Background: SirT1 regulates mitochondrial biogenesis in various tissues.
Results: Exercise combined with resveratrol has a SirT1-dependent synergistic effect on mitochondrial biogenesis, despite individual treatments being SirT1-independent.
Conclusion: SirT1 is important for maintaining muscle mitochondrial content and function.
Significance: The dependence of muscle mitochondrial biogenesis on SirT1 depends on the metabolic state of the muscle.
Keywords: Exercise, Reactive Oxygen Species (ROS), Resveratrol, Sirt1, Skeletal Muscle, Nampt, PGC-1α, Cytochrome c Oxidase Activity, Mitochondrial Biogenesis, Mitochondrial Respiration
Abstract
The purpose of this study was to evaluate the role of sirtuin 1 (SirT1) in exercise- and resveratrol (RSV)-induced skeletal muscle mitochondrial biogenesis. Using muscle-specific SirT1-deficient (KO) mice and a cell culture model of differentiated myotubes, we compared the treatment of resveratrol, an activator of SirT1, with that of exercise in inducing mitochondrial biogenesis. These experiments demonstrated that SirT1 plays a modest role in maintaining basal mitochondrial content and a larger role in preserving mitochondrial function. Furthermore, voluntary exercise and RSV treatment induced mitochondrial biogenesis in a SirT1-independent manner. However, when RSV and exercise were combined, a SirT1-dependent synergistic effect was evident, leading to enhanced translocation of PGC-1α and SirT1 to the nucleus and stimulation of mitochondrial biogenesis. Thus, the magnitude of the effect of RSV on muscle mitochondrial biogenesis is reliant on SirT1, as well as the cellular environment, such as that produced by repeated bouts of exercise.
Introduction
As the largest organ in the body, skeletal muscle has demonstrated great adaptive potential in response to physiological stressors. The most potent example of muscle adaptation occurs in mitochondria following exercise training. The biogenesis of new mitochondria, and the clearance of damaged mitochondria, promotes healthy muscle. This turnover can prevent imbalances in energy homeostasis that lead to obesity, diabetes, cardiovascular disease, and aging (1, 2). Mitochondrial biogenesis in response to exercise is reliant, in part, on adaptive changes in the expression of the transcriptional coactivator PGC-1α, along with its phosphorylation by AMP kinase (AMPK)3 (3). In addition, the histone deacetylase enzyme SirT1 has been shown to activate PGC-1α (4–6). The activity of SirT1 is reliant on the provision of NAD+, a necessary coenzyme synthesized by the rate-limiting enzyme for NAD+ biosynthesis, nicotinamide phosphoribosyltransferase (Nampt) (7, 8). In turn, this protein is regulated by both AMPK and SirT1 (9, 10). The integration of these reactions has been shown to induce the metabolic adaptations that occur during states of energy deprivation such as fasting or exercise (11). We suspect that this Nampt-SirT1-AMPK metabolic sensing pathway plays a dominant role in mitochondrial turnover by influencing organelle biogenesis and autophagy following cellular stress (12). During acute bouts of exercise, AMPK can be activated through the modulation of the AMP/ATP ratio, whereas SirT1 has the potential to be activated via changes in NAD+ levels within the cell (9). Therefore, the metabolic alterations that occur with acute exercise activate these enzyme systems to regulate the phosphorylation and deacetylation of PGC-1α, the downstream autoregulatory loop controlling PGC-1α expression (13), and the subsequent mitochondrial biogenesis.
The natural polyphenolic compound resveratrol (RSV) is well known for its phytoestrogenic and antioxidant properties (14). RSV became widely researched following indications that this compound could increase longevity in lower organisms and increase the health and survival of mice (15–17). RSV has also been described as an exercise mimetic through its activation of SirT1 and AMPK (18). Mice treated with RSV demonstrate elevations in AMPK activation and PGC-1α expression, along with increases in mitochondria in animals fed a high fat diet (19). PGC-1α acts as a transcriptional coactivator for nuclear genes encoding mitochondrial proteins (20). As a result, RSV treatment appears to induce a higher aerobic capacity in mice, as shown by increased running time and oxygen consumption in muscle fibers (21). Thus, these findings have generated interest in the therapeutic potential of RSV, or of similar analogues, for the treatment of type 2 diabetes and obesity (22, 23). However, there is still some controversy regarding how RSV activates mitochondrial biogenesis and the dependence of this effect on SirT1 and AMPK. Studies have contended that RSV may rely on pathways upstream of either calcium-calmodulin kinase II (24) or LKB1 (25) to activate AMPK-dependent increases in the NAD-to-NADH ratio for the induction of SirT1 activity. Because the effect of RSV on SirT1 activation is controversial, we have examined the role of SirT1 from the viewpoint that this protein may be required, in part, during muscle mitochondrial biogenesis. We predicted that chronic treatment with RSV may have both SirT1-dependent and -independent effects on the induction of mitochondrial biogenesis that are dependent on the metabolic status of the tissue.
To address how SirT1 is involved in muscle mitochondrial function and biogenesis, we compared WT mice with those that express a deacetylase-deficient SirT1 protein driven by a muscle-specific MLCf1-Cre promoter. These mice were either treated with a control or RSV-supplemented diet, while being either maintained sedentary or subjected to exercise training via voluntary wheel running. Similarly, we used chronic electrical stimulation of C2C12 mouse muscle myotubes to examine the differences in mitochondrial biogenesis when induced by RSV, chronic stimulation, or the combination of these treatments. Our study demonstrates that exercise, or chronic contractile activity (CCA), has more robust effects on the induction of mitochondrial biogenesis compared with RSV treatment in either mice or in C2C12 myotubes. However, RSV was shown to act synergistically with exercise to increase mitochondrial content, an effect that was dependent on the presence of SirT1.
EXPERIMENTAL PROCEDURES
Cell Culture and CCA
Embryonic murine skeletal muscle C2C12 myoblasts (ATCC) were differentiated into myotubes and stimulated to induce CCA as described earlier (20). CCA was performed using either one or four successive 3-h bouts (1- and 4-day protocols) of electrical stimulation (5 Hz, 7 V) with an intervening 21-h recovery period following each 3-h bout. Myotubes were treated separately with CCA or in combination with 100 μm RSV, 50 μm quercetin, or the SirT1 inhibitor nicotinamide (NAM; 5 mm) during both the stimulation and recovery periods.
Animal Models
SirT1loxP/loxP mice (generated with heterozygous B6;129-SirT1tm1Ygu/J mice; JAX Laboratory) have a loxP-flanked neomycin cassette upstream of exon 4 and another loxP site downstream of exon 4 that was inserted to create this targeted mutant SirT1 allele. These mice were then bred with MLC1fCre recombinase mice to create a Cre/loxP recombination. MLC1f is a splice variant, along with MLC3f, of the Myl1 gene expressed in fast skeletal muscle fibers. The resulting offspring have exon 4 of the SirT1 gene, encoding 51 amino acids of the SirT1 catalytic domain, deleted in Cre-expressing tissues. We describe our data by referring to the Cre+-SirT1loxP/loxP animals as muscle-specific “SirT1-KO” mice. All experimental mice were paired to the WT Cre-negative SirT1loxP/loxP littermates of the same age and used at ∼2–3 months.
SirtT1 Activity
Muscle-specific SirT1-KO quadriceps exhibited 50% lower SirT1 activity than WT mice. Residual SirT1 activity was expected from any nonmuscular tissues and muscle fibers not expressing MLCf1. It has been estimated that 45% of the nuclei within skeletal muscle tissue is within muscle cells, whereas the remaining nuclei are composed of other tissue types such as adipocytes, fibroblasts, and Schwann cells (26). SirT1 activity was measured using an in vitro SirT1 fluorometric assay (Biomol) as described by the manufacturer and as done previously (27).
Acute in Situ Muscle Stimulation
The stimulation protocol was performed as described previously (28). The sciatic nerve from the gastrocnemius muscle of one leg was stimulated at 1 and 3 tetanic contractions/s for 2 min each, intensities that are sufficient to cause moderate and more severe muscle fatigue, respectively. Following the in situ stimulation protocol, the gastrocnemius muscles from the electrically stimulated leg and the nonstimulated contralateral leg of the animal were excised and weighed.
RSV Treatment and Voluntary Wheel Running
SirT1-KO and WT mice were treated for 9 weeks with RSV. RSV was reconstituted in mouse chow at a concentration of 1 g/kg in a phytoestrogen-free control diet (29, 30). This treatment was performed in conjunction with mice that were placed in cages with voluntary running wheels and trained over a 9-week period. Based on their food consumption and body weights, the dose of RSV administered was ∼100 mg of RSV/kg of body mass/day. RSV-treated mice were compared with untrained or trained mice fed control diets.
