proteasome (22), we evaluated the effect of proteasome inhibition on its levels. PSI-treated platelets were found to possess significantly higher levels of functionally active Bax than their control (untreated) counterparts although there was no significant difference in levels of total Bax. Further, we found that active Bax was being constitutively polyubiquitinated in resting platelets and thus targeted to proteasome. We were also able to establish Bax as a mediator of platelet cell death induced upon proteasomal inhibition using its peptide inhibitor V5. It reversed the apoptotic features in washed platelets as well as the drop in platelet life span in mice that were induced by PSI. The accumulation of conformationally active Bax also led to its increased localization to mitochondrial membrane. However, this did not translate into release of mitochondrial cytochrome c into cytosol or activation of caspase-3. These results are consistent with existence of caspase-independent cell death pathways that have been described in white blood cells as well as platelets (19, 29, 30, 31, 41). There has also been evidence of calpain, a calcium-dependent protease, to be activated in stored platelets undergoing caspase-independent apoptosis reflective of its role in mediating cell death (31). In concurrence with these findings we found that platelets exhibited higher calpain activity upon treatment with proteasome inhibitors. Because Bax is known to induce release of calcium stores in endoplasmic reticulum into the cytoplasm (32) we examined intracellular calcium levels that determine calpain activity and found it to be significantly elevated after treating platelets with proteasome inhibitors (Fig. 4D). Calpeptin, a specific calpain inhibitor, partially reversed the effects of proteasome inhibition on plalelet Δψm and PS exposure (Fig. 4, E and F), consistent with a causal role of calpain in mediating cell death.
We explored whether proteasome inhibition would adversely affect platelet life span in vivo in a rodent model. Administration of mice with PSI led to thrombocytopenia. This was associated with reduced half-life of platelets and increased number of young reticulated cells in circulation, indicative of enhanced rate of platelet clearance in PSI-administered mice. Also, administration of oleuropien, a small molecule proteasome activator, brought about a modest increase in platelet half-life. Consistent with this, PSI-treated human platelets were found to be phagocytosed more efficiently by macrophages, as demonstrated in vitro from flow cytometry and epifluorescence microscopy studies.
The present study indicates that proteasomal peptidase activity promotes platelet survival through constitutive elimination of the conformationally active Bax. Proteasome enzymic function is known to decline with cell aging (42, 43), which would lead to accumulation of pro-apoptotic proteins like Bax in platelets. Thus, progressive attenuation in activities of either Bcl-XL (7, 8) or proteasome in aging cells would result in unrestrained activity of pro-apoptotic Bax/Bak, eventually leading to platelet cell death and phagocytic clearance. Apart from the obvious relevance to platelet physiology, this study would also have translational implications. Pharmacological intervention using activators such as oleuropein (44) or genetic modulation to overexpress proteasome activator subunits to promote proteasomal activity could prove clinically beneficial as it would favor platelet survival in conditions like thrombocytopenia and transfusion of stored platelets. On the other hand, proteasome inhibitors may be exploited as therapeutic strategy to induce platelet apoptosis and thus to reduce severity of thrombosis. Our observations also call for careful consideration of potential adverse effect on platelet life span while exploiting the benefits of proteasomal inhibition in cancer therapeutics.
This work was supported by grants from the Council of Scientific and Industrial Research, the Department of Biotechnology, and the Department of Science and Technology, Government of India (all to D. D.).
- PSI
- (Z-Ile-Glu(OtBu)-Ala-Leu-CHO)
- PSII
- (Z-Leu-Leu-Phe-CHO)
- AC-DEVD-AMC
- acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin
- BSA
- bovine serum albumin
- CCCP
- carbonyl cyanide 3-chlorophenylhydrazone
- DMSO
- dimethyl sulfoxide
- EDTA
- ethylenediamine tetraacetic acid
- EGTA
- ethyleneglycol tetraacetic acid
- H2DCFDA
- 6-carboxy-2′,7′-dichlorodihydrofluorescein
- JC-1
- 5,5′-6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- MDMs
- monocyte-derived macrophages
- NHS-biotin
- N-hydroxysuccinimidobiotin
- PBS
- phosphate buffered saline
- PBMCs
- peripheral blood mononuclear cells
- PRP
- platelet-rich plasma
- PS
- phosphatidylserine
- Δψm
- mitochondrial transmembrane potential.
