Abstract
Mucin-type O-glycosylation is an evolutionarily conserved protein modification present on membrane-bound and secreted proteins. Aberrations in O-glycosylation are responsible for certain human diseases and are associated with disease risk factors. Recent studies have demonstrated essential roles for mucin-type O-glycosylation in protein secretion, stability, processing, and function. Here, we summarize our current understanding of the diverse roles of mucin-type O-glycosylation during eukaryotic development. Appreciating how this conserved modification operates in developmental processes will provide insight into its roles in human disease and disease susceptibilities.
Keywords: Development, Glycosylation, Golgi, Mucins, Secretion, O-Glycosylation
Introduction
Glycosylation, or the addition of sugar chains to proteins, is a ubiquitous and highly conserved type of protein modification. Two of the most abundant forms of glycosylation occurring on proteins destined to be secreted or membrane-bound are N-linked (to asparagine) and mucin-type O-linked (to serine or threonine). (Other types of O-linked glycosylation exist but will not be covered in this minireview). Mucin-type O-glycosylation (hereafter referred to as O-glycosylation) is an evolutionarily conserved protein modification found in mammals, echinoderms, worms, insects, protozoa, and certain types of fungi. This modification is characterized by the initial addition of a GalNAc sugar to the hydroxyl group of serine or threonine residues. These glycans are abundant on mucins, proteins typified by repeating domains rich in proline, threonine, and serine (PTS domains). The extensive O-glycosylation present within these repeating domains serves to extend the protein backbone of mucins, transforming it from a globular structure to an extended rod-like structure (1). Additionally, these clustered regions of O-glycosylation confer both protection from proteolysis and unique rheological properties to the mucin protein core (2). Mucin-type O-glycans are also found on many other cell surface and secreted proteins and thus are uniquely positioned to modulate recognition, adhesion, and communication events occurring between cells and their surrounding environments. Indeed, roles for mucin-type O-glycans in selectin-carbohydrate-mediated cell adhesion events are well documented within the immune system (3–7). However, deciphering the developmental role of these glycans has been difficult due to the complexity associated with their synthesis. Because a large family of enzymes is responsible for catalyzing the initial addition of the GalNAc sugar, functional redundancy built into O-glycan biosynthesis has made single gene knock-outs in mammals challenging to analyze. Additionally, prediction, identification, and characterization of O-glycosylated substrates have been arduous given that there is no consensus sequence for O-GalNAc addition, no single reagent that will detect all O-glycans, and no enzyme that will efficiently remove all O-glycans regardless of their length or complexity. However, recent analytical advances have facilitated the identification of O-glycosylated proteins, and studies in model organisms have demonstrated essential roles for O-glycans in embryonic development, organogenesis, and tissue homeostasis. This minireview summarizes our current understanding of the roles of mucin-type O-glycosylation during development.
Mucin-type O-Glycosylation Is Initiated by a Family of Enzymes
Mucin-type O-glycosylation is unique among various types of glycosylation in that it is initiated by a large family of enzymes (UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (ppGalNAc-Ts)2 or GalNAc transferases in mammals and PGANTs in Drosophila; EC 2.4.1.41). Each member of this family is capable of catalyzing the addition of GalNAc (via an O-glycosidic linkage) to the hydroxyl groups of serines or threonines in protein substrates (Fig. 1) (reviewed in Refs. 8–10). There are 20 ppGalNAc-Ts in humans (11), 19 in mice, and as many as 12 in Drosophila. All family members are type II transmembrane proteins, having a short N-terminal cytoplasmic tail, a hydrophobic region that spans the Golgi membrane, and a conserved catalytic region that lies within the Golgi lumen.
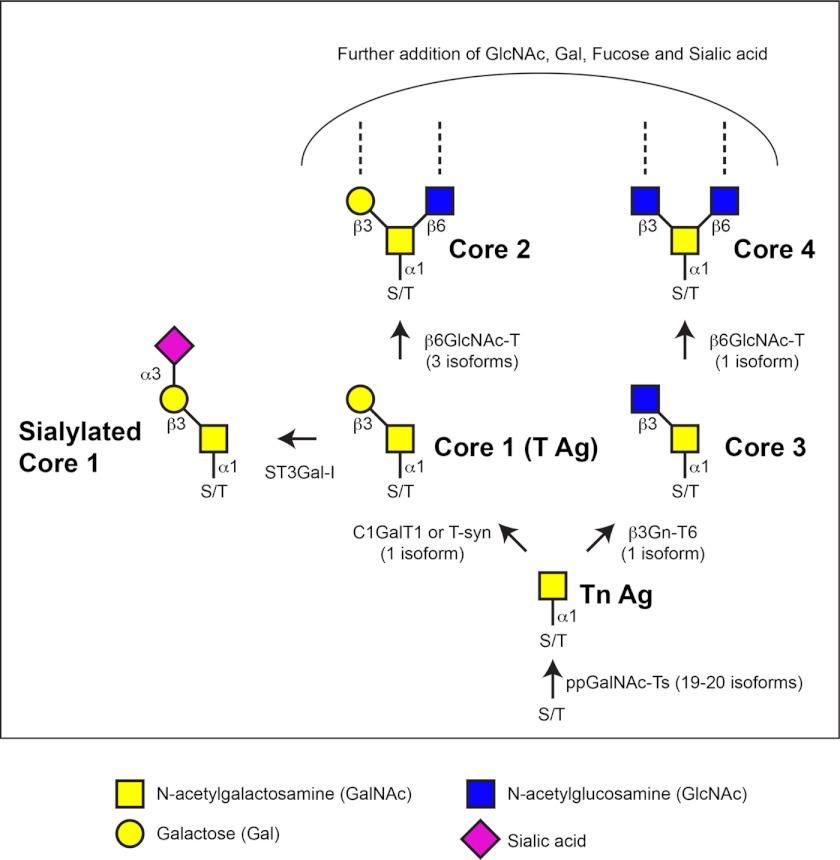
FIGURE 1.
Biosynthesis of mucin-type O-glycans. The initiation of mucin-type O-glycosylation is catalyzed by the addition of GalNAc to the hydroxyl groups of serine or threonine in protein substrates destined to be membrane-bound or secreted, forming the Tn antigen (Tn Ag). The addition of other sugars results in the formation of the core structures. Enzymes responsible for the synthesis of the Tn antigen, core 1 (T antigen (T Ag)), core 2, core 3, core 4, and sialylated core 1 structures are shown. The number of isoforms present in mammals is shown in parentheses. Additional extensions of O-glycans are not shown. C1GalT1 or T-syn, core 1 β1,3-galactosyltransferase; β3Gn-T6, β1,3-N-acetylglucosaminyltransferase 6; β6GlcNAc-T, β1,6-N-acetylglucosaminyltransferase; ST3Gal-I, α2,3-sialyltransferase I.