Histochemistry
COX and succinate dehydrogenase (SDH) staining was performed on 10-μm serial sections of gastrocnemius muscle. After drying for 5 min at room temperature, these sections were immersed in either COX or SDH reaction solutions for 30 min in the dark at 25 °C. COX reaction solution (pH 7.4) contained 16.75 mm sodium phosphate monobasic, 81 mm sodium phosphate biphasic, 5 mm 3,3′-Diaminobenzidine, 90 μm cytochrome c, and 6000 units catalase. The SDH reaction solution (pH 7.6) consisted of 11.5 mm sodium phosphate monobasic, 87 mm sodium phosphate biphasic, 50 mm succinate, 200 μm 1-Methoxy-5-methylphenazinium methosulfate, 1.5 mm nitro blue tetrazolium, and 1 mm sodium azide.
Muscle Isolation and Preparation
The tibialis anterior, triceps, and gastrocnemius muscles from both sides of the animal were quickly harvested, weighed, and placed in ice-cold mitochondrial isolation buffer. The quadriceps muscles were sectioned, freeze-clamped with aluminum tongs pre-cooled in liquid nitrogen, and stored at −70 °C for subsequent use in cytochrome c oxidase (COX) enzyme activity measurements and Western blotting analyses.
Mitochondrial Respiration and Cytochrome c Oxidase (COX) Activity
Samples of isolated mitochondria were examined as done previously (28). Briefly, respiration rates driven by complex I in the mitochondrial electron transport chain were evaluated in the presence of 10 mm glutamate (state 4 respiration) and glutamate with 0.44 mm ADP (state 3 respiration) using the Mitocell S200 Micro Respirometry System (Strathkelvin Instruments). The addition of NADH during state 3 measurements had no substantial effect on the respiration rate, indicating good inner mitochondrial membrane integrity. In addition, COX activity of the mixed powdered muscle from control and muscle-specific SirT1-KO animals was evaluated as described previously (20). Enzyme activity was determined by measuring the maximal rate of oxidation of reduced cytochrome c using the change in absorbance at 550 nm on a Bio-Tek Synergy HT microplate reader.
Mitochondrial ROS Production
ROS were measured as described previously (31). Briefly, mitochondria from WT and muscle-specific SirT1-KO animals were incubated with VO2 buffer in a 96-well plate. ROS production was assessed at 37 °C for 30 min during state 4 and state 3 respiration by adding 10 mm glutamate or glutamate with 0.44 mm ADP, respectively, immediately prior to the addition of 50 μm 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, Sigma). The fluorescence emission between 480 and 520 nm, measured with a multidetection micro-plate reader, is directly related to ROS production. ROS production measured in absolute fluorescence units was linear over the entire measurement period. ROS levels were expressed per nanoatom of O2 consumed, measured during the mitochondrial respiration assay.
Immunoblotting
Protein extractions from frozen quadriceps sections were performed as described previously (32). Protein extracts of C2C12 myotubes or muscle homogenates were separated via electrophoresis through SDS-polyacrylamide gels and were then transferred to nitrocellulose membranes (Amersham Biosciences). Membranes were incubated overnight with antibodies against aciculin, α-tubulin (Calbiochem, CP06), COX-IV (Invitrogen, A21348), phospho-AMP-activated protein kinase (pAMPK; Thr-172; Cell Signaling, 25315), total-AMPKα (Cell Signaling, 2532), phospho-p38 (Thr-180/Thr-182; Cell Signaling, 9211S), total p38 (Cell Signaling, 9212), PGC-1α (Millipore, AB3242), cytochrome c, Nampt (Bethyl; 779A), and SirT1 (Upstate, 05-707). This was followed by a 2-h incubation at room temperature with the appropriate secondary antibodies. Western blot signals are semiquantitative and obtained within the linear range of the film. The specificity of the PGC-1α antibody was previously established in our laboratory using siRNA and HA-tagged experiments (20).
Mitochondrial Mass and ROS
Mitochondrial mass was examined using MTGFM. Cells were stained with 20 nm MTGFM for 45 min at 37 °C in differentiation media. ROS were monitored using H2DCFDA. Cells were preincubated with 5 μm of H2DCFDA for 45 min at 37 °C in differentiation media. Staining for both of these fluorescent probes was performed in 6-well cell culture plates and then quantified using a Bio-Tek Synergy HT microplate reader. Each well was then scraped for protein and quantified using a Bradford assay. All fluorescent values were expressed per microgram of protein.
Immunofluorescence
Cells were grown in a 6-well cell culture dish, fixed with 4% paraformaldehyde in PBS for 7 min at room temperature, and permeabilized with a 0.1% solution of Triton X-100 in PBS for 15 min at 4 °C. Cells were then incubated overnight at 4 °C with SirT1 and PGC-1α antibodies. Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies (Invitrogen) were coincubated for 1 h at room temperature. To visualize the nuclei within the myotubes, DAPI was included with the 1-h secondary antibody incubation at a dilution of 0.5 μg/ml. Images were taken using a Nikon Eclipse fluorescent microscope.
Statistics
Comparisons between either control or CCA-treated cells or WT and SirT1-KO animals were evaluated using two-way analyses of variance on each of the treatment conditions. Bonferroni post-tests were performed when applicable, and all error bars represent mean ± S.E. Additionally, the interaction term of a two-factor analysis of variance was used to test for synergism when examining a combined treatment of RSV and CCA or exercise, as described previously (33), with significance defined as p < 0.05.
RESULTS
RSV Induces AMPK and p38 Activity in a Temporal Manner That Is Distinct from That of Contractile Activity (CA) in C2C12 Myotubes
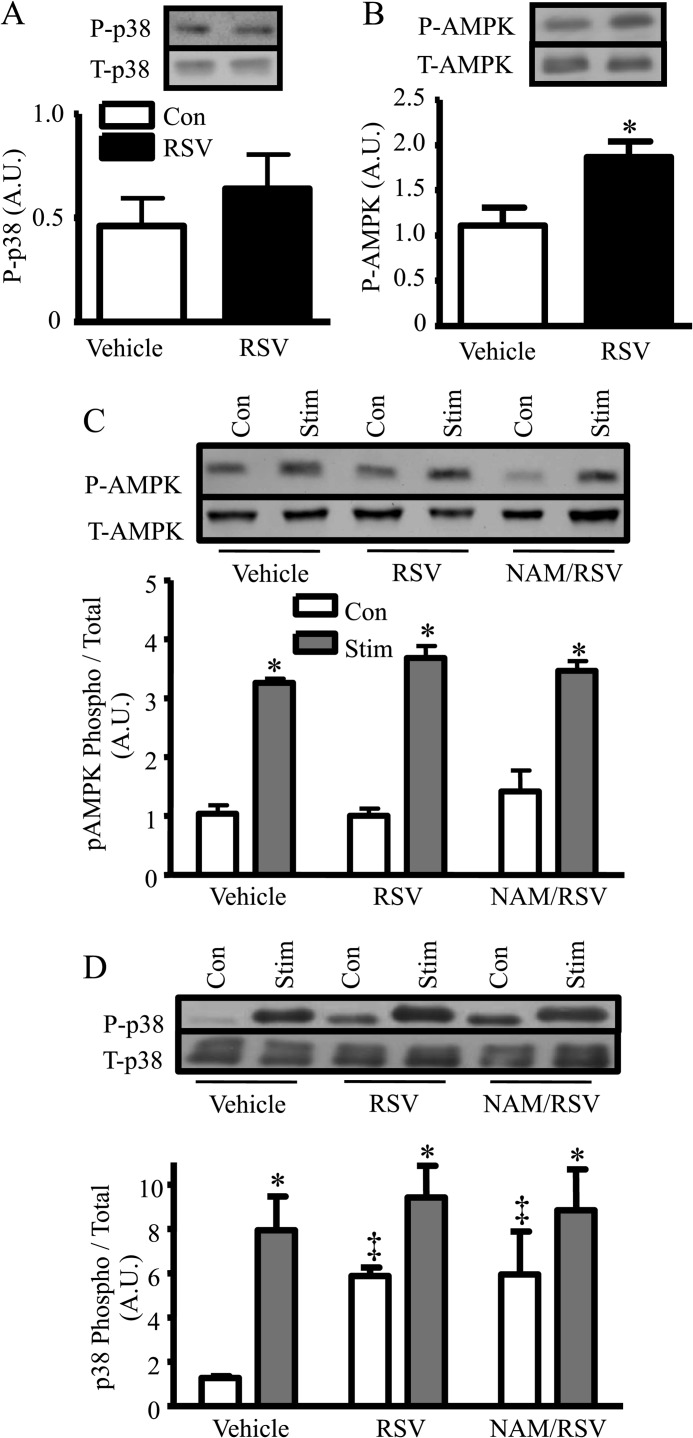
To document the effectiveness of RSV in differentiated muscle cells, we examined the phosphorylation of AMPK and p38 as early indicators of the onset of downstream metabolic effects, as is seen with acute contractile activity (32). Following short term RSV treatment (1 h), AMPK phosphorylation increased by 1.7-fold (p < 0.05), although p38 phosphorylation was not significantly increased (Fig. 1, A and B).