REFERENCES
- 1. Richardson J. L., Shivdasani R. A., Boers C., Hartwig J. H., Italiano J. E., Jr. (2005) Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood 106, 4066–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falcieri E., Bassini A., Pierpaoli S., Luchetti F., Zamai L., Vitale M., Guidotti L., Zauli G. (2000) Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat. Rec. 258, 90–99 [DOI] [PubMed] [Google Scholar]
- 3. Harker L. A. (1977) The kinetics of platelet production and destruction in man. Clin. Haematol. 6, 671–693 [PubMed] [Google Scholar]
- 4. Mustard J. F., Rowsell H. C., Murphy E. A. (1966) Platelet economy (platelet survival and turnover). Br. J. Haematol. 12, 1–24 [DOI] [PubMed] [Google Scholar]
- 5. Murphy E. A., Francis M. E. (1971) The estimation of blood platelet survival. II. The multiple hit model. Thromb. Diath. Haemorrh. 25, 53–80 [PubMed] [Google Scholar]
- 6. Murphy E. A. (1971) The estimation of blood platelet survival. III. The robustness of the basic models. Thromb. Diath. Haemorrh. 26, 431–448 [PubMed] [Google Scholar]
- 7. Mason K. D., Carpinelli M. R., Fletcher J. I., Collinge J. E., Hilton A. A., Ellis S., Kelly P. N., Ekert P. G., Metcalf D., Roberts A. W., Huang D. C., Kile B. T. (2007) Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 [DOI] [PubMed] [Google Scholar]
- 8. Dowling M. R., Josefsson E. C., Henley K. J., Hodgkin P. D., Kile B. T. (2010) Platelet senescence is regulated by an internal timer, not damage inflicted by hits. Blood 116, 1776–1778 [DOI] [PubMed] [Google Scholar]
- 9. Nussbaum A. K., Dick T. P., Keilholz W., Schirle M., Stevanović S., Dietz K., Heinemeyer W., Groll M., Wolf D. H., Huber R., Rammensee H. G., Schild H. (1998) Cleavage motifs of the yeast 20 S proteasome β subunits deduced from digests of enolase 1. Proc. Natl. Acad. Sci. U.S.A. 95, 12504–12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kourtis N., Tavernarakis N. (2011) Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 30, 2520–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avcu F., Ural A. U., Cetin T., Nevruz O. (2008) Effects of bortezomib on platelet aggregation and ATP release in human platelets, in vitro. Thromb. Res. 121, 567–571 [DOI] [PubMed] [Google Scholar]
- 12. Yukawa M., Sakon M., Kambayashi J., Shiba E., Kawasaki T., Uemura Y., Murata K., Tanaka T., Nakayama T., Shibata H. (1993) Purification and characterization of endogenous protein activator of human platelet proteasome. J. Biochem. 114, 317–323 [DOI] [PubMed] [Google Scholar]
- 13. Nayak M. K., Kumar K., Dash D. (2011) Regulation of proteasome activity in activated human platelets. Cell Calcium 49, 226–232 [DOI] [PubMed] [Google Scholar]
- 14. Nayak M. K., Singh S. K., Roy A., Prakash V., Kumar A., Dash D. (2011) Anti-thrombotic effects of selective estrogen receptor modulator tamoxifen. Thromb. Haemost. 106, 624–635 [DOI] [PubMed] [Google Scholar]
- 15. Ostrowska J. K., Wojtukiewicz M. Z., Chabielska E., Buczko W., Ostrowska H. (2004) Proteasome inhibitor prevents experimental arterial thrombosis in renovascular hypertensive rats. Thromb. Haemost. 92, 171–177 [DOI] [PubMed] [Google Scholar]
- 16. Lopez J. J., Salido G. M., Gómez-Arteta E., Rosado J. A., Pariente J. A. (2007) Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J. Thromb. Haemost. 5, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 17. Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Kanto T., Hiramatsu N., Yin X. M., Hayashi N. (2011) BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: molecular requisites for the mitochondrial pathway to apoptosis in platelets. J. Biol. Chem. 286, 13905–13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C., Chen S., Yue P., Deng X., Lonial S., Khuri F. R., Sun S. Y. (2010) Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IκBα degradation. J. Biol. Chem. 285, 16096–16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown S. B., Clarke M. C., Magowan L., Sanderson H., Savill J. (2000) Constitutive death of platelets leading to scavenger receptor mediated phagocytosis: a caspase-independent cell clearance program. J. Biol. Chem. 275, 5987–5996 [DOI] [PubMed] [Google Scholar]
- 20. Breitschopf K., Haendeler J., Malchow P., Zeiher A. M., Dimmeler S. (2000) Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol. Cell. Biol. 20, 1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji L., Chen Y., Liu T., Wang Z. (2008) Involvement of Bcl-xL degradation and mitochondrial-mediated apoptotic pathway in pyrrolizidine alkaloids-induced apoptosis in hepatocytes. Toxicol. Appl. Pharmacol. 231, 393–400 [DOI] [PubMed] [Google Scholar]
- 22. Fu N. Y., Sukumaran S. K., Kerk S. Y., Yu V. C. (2009) Baxβ: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol. Cell 33, 15–29 [DOI] [PubMed] [Google Scholar]