Why are there so many enzymes responsible for the initiation of O-glycosylation? Studies examining the expression of each ppGalNAc-T family member during both mammalian and Drosophila development have demonstrated that many members have unique tissue- and stage-specific expression patterns (12–14). Although some members are expressed widely across many developing tissues, others have very restricted expression in both space and time, suggesting that they may be performing unique functional roles (13, 15). In support of this, in vitro studies have demonstrated that certain members of this family have unique protein substrate preferences as well as specific sites of GalNAc addition within those substrates (14, 16–25). Interestingly, the substrate specificities of individual isoforms appear to be evolutionarily conserved, as mammalian and Drosophila orthologs display similar substrate preferences and preferred sites of GalNAc addition in vitro (12, 25). This conservation of enzyme specificity further suggests that individual family members may be performing conserved enzymatic and biological functions. Finally, biochemical studies have revealed that a hierarchy of activity exists within this enzyme family; certain enzymes perform the initial addition of GalNAc to unmodified substrates (initiating or peptide transferases), and others glycosylate only substrates that contain a pre-existing GalNAc (glycopeptide transferases), in many cases adding GalNAc vicinal to the site of the extant GalNAc (12, 14, 16–20, 22, 26, 27). These results suggest that an elaborate network of ppGalNAc-T activity exists to ensure the appropriate glycosylation of endogenous substrates.
After the initial addition of GalNAc, extension of the sugar chain occurs in a stepwise manner, yielding several higher order glycan structures (Fig. 1). The most common extension is known as the core 1 or T antigen structure. This structure is catalyzed by the core 1 β1,3-galactosyltransferase (T-synthase or C1GalT1), which adds galactose in a β1,3-linkage to the extant GalNAc (28, 29). The gene encoding the C1GalT1 enzyme in mammals is evolutionarily conserved; one is present in Caenorhabditis elegans (30), and as many as nine are present in Drosophila (31, 32). Although the single C1galt1 gene (in mammals and C. elegans) is widely expressed across many tissues and stages of development (28–30), the C1GalT genes in Drosophila have specific temporal and spatial patterns of expression, suggesting unique developmental roles for individual members of this family (31, 32). Interestingly, the proper folding and activity of the mammalian C1GalT1 enzyme are dependent upon an endoplasmic reticulum (ER) chaperone known as Cosmc (33). The chaperoning activity of Cosmc appears to be specific to the C1GalT1 enzyme, as loss of Cosmc affects only the synthesis of core 1 O-glycans in mammals (33–35). In contrast, the C. elegans and Drosophila C1GalT enzymes do not require such a chaperone for activity (30, 32).
The core 3 structure is catalyzed by β1,3-N-acetylglucosaminyltransferase 6, which adds a GlcNAc in a β1,3-linkage to the extant GalNAc (Fig. 1). One β1,3-N-acetylglucosaminyltransferase 6 isoform is present in mammals and expressed most abundantly in the digestive tract. Core 1 and core 3 structures can be further modified to form core 2 and core 4 structures, respectively. These structures are catalyzed by the β1,6-N-acetylglucosaminyltransferases. There are three β1,6-N-acetylglucosaminyltransferases in mammals, two of which catalyze the formation of the core 2 structure and one that can catalyze either the core 2 or core 4 structure (Fig. 1). In addition to the core structures described above, other less common core structures exist but will not be discussed in this minireview.
As illustrated in Fig. 1, the core O-glycan structures can be further modified or extended with the addition of other sugars such as galactose, GlcNAc, fucose, and sialic acid, creating extended linear or branched structures. Such branched structures are typically seen in mammals and often terminate with the negatively charged sugar sialic acid. However, O-glycans in Drosophila tend to be shorter, consisting primarily of Tn antigen and T antigen (36, 37). Additionally, Drosophila O-glycans have not been found to contain sialic acid but rather contain glucuronic acid as a terminal negatively charged sugar (36).
Mucin-type O-Glycans Are Present in Many Developing Tissues and Organs
Antibodies and sugar-binding proteins (lectins) specific for mucin-type O-linked glycans have been used to examine the presence of O-glycoproteins in situ during both mammalian and Drosophila development. Mucin-type O-glycans are present in most developing Drosophila embryonic tissues and larval imaginal discs (38). Within those tissues, O-linked glycoproteins are located in very specific regions (Fig. 2) (38–41). In epithelial tubes such as the respiratory system, the salivary glands, and the digestive tract, O-glycans are found predominantly along the apical and luminal surfaces. In other tissues, O-glycans are clustered within the basement membrane, where many extracellular matrix (ECM) proteins that mediate cell adhesion and signaling are present (Fig. 2) (40, 41). Similarly, in mammalian organs, O-linked glycans are found in distinct regions of developing organs and tissues (Fig. 2) (42, 43). Studies examining O-glycan structures present during development have further demonstrated stage-specific changes in the types and extent of O-glycan branching. Additionally, different regions of developing tissues can also express very different subsets of O-glycans, suggesting unique roles for different glycan structures as well as unique regulation of glycan synthesis in particular cells (36).
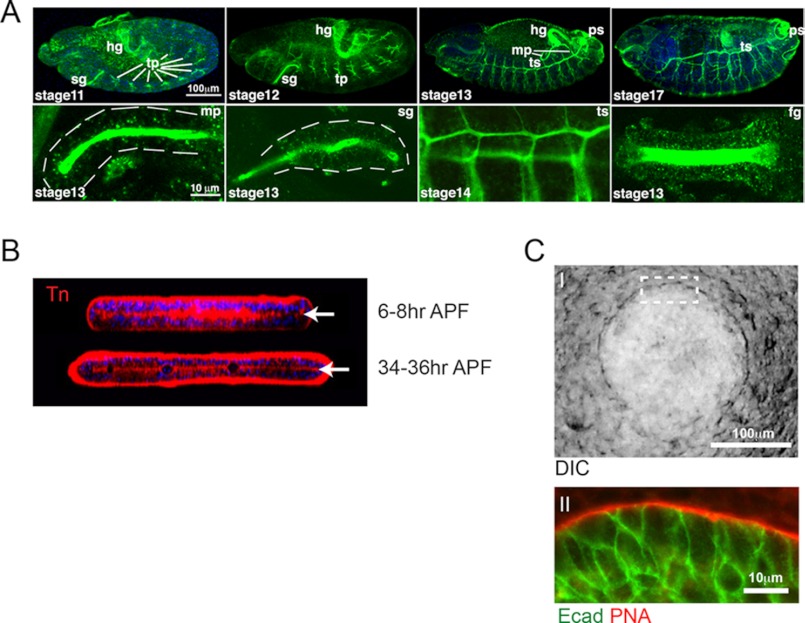
FIGURE 2.
Mucin-type O-glycan expression during development. A, Tn antigen (GalNAcα1-Ser/Thr) expression during Drosophila embryonic development (as detected by the anti-Tn antibody, green). The upper panels show whole embryos at different stages of development (stages 11–13 and 17), with the salivary glands (sg), hindgut (hg), tracheal placodes (tp), tracheal system (ts), Malpighian tubules (mp), and posterior spiracles (ps) indicated. The lower panels show magnified views of the Malpighian tubules, salivary glands, tracheal placodes, and foregut (fg). The dashed white lines outline the outer edges of specific organs. This figure is adapted from Ref. 38. B, Tn antigen expression in Drosophila pupal wings at 6–8 and 34–36 h after puparium formation (APF). X-Z optical sections are shown and demonstrate expression of Tn antigen structures at the interface (white arrows) of the dorsal and ventral cell layers of the developing wing. This figure is adapted from Ref. 40. C, expression of core 1 structures along the basement membrane during mouse embryonic SMG development. The phase-contrast image of an embryonic day 12 SMG (panel I) indicates the region (dashed white box) of higher magnification shown below (panel II). Arachis hypogaea (PNA) lectin staining (red) shows abundant core 1 structures present within the basement membrane of the developing SMG (panel II). E-cadherin (Ecad) staining of epithelial cells of the gland is shown in green. This figure is adapted from Ref. 43. DIC, differential interference contrast.
Roles for Mucin-type O-Glycosylation during Development
The first evidence that O-linked glycans are essential came from studies in the model organism Drosophila melanogaster. Mutations in one pgant family member (pgant35A) resulted in lethality (Table 1) (18, 21), and loss of both maternal and zygotic pgant35A resulted in defective respiratory system development, characterized by loss of apical-basal polarity and compromised diffusion barrier formation (39). These studies demonstrated an essential role for this modification during development as well as a more specific role in the establishment of polarity and tight junction formation within the epithelial tubes of the fly respiratory tract.
TABLE 1.
Developmental phenotypes associated with mutation or knockdown of genes directly involved in mucin-type O-glycan biosynthesis
| Gene | Protein | Species | Structure formed | Mutant phenotypes | Refs. |
|---|---|---|---|---|---|
| Galnt1 | ppGalNAcT-1 | Mouse | Tn Aga | Altered salivary gland morphogenesis due to decreased secretion of ECM components and reduced FGF signaling; induction of ER stress; lymphocyte homing and bleeding disorders | 43, 46 |
| Galnt3 | ppGalNAcT-3 | Mouse | Tn Ag | Growth retardation in males; infertility in males; decreased circulating intact FGF23; hyperphosphatemia; increased bone density | 56–58 |
| pgant3 | PGANT3 | Drosophila | Tn Ag | Decreased secretion of ECM components; loss of integrin-mediated cell adhesion during development | 40, 41 |
| pgant4 | PGANT4 | Drosophila | Tn Ag | Lethal during development | 44 |
| pgant5 | PGANT5 | Drosophila | Tn Ag | Lethal during development; loss of gut acidification | 44 |
| pgant7 | PGANT7 | Drosophila | Tn Ag | Lethal during development | 44 |
| pgant35A | PGANT35A | Drosophila | Tn Ag | Lethal during development; irregular formation of embryonic respiratory system; loss of cell polarity and diffusion barrier in respiratory system | 18, 21, 39 |
| CG30463 | CG30463 | Drosophila | Tn Ag | Lethal during development | 44 |
| xGalntl-1 | xGalntl-1 | Xenopus | Tn Ag | Smaller brain and spinal cord | 47 |
| galnt11 | galnt11 | Xenopus | Tn Ag | LR patterning defects | 48 |
| C1galt1 or T-syn | C1GalT1 or T-synthase | Mouse | Core 1 | Systemic deficiency is lethal by E14 with defective angiogenesis and brain hemorrhages; hypomorphic mutants die by day 200 and display thrombocytopenia and kidney failure with distorted glomeruli and proximal tubules; targeted disruption in endothelial cells causes embryonic/neonatal lethality with blood/lymphatic misconnections; targeted disruption in intestinal epithelium causes spontaneous colitis | 59, 60, 62, 64 |
| Cosmc | Cosmc | Mouse | Core 1 | Systemic deficiency is lethal by E12.5 with brain and spinal cord hemorrhages; targeted disruption in endothelial cells causes perinatal lethality with hemorrhages and defects in platelet biogenesis | 35, 63 |
| C1GalTA | C1GalTA | Drosophila | Core 1 | Elongated ventral nerve cord; distorted brain hemispheres; decreased number of circulating hemocytes | 31, 76 |
| Gne | GNE/MNK | Mouse | Sialylated glycans | GneM712T/M712T mutant displays loss of sialylated O-glycans in developing kidney along with glomerulopathy and podocyte effacement | 42 |
a Ag, antigen; E, embryonic day.
Recent work in Drosophila has identified four additional pgant genes that are essential for viability (44). RNAi studies identified pgant4, pgant5, pgant7, and CG30463 as essential genes during Drosophila development (Table 1) (44). Moreover, tissue-specific RNAi demonstrated essential roles for each gene in various developing organ systems. One essential gene (pgant5) is required in specialized cells of the digestive system that regulate proper gut acidification (44). Additionally, specific tissues (the mesoderm, digestive system, and respiratory system) require more than one pgant gene, suggesting a collaborative or hierarchical network of PGANT activity in vivo.
Specific roles for O-glycosylation in cell-cell interactions occurring during development have also been documented (40, 41). Loss of pgant3 resulted in loss of proper integrin-mediated cell adhesion during wing development in Drosophila (Table 1) (40). PGANT3 was found to glycosylate an integrin-binding ECM protein that is normally secreted to the basement membrane, where it coordinates cell adhesion. In the absence of PGANT3, this matrix protein was not secreted, resulting in disrupted cell adhesion. The role of O-glycosylation in secretion was further supported by cell culture studies showing that the loss of PGANT3 resulted in decreased secretion of a reporter protein and changes in the organization of the Golgi apparatus (45). Taken together, these results support a model in which O-glycosylation is required for the proper secretion of components of the ECM responsible for mediating cell-cell interactions during development.