FIGURE 1.
A and B, acute effect of RSV (1 h treatment) versus vehicle on P-p38 and P-AMPK protein expression. C and D, longer term (total 24 h) effect of RSV or NAM/RSV versus vehicle on AMPK and p38 (phospho/total) phosphorylation in quiescent or contracting (3 h followed by 21 h of recovery) myotubes (n = 9, arbitrary scanner units (A.U.) corrected for loading using GAPDH; *, p < 0.05, versus vehicle; ‡, p < 0.05, overall effect of treatment with no stimulation (Stim) versus control (Con)).
To examine the persistence of the RSV effect, and to compare this with contractile activity, a model of exercise, we electrically stimulated C2C12 myotubes for 3 h followed by 21 h of recovery in the presence or absence of RSV for the full 24 h (1-day protocol). The acute RSV effect on AMPK phosphorylation observed at 1 h (Fig. 1B) dissipated by 24 h (Fig. 1C). However, CA followed by recovery had a pronounced 3-fold impact on AMPK phosphorylation. These effects were not influenced by RSV or by the SirT1 inhibitor NAM. In contrast, the phosphorylation levels of p38 increased 6-fold with RSV treatment alone, 8-fold with CA alone, and 9.5-fold with the combined treatment of RSV and CA (p < 0.05; Fig. 1D). The effects of RSV and CA on p38 phosphorylation were SirT1-independent, as NAM did not attenuate the RSV- or CA-induced effects. These data indicate that the response to RSV is kinase-specific. RSV is clearly effective in triggering several early signaling pathways associated with mitochondrial biogenesis in C2C12 myotubes, but it acts on these kinases in a temporally divergent manner. Because the effects of RSV and CA on early signaling pathways linked to mitochondrial biogenesis were distinct, we combined these treatments in subsequent long term experiments (4-day protocol) to see if they exhibited an additive effect on mitochondrial content and function.
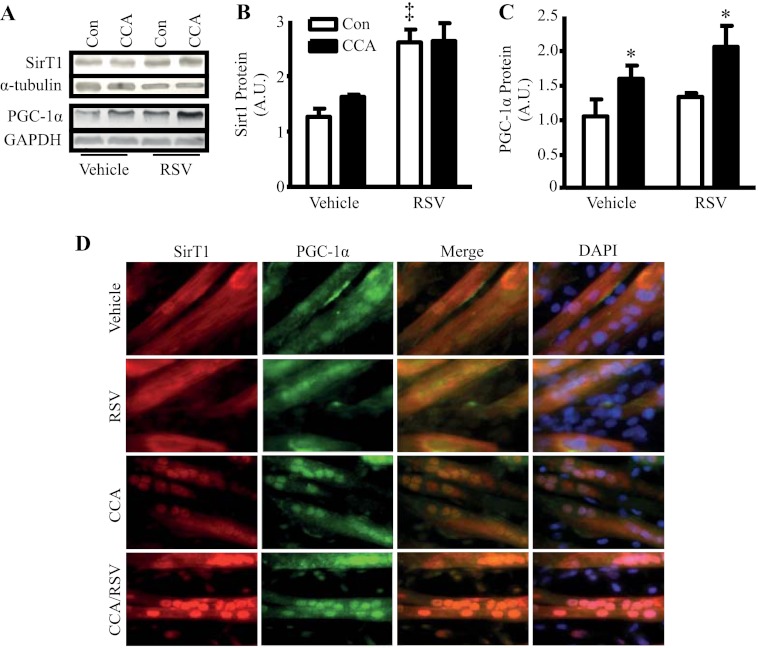
RSV and CCA Elevate Both SirT1 and PGC-1α Protein and Increase Nuclear Localization
It has been previously demonstrated that the coordination between SirT1 and PGC-1α expression/activity is important for the induction of genes related to mitochondrial biogenesis and fatty acid oxidation (6, 34). To investigate whether chronic treatment of RSV, or chronic contractile activity (CCA), could induce this coordinated response, C2C12 myotubes were treated with CCA and RSV over a 4-day period. RSV treatment induced a 2-fold increase in SirT1 protein expression (p < 0.05; Fig. 2, A and B). In contrast, CCA resulted in no increase in SirT1 expression. Alternatively, RSV did not alter PGC-1α protein levels, but PGC-1α expression increased 1.6-fold following 4 days of CCA (p < 0.05; Fig. 2, A and C). The basis for increases in PGC-1 with CCA may be related to the transient elevations in p38 and AMPK phosphorylation, as shown in Fig. 1, as both kinases are known to be important regulators of PGC-1 expression (35, 36). However, this remains to be tested in this context. It is known that the activation of these kinases can facilitate mitochondrial biogenesis in mouse skeletal muscle (3, 37). Interestingly, the combination of both RSV and CCA was the only treatment that produced both an increase in SirT1 and PGC-1α protein expression, but no additional effect on PGC-1α expression was observed in comparison with the CCA treatment.
FIGURE 2.
A, SirT1 and PGC-1α protein expression in vehicle and RSV-treated C2C12 myotubes, with or without stimulation using the 4-day protocol. B and C, SirT1 and PGC-1α protein expression data shown graphically. D, immunofluorescence of SirT1 (red) and PGC-1α (green) with DAPI (blue)-stained nuclei. Images examine SirT1 and PGC-1α nuclear localization in C212 myotubes treated with vehicle or RSV, with and without CCA using the 4-day protocol (n = 9, arbitrary scanner units (A.U.) corrected for loading using α-tubulin; *, p < 0.05, overall effect of stimulation versus control (Con); ‡, p < 0.05, overall effect of RSV versus control).
In addition to expression, we utilized immunofluorescent measures to identify the subcellular location of PGC-1α and SirT1 in response to RSV and CCA. In vehicle-treated myotubes, both SirT1 and PGC-1α were found to be dispersed evenly throughout the cell (Fig. 2D). With CCA, SirT1 and PGC-1α appeared to translocate and colocalize to myotube nuclei. This was not evident with RSV treatment. However, the combined treatment of RSV and CCA resulted in the most prominent translocation and colocalization of SirT1 and PGC-1α to the nucleus. These data support a mechanism for SirT1 to directly influence PGC-1α cotranscriptional activity within the nucleus (34), an effect that may be mediated by ROS, signaling molecules elevated during exercise (38).
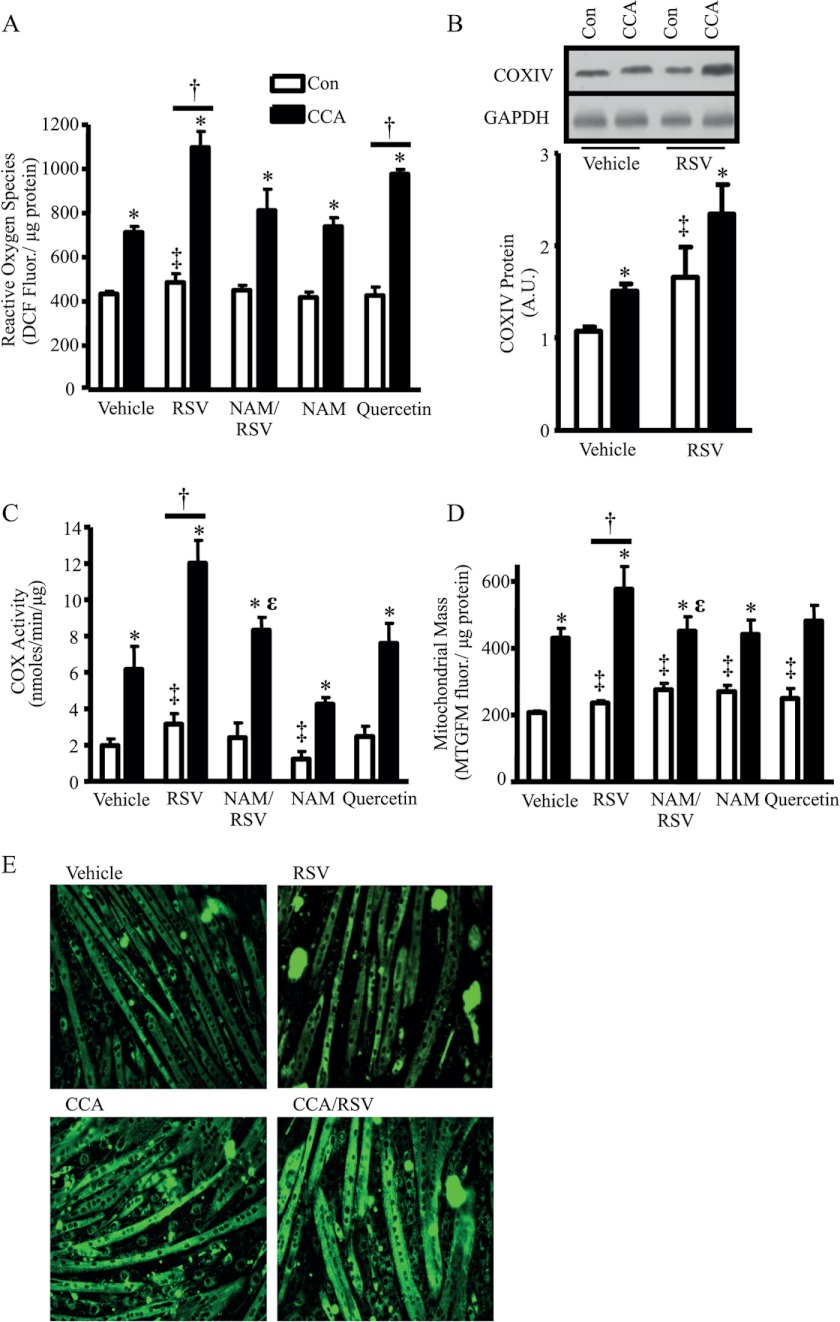
Combined CCA and RSV Treatment Synergistically Increase ROS Signaling and the Induction of Mitochondrial Content in C2C12 Myotubes
ROS-mediated signaling has been shown to be important for the induction of mitochondrial biogenesis (39, 40). As Irrcher et al. (40) revealed, ROS signaling can induce PGC-1α transcription and a resulting increase in mitochondrial biogenesis. Here, we demonstrated that CCA and RSV treatments produced 1.6- and 1.2-fold increases in ROS above the vehicle-treated myotubes (p < 0.05; Fig. 3A). The combined treatments of RSV or quercetin, another polyphenolic compound, with CCA resulted in 2.6- and 2.3-fold changes in ROS production, respectively, compared with the vehicle-treated control cells (p < 0.05). The effect of the RSV/CCA combination treatment on ROS generation appeared to be dependent on SirT1 activity, as indicated by the reduced ROS production when combined with NAM treatment in myotubes (p < 0.05).
FIGURE 3.
A, ROS production measured using 2′,7′-dichlorofluorescein (DCF) fluorescence with and without CCA and treated with vehicle, RSV, or quercetin using the 4-day protocol. Con, control. B, COX-IV protein expression in vehicle and RSV-treated C2C12 myotubes, with or without CCA, using the 4-day protocol is shown graphically. C, COX activity in vehicle, RSV, quercetin- or NAM-treated myotubes, with or without the 4-day protocol of CCA. D, mitochondrial mass measured using MTGFM in a 6-well cell culture plate. E, MTGFM-stained C2C12 myotubes that were treated with CCA, vehicle, or RSV using the 4-day protocol. Images were taken using a fluorescent microscope (n = 9–12, arbitrary scanner units (A.U.) corrected for loading using GAPDH; *, p < 0.05, overall effect of CCA; ‡, p < 0.05, overall effect of treatment versus vehicle-treated control; †, p < 0.05, interaction of treatment versus vehicle-treated myotubes; ϵ, p < 0.05, versus myotubes treated with CCA/RSV).
With the complementary, yet asymmetrical, effects of RSV and CCA on SirT1 and PGC-1α expression, respectively, we hypothesized that there may be the potential for an additive or synergistic effect on mitochondrial biogenesis with a combined treatment. This hypothesis would also be supported by the increase in nuclear localization of both PGC-1α and SirT1 following the combination of RSV and CCA. We assessed mitochondrial content in C2C12 myotubes using COX subunit IV (COX-IV) protein expression, COX activity as a functional measurement for complex IV (41), and MTGFM fluorescence as an indicator of organelle mass. CCA resulted in a 1.4-fold increase in COX-IV protein, whereas RSV treatment alone also induced a similar 1.5-fold increase (p < 0.05; Fig. 3B). In support of our hypothesis, when RSV and CCA were combined, an additive 2.2-fold effect was observed on COX-IV protein levels. COX activity was 3.1-fold higher following CCA (p < 0.05; Fig. 3C), whereas RSV treatment alone produced a smaller but significant 1.6-fold increase in COX activity in comparison with vehicle-treated myotubes. In addition, the higher cellular ROS produced with CCA in comparison with RSV treatment not only coordinates well with the increase in mitochondrial biogenesis but may also prove to be a major distinction for the differences in the induction and translocation of PGC-1α and the subsequent triggering of mitochondrial biogenesis between these two treatments. The combination of RSV and CCA treatment induced a synergistic 6.1-fold response (p < 0.05), which matches the response seen with ROS generation. These data were also largely corroborated by measures of mitochondrial mass (Fig. 3, D and E). Treatment with NAM attenuated the combined treatment response, indicating that this synergistic effect occurred in a SirT1-dependent manner. Interestingly, treatment of the cells with quercetin did not increase COX activity in the presence or absence of CCA, indicating that RSV is a more potent inducer of mitochondrial biogenesis when combined with CCA (Fig. 3, C and D). We attribute this potentiation effect to the consequences of independent elevations in the expression of PGC-1α via CCA and SirT1 via RSV treatment. Along with enhanced nuclear localization, there is a greater potential for PGC-1α to be deacetylated and to coactivate the transcription of nuclear genes encoding mitochondrial proteins. However, this effect relies on the deacetylase activity of SirT1. Therefore, to directly examine the SirT1-dependent effect on basal mitochondrial biogenesis under basal conditions, and in response to exercise, we created a muscle-specific SirT1-KO animal and examined the effect of RSV treatment in vivo.
Muscle-specific SirT1-KO Animals Have Reduced Mitochondrial Content and Function
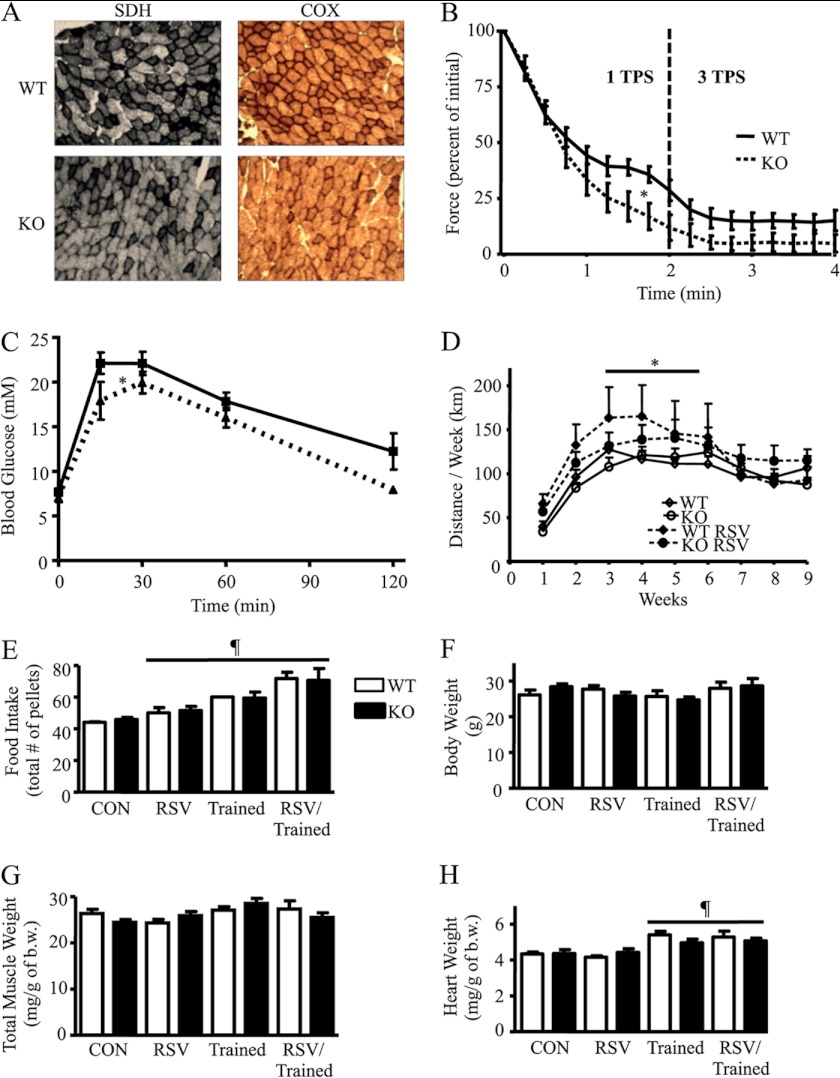
SirT1-KO mice demonstrated no change in body, muscle, or heart weight, compared with control WT mice, as seen in Table 1. In addition, SirT1-KO and WT animals consumed the same amount of food. Muscle mitochondrial content was 23% lower in control SirT1-KO mice compared with WT, as determined by COX activity measurements (Table 1). In support of this finding, SirT1-KO animals exhibited reduced COX and SDH staining of muscle fibers from serial sections compared with WT animals (Fig. 4A). These data indicate that the basal mitochondrial content of muscle relies, in part, on SirT1 expression and/or activity. The absence of SirT1 in muscle also had a pronounced effect on mitochondrial function, as reflected by marked decrements in both state 3 and 4 respiration rates in SirT1-KO animals. Glutamate-stimulated state 4 (basal) and 3 (active) mitochondrial oxygen consumption (VO2) was 47 and 30% lower in the SirT1-KO animals compared with the WT control animals, respectively (Table 1). In addition, ROS production from isolated SirT1-KO mouse mitochondria was 1.5- and 3.1-fold higher than the WT control mice during both state 4 and 3 respiration, respectively (Table 1). These data demonstrate that SirT1 is important for maintaining mitochondrial content and function in skeletal muscle.