- 23. Berger G., Hartwell D. W., Wagner D. D. (1998) P-selectin and platelet clearance. Blood 92, 4446–4452 [PubMed] [Google Scholar]
- 24. Bross P. F., Kane R., Farrell A. T., Abraham S., Benson K., Brower M. E., Bradley S., Gobburu J. V., Goheer A., Lee S. L., Leighton J., Liang C. Y., Lostritto R. T., McGuinn W. D., Morse D. E., Rahman A., Rosario L. A., Verbois S. L., Williams G., Wang Y. C., Pazdur R. (2004) Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 10, 3954–3964 [DOI] [PubMed] [Google Scholar]
- 25. Zhang W., Zhao L., Liu J., Du J., Wang Z., Ruan C., Dai K. (2012) Cisplatin induces platelet apoptosis through the ERK signaling pathway. Thromb. Res. 130, 81–91 [DOI] [PubMed] [Google Scholar]
- 26. Circu M. L., Aw T. Y. (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48, 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Er E., Oliver L., Cartron P. F., Juin P., Manon S., Vallette F. M. (2006) Mitochondria as the target of the pro-apoptotic protein Bax. Biochim. Biophys. Acta 1757, 1301–1311 [DOI] [PubMed] [Google Scholar]
- 28. Wolf B. B., Goldstein J. C., Stennicke H. R., Beere H., Amarante-Mendes G. P., Salvesen G. S., Green D. R. (1999) Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood 94, 1683–1692 [PubMed] [Google Scholar]
- 29. Perfettini J. L., Reed J. C., Israël N., Martinou J. C., Dautry-Varsat A., Ojcius D. M. (2002) Role of Bcl-2 family members in caspase-independent apoptosis during Chlamydia infection. Infect. Immun. 70, 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiang J., Chao D. T., Korsmeyer S. J. (1996) Bax-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. U.S.A. 93, 14559–14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wadhawan V., Karim Z. A., Mukhopadhyay S., Gupta R., Dikshit M., Dash D. (2004) Platelet storage under in vitro condition is associated with calcium-dependent apoptosis-like lesions and novel reorganization in platelet cytoskeleton. Arch. Biochem. Biophys. 422, 183–190 [DOI] [PubMed] [Google Scholar]
- 32. Oakes S. A., Scorrano L., Opferman J. T., Bassik M. C., Nishino M., Pozzan T., Korsmeyer S. J. (2005) Proapoptotic Bax and Bak regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 102, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pereira J., Palomo I., Ocqueteau M., Soto M., Aranda E., Mezzano D. (1999) Platelet aging in vivo is associated with loss of membrane phospholipid asymmetry. Thromb. Haemost. 82, 1318–1321 [PubMed] [Google Scholar]
- 34. Ciechanover A., Finley D., Varshavsky A. (1984) Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell 37, 57–66 [DOI] [PubMed] [Google Scholar]
- 35. Varshavsky A., Bachmair A., Finley D., Gonda D. K., Wünning I. (1989) Targeting of proteins for degradation. BioTechnology 13, 109–143 [PubMed] [Google Scholar]
- 36. Hideshima T., Mitsiades C., Akiyama M., Hayashi T., Chauhan D., Richardson P., Schlossman R., Podar K., Munshi N. C., Mitsiades N., Anderson K. C. (2003) Molecular mechanisms mediating anti-myeloma activity of proteasome inhibitor PS-341. Blood 101, 1530–1534 [DOI] [PubMed] [Google Scholar]
- 37. Lin A., Karin M. (2003) NF-κB in cancer: a marked target. Semin. Cancer Biol. 13, 107–114 [DOI] [PubMed] [Google Scholar]
- 38. Sunwoo J. B., Chen Z., Dong G. (2001) Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κB, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 7, 1419–1428 [PubMed] [Google Scholar]
- 39. Zhang H., Nimmer P. M., Tahir S. K., Chen J., Fryer R. M., Hahn K. R., Iciek L. A., Morgan S. J., Nasarre M. C., Nelson R., Preusser L. C., Reinhart G. A., Smith M. L., Rosenberg S. H., Elmore S. W., Tse C. (2007) Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 40. Vanags D. M., Orrenius S., Aguilar-Santelises M. (1997) Alterations in Bcl-2/Bax protein levels in platelets form part of an ionomycin-induced process that resembles apoptosis. Br. J. Haematol. 99, 824–831 [DOI] [PubMed] [Google Scholar]
- 41. Ferraro-Peyret C., Quemeneur L., Flacher M., Revillard J. P., Genestier L. (2002) Caspase-independent phosphatidylserine exposure during apoptosis of primary T lymphocytes. J. Immunol. 169, 4805–4810 [DOI] [PubMed] [Google Scholar]
- 42. Vernace V. A., Arnaud L., Schmidt-Glenewinkel T., Figueiredo-Pereira M. E. (2007) Aging perturbs 26 S proteasome assembly in Drosophila melanogaster. FASEB J. 21, 2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaczynska M., Osmulski P. A., Ward W. F. (2001) Caretaker or undertaker? The role of the proteasome in aging. Mech. Ageing Dev. 122, 235–254 [DOI] [PubMed] [Google Scholar]
- 44. Huang L., Chen C. H. (2009) Proteasome regulators: activators and inhibitors. Curr. Med. Chem. 16, 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]