Background: Nicotinic receptors are important pharmaceutical targets.
Results: Several drug-receptor hydrogen bonding interactions seen in structural models are not functionally relevant, with variations seen across different ligands and receptor subtypes.
Conclusion: Caution is necessary when extrapolating from structures of model systems to functional receptors.
Significance: Functional validation of drug-receptor interactions can aid drug discovery efforts.
Keywords: Ion Channels, Mutagenesis Site-specific, Nicotinic Acetylcholine Receptors, Pharmacology, Protein-Drug Interactions
Abstract
The agonist-binding site of nicotinic acetylcholine receptors (nAChRs) spans an interface between two subunits of the pentameric receptor. The principal component of this binding site is contributed by an α subunit, and it binds the cationic moiety of the nicotinic pharmacophore. The other part of the pharmacophore, a hydrogen bond acceptor, has recently been shown to bind to the complementary non-α subunit via the backbone NH of a conserved Leu. This interaction was predicted by studies of ACh-binding proteins and confirmed by functional studies of the neuronal (CNS) nAChR, α4β2. The ACh-binding protein structures further suggested that the hydrogen bond to the backbone NH is mediated by a water molecule and that a second hydrogen bonding interaction occurs between the water molecule and the backbone CO of a conserved Asn, also on the non-α subunit. Here, we provide new insights into the nature of the interactions between the hydrogen bond acceptor of nicotinic agonists and the complementary subunit backbone. We studied both the nAChR of the neuromuscular junction (muscle-type) and a neuronal subtype, (α4)2(β4)3. In the muscle-type receptor, both ACh and nicotine showed a strong interaction with the Leu NH, but the potent nicotine analog epibatidine did not. This interaction was much attenuated in the α4β4 receptor. Surprisingly, we found no evidence for a functionally significant interaction with the backbone carbonyl of the relevant Asn in either receptor with an array of agonists.
Introduction
Nicotinic acetylcholine receptors (nAChRs)2 are ligand-gated ion channels that propagate neurotransmission in the central and peripheral nervous systems (1–4). They are activated by the neurotransmitter acetylcholine (ACh) and also by nicotine and related compounds. The nAChRs are members of a superfamily of ligand-gated ion channels called the Cys-loop (or pentameric) receptors, which also includes receptors for the neurotransmitters γ-aminobutyric acid (GABAA and GABAC), glycine, and serotonin (5, 6). These receptors are implicated in an assortment of neurological disorders, including Alzheimer disease, Parkinson disease, schizophrenia, and depression, and are also essential for learning, memory, and sensory perception.
nAChRs are pentamers, composed of five subunits arranged symmetrically around a central ion-conducting pore. There are 16 mammalian genes that encode 16 homologous but functionally distinct nAChR subunits (α1–α7, α9, α10, β1–β4, γ, δ, and ϵ). From various combinations of these subunits, >20 active nAChR subtypes have been established (7). Nicotinic agonists bind at subunit interfaces, and a combination of structure-function studies and structural studies of the acetylcholine-binding proteins (AChBPs), which share considerable sequence homology with the ligand-binding domain of the nAChR, has established a detailed binding model (8–10). The α subunits contribute the principal component of the agonist-binding site, which binds to the cationic end of agonists. This binding site is well characterized, consisting of a cation-π interaction with one of several conserved aromatic residues and typically a hydrogen bond from the N+H of the drug to a backbone carbonyl (11–13). The natural agonist ACh, which lacks the crucial N+H, does not participate in the latter interaction.
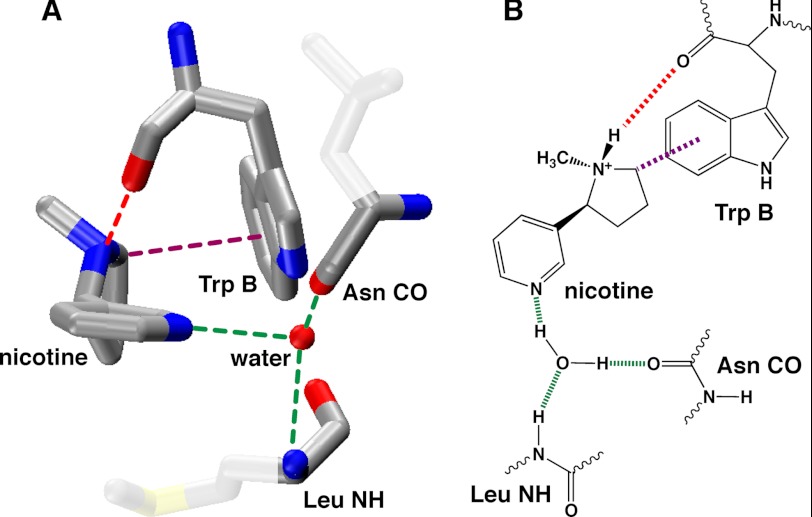
The complementary component of the agonist-binding site is formed by non-α subunits, and recent work has shown that it involves a hydrogen bonding interaction with the hydrogen bond acceptor of agonists (e.g. the C=O of ACh or the pyridine nitrogen of nicotine) (Figs. 1 and 2). Crystal structures of AChBPs with several drugs bound produced a binding model in which two backbone features, a CO and an NH from amino acids that are 12 residues apart, coordinate a water molecule, which in turn hydrogen bonds to the hydrogen bond acceptor of agonists (Fig. 1) (9, 14, 15). In nAChRs, the particular residues are Asn and Leu, and they are conserved across the family (Fig. 3; they are Leu and Met, respectively, in the AChBP structure of Fig. 1A). Because residue numbering varies among different receptors, we will refer to them simply as the Asn and Leu sites, the former contributing a CO and the latter an NH to the proposed hydrogen bonding array; specific residue numbers are noted under “Experimental Procedures.” Recent studies of the neuronal α4β2 nAChR confirmed that the Leu NH of the β2 subunit does hydrogen bond to the pyridine nitrogen of nicotine and to the carbonyl oxygen of ACh (16).