TABLE 1.
Physiological and mitochondrial measurements of WT and SirT1-KO mice
AFU indicates arbitrary fluorescence units.
| WT | SirT1-KO | |
|---|---|---|
| Physiological measurements | ||
| Body weight (g) | 26.1 ± 1.4 | 28.4 ± 0.9 |
| Combined muscle weight (tibialis anterior, gastrocnemius, triceps in mg/g body weight) | 26.4 ± 0.9 | 24.5 ± 0.6 |
| Heart weight (mg/g body weight) | 4.3 ± 0.1 | 4.4 ± 0.2 |
| Food intake (total no. of pellets) | 44 ± 0.4 | 46 ± 1.4 |
| COX activity (nmol/min/μg muscle protein) | 6.7 ± 0.3 | 5.1 ± 0.2a |
| Isolated mitochondrial measurements | ||
| State 3 respiration (no. of atoms O2/mg/min) | 73.1 ± 3.1 | 50.9 ± 5.8a |
| State 4 respiration (no. of atoms O2/mg/min) | 15.0 ± 1.0 | 8.0 ± 1.1a |
| ROS production, state 3 (AFU/no. of atoms O2 consumed) | 116 ± 11 | 61 ± 62a |
| ROS production, state 4 (AFU/no. of atoms O2 consumed) | 430 ± 50 | 664 ± 116a |
a p < 0.05 SirT1-KO versus WT.
FIGURE 4.
A, COX and SDH histochemical staining of serial sections of gastrocnemius muscle from WT and SirT1-KO mice. B, fatigue response from the in situ stimulation of WT or SirT1-KO gastrocnemius muscle as a percent of initial twitch force. TPS, tetanic contraction/s. C, intraperitoneal glucose tolerance test was performed on WT and SirT1-KO animals. Mice were given an intraperitoneal injection of d-glucose (2 g/kg of body weight) following 6 h of fasting. D, voluntary wheel running distance by mice over 9 weeks fed control or RSV diets. E, total number of pellets of RSV or control diet consumed throughout the treatment. Con, control. F, body weight of animals. G, total wet muscle weight (tibialis anterior, gastrocnemius, and triceps)/g of body weight (b.w.) for WT and SirT1-KO animals. H, heart weight/g of body weight (n = 9–12, *, p < 0.05, effect of RSV versus control; ¶, p < 0.05, overall effect of each treatment under the line versus control).
SirT1-KO Mice Exhibit Greater in Situ Fatigue Rates and Altered Glucose Homeostasis
To examine the physiological consequence of reduced mitochondrial content and function in SirT1-KO animals, we investigated muscle function during acute contractile activity using an in situ hindlimb muscle fatigue test. Maximal, indirect stimulation of the gastrocnemius muscle via the sciatic nerve resulted in greater fatigability of SirT1-KO animals compared with WT animals, during both moderate and severe contraction intensities (Fig. 4B). This indicates that the absence of SirT1 results in muscle metabolic derangements, including decrements in mitochondrial function and content, leading to greater muscle fatigue. Interestingly, SirT1-KO mice displayed a modest improvement in glucose tolerance (Fig. 4C). Similar findings have previously been observed with the muscle-specific ablation of proteins, leading to oxidative phosphorylation defects (42). In comparison with PGC-1α, muscle-specific PGC-1α-KO mice developed the opposite effect, which is impaired glucose tolerance (43), while overexpression of PGC-1α in muscle of exercising mice led to improvements in glucose tolerance (44). Thus, the functions of SirT1 and PGC-1α are not inextricably tied to one another with respect to glucose homeostasis.
RSV Improves Voluntary Running Distance in Both WT and SirT1-KO Animals
To further examine the SirT1-dependent effects of exercise or RSV on mitochondrial biogenesis, mice were trained for 9 weeks on a voluntary running wheel in the presence or absence of an RSV diet. It should be noted that the submaximal performance of WT and muscle-specific SirT1-KO mice was similar over this treatment period (Fig. 4D). These results suggest that the deficiencies brought about by knock-out of SirT1 are not manifest during mild, voluntary submaximal exercise conditions but are revealed during the high energy demand of more severe contractile activity conditions (Fig. 4B). Interestingly, animals fed an RSV-supplemented diet exhibited a 30% higher (p < 0.05) average running distance per week during weeks 3–6 for both WT and SirT1-KO animals. The quantity of food consumed increased with both RSV treatment and voluntary running, and this was similar in both the WT and SirT1-KO animals (Fig. 4E). Despite differences in food consumption, all treatment groups had similar body weights (Fig. 4F) suggesting concomitant elevations in energy expenditure with either RSV treatment or training. RSV has previously been shown to result in an elevation in basal metabolic rate in both mice and primates (24, 45). There were no differences in muscle mass with any treatment, although the trained animals exhibited a modest cardiac hypertrophy (Fig. 4, G and H), as expected with endurance training.
Synergy of Exercise and RSV on Mitochondrial Biogenesis Is Lost in SirT1-KO Animals
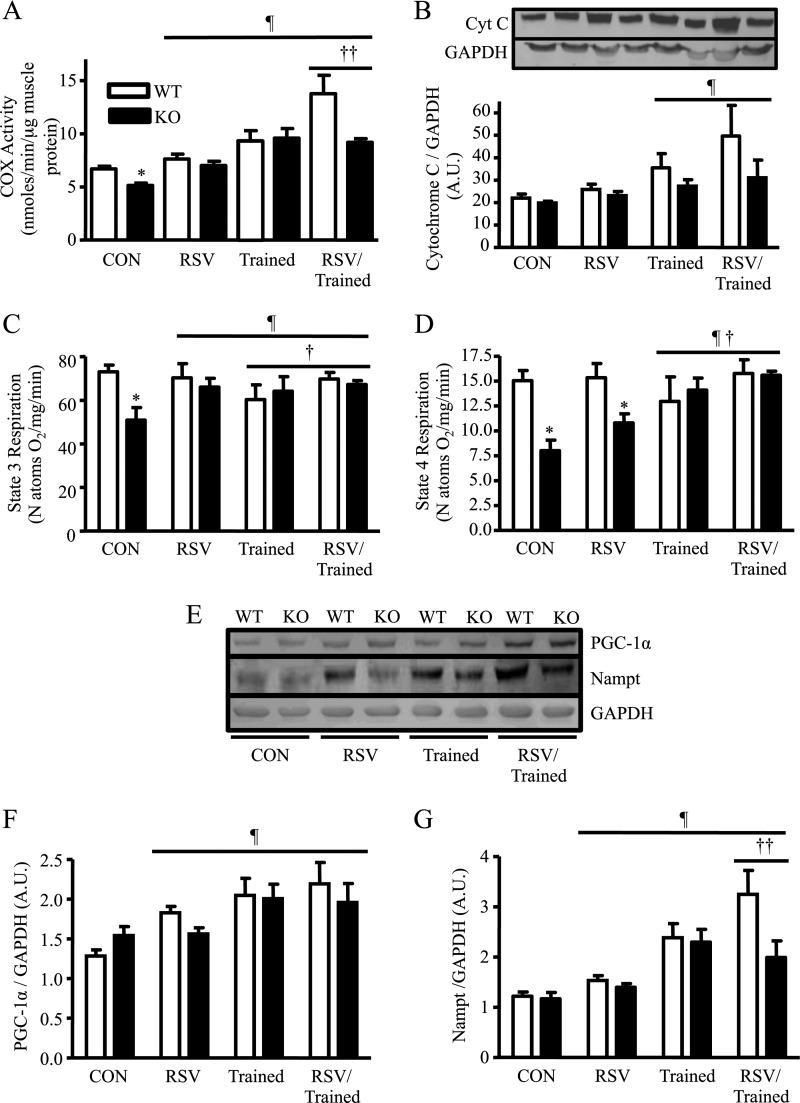
We set out to determine the SirT1 dependency of the individual or combined effects of exercise and RSV on mitochondrial biogenesis. Although WT animals treated with RSV did not demonstrate a significant change in COX activity, SirT1-KO animals fed an RSV diet had restored COX activity values that approximated those of the WT control-fed animals (Fig. 5A). Voluntary wheel training increased COX activity by 1.4- and 1.8-fold in both the WT and SirT1-KO mice, respectively, to similar absolute levels. Thus, the effects of voluntary endurance exercise do not rely on SirT1 activity to produce an increase in mitochondrial content. In addition, RSV was effective in improving mitochondrial content in muscle during conditions of organelle impairment, as seen in the SirT1-KO animals. Measurements of cytochrome c protein, another mitochondrial marker, largely supported the changes seen for each of the treatment groups evaluated (Fig. 5B). Similar to C2C12 myotubes treated with the combination of CCA and RSV (Fig. 3B), the most potent treatment response for COX activity (2.1-fold) occurred when WT mice were both trained and fed an RSV-supplemented diet. Strikingly, these synergistic responses were not duplicated for the combined treatment in SirT1-KO mice. Thus, mitochondrial biogenesis induced by RSV or submaximal exercise does not require SirT1, but interestingly, SirT1 is required for the synergistic enhancement of mitochondrial content produced by both treatments.
FIGURE 5.
A, skeletal muscle COX activity following training, RSV, and combined treatments in skeletal muscle from WT and SirT1-KO mice. B, cytochrome c (Cyt C) protein expression. C and D, state 4 and state 3 respirations/nanoatom oxygen consumed in subsarcolemmal mitochondria. E, PGC-1α and Nampt protein expression following training, RSV, and combined treatments in skeletal muscle from WT and SirT1-KO mice. F, and G, PGC-1α and Nampt protein expression is shown graphically (n = 7–12, arbitrary scanner units (A.U.) corrected for loading using GAPDH; ¶, p < 0.05, overall effect of each treatment under the line versus control; *, p < 0.05, versus control (Con) WT mice; †, p < 0.05, interaction of each treatment under the line versus control; ††, p < 0.05, interaction of RSV/Trained versus the trained mouse).
SirT1-dependent Deficiencies in Mitochondrial Respiration Are Improved with Exercise
We examined the influence of exercise training or RSV on improving mitochondrial function in SirT1-KO animals by measuring oxygen consumption using isolated muscle mitochondrial fractions. However, it is important to note that, with exercise training, mitochondrial respiration is not usually altered despite elevations in cellular mitochondrial biogenesis (28). Similarly, mitochondria isolated from WT animals exhibited no changes in state 3 and 4 respiration rates for any of the treatment conditions (Fig. 5, C and D). In contrast, the impaired state 3 respiration evident in SirT1-KO animals was restored to control levels with an RSV diet, with exercise, and with the combination of these treatments (Fig. 5C). State 4 respiration was not altered with RSV treatment alone, but did recover with training and the combined treatment (Fig. 5D). Thus, improvements in mitochondrial respiratory function with either RSV or training are largely independent of SirT1.
Exercise Combined with RSV Induced a SirT1-dependent Synergistic Elevation in Nampt but Not PGC-1α in WT Animals
To examine the upstream events leading to the observed changes in mitochondrial content and function, we measured PGC-1α expression, a coactivator of genes that encode for mitochondrial proteins as well as Nampt, an enzyme responsible for the generation of the SirT1 cofactor NAD+. The dependence of PGC-1α or Nampt expression on SirT1 activity was first examined under basal conditions and then following exercise and RSV treatment. Both PGC-1α and Nampt protein expressions were not altered in SirT1-KO animals compared with WT animals (Fig. 5, E and F). Each treatment resulted in a significant increase in PGC-1α expression compared with sedentary control WT mice. However, Nampt protein was not influenced by RSV treatment but increased in both the WT and SirT1-KO animals following training (Fig. 5, E and G). Notably, in WT animals, a synergistic increase in Nampt expression was observed with both training and RSV treatment that was not found in the SirT1-KO animals.
Elevated ROS Production Observed in the Absence of SirT1 Is Improved with Exercise and RSV Treatment
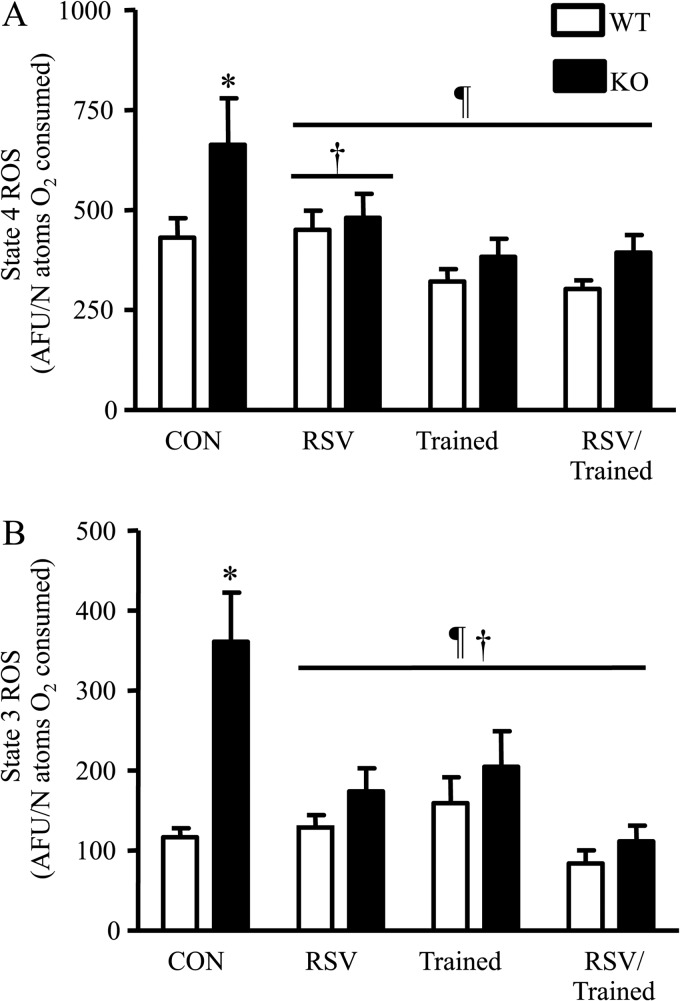
In mitochondria, ROS production is a metabolic by-product of respiration that occurs at complex I and III of the electron transport chain (46). During both state 3 and state 4 of respiration, ROS production was higher in SirT1-KO mice (Fig. 6, A and B), which corresponds to the decrements observed in mitochondrial function (Fig. 5, C and D). Defects in respiration are known to result in an increase in ROS generation (47–49). Following RSV feeding or training, ROS production was reduced in the SirT1-KO mice to levels that were equal to those of the similarly treated WT mice. In contrast, there were no effects of training or RSV on ROS production in WT animals in which respiratory function was normal.
FIGURE 6.
A and B, state 4 and state 3 ROS production per nanoatom oxygen consumed in SS mitochondria (n = 7–12, *, p < 0. 05, versus control WT mice, ¶, p < 0.05, overall effect of treatment versus control mice, †, p < 0.05, interaction of each treatment under the line versus control mice).
DISCUSSION
The goal of this study was to delineate the role of SirT1 on mitochondrial function and biogenesis in both resting and exercising muscle and the consequent effects of the lack of SirT1 on muscle performance. This is particularly relevant given the controversy surrounding the effects of exercise on SirT1 protein and activity (12). Here, we examined the necessity of SirT1 to maintain basal muscle mitochondrial content and function and to induce mitochondrial biogenesis following voluntary endurance exercise or CCA. We also provide evidence for a switch from the SirT1-independent to SirT1-dependent effects of dietary RSV treatment on mitochondria in muscle with an increase in cellular energy demand, as produced by exercise.
The absence of SirT1 in muscle resulted in a reduction in mitochondrial content as seen with decreased COX activity, COX and SDH fiber staining, and cytochrome c protein expression. These data indicate that the basal mitochondrial content of muscle relies, in part, on SirT1 expression and/or activity. However, the absence of SirT1 had a more pronounced effect on mitochondrial function, as reflected by marked reductions in respiration rates. This SirT1-dependent reduction in muscle mitochondrial function is supported by data demonstrating the necessity of SirT1 for the maintenance of fatty acid oxidation in C2C12 mouse muscle cells (34). Likewise, isolated liver mitochondria from SirT1-null mice also showed reduced mitochondrial function (50). Decreases in muscle mitochondrial function are further affirmed by increases in ROS generation from SirT1-KO-isolated mitochondria during respiration compared with WT animals. Although ROS are important signaling molecules in the cell, in large concentrations they can cause oxidative damage to proteins, DNA, and other cellular macromolecules. SirT1 is therefore important for muscle oxidative stress homeostasis, as well as basal mitochondrial function and content. These reductions in mitochondrial content and function had no effect on the performance of the SirT1-KO animals during voluntary wheel running. However, the physiological decrements were quite apparent with both moderate and severe contraction intensities, when the muscle was challenged more strenuously in situ.
As the most well known inducer of muscle mitochondrial biogenesis (51), exercise training was used as a treatment for both WT and SirT1-KO mice. Voluntary wheel exercise produced an equal and substantial elevation of mitochondrial content in both the SirT1-KO animals and WT animals. This illustrates that the effects of voluntary exercise training do not rely on SirT1 activity to produce an increase in mitochondrial content, as was also demonstrated by Philp et al. (52). Similarly, CCA-induced mitochondrial biogenesis in C2C12 myotubes was not attenuated by the SirT1 inhibitor NAM when examining mitochondrial mass and enzyme activity. In addition, mitochondrial respiration in SirT1-KO mice following voluntary exercise was restored to WT levels, and ROS production was markedly reduced. Thus, exercise can serve to ameliorate mitochondrial content and function in a SirT1-independent manner.
RSV acts in a manner distinct from that provided by exercise or CCA. Unlike CCA of C2C12 myotubes, RSV treatment resulted in an increase of SirT1 protein and no change in PGC-1α expression using the 4-day protocol. Despite the lack of change in PGC-1α expression, the increase in SirT1 protein could contribute to an enhancement of PGC-1α coactivator activity (5). Our results show that RSV can activate both AMPK and p38 in temporally distinct stages, which could promote post-translational changes in PGC-1α, thereby altering its activity (3). Whether these transient modifications of PGC-1α have an impact on RSV-mediated mitochondrial biogenesis remains to be determined. There is also considerable contention as to whether PGC-1α acts as a sole regulator of the induction of organelle synthesis in muscle, at least in response to exercise (20, 53–55). Thus, this raises the possibility that RSV may act through other mediators of mitochondrial biogenesis such as PGC-β or PGC-1α-related coactivator. Our data in myotubes show that RSV can induce increases in COX activity, either alone or in combination with CCA, and that this effect was attenuated with the addition of NAM, suggesting the involvement of a SirT1-mediated pathway. Because there has been some contention as to whether RSV requires SirT1 for an improvement in mitochondrial biogenesis (24, 25), we also compared the effects of RSV on mitochondrial content in the presence and absence of SirT1 in vivo. RSV had no effect on mitochondrial content and function in WT animals. However, this polyphenol was successful in both elevating mitochondrial content and improving respiration in the direction of WT levels in SirT1-KO mice. These SirT1-independent effects of RSV support the idea that RSV can mediate its effects on mitochondrial biogenesis via other pathways (24), at least at the dose employed in this study. It would be interesting to examine whether some of these SirT1-independent effects are mediated by reductions in the activity of GCN5 acetyltransferase activity on PGC-1α (52, 56), which would normally serve to acetylate and deactivate PGC-1α.
With the complementary, yet asymmetrical, effects of RSV and CCA on PGC-1α and SirT1 expression using the 4-day protocol in C2C12 myotubes, we hypothesized that there may be the potential for an additive or synergistic effect with a combined treatment. This combination induced a distinctly synergistic effect on both COX activity and mitochondrial mass, as indicated by statistical interactions. The synergistic response was dependent on SirT1, because the effect on mitochondrial biogenesis was attenuated with SirT1 inhibition in myotubes. Congruent with these data, RSV treatment of mice resulted in a significantly stronger influence on mitochondrial biogenesis when the muscle was subjected to the repeated metabolic demands of voluntary exercise training. This suggests that the effect of exercise on mitochondrial biogenesis cannot be further enhanced by RSV treatment without the presence of the SirT1 protein. Thus, the maximal effect of RSV requires both SirT1 and a condition of energy demand in muscle that would be high in NAD+ and AMP, cofactors which activate SirT1 and AMPK, respectively. These SirT1-dependent effects support the proposal by Price et al. (25) that RSV requires SirT1 for mitochondrial biogenesis. However, we also propose that the maximal effect of RSV may depend on the energy state of the muscle, as altered by exercise, and the dose of RSV employed. An additional influence may be the presence or absence of SirT1 in tissues other than muscle. In contrast to our work using muscle-specific SirT1 ablation, Price et al. (25) employed a whole body conditional knock-out of SirT1. Evidence brought forth by Ramadori et al. (57) has demonstrated that even neuronal SirT1 expression can influence muscle insulin sensitivity.
Our study demonstrates that SirT1 protein is responsible for the partial maintenance of basal mitochondrial content and function, in addition to lowering mitochondrial ROS generation and improving muscle fatigue rates in skeletal muscle. These findings are somewhat discordant with those observed by Philp et al. (52) that showed no change in basal mitochondrial biogenesis and fatigue rates of the extensor digitorum longus muscle of SirT1-KO mice. This may be a result of differences in the efficacy and fiber type distribution of MCK-driven (52) and our MLC1f (this study) promoter-driven Cre-LoxP recombination of the SirT1 gene. Nonetheless, the SirT1-independent increase in mitochondrial content with endurance exercise as shown in our study was also demonstrated by Philp et al. (52).
The lesser effect of dietary RSV on mitochondrial biogenesis and function in comparison with exercise in WT animals is likely a consequence of the lack of metabolic stress evoked by RSV, elevations in NAD+ and AMP, the metabolites necessary for activating AMPK and SirT1, respectively. It may be for this reason that SirT1-KO animals treated with RSV and subjected to exercise do not induce a synergistic mitochondrial response compared with WT animals. When RSV is combined with the metabolic stress of CCA in myotubes, or exercise in vivo, there is a synergistic response that relies on SirT1. In addition, elevations in SirT1 and PGC-1α nuclear localization following CCA of myotubes in combination with RSV treatment, when compared with RSV alone, may help to explain the synergistic results of the combined treatments in vivo. Therefore, this study reveals that RSV does not induce as robust an effect as exercise training on mitochondrial biogenesis and function, but when combined with training, RSV may induce a SirT1-dependent synergistic response. These findings demonstrate the following: 1) exercise can induce mitochondrial biogenesis independent of SirT1, and 2) the optimal therapeutic potential of RSV for the induction of mitochondrial biogenesis and function may rely on both the presence of SirT1 as well as a cellular environment created by the repeated energy demands of exercise.
Acknowledgment
We are grateful for the expert technical assistance of Steve Pastore.
This work was supported in part by grants from the Canadian Institutes of Health Research (to D. A. H.).
- AMPK
- AMP kinase
- Nampt
- nicotinamide phosphoribosyltransferase
- RSV
- resveratrol
- ROS
- reactive oxygen species
- CCA
- chronic contractile activity
- CA
- contractile activity
- NAM
- nicotinamide
- H2DCFDA
- 2′,7′-dichlorodihydrofluorescein diacetate
- MTGFM
- MitoTracker Green FM.
REFERENCES
- 1. Joseph A. M., Joanisse D. R., Baillot R. G., Hood D. A. (2012) Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp. Diabetes Res. 2012:642038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patti M. E., Butte A. J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., Landaker E. J., Goldfine A. B., Mun E., DeFronzo R., Finlayson J., Kahn C. R., Mandarino L. J. (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 100, 8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 5. Nemoto S., Fergusson M. M., Finkel T. (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 [DOI] [PubMed] [Google Scholar]
- 6. Amat R., Planavila A., Chen S. L., Iglesias R., Giralt M., Villarroya F. (2009) SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) gene in skeletal muscle through the PGC-1α autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284, 21872–21880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Revollo J. R., Grimm A. A., Imai S. (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- 8. Zhang T., Berrocal J. G., Frizzell K. M., Gamble M. J., DuMond M. E., Krishnakumar R., Yang T., Sauve A. A., Kraus W. L. (2009) Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J. Biol. Chem. 284, 20408–20417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fulco M., Cen Y., Zhao P., Hoffman E. P., McBurney M. W., Sauve A. A., Sartorelli V. (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 14, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cantó C., Jiang L. Q., Deshmukh A. S., Mataki C., Coste A., Lagouge M., Zierath J. R., Auwerx J. (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menzies K. J., Hood D. A. (2012) The role of SirT1 in muscle mitochondrial turnover. Mitochondrion 12, 5–13 [DOI] [PubMed] [Google Scholar]
- 13. Handschin C., Rhee J., Lin J., Tarr P. T., Spiegelman B. M. (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 7111–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aparicio O. M., Billington B. L., Gottschling D. E. (1991) Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66, 1279–1287 [DOI] [PubMed] [Google Scholar]
- 15. Wood J. G., Rogina B., Lavu S., Howitz K., Helfand S. L., Tatar M., Sinclair D. (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 [DOI] [PubMed] [Google Scholar]
- 16. Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 17. Valenzano D. R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. (2006) Resveratrol prolongs life span and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 [DOI] [PubMed] [Google Scholar]
- 18. Narkar V. A., Downes M., Yu R. T., Embler E., Wang Y. X., Banayo E., Mihaylova M. M., Nelson M. C., Zou Y., Juguilon H., Kang H., Shaw R. J., Evans R. M. (2008) AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uguccioni G., Hood D. A. (2011) The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 300, E361–E371 [DOI] [PubMed] [Google Scholar]
- 21. Boulton S. J., Jackson S. P. (1998) Components of the Ku-dependent nonhomologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17, 1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baur J. A., Sinclair D. A. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 [DOI] [PubMed] [Google Scholar]
- 23. Timmers S., Konings E., Bilet L., Houtkooper R. H., van de Weijer T., Goossens G. H., Hoeks J., van der Krieken S., Ryu D., Kersten S., Moonen-Kornips E., Hesselink M. K., Kunz I., Schrauwen-Hinderling V. B., Blaak E. E., Auwerx J., Schrauwen P. (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park S. J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A. L., Kim M. K., Beaven M. A., Burgin A. B., Manganiello V., Chung J. H. (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price N. L., Gomes A. P., Ling A. J., Duarte F. V., Martin-Montalvo A., North B. J., Agarwal B., Ye L., Ramadori G., Teodoro J. S., Hubbard B. P., Varela A. T., Davis J. G., Varamini B., Hafner A., Moaddel R., Rolo A. P., Coppari R., Palmeira C. M., de Cabo R., Baur J. A., Sinclair D. A. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bothe G. W., Haspel J. A., Smith C. L., Wiener H. H., Burden S. J. (2000) Selective expression of Cre recombinase in skeletal muscle fibers. Genesis 26, 165–166 [PubMed] [Google Scholar]
- 27. Chabi B., Adhihetty P. J., O'Leary M. F., Menzies K. J., Hood D. A. (2009) Relationship between Sirt1 expression and mitochondrial proteins during conditions of chronic muscle use and disuse. J. Appl. Physiol. 107, 1730–1735 [DOI] [PubMed] [Google Scholar]
- 28. Saleem A., Adhihetty P. J., Hood D. A. (2009) Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol. Genomics 37, 58–66 [DOI] [PubMed] [Google Scholar]
- 29. Harper C. E., Patel B. B., Wang J., Arabshahi A., Eltoum I. A., Lamartiniere C. A. (2007) Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 28, 1946–1953 [DOI] [PubMed] [Google Scholar]
- 30. Horn T. L., Cwik M. J., Morrissey R. L., Kapetanovic I., Crowell J. A., Booth T. D., McCormick D. L. (2007) Oncogenicity evaluation of resveratrol in p53+/− (p53 knockout) mice. Food Chem. Toxicol. 45, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adhihetty P. J., Ljubicic V., Hood D. A. (2007) Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 292, E748–E755 [DOI] [PubMed] [Google Scholar]
- 32. Ljubicic V., Hood D. A. (2008) Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am. J. Physiol. Endocrinol. Metab. 295, E195–E204 [DOI] [PubMed] [Google Scholar]
- 33. Slinker B. K. (1998) The statistics of synergism. J. Mol. Cell. Cardiol. 30, 723–731 [DOI] [PubMed] [Google Scholar]
- 34. Gerhart-Hines Z., Rodgers J. T., Bare O., Lerin C., Kim S. H., Mostoslavsky R., Alt F. W., Wu Z., Puigserver P. (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 26, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gibala M. J., McGee S. L., Garnham A. P., Howlett K. F., Snow R. J., Hargreaves M. (2009) Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J. Appl. Physiol. 106, 929–934 [DOI] [PubMed] [Google Scholar]
- 36. Irrcher I., Ljubicic V., Kirwan A. F., Hood D. A. (2008) AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS ONE 3, e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pogozelski A. R., Geng T., Li P., Yin X., Lira V. A., Zhang M., Chi J. T., Yan Z. (2009) p38γ mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE 4, e7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nasrin N., Kaushik V. K., Fortier E., Wall D., Pearson K. J., de Cabo R., Bordone L. (2009) JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS ONE 4, e8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silveira L. R., Pilegaard H., Kusuhara K., Curi R., Hellsten Y. (2006) The contraction-induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1α (PGC-1α), mitochondrial uncoupling protein 3 (UCP3), and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim. Biophys. Acta 1763, 969–976 [DOI] [PubMed] [Google Scholar]
- 40. Irrcher I., Ljubicic V., Hood D. A. (2009) Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 296, C116–C123 [DOI] [PubMed] [Google Scholar]
- 41. Li Y., Park J. S., Deng J. H., Bai Y. (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 38, 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pospisilik J. A., Knauf C., Joza N., Benit P., Orthofer M., Cani P. D., Ebersberger I., Nakashima T., Sarao R., Neely G., Esterbauer H., Kozlov A., Kahn C. R., Kroemer G., Rustin P., Burcelin R., Penninger J. M. (2007) Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131, 476–491 [DOI] [PubMed] [Google Scholar]
- 43. Handschin C., Choi C. S., Chin S., Kim S., Kawamori D., Kurpad A. J., Neubauer N., Hu J., Mootha V. K., Kim Y. B., Kulkarni R. N., Shulman G. I., Spiegelman B. M. (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic beta cell cross-talk. J. Clin. Invest. 117, 3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Summermatter S., Shui G., Maag D., Santos G., Wenk M. R., Handschin C. (2013) PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dal-Pan A., Pifferi F., Marchal J., Picq J. L., Aujard F. (2011) Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE 6, e16581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finkel T., Holbrook N. J. (2000) Oxidants, oxidative stress, and the biology of ageing. Nature 408, 239–247 [DOI] [PubMed] [Google Scholar]
- 47. O'Leary M. F., Hood D. A. (2008) Effect of prior chronic contractile activity on mitochondrial function and apoptotic protein expression in denervated muscle. J. Appl. Physiol. 105, 114–120 [DOI] [PubMed] [Google Scholar]
- 48. Chen Q., Moghaddas S., Hoppel C. L., Lesnefsky E. J. (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am. J. Physiol. Cell Physiol. 294, C460–C466 [DOI] [PubMed] [Google Scholar]
- 49. Zuin A., Gabrielli N., Calvo I. A., García-Santamarina S., Hoe K. L., Kim D. U., Park H. O., Hayles J., Ayté J., Hidalgo E. (2008) Mitochondrial dysfunction increases oxidative stress and decreases chronological life span in fission yeast. PLoS ONE 3, e2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boily G., Seifert E. L., Bevilacqua L., He X. H., Sabourin G., Estey C., Moffat C., Crawford S., Saliba S., Jardine K., Xuan J., Evans M., Harper M. E., McBurney M. W. (2008) SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE 3, e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Irrcher I., Adhihetty P. J., Joseph A. M., Ljubicic V., Hood D. A. (2003) Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 33, 783–793 [DOI] [PubMed] [Google Scholar]
- 52. Philp A., Chen A., Lan D., Meyer G. A., Murphy A. N., Knapp A. E., Olfert I. M., McCurdy C. E., Marcotte G. R., Hogan M. C., Baar K., Schenk S. (2011) Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J. Biol. Chem. 286, 30561–30570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zechner C., Lai L., Zechner J. F., Geng T., Yan Z., Rumsey J. W., Collia D., Chen Z., Wozniak D. F., Leone T. C., Kelly D. P. (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 12, 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rowe G. C., El-Khoury R., Patten I. S., Rustin P., Arany Z. (2012) PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE 7, e41817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leick L., Wojtaszewski J. F., Johansen S. T., Kiilerich K., Comes G., Hellsten Y., Hidalgo J., Pilegaard H. (2008) PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab 294, E463–E474 [DOI] [PubMed] [Google Scholar]
- 56. Lerin C., Rodgers J. T., Kalume D. E., Kim S. H., Pandey A., Puigserver P. (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab. 3, 429–438 [DOI] [PubMed] [Google Scholar]
- 57. Ramadori G., Fujikawa T., Anderson J., Berglund E. D., Frazao R., Michán S., Vianna C. R., Sinclair D. A., Elias C. F., Coppari R. (2011) SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 14, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]