Mice deficient in a number of ppGalNAc-T family members have been created, but many show no obvious phenotypes, likely due to the functional redundancy present among the 19 murine isoforms (reviewed in Refs. 8 and 9). However, mice deficient for Galnt1 (which have defects in lymphocyte homing and blood coagulation) were underrepresented in Mendelian ratios from crosses of heterozygotes, suggesting non-redundant developmental roles for this glycosyltransferase (46). Recent studies examining the role of Galnt1 in organs in which it is abundantly expressed during early development demonstrated specific defects in the growth and development of the embryonic heart3 and submandibular glands (SMGs) (Table 1) (43). Loss of Galnt1 resulted in defects in the secretion of basement membrane (BM) proteins normally present during early stages of SMG development (43). Loss of these proteins along the BM region resulted in decreased FGF and integrin signaling, decreased AKT and MAPK phosphorylation, and decreased epithelial cell proliferation within the SMGs. SMGs from Galnt1-deficient mice grew at a slower rate and remained smaller into adulthood. SMG growth and FGF signaling could be rescued by the addition of exogenous BM components. Additionally, the loss of Galnt1 resulted in induction of an ER stress response, suggesting that O-glycosylation may be required for efficient secretory apparatus function during certain stages of development. This study demonstrated that O-glycosylation influences the composition of the ECM during mammalian development, similar to what was found in Drosophila. In this instance, changes in the ECM affected conserved signaling events involved in cell proliferation and organogenesis (43). Additionally, this study provided evidence that loss of O-glycosylation, like N-glycosylation, can result in an ER stress response, suggesting a role for O-glycosylation in the maintenance and regulation of the proteostasis network.
Studies in Xenopus have suggested roles for O-glycosylation in TGF-β signaling during vertebrate development. Overexpression of xGalntl-1 (the ortholog of human GALNT16) resulted in inhibition of Nodal/activin and BMP signaling during Xenopus development (47). Morpholino knockdown of xGalntl-1 expression was less dramatic but did result in reduced brain and spinal cord size, indicative of changes in TGF-β signaling (Table 1) (47). It was proposed that O-glycosylation of the TGF-β receptor ActR-IIB may be responsible for the effects observed. However, the identification of the xGalntl-1 substrate(s) remains to be determined.
Studies implicating O-glycosylation in human development arose from high-resolution genotyping of patients with heterotaxy, a congenital heart defect resulting from abnormalities in left-right (LR) body patterning (48). GALNT11 was identified as one of the gene copy number variants seen in heterotaxy patients. Evidence for a causal role for GALNT11 in heterotaxy was obtained by morpholino knockdown of the GALNT11 ortholog in Xenopus, which resulted in striking disruption of LR patterning (Table 1). Although the exact mechanism by which the loss of GALNT11 affects LR patterning is not known, it was speculated that it may also involve changes in TGF-β signaling (48).
The importance of O-glycosylation in mammalian development and disease was further demonstrated by mutations in another member of the ppGalNAc-T family. Previous studies identified inactivating mutations in either GALNT3 or FGF23 as the cause of the human disease familial tumoral calcinosis, a metabolic disorder characterized by ectopic calcifications in soft tissues, bone density changes, and hyperphosphatemia (Table 1) (49–52). FGF23, a hormone responsible for regulating phosphate reabsorption in the proximal tubules of the kidney, exists as an intact active form (iFGF23) as well as an inactive cleaved form (cFGF23) (53). In the case of GALNT3 mutations, patients presented with low ratios of iFGF23/cFGF23, increased circulating phosphate levels, increased bone density, and ectopic calcifications (52). In vitro studies suggest that glycosylation of FGF23 by GALNT3 (ppGalNAc-T3) may block furin-mediated cleavage and thus alter the iFGF23/cFGF23 ratios (54). Additional studies have provided evidence that GALNT3 transcription may itself be regulated by phosphate levels, thus potentially establishing a feedback loop involving the activity of this glycosyltransferase to maintain proper phosphate homeostasis and bone density during normal growth and development (55). In support of this, recent genome-wide association studies have identified GALNT3 as one gene associated with changes in bone mineral density and fracture risk in human populations (56). Mice deficient for Galnt3 have confirmed its effect on iFGF23/cFGF23 ratios, phosphate levels, and bone mineral density (56, 57), supporting a role for O-glycosylation in regulating the proteolytic processing and bioactivity of FGF23. Moreover, Galnt3-deficient male mice also displayed growth retardation and infertility (the result of defective acrosome formation in spermatids), indicating additional roles for Galnt3 in normal growth/development and spermatogenesis, respectively (Table 1) (57, 58).
The developmental importance of mucin-type O-glycan chain extensions was first discovered in mice deficient for C1galt1 (or T-syn), the gene encoding the enzyme responsible for synthesis of the core 1 structure (Fig. 1 and Table 1). Homozygous C1galt1-deficient mice died by embryonic day 14 and suffered fatal brain hemorrhages (59) due to irregular vasculature formation. Specifically, vessels had irregular lumens, and endothelial cells displayed defects in cell adhesion, failing to properly attach to pericytes and the ECM. Mice deficient for the C1GalT1 chaperone Cosmc displayed similar hemorrhages, although Cosmc null mice died 1–2 days earlier than C1galt1 null mice (35). Mice mosaic for loss of Cosmc displayed a variety of additional phenotypes, including growth retardation and splenomegaly (35). Altogether, these studies indicate essential roles for the core 1 structure of O-glycans during mammalian development.
Additional studies have examined the effects of the core 1 structure in specific tissues. Hypomorphic mutations in C1galt1 did not cause embryonic lethality but did result in mice that were smaller than wild-type mice and had thrombocytopenia and kidney disease (60). Specifically, the kidneys in these mice had distorted glomeruli and proximal tubules, resulting in eventual renal failure and death by 200 days of age (60). It was proposed that O-glycosylation of podocalyxin, a sialylated O-glycoprotein present on the renal podocyte foot process, may be necessary for proper filtration slit formation and renal function, as mice deficient for podocalyxin (podx1−/−) also displayed similar kidney defects (61). This hypothesis was further supported by recent studies examining mice mutant for an enzyme involved in sialic acid biosynthesis, N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE/MNK), which is encoded by the Gne gene (42). Mice homozygous for the GneM712T/M712T mutation displayed loss of sialylated O-glycans in the developing kidneys, podocyte effacement, and death from renal failure by postnatal day 3 (Table 1). Hyposialylated O-glycoproteins present in the developing kidneys of these mice included podocalyxin and nephrin. Interestingly, dietary supplementation with N-acetylmannosamine, a sialic acid precursor that bypasses the GneM712T mutation, partially restored O-glycan sialylation, podocyte structure, and renal function (42). Taken together, these findings support the proposal that negatively charged sialic acids on O-linked glycoproteins (such as podocalyxin) in the developing kidneys are necessary for proper repulsion of adjacent podocyte foot processes to form functional filtration slits. This study also highlights the potential for therapeutic interventions in certain disorders of O-glycan biosynthesis.
Endothelial cell-specific deletion of C1galt1 resulted in misconnections between the blood and lymphatic systems. These mice died during embryogenesis and neonatal development and displayed vascular abnormalities similar to (but less severe than) those seen in the systemic C1galt1 deficiency (62). Endothelial cell-specific deletion of the C1galt1 chaperone Cosmc also resulted in perinatal lethality with similar venous system abnormalities (63). Interestingly, inducible loss of C1galt1 during postnatal development also resulted in the development of misconnections between venous and lymphatic vessels, especially in tissues wherein vessel remodeling was actively taking place (62). Pdpn (podoplanin), an endothelial O-glycoprotein, was partially degraded and dramatically reduced on the endothelial cell surface of C1galt1-deficient mice. Additionally, pdpn-deficient mice displayed similar vascular misconnections (62). It was proposed that core 1 O-glycans regulate the stability/cell surface expression of podoplanin, which then serves to maintain lymphatic cell identity during both embryonic and postnatal development (62).
Core 1 O-glycans also play roles in the integrity and maintenance of the colonic epithelium. Loss of C1galt1 specifically within the developing intestinal epithelium resulted in spontaneous colitis shortly after birth (64). C1galt1-deficient mice produced less intestinal mucin (Muc2) and had impaired mucosal barrier formation and integrity. Interestingly, induced loss of C1galt1 in adults also resulted in spontaneous colitis. Additionally, loss of the β1,3-N-acetylglucosaminyltransferase 6 gene (responsible for formation of the core 3 structure) caused decreased mucosal integrity and increased susceptibility to induced colitis and colon cancer (65). These studies reveal a role for O-glycans in the formation and maintenance of the protective intestinal mucosal barrier throughout the life of the animal.
Finally, studies in Drosophila have demonstrated a role for core 1 O-glycans in proper nervous system formation (31). Null mutations in one member of the Drosophila core 1 galactosyltransferase family, C1GalTA, which is expressed in the developing central nervous system, resulted in irregular elongation of the ventral nerve cord and misshapen brain hemispheres (Table 1). Although substrates for this enzyme have not been identified, it was proposed that C1GalTA may modify components of the ECM that are responsible for proper condensation of the ventral nerve cord (31).
Summary and Perspectives
Alterations in mucin-type O-linked glycosylation are known to be responsible for the human diseases familial tumoral calcinosis (51, 52) and Tn syndrome (a disorder characterized by thrombocytopenia and hemolytic anemia) (66). Additionally, recent linkage and genome-wide association studies have identified associations between components of the O-glycosylation machinery and other human conditions and diseases (48, 67–72). For example, GALNT2 is among the genes associated with variations in plasma lipid levels and thus cardiovascular disease risk (67, 72); GALNT3 is associated with bone density and fracture risk (56); and GALNT4 is associated with acute coronary disease risk (73). Changes in O-glycosylation, as well as inactivating mutations in the genes controlling O-glycosylation, are also associated with tumor formation and progression (68–71). These studies show the potential wide-ranging effects of this conserved protein modification in human health and disease and illustrate why a clear functional understanding of O-glycosylation is paramount.
The studies summarized here highlight essential roles played by mucin-type O-glycosylation across species and in many developing systems (Table 1). We have evidence that O-glycosylation impacts the cell surface presentation and function of proteins, altering the polarity, morphology, and differentiation status of cells and tissues. Additionally, O-glycosylation modulates proteolytic processing, thus regulating the bioactivity of proteins involved in developmental and homeostatic processes. Finally, O-glycosylation influences the secretion and composition of the ECM in a number of systems, thereby affecting cell adhesion and conserved signaling pathways during development. These and other studies have further suggested that O-glycosylation may also be involved in sensing the status of the secretory apparatus and regulating ER stress-related processes (43, 74). In support of this, a number of proteins involved in sensing and responding to ER stress have recently been shown to be O-glycosylated (75), suggesting that proper glycosylation of these proteins may relay the status of the Golgi apparatus and protein transport to the proteostasis network during crucial stages of development. If O-glycosylation acts as a type of sensor during times of increased secretory burden, it may play roles that are more widespread than previously thought.
Future progress in deciphering how O-glycans influence dynamic developmental processes will require additional methodologies to predict, detect, characterize, and track O-glycosylated proteins in vivo. In particular, facile tools for identification and characterization of all O-glycoproteins present within limited amounts of developing tissues are needed. If we are to understand how O-glycans work, we must know the total complement of substrate proteins to which they are attached. In addition, the sites of glycan addition within those proteins and the specific glycan structures present will need to be determined to gain a complete understanding of the role these modifications play in protein stability, recognition, transport, and function. Real-time imaging of O-glycans and the proteins to which they are attached will allow us to investigate dynamic cellular and subcellular changes occurring during development as a result of O-glycosylation. Finally, robust predictive tools are needed to bioinformatically screen for O-glycosylated substrates and sites of addition to take advantage of the vast amounts of information present in various model organism databases. The combination of genetic, cell biological, analytical, biochemical, and bioinformatic tools should provide mechanistic insight into how specific O-glycan structures affect the dynamic properties of their substrates in developing tissues and organs.
In summary, studies in model organisms have demonstrated that mucin-type O-glycosylation is essential for eukaryotic development. Future work capitalizing on insights gained from these systems will enable us to decipher the dynamic roles of O-glycans in many cellular and developmental processes. Knowing how O-glycosylation functions during development will afford us a clearer understanding of its dysfunction in disease.
Acknowledgments
We thank the many members of the community who have contributed to the work mentioned herein.
This work was supported by the Intramural Research Program of the NIDCR, National Institutes of Health. This is the third article in the Thematic Minireview Series on Glycobiology and Extracellular Matrices: Glycan Functions Pervade Biology at All Levels.
Y. Guan and L. A. Tabak, personal communication.
- ppGalNAc-T
- UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase
- ER
- endoplasmic reticulum
- ECM
- extracellular matrix
- SMG
- submandibular gland
- BM
- basement membrane
- LR
- left-right
- iFGF23
- intact FGF23
- cFGF23
- cleaved FGF23.
REFERENCES
- 1. Shogren R., Gerken T. A., Jentoft N. (1989) Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry 28, 5525–5536 [DOI] [PubMed] [Google Scholar]
- 2. Tabak L. A. (1995) In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu. Rev. Physiol. 57, 547–564 [DOI] [PubMed] [Google Scholar]
- 3. Broide D. H., Miller M., Castaneda D., Nayar J., Cho J. Y., Roman M., Ellies L. G., Sriramarao P. (2002) Core 2 oligosaccharides mediate eosinophil and neutrophil peritoneal but not lung recruitment. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L259–L266 [DOI] [PubMed] [Google Scholar]
- 4. Ellies L. G., Tsuboi S., Petryniak B., Lowe J. B., Fukuda M., Marth J. D. (1998) Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity 9, 881–890 [DOI] [PubMed] [Google Scholar]
- 5. Homeister J. W., Thall A. D., Petryniak B., Malý P., Rogers C. E., Smith P. L., Kelly R. J., Gersten K. M., Askari S. W., Cheng G., Smithson G., Marks R. M., Misra A. K., Hindsgaul O., von Andrian U. H., Lowe J. B. (2001) The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 [DOI] [PubMed] [Google Scholar]
- 6. Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J. M., Yu S. Y., Kawashima H., Saito H., Ohtsubo K., Marth J. D., Khoo K. H., von Andrian U. H., Lowe J. B., Fukuda M. (2007) Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat. Immunol. 8, 409–418 [DOI] [PubMed] [Google Scholar]
- 7. Priatel J. J., Chui D., Hiraoka N., Simmons C. J., Richardson K. B., Page D. M., Fukuda M., Varki N. M., Marth J. D. (2000) The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity 12, 273–283 [DOI] [PubMed] [Google Scholar]
- 8. Bennett E. P., Mandel U., Clausen H., Gerken T. A., Fritz T. A., Tabak L. A. (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabak L. A. (2010) The role of mucin-type O-glycans in eukaryotic development. Semin. Cell Dev. Biol. 21, 616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tian E., Ten Hagen K. G. (2009) Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 26, 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raman J., Guan Y., Perrine C. L., Gerken T. A., Tabak L. A. (2012) UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferases: completion of the family tree. Glycobiology 22, 768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ten Hagen K. G., Tran D. T., Gerken T. A., Stein D. S., Zhang Z. (2003) Functional characterization and expression analysis of members of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family from Drosophila melanogaster. J. Biol. Chem. 278, 35039–35048 [DOI] [PubMed] [Google Scholar]
- 13. Tian E., Ten Hagen K. G. (2006) Expression of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family is spatially and temporally regulated during Drosophila development. Glycobiology 16, 83–95 [DOI] [PubMed] [Google Scholar]
- 14. Zara J., Hagen F. K., Ten Hagen K. G., Van Wuyckhuyse B. C., Tabak L. A. (1996) Cloning and expression of mouse UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-T3. Biochem. Biophys. Res. Commun. 228, 38–44 [DOI] [PubMed] [Google Scholar]
- 15. Kingsley P. D., Hagen K. G., Maltby K. M., Zara J., Tabak L. A. (2000) Diverse spatial expression patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family member mRNAs during mouse development. Glycobiology 10, 1317–1323 [DOI] [PubMed] [Google Scholar]
- 16. Bennett E. P., Hassan H., Mandel U., Hollingsworth M. A., Akisawa N., Ikematsu Y., Merkx G., van Kessel A. G., Olofsson S., Clausen H. (1999) Cloning and characterization of a close homologue of human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J. Biol. Chem. 274, 25362–25370 [DOI] [PubMed] [Google Scholar]
- 17. Nehrke K., Hagen F. K., Tabak L. A. (1998) Isoform-specific O-glycosylation by murine UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-T3 in vivo. Glycobiology 8, 367–371 [DOI] [PubMed] [Google Scholar]
- 18. Schwientek T., Bennett E. P., Flores C., Thacker J., Hollmann M., Reis C. A., Behrens J., Mandel U., Keck B., Schäfer M. A., Haselmann K., Zubarev R., Roepstorff P., Burchell J. M., Taylor-Papadimitriou J., Hollingsworth M. A., Clausen H. (2002) Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila. J. Biol. Chem. 277, 22623–22638 [DOI] [PubMed] [Google Scholar]
- 19. Takeuchi H., Kato K., Hassan H., Clausen H., Irimura T. (2002) O-GalNAc incorporation into a cluster acceptor site of three consecutive threonines. Distinct specificity of GalNAc-transferase isoforms. Eur. J. Biochem. 269, 6173–6183 [DOI] [PubMed] [Google Scholar]
- 20. Ten Hagen K. G., Hagen F. K., Balys M. M., Beres T. M., Van Wuyckhuyse B., Tabak L. A. (1998) Cloning and expression of a novel, tissue specifically expressed member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family. J. Biol. Chem. 273, 27749–27754 [DOI] [PubMed] [Google Scholar]
- 21. Ten Hagen K. G., Tran D. T. (2002) A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is essential for viability in Drosophila melanogaster. J. Biol. Chem. 277, 22616–22622 [DOI] [PubMed] [Google Scholar]
- 22. Wandall H. H., Hassan H., Mirgorodskaya E., Kristensen A. K., Roepstorff P., Bennett E. P., Nielsen P. A., Hollingsworth M. A., Burchell J., Taylor-Papadimitriou J., Clausen H. (1997) Substrate specificities of three members of the human UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J. Biol. Chem. 272, 23503–23514 [DOI] [PubMed] [Google Scholar]
- 23. Gerken T. A., Jamison O., Perrine C. L., Collette J. C., Moinova H., Ravi L., Markowitz S. D., Shen W., Patel H., Tabak L. A. (2011) Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem. 286, 14493–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerken T. A., Raman J., Fritz T. A., Jamison O. (2006) Identification of common and unique peptide substrate preferences for the UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. J. Biol. Chem. 281, 32403–32416 [DOI] [PubMed] [Google Scholar]
- 25. Gerken T. A., Ten Hagen K. G., Jamison O. (2008) Conservation of peptide acceptor preferences between Drosophila and mammalian polypeptide-GalNAc transferase ortholog pairs. Glycobiology 18, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ten Hagen K. G., Bedi G. S., Tetaert D., Kingsley P. D., Hagen F. K., Balys M. M., Beres T. M., Degand P., Tabak L. A. (2001) Cloning and characterization of a ninth member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family, ppGaNTase-T9. J. Biol. Chem. 276, 17395–17404 [DOI] [PubMed] [Google Scholar]
- 27. Ten Hagen K. G., Tetaert D., Hagen F. K., Richet C., Beres T. M., Gagnon J., Balys M. M., VanWuyckhuyse B., Bedi G. S., Degand P., Tabak L. A. (1999) Characterization of a UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase that displays glycopeptide N-acetylgalactosaminyltransferase activity. J. Biol. Chem. 274, 27867–27874 [DOI] [PubMed] [Google Scholar]
- 28. Ju T., Brewer K., D'Souza A., Cummings R. D., Canfield W. M. (2002) Cloning and expression of human core 1 β1,3-galactosyltransferase. J. Biol. Chem. 277, 178–186 [DOI] [PubMed] [Google Scholar]
- 29. Ju T., Cummings R. D., Canfield W. M. (2002) Purification, characterization, and subunit structure of rat core 1 β1,3-galactosyltransferase. J. Biol. Chem. 277, 169–177 [DOI] [PubMed] [Google Scholar]
- 30. Ju T., Zheng Q., Cummings R. D. (2006) Identification of core 1 O-glycan T-synthase from Caenorhabditis elegans. Glycobiology 16, 947–958 [DOI] [PubMed] [Google Scholar]
- 31. Lin Y. R., Reddy B. V., Irvine K. D. (2008) Requirement for a core 1 galactosyltransferase in the Drosophila nervous system. Dev. Dyn. 237, 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Müller R., Hülsmeier A. J., Altmann F., Ten Hagen K., Tiemeyer M., Hennet T. (2005) Characterization of mucin-type core-1 β1–3-galactosyltransferase homologous enzymes in Drosophila melanogaster. FEBS J. 272, 4295–4305 [DOI] [PubMed] [Google Scholar]
- 33. Ju T., Cummings R. D. (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. U.S.A. 99, 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narimatsu Y., Ikehara Y., Iwasaki H., Nonomura C., Sato T., Nakanishi H., Narimatsu H. (2008) Immunocytochemical analysis for intracellular dynamics of C1GalT associated with molecular chaperone, Cosmc. Biochem. Biophys. Res. Commun. 366, 199–205 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y., Ju T., Ding X., Xia B., Wang W., Xia L., He M., Cummings R. D. (2010) Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. U.S.A. 107, 9228–9233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aoki K., Porterfield M., Lee S. S., Dong B., Nguyen K., McGlamry K. H., Tiemeyer M. (2008) The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J. Biol. Chem. 283, 30385–30400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. North S. J., Koles K., Hembd C., Morris H. R., Dell A., Panin V. M., Haslam S. M. (2006) Glycomic studies of Drosophila melanogaster embryos. Glycoconj. J. 23, 345–354 [DOI] [PubMed] [Google Scholar]
- 38. Tian E., Ten Hagen K. G. (2007) O-Linked glycan expression during Drosophila development. Glycobiology 17, 820–827 [DOI] [PubMed] [Google Scholar]
- 39. Tian E., Ten Hagen K. G. (2007) A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is required for epithelial tube formation. J. Biol. Chem. 282, 606–614 [DOI] [PubMed] [Google Scholar]
- 40. Zhang L., Tran D. T., Ten Hagen K. G. (2010) An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J. Biol. Chem. 285, 19491–19501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L., Zhang Y., Hagen K. G. (2008) A mucin-type O-glycosyltransferase modulates cell adhesion during Drosophila development. J. Biol. Chem. 283, 34076–34086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kakani S., Yardeni T., Poling J., Ciccone C., Niethamer T., Klootwijk E. D., Manoli I., Darvish D., Hoogstraten-Miller S., Zerfas P., Tian E., Ten Hagen K. G., Kopp J. B., Gahl W. A., Huizing M. (2012) The GneM712T mouse as a model for human glomerulopathy. Am. J. Pathol. 180, 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tian E., Hoffman M. P., Ten Hagen K. G. (2012) O-Glycosylation modulates integrin and FGF signalling by influencing the secretion of basement membrane components. Nat. Commun. 3, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tran D. T., Zhang L., Zhang Y., Tian E., Earl L. A., Ten Hagen K. G. (2012) Multiple members of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family are essential for viability in Drosophila. J. Biol. Chem. 287, 5243–5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang L., Ten Hagen K. G. (2010) Dissecting the biological role of mucin-type O-glycosylation using RNA interference in Drosophila cell culture. J. Biol. Chem. 285, 34477–34484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tenno M., Ohtsubo K., Hagen F. K., Ditto D., Zarbock A., Schaerli P., von Andrian U. H., Ley K., Le D., Tabak L. A., Marth J. D. (2007) Initiation of protein O-glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol. Cell. Biol. 27, 8783–8796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Herr P., Korniychuk G., Yamamoto Y., Grubisic K., Oelgeschläger M. (2008) Regulation of TGF-β signalling by N-acetylgalactosaminyltransferase-like 1. Development 135, 1813–1822 [DOI] [PubMed] [Google Scholar]
- 48. Fakhro K. A., Choi M., Ware S. M., Belmont J. W., Towbin J. A., Lifton R. P., Khokha M. K., Brueckner M. (2011) Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc. Natl. Acad. Sci. U.S.A. 108, 2915–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Araya K., Fukumoto S., Backenroth R., Takeuchi Y., Nakayama K., Ito N., Yoshii N., Yamazaki Y., Yamashita T., Silver J., Igarashi T., Fujita T. (2005) A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J. Clin. Endocrinol. Metab. 90, 5523–5527 [DOI] [PubMed] [Google Scholar]
- 50. Benet-Pagès A., Orlik P., Strom T. M., Lorenz-Depiereux B. (2005) An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 14, 385–390 [DOI] [PubMed] [Google Scholar]
- 51. Ichikawa S., Lyles K. W., Econs M. J. (2005) A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J. Clin. Endocrinol. Metab. 90, 2420–2423 [DOI] [PubMed] [Google Scholar]
- 52. Topaz O., Shurman D. L., Bergman R., Indelman M., Ratajczak P., Mizrachi M., Khamaysi Z., Behar D., Petronius D., Friedman V., Zelikovic I., Raimer S., Metzker A., Richard G., Sprecher E. (2004) Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 36, 579–581 [DOI] [PubMed] [Google Scholar]
- 53. Shimada T., Muto T., Urakawa I., Yoneya T., Yamazaki Y., Okawa K., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. (2002) Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 [DOI] [PubMed] [Google Scholar]
- 54. Kato K., Jeanneau C., Tarp M. A., Benet-Pagès A., Lorenz-Depiereux B., Bennett E. P., Mandel U., Strom T. M., Clausen H. (2006) Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 [DOI] [PubMed] [Google Scholar]
- 55. Chefetz I., Sprecher E. (2009) Familial tumoral calcinosis and the role of O-glycosylation in the maintenance of phosphate homeostasis. Biochim. Biophys. Acta 1792, 847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duncan E. L., Danoy P., Kemp J. P., Leo P. J., McCloskey E., Nicholson G. C., Eastell R., Prince R. L., Eisman J. A., Jones G., Sambrook P. N., Reid I. R., Dennison E. M., Wark J., Richards J. B., Uitterlinden A. G., Spector T. D., Esapa C., Cox R. D., Brown S. D., Thakker R. V., Addison K. A., Bradbury L. A., Center J. R., Cooper C., Cremin C., Estrada K., Felsenberg D., Glüer C. C., Hadler J., Henry M. J., Hofman A., Kotowicz M. A., Makovey J., Nguyen S. C., Nguyen T. V., Pasco J. A., Pryce K., Reid D. M., Rivadeneira F., Roux C., Stefansson K., Styrkarsdottir U., Thorleifsson G., Tichawangana R., Evans D. M., Brown M. A. (2011) Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 7, e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ichikawa S., Sorenson A. H., Austin A. M., Mackenzie D. S., Fritz T. A., Moh A., Hui S. L., Econs M. J. (2009) Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 150, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miyazaki T., Mori M., Yoshida C. A., Ito C., Yamatoya K., Moriishi T., Kawai Y., Komori H., Kawane T., Izumi S. I., Toshimori K., Komori T. (2012) Galnt3 deficiency disrupts acrosome formation and leads to oligoasthenoteratozoospermia. Histochem. Cell Biol., in press [DOI] [PubMed] [Google Scholar]
- 59. Xia L., Ju T., Westmuckett A., An G., Ivanciu L., McDaniel J. M., Lupu F., Cummings R. D., McEver R. P. (2004) Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J. Cell Biol. 164, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alexander W. S., Viney E. M., Zhang J. G., Metcalf D., Kauppi M., Hyland C. D., Carpinelli M. R., Stevenson W., Croker B. A., Hilton A. A., Ellis S., Selan C., Nandurkar H. H., Goodnow C. C., Kile B. T., Nicola N. A., Roberts A. W., Hilton D. J. (2006) Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc. Natl. Acad. Sci. U.S.A. 103, 16442–16447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Doyonnas R., Kershaw D. B., Duhme C., Merkens H., Chelliah S., Graf T., McNagny K. M. (2001) Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 194, 13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fu J., Gerhardt H., McDaniel J. M., Xia B., Liu X., Ivanciu L., Ny A., Hermans K., Silasi-Mansat R., McGee S., Nye E., Ju T., Ramirez M. I., Carmeliet P., Cummings R. D., Lupu F., Xia L. (2008) Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J. Clin. Invest. 118, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y., Jobe S. M., Ding X., Choo H., Archer D. R., Mi R., Ju T., Cummings R. D. (2012) Platelet biogenesis and functions require correct protein O-glycosylation. Proc. Natl. Acad. Sci. U.S.A. 109, 16143–16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fu J., Wei B., Wen T., Johansson M. E., Liu X., Bradford E., Thomsson K. A., McGee S., Mansour L., Tong M., McDaniel J. M., Sferra T. J., Turner J. R., Chen H., Hansson G. C., Braun J., Xia L. (2011) Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. An G., Wei B., Xia B., McDaniel J. M., Ju T., Cummings R. D., Braun J., Xia L. (2007) Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 204, 1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ju T., Cummings R. D. (2005) Protein glycosylation: chaperone mutation in Tn syndrome. Nature 437, 1252. [DOI] [PubMed] [Google Scholar]
- 67. Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., Wahlstrand B., Hedner T., Corella D., Tai E. S., Ordovas J. M., Berglund G., Vartiainen E., Jousilahti P., Hedblad B., Taskinen M. R., Newton-Cheh C., Salomaa V., Peltonen L., Groop L., Altshuler D. M., Orho-Melander M. (2008) Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim Y. J., Varki A. (1997) Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 14, 569–576 [DOI] [PubMed] [Google Scholar]
- 69. Kim Y. S., Gum J., Jr., Brockhausen I. (1996) Mucin glycoproteins in neoplasia. Glycoconj. J. 13, 693–707 [DOI] [PubMed] [Google Scholar]
- 70. Ono M., Hakomori S. (2004) Glycosylation defining cancer cell motility and invasiveness. Glycoconj. J. 20, 71–78 [DOI] [PubMed] [Google Scholar]
- 71. Springer G. F. (1997) Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 75, 594–602 [DOI] [PubMed] [Google Scholar]
- 72. Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., Strait J., Duren W. L., Maschio A., Busonero F., Mulas A., Albai G., Swift A. J., Morken M. A., Narisu N., Bennett D., Parish S., Shen H., Galan P., Meneton P., Hercberg S., Zelenika D., Chen W. M., Li Y., Scott L. J., Scheet P. A., Sundvall J., Watanabe R. M., Nagaraja R., Ebrahim S., Lawlor D. A., Ben-Shlomo Y., Davey-Smith G., Shuldiner A. R., Collins R., Bergman R. N., Uda M., Tuomilehto J., Cao A., Collins F. S., Lakatta E., Lathrop G. M., Boehnke M., Schlessinger D., Mohlke K. L., Abecasis G. R. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40, 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O'Halloran A. M., Patterson C. C., Horan P., Maree A., Curtin R., Stanton A., McKeown P. P., Shields D. C. (2009) Genetic polymorphisms in platelet-related proteins and coronary artery disease: investigation of candidate genes, including N-acetylgalactosaminyltransferase 4 (GALNT4) and sulphotransferase 1A1/2 (SULT1A1/2). J. Thromb. Thrombolysis 27, 175–184 [DOI] [PubMed] [Google Scholar]
- 74. Xu Y. X., Liu L., Caffaro C. E., Hirschberg C. B. (2010) Inhibition of Golgi apparatus glycosylation causes endoplasmic reticulum stress and decreased protein synthesis. J. Biol. Chem. 285, 24600–24608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Steentoft C., Vakhrushev S. Y., Vester-Christensen M. B., Schjoldager K. T., Kong Y., Bennett E. P., Mandel U., Wandall H., Levery S. B., Clausen H. (2011) Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 [DOI] [PubMed] [Google Scholar]
- 76. Yoshida H., Fuwa T. J., Arima M., Hamamoto H., Sasaki N., Ichimiya T., Osawa K., Ueda R., Nishihara S. (2008) Identification of the Drosophila core 1 beta1,3-galactosyltransferase gene that synthesizes T antigen in the embryonic central nervous system and hemocytes. Glycobiology 18, 1094–1104 [DOI] [PubMed] [Google Scholar]