FIGURE 1.
Proposed binding model for nicotine at nAChRs. A, crystal structure of nicotine bound to AChBP (Protein Data Bank code 1UW6). B, schematic of binding model denoting the key interactions probed here.
FIGURE 2.
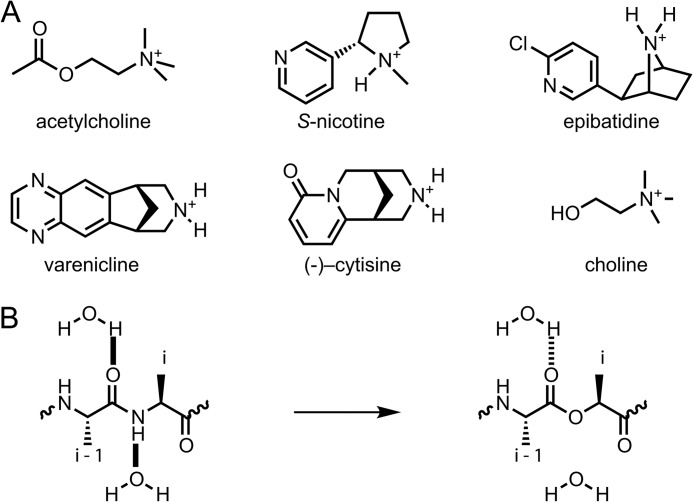
A, agonists used in this study. B, illustration of amide-to-ester mutation. Introduction of an α-hydroxy acid in place of an amino acid eliminates the hydrogen bond donor (backbone NH) of the i residue and attenuates the hydrogen bond-accepting ability of the i−1 carbonyl. The attenuated hydrogen bond is represented by dashed lines.
FIGURE 3.

Sequences of the complementary subunits considered here. The hydrogen bond-donating Leu and hydrogen bond-accepting Asn are highlighted. The key residues are highly conserved in other orthologs. Residue numbering is for the β2 subunit.
In this work, we expanded these studies of hydrogen bonding interactions involving the complementary subunit in two ways. First, we considered two new receptor subtypes: a second neuronal form, (α4)2(β4)3, henceforth referred to as α4β4; and the form found at the neuromuscular junction of the peripheral nervous system, (α1)2β1γδ (the fetal form; in the adult variant, the ϵ subunit replaces the γ subunit), which we refer to as the muscle-type receptor. Note that the pharmacology of the muscle-type receptor is quite distinct from that of neuronal receptors such as α4β2 and α4β4, most importantly in the fact that nicotine is quite potent at these neuronal receptors but not at the receptors of the neuromuscular junction. This distinction allows smokers to become addicted to nicotine without adverse peripheral effects. Second, we evaluated the other component of the proposed hydrogen bonding model, the water-mediated hydrogen bond to the Asn carbonyl, in both the α4β4 and muscle-type receptors. Efforts to probe the Asn backbone carbonyl in the previously studied α4β2 receptor were thwarted by technical issues; the nonsense suppression methodology necessary for these studies was not selective/efficient enough for our purposes.
Using unnatural amino acid mutagenesis, we found key differences in the hydrogen bonding properties of specific drug-receptor combinations. Interestingly, we found no evidence for a functionally significant hydrogen bond to the Asn backbone carbonyl.
EXPERIMENTAL PROCEDURES
Mutagenesis
Nonsense suppression was performed using techniques described previously on the embryonic mouse muscle-type nAChR ((α1)2β1γδ) in the pAMV vector (12) and the human α4β4 receptor in the pGEMhe vector. For nonsense suppression experiments, a TAG (for mutation at γVal-108/δVal-110) or TGA (for mutation at γLeu-119/δLeu-121, β4Leu-108, and β4Leu-119) stop codon was introduced at the site of interest using the standard Stratagene QuikChange protocol and verified through sequencing. The β1 subunit contains a background mutation in the transmembrane M2 helix (β1L9′S) that is known to lower whole-cell EC50 values. The α1 subunit contains a hemagglutinin epitope in the M3-M4 cytoplasmic loop that does not alter EC50 values in control experiments. cDNA was linearized with the restriction enzyme NotI for muscle-type receptor subunits and with NheI for α4 and β4 subunits. mRNA was prepared by in vitro transcription using the mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX).
Stage V-VI Xenopus laevis oocytes were injected with mRNA at an α1:β1:γ:δ ratio of 10:1:1:1 or 1:1:5:5 for wild-type/conventional mutagenesis or nonsense suppression experiments, respectively, with the muscle-type receptor. An α4:β4 mRNA ratio of 1:20 was used for nonsense suppression experiments with the α4β4 receptor. Control nonsense suppression experiments confirmed that this ratio ensures a 2:3 subunit stoichiometry. α-Hydroxy acids and amino acids were appended to the dinucleotide dCA and enzymatically ligated to the truncated 74-mer amber suppressor tRNA THG73 or opal suppressor tRNA TQOpS′ as previously described (12). For wild-type or conventional mutagenesis experiments with the muscle-type receptor, 1–2 ng of mRNA was injected per oocyte in a single 75-nl injection. For nonsense suppression experiments with the muscle-type receptor, each cell was injected with 75 nl of a 1:1 mixture of mRNA (20–25 ng of total mRNA) and tRNA (10–25 ng). For nonsense suppression experiments with the α4β4 receptor, each cell was injected with 50 nl of a 1:1 mixture of mRNA (50 ng of total mRNA) and tRNA (∼25 ng). Amino acids bearing a 6-nitroveratryloxycarbonyl protecting group were deprotected prior to injection via irradiation with a 500-watt mercury/xenon arc lamp, filtered with WG-334 and UG-11 filters prior to injection. Oocytes were incubated at 18 °C for 16–20 or 24–48 h after injection for the wild-type/conventional mutagenesis or nonsense suppression experiments, respectively, with the muscle-type receptor. Oocytes were incubated for 48 h after injection for nonsense suppression experiments with the α4β4 receptor. Wild-type recovery control experiments (injection of tRNA appended to the natural amino acid) were performed to evaluate the fidelity of the nonsense suppression experiments. In additional control experiments with the muscle-type receptor, injections of mRNA only and mRNA with 76-mer THG73 or TQOpS′ gave minimal currents in electrophysiology experiments (∼100 nA or less for controls compared with ≫2 μA for nonsense suppression experiments). For the α4β4 receptor, injections of mRNA with 76-mer TQOpS′ gave no detectable currents.
Electrophysiology
Two-electrode voltage clamp electrophysiology was used to measure the functional effects of each mutation. Electrophysiology recordings were performed after injection and incubation as described above using the OpusXpress 6000A system (Axon Instruments) at a holding potential of −60 mV. The running buffer was a Ca2+-free ND96 solution (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, and 5 mm HEPES, pH 7.5). Agonist doses in Ca2+-free ND96 were applied for 15 s, followed by a 116-s wash with the running buffer. Cells expressing muscle-type receptors containing the α1G153K mutation were given longer exposures to agonist (90 s). Acetylcholine chloride, (−)-nicotine tartrate, and (−)-cytisine were purchased from Sigma-Aldrich/RBI, (±)-epibatidine was purchased from Tocris, and varenicline tartrate was a generous gift from Pfizer. Dose-response data were obtained for eight or more agonist concentrations on eight or more cells. Dose-response relations were fit to the Hill equation to obtain EC50 and Hill coefficient values, which are reported as means ± S.E. of the fit.
RESULTS
General Strategy
The two hydrogen bonding interactions being considered here involve the protein backbone, and such interactions can be probed by incorporating α-hydroxy analogs of amino acids at appropriate locations (Fig. 2B). As a probe of the Leu NH, the strategy is straightforward: the backbone NH is replaced with an oxygen. Concerning the backbone CO, α-hydroxy substitution attenuates the hydrogen bonding ability of the i−1 carbonyl by converting it to an ester carbonyl. It is well established that the carbonyls of esters are much poorer hydrogen bond acceptors than those of amides. Interestingly, in many studies, both quantitative and qualitative, it has been shown that the two effects associated with backbone ester incorporation, removal of the NH hydrogen bond donor and attenuation of the CO hydrogen bond acceptor, can have similar energetic consequences (17–21). As such, to perturb the Asn CO, we actually mutated the i+1 residue, which is Leu in the α4β4 receptor and Val in the muscle-type receptor. Backbone ester mutations can be efficiently incorporated site-specifically into nAChRs expressed in Xenopus oocytes by nonsense suppression methodology (22, 23). Typical experimental traces and dose-response relations for unnatural amino acid mutagenesis experiments with these receptors have been reported previously (12, 13).
These studies use EC50, the effective agonist concentration needed to reach a half-maximal response, as a readout of the functional impact of each mutation. It is well recognized that EC50 is a composite measure, reflecting multiple equilibria that include both “binding” events (drug entering/exiting the agonist-binding site) and “gating” events (the equilibria between open and closed states of the channel). It is typical in an EC50 study to note an ambiguity as to whether a given mutation affects binding or gating. We would argue that, in the present system, as in many similar previous studies from our laboratory, the ambiguity is of a different kind. Given the subtlety and precision of the modifications enabled by unnatural amino acid mutagenesis, combined with our structural knowledge of the binding site and the location of the mutations made, it is clear that we are perturbing a hydrogen bonding interaction between the drug and the receptor, a binding interaction. To see a change in EC50, it must be true that the hydrogen bonding interaction is diminished (or enhanced) in one or more of the multiple equilibria noted above. In the studies presented here, the ambiguity in the EC50 measurement concerns which equilibrium is perturbed, not the nature of the perturbation, which is clearly an attenuated binding interaction.
Detailed kinetic analyses, typically at the single-channel level, can often determine which equilibrium step(s) is being perturbed. However, we consider EC50 to be an appropriate metric here for two reasons. First, detailed single-channel studies are not feasible for the large number of drug-receptor combinations that we have considered. This is especially so, given the protein expression limitations that are sometimes seen with unnatural amino acid mutagenesis. Second and more importantly, our goal is to make pharmacological comparisons among closely related systems in response to subtle structural changes. We also wish to compare these results to those of previous studies on related systems. EC50 is a good measure of pharmacological activity. Given our experience with these systems and unnatural amino acid mutagenesis in particular, we consider EC50 differences of less than a factor of 2 to be not interpretable.
For studies of the muscle-type receptor, we used the known L9′S mutation in the M2 transmembrane helix of the β1 subunit (where 9′ is the ninth amino acid from the cytoplasmic end of the M2 α helix) (24, 25). This mutation was introduced to generically increase the sensitivity of the protein to agonists, and it resulted in a systematic ∼40-fold decrease in EC50. Given that the 9′ position is ∼60 Å away from the agonist-binding site, this mutation is generally expected to affect primarily gating and not agonist binding, although complications can arise (26). We performed backbone ester mutagenesis of the Leu NH in the muscle-type receptor in both the absence and presence of the L9′S background mutation, and similar shifts in EC50 were seen for ACh (see Table 3). This confirms the viability of this strategy in the present system. The agonist concentrations that were required to obtain a dose-response relation for epibatidine, nicotine, and choline in the absence of the L9′S mutation were high enough that channel block by the agonist became a problem with some mutants, so all comparisons for this receptor were done using the L9′S mutation. An analogous mutation was also used in the studies of α4β2 (12). No such modification was necessary for the α4β4 receptor.
TABLE 3.
EC50 and Hill coefficient values (mean ± S.E.) for mutations in the muscle-type receptor
Mutations identified as Leu and Val represent recovery of the wild-type receptor by nonsense suppression. Mutations denoted with an asterisk have the βL9′S mutation. Lah, α-hydroxyleucine; Vah, α-hydroxyvaline.
| Mutation | Drug | EC50 | Hill |
|---|---|---|---|
| μm | |||
| Wild type | ACh | 16.0 ± 0.3 | 1.3 ± 0.1 |
| γL119Leu/δL121Leu | ACh | 16.0 ± 0.5 | 1.5 ± 0.1 |
| γL119Lah/δL121Lah | ACh | 230 ± 6 | 1.5 ± 0.1 |
| Wild type* | ACh | 0.61 ± 0.04 | 1.4 ± 0.1 |
| γL119Leu/δL121Leu* | ACh | 0.31 ± 0.02 | 1.5 ± 0.1 |
| γL119Lah/δL121Lah* | ACh | 9.1 ± 0.7 | 1.6 ± 0.2 |
| Wild type* | Choline | 840 ± 20 | 1.6 ± 0.1 |
| γL119Leu/δL121Leu* | Choline | 780 ± 30 | 1.7 ± 0.1 |
| γL119Lah/δL121Lah* | Choline | 1000.00 ± 0.05 | 1.8 ± 0.1 |
| Wild type* | (±)-Epibatidine | 0.32 ± 0.02 | 1.5 ± 0.1 |
| γL119Leu/δL121Leu* | (±)-Epibatidine | 0.40 ± 0.02 | 1.5 ± 0.1 |
| γL119Lah/δL121Lah* | (±)-Epibatidine | 0.52 ± 0.03 | 1.6 ± 0.1 |
| Wild type* | S-Nicotine | 22.0 ± 0.8 | 1.6 ± 0.1 |
| γL119Leu/δL121Leu* | S-Nicotine | 23 ± 0.7 | 1.7 ± 0.1 |
| γL119Lah/δL121Lah* | S-Nicotine | 230 ± 30 | 2.2 ± 0.5 |
| γV108Val/δV110Val* | ACh | 0.29 ± 0.01 | 1.3 ± 0.1 |
| γV108Vah/δV110Vah* | ACh | 0.41 ± 0.05 | 1.2 ± 0.2 |
| γV108Val/δV110Val* | Choline | 620 ± 20 | 1.4 ± 0.1 |
| γV108Vah/δV110Vah* | Choline | 790 ± 60 | 1.4 ± 0.1 |
| γV108Val/δV110Val* | (±)-Epibatidine | 0.230 ± 0.006 | 1.4 ± 0.1 |
| γV108Vah/δV110Vah* | (±)-Epibatidine | 0.240 ± 0.006 | 1.5 ± 0.1 |
| γV108Val/δV110Val* | S-Nicotine | 15 ± 1 | 1.2 ± 0.1 |
| γV108Vah/δV110Vah* | S-Nicotine | 33 ± 2 | 1.6 ± 0.1 |
| α1G153K* | ACh | 0.0072 ± 0.0007 | 1.3 ± 0.1 |
| α1G153K/γL119Leu/δL121Leu* | ACh | 0.0076 ± 0.0007 | 1.8 ± 0.3 |
| α1G153K/γL119Lah/δL121Lah* | ACh | 0.18 ± 0.02 | 1.3 ± 0.1 |
| α1G153K* | Choline | 30 ± 2 | 1.0 ± 0.1 |
| α1G153K/γL119Leu/δL121Leu* | Choline | 27 ± 2 | 1.1 ± 0.1 |
| α1G153K/γL119Lah/δL121Lah* | Choline | 68 ± 3 | 1.3 ± 0.1 |
| α1G153K* | (±)-Epibatidine | 0.0043 ± 0.0005 | 0.77 ± 0.5 |
| α1G153K/γL119Leu/δL121Leu* | (±)-Epibatidine | 0.0023 ± 0.0004 | 0.74 ± 0.1 |
| α1G153K/γL119Lah/δL121Lah* | (±)-Epibatidine | 0.0096 ± 0.0004 | 1.1 ± 0.1 |
| α1G153K* | S-Nicotine | 0.32 ± 0.03 | 1.4 ± 0.2 |
| α1G153K/γL119Leu/δL121Leu* | S-Nicotine | 0.36 ± 0.04 | 0.95 ± 0.1 |
| α1G153K/γL119Lah/δL121Lah* | S-Nicotine | 6.5 ± 0.5 | 1.3 ± 0.1 |
To facilitate discussion, we collected the key data (the -fold shifts in EC50 values for backbone mutants) into Tables 1 and 2. Full EC50 values and Hill coefficients are given in Tables 3 and 4.
TABLE 1.
Shifts in EC50 values for backbone mutants of the muscle-type receptor
Values are EC50(mutant)/EC50(wild type). Lah, α-hydroxyleucine; Vah, α-hydroxyvaline.
| ACh | Nicotine | Epibatidine | Choline | |
|---|---|---|---|---|
| Probing Leu NH−Leu-Lah mutation | 29 | 10 | 1.3 | 1.3 |
| Probing Asn CO−Val-Vah mutation | 1.4 | 2.2 | 1.0 | 1.3 |
TABLE 2.
Shifts in EC50 values for backbone mutants of the α4β4 receptor
Values are EC50(mutant)/EC50(wild type). Lah, α-hydroxyleucine.
| ACh | Nicotine | Epibatidine | Varenicline | Cytisine | Choline | |
|---|---|---|---|---|---|---|
| Probing Leu NH−Leu-Lah mutation | 2.9 | 2.8 | 1.9 | 0.38 | 14 | 1.4 |
| Probing Asn CO−Leu-Lah mutation | 0.86 | 0.89 | 1.3 | 2.0 | 0.61 | 0.92 |
TABLE 4.
EC50 and Hill coefficient values (mean ± S.E.) for mutations made to the the α4β4 receptor
Mutations identified as Leu represent recovery of the wild-type receptor by nonsense suppression. Lah, α-hydroxyleucine.
| Mutation | Drug | EC50 | Hill |
|---|---|---|---|
| μm | |||
| β4L108Leu | ACh | 15.2 ± 0.9 | 1.43 ± 0.09 |
| β4L108Lah | ACh | 13 ± 2 | 1.2 ± 0.2 |
| β4L108Leu | S-Nicotine | 1.9 ± 0.2 | 1.3 ± 0.1 |
| β4L108Lah | S-Nicotine | 1.7 ± 0.2 | 1.4 ± 0.1 |
| β4L108Leu | (±)-Epibatidine | 0.0050 ± 0.0002 | 1.70 ± 0.08 |
| β4L108Lah | (±)-Epibatidine | 0.0065 ± 0.0002 | 1.71 ± 0.08 |
| β4L108Leu | Varenicline | 0.120 ± 0.004 | 1.38 ± 0.05 |
| β4L108Lah | Varenicline | 0.24 ± 0.01 | 1.19 ± 0.05 |
| β4L108Leu | (−)-Cytisine | 0.227 ± 0.005 | 1.42 ± 0.04 |
| β4L108Lah | (−)-Cytisine | 0.139 ± 0.007 | 1.40 ± 0.08 |
| β4L108Leu | Choline | 1200 ± 70 | 1.62 ± 0.09 |
| β4L108Lah | Choline | 1100 ± 100 | 1.4 ± 0.1 |
| β4L119Leu | ACh | 15.0 ± 0.7 | 1.42 ± 0.08 |
| β4L119Lah | ACh | 43 ± 4 | 1.5 ± 0.2 |
| β4L119Leu | S-Nicotine | 2.1 ± 0.2 | 1.3 ± 0.1 |
| β4L119Lah | S-Nicotine | 5.8 ± 0.4 | 1.4 ± 0.1 |
| β4L119Leu | (±)-Epibatidine | 0.0055 ± 0.0001 | 1.80 ± 0.05 |
| β4L119Lah | (±)-Epibatidine | 0.01018 ± 0.00009 | 1.63 ± 0.02 |
| β4L119Leu | Varenicline | 0.133 ± 0.002 | 1.37 ± 0.02 |
| β4L119Lah | Varenicline | 0.050 ± 0.003 | 1.5 ± 0.1 |
| β4L119Leu | (−)-Cytisine | 0.229 ± 0.004 | 1.37 ± 0.02 |
| β4L119Lah | (−)-Cytisine | 3.1 ± 0.1 | 1.37 ± 0.05 |
| β4L119Leu | Choline | 1400 ± 300 | 1.4 ± 0.2 |
| β4L119Lah | Choline | 2000 ± 700 | 1.1 ± 0.2 |
Mutagenesis Studies of the Leu NH
To probe for the presumed hydrogen bond to the Leu backbone NH, the leucine was replaced with its α-hydroxy acid analog (α-hydroxyleucine). In the muscle-type receptor, ACh and nicotine both showed substantial increases in EC50 (Table 1), confirming that the backbone NH is important for receptor activation by these agonists. Surprisingly, epibatidine, a nicotine analog that is quite potent at the muscle-type nAChR (although ∼300-fold less so than at the α4β2 subtype), was unresponsive to the backbone ester mutation. This contrasts with the 5-fold increase in EC50 seen in the α4β2 receptor for the analogous mutation with epibatidine as agonist. As expected, choline, which lacks the CO that serves as the hydrogen bond acceptor, was unresponsive to the backbone mutation, giving no shift in EC50 upon incorporation of the α-hydroxy acid.
We have shown previously that introduction of a single mutation in the α1 subunit of the muscle-type receptor, α1G153K, has dramatic effects on the EC50 for nicotine and that the increased potency of nicotine is, at least in part, a consequence of an enhanced cation-π interaction with nicotine in the mutant muscle-type receptor (12). In the present study, we found that the α1G153K mutation had little effect on the magnitude of the EC50 -fold shifts seen for ACh or choline in response to the backbone mutation at Leu (Table 3). A small increase in the -fold shift in EC50 was seen for nicotine and epibatidine, suggesting that perhaps the α1G153K mutation has a modest effect on agonist binding to the Leu residue for these agonists.
Surprisingly, the analogous mutation of Leu to α-hydroxyleucine in the α4β4 receptor showed small to negligible effects for ACh, nicotine, epibatidine, varenicline, and (as expected) choline (Table 2). Cytisine showed a large response, establishing that the Leu NH can function as a hydrogen bond donor to an agonist in the α4β4 receptor.
Mutagenesis Studies of the Asn CO
The second hydrogen bond predicted by the AChBP structures is to the backbone CO of a conserved Asn. To probe for a hydrogen bond to this backbone CO, the i+1 residue (Val in the muscle-type receptor and Leu in the α4β4 receptor) was replaced with its α-hydroxy acid analog (α-hydroxyvaline for Val or α-hydroxyleucine for Leu). As discussed above, this converts a backbone amide to a backbone ester, thereby attenuating the hydrogen bond-accepting ability of this moiety.
Early efforts to probe the CO of the relevant Asn residue in the α4β2 receptor gave inconsistent results that led us to question whether we could reliably control the stoichiometry of the mutant receptor (27). Because the muscle-type receptor reliably assembles into just one stoichiometry ((α1)2β1γδ), we anticipated that comparable experiments would experience fewer complications, and indeed, nonsense suppression studies at the appropriate Val gave functional mutant receptors. However, ACh, nicotine, epibatidine, and choline were not significantly impacted by the backbone ester mutation (Table 1).
With the experience gained from the muscle-type receptor, we were able to probe the key Asn carbonyl in a neuronal receptor, α4β4. Again, we found no evidence for a meaningful interaction with the carbonyl for the agonists ACh, nicotine, epibatidine, varenicline, cytisine, and choline (Table 2).
DISCUSSION
In recent years, the well studied nicotinic pharmacophore, composed of a cationic nitrogen and a hydrogen bond acceptor (28), has been mapped onto specific binding interactions in the nAChR (Fig. 1). The cationic nitrogen binds to the principal component of the agonist-binding site in the α subunit, and the hydrogen bond acceptor binds to the complementary non-α subunit. Guided by structures of AChBP, backbone mutagenesis studies established a hydrogen bond between the pharmacophore acceptor (the pyridine nitrogen of nicotine and the carbonyl oxygen of ACh) and a Leu backbone NH in the neuronal α4β2 nAChR. It is important to note that it is not just the rise in EC50 resulting from the backbone mutation that establishes a hydrogen bond. In all cases, choline, which lacks the hydrogen bond acceptor of the other agonists, is unaffected by the backbone mutation, proving a direct link between the mutation and the hydrogen bond acceptor of agonists. In our previous experiments with the α4β2 subtype, we also studied the nicotine analog S-N-methyl-2-phenylpyrrolidine (16). In this structure, the pyridyl ring of nicotine is replaced with a phenyl ring, providing an alternative way to probe the hydrogen bond-accepting pyridine nitrogen. This is a more subtle probe than the ACh/choline comparison, and it provided a compelling link between the hydrogen bond acceptor of nicotine and the backbone NH in the α4β2 receptor. In the present systems, we were unable to perform comparable studies with S-N-methyl-2-phenylpyrrolidine because the low potency of this compound at the receptors considered here required agonist concentrations that produced competing channel block of the receptor in dose-response studies. Nevertheless, the studies of the α4β2 receptor provide support for the idea that mutations of the Leu NH are perturbing a hydrogen bond to the agonist.
It is worth emphasizing that, although we consider the present work as probing hydrogen bonding interactions, we are in fact probing the functional significance of particular hydrogen bonds. Thus, it is possible that a structural study could show the presence of a hydrogen bond, but if deleting/attenuating that hydrogen bond has no functional consequence, it would show up as no hydrogen bond in our assay. We first discuss our findings concerning the contribution of the Leu backbone NH.
ACh and nicotine both show a strong hydrogen bonding interaction with the Leu backbone NH in the muscle-type receptor. Nicotine shows very poor potency at the wild-type muscle receptor, and so we were surprised to find that nicotine is very sensitive to the Leu backbone ester mutation, more sensitive than it is to the corresponding mutation in the α4β2 receptor, in which nicotine is a very potent agonist. This mutation also impacts ACh potency much more in the muscle-type receptor than in the α4β2 receptor. We have performed similar backbone mutations at locations throughout the nAChR to probe for various hydrogen bonds, and we typically saw informative but modest increases in EC50 of ∼5–20-fold. The 29-fold increase in EC50 seen for ACh in the muscle-type receptor is among the largest responses we have seen for a backbone ester mutation. It is also much larger than the 7-fold increase seen for the equivalent mutation in the α4β2 receptor (16). These results may suggest that the hydrogen bond to the Leu NH is stronger in the muscle-type receptor, and it is possible that ACh and nicotine sit more closely to this residue in the muscle-type receptor than they do in the α4β2 subtype. As expected, choline as an agonist is unresponsive to this mutation.
Surprisingly, epibatidine, a potent agonist at the muscle-type receptor, is unresponsive to the Leu backbone NH mutation. In crystal structures of AChBP binding nicotine or epibatidine, the relative positioning of all relevant atoms, the pyridine nitrogen and the backbone NH and CO, is essentially identical. As such, it has been assumed that these two closely related molecules bind in the same way, even though the bridging water is not observable in the epibatidine structure, presumably because it is not ordered enough for the relatively low resolution structure.
One possible explanation for the lack of a functionally significant hydrogen bonding interaction with the pyridine nitrogen of epibatidine in the muscle-type receptor is that the nitrogen is a relatively poor hydrogen bond acceptor. When considering closely related systems, pKa is an excellent predictor of hydrogen bonding ability. Pyridine, a good model for nicotine, has a pKa of 5.2, but 2-chloropyridine, a model for epibatidine, has a much lower pKa of 0.5 (29). Thus, epibatidine is expected to be a poorer base than nicotine by ∼5 orders of magnitude, and it is safe to conclude that epibatidine would also be a much poorer hydrogen bond acceptor. Recently, we showed that varenicline, the smoking cessation compound marketed as Chantix®, is similarly unresponsive to the analogous backbone NH mutation in the α4β2 receptor (30). The quinoxaline nitrogens of varenicline have a pKa of 0.8, quite similar to that for epibatidine. Thus, in these two very potent nicotinic agonists, epibatidine and varenicline, the strength of the hydrogen bond acceptor is expected to be greatly attenuated, and our functional assay for this hydrogen bond appears to reflect this property.
Earlier studies showed that the identity of the residue at position 153 of the α1 subunit strongly impacts receptor function in the muscle-type receptor. An α1G153K mutation in the muscle-type receptor greatly increases nicotine potency, and it does so by facilitating a strong cation-π interaction with TrpB that is absent in the wild-type receptor. We have now found that the α1G153K mutation does not have a substantial impact on the Leu backbone NH interaction. This is perhaps not surprising, given that the α1Gly-153 residue is located in the principal component of the agonist-binding site, whereas the Leu residue lies across the subunit interface in the complementary component of the binding site.
When we probed the Leu backbone NH interaction in the α4β4 receptor, we found a much smaller impact of the backbone mutation. ACh and nicotine showed meaningful but much smaller effects than is usual; in α4β2 receptors, the effect ranged from 5.6- to 8.5-fold for the same agonists. Again, epibatidine showed no meaningful effect, and varenicline actually showed a small gain of function. Cytisine, another compound that is marketed for smoking cessation under the brand name Tabex®, showed a large effect. The hydrogen bond acceptor in cytisine is not a pyridine-type nitrogen but is rather the oxygen of an amide carbonyl. Amides are much stronger hydrogen bond acceptors, and indeed, we saw very large effects for cytisine at the α4β2 receptor. For the two stoichiometries of α4β2, 2:3 and 3:2, the backbone NH mutation led to perturbations of 62- and 14-fold, respectively, with cytisine as the agonist.
The second component of the interaction with the hydrogen bond acceptor of nicotinic agonists predicted by AChBP structures is the water-mediated hydrogen bond to the Asn backbone CO. Using the backbone ester strategy to perturb this proposed interaction, we measured nine different drug-receptor interactions involving two different receptors, and in no case, did we see a meaningful interaction (not including choline, for which no effect is expected). The strongest effects were for varenicline at the α4β4 receptor, with a ratio of 2.0, and for nicotine at the muscle-type receptor, with a ratio of 2.2, barely what we consider to be meaningful. All other effects were less than a factor of 2.
Note that the strategy employed here to probe a hydrogen bond to a backbone carbonyl can produce large effects. In nicotinic receptors, when we used the strategy to probe the interaction of the N+H of the drug with the backbone carbonyl of the key Trp residue of the binding site (Fig. 1), EC50 shifts ranged from 9- to 27-fold for potent drug-receptor combinations.
We thus conclude that the water-mediated interaction between the hydrogen bond acceptor of agonists and the Asn backbone CO seen in AChBP structures is not functionally significant in nAChRs in general. We note that there is a fundamental distinction between the two hydrogen bonds seen in AChBP. If the water molecule were not present, the Leu backbone NH could donate a hydrogen bond directly to the hydrogen bond acceptor of agonists. In contrast, the Asn backbone CO is itself a hydrogen bond acceptor, and so it can interact with the hydrogen bond acceptor of agonists only through an intermediary water. Our results thus open up the possibility that the water molecule that is seen in essentially all AChBP structures is not present in actual receptors. Whether the water molecule is or is not present, we found that perturbing its putative hydrogen bonding partner has little consequence on receptor function.
In the pharmacology of nicotinic receptors, it has often been suggested that the complementary non-α subunit plays a key role in establishing subtype selectivity for various drugs. We have now probed the complementary binding site for four nicotinic subtypes (muscle-type, α4β4, and both stoichiometries of α4β2) and observed interesting variations for particular drug-receptor combinations. We believe that this information will be of value to efforts to develop more selective drugs that target nicotinic receptors.
We have shown that ACh and nicotine both engage in a functionally important hydrogen bond to the complementary subunit Leu backbone NH in the muscle-type nAChR, but the nicotine analog epibatidine does not. In the α4β4 receptor, interactions with the Leu backbone NH are surprisingly weak. We also found no evidence for a functionally important water-mediated hydrogen bond to the Asn backbone CO. Our results highlight the necessity of functional studies on intact receptors to probe interactions suggested by structural studies of model systems.
Acknowledgment
We thank Pfizer for the generous gift of varenicline.
This work was supported, in whole or in part, by National Institutes of Health Grants NS34407 and NS11756. This work was also supported by Tobacco-Related Disease Research Program Award 19XT-0102 from the University of California.
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- AChBP
- acetylcholine-binding protein.
REFERENCES
- 1. Corringer P. J., Le Novère N., Changeux J. P. (2000) Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458 [DOI] [PubMed] [Google Scholar]
- 2. Grutter T., Changeux J. P. (2001) Nicotinic receptors in wonderland. Trends Biochem. Sci. 26, 459–463 [DOI] [PubMed] [Google Scholar]
- 3. Karlin A. (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 [DOI] [PubMed] [Google Scholar]
- 4. Corringer P. J., Poitevin F., Prevost M. S., Sauguet L., Delarue M., Changeux J. P. (2012) Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure 20, 941–956 [DOI] [PubMed] [Google Scholar]
- 5. Lester H. A., Dibas M. I., Dahan D. S., Leite J. F., Dougherty D. A. (2004) Cys-loop receptors: new twists and turns. Trends Neurosci. 27, 329–336 [DOI] [PubMed] [Google Scholar]
- 6. Dougherty D. A. (2008) Cys-loop neuroreceptors: structure to the rescue? Chem. Rev. 108, 1642–1653 [DOI] [PubMed] [Google Scholar]
- 7. Jensen A. A., Frølund B., Liljefors T., Krogsgaard-Larsen P. (2005) Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48, 4705–4745 [DOI] [PubMed] [Google Scholar]
- 8. Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van Der Oost J., Smit A. B., Sixma T. K. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 9. Celie P. H., van Rossum-Fikkert S. E., van Dijk W. J., Brejc K., Smit A. B., Sixma T. K. (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 [DOI] [PubMed] [Google Scholar]
- 10. Sixma T. K., Smit A. B. (2003) Acetylcholine-binding protein (AChBP): a secreted glial protein that provides a high-resolution model for the extracellular domain of pentameric ligand-gated ion channels. Annu. Rev. Biophys. Biomol. Struct. 32, 311–334 [DOI] [PubMed] [Google Scholar]
- 11. Cashin A. L., Petersson E. J., Lester H. A., Dougherty D. A. (2005) Using physical chemistry to differentiate nicotinic from cholinergic agonists at the nicotinic acetylcholine receptor. J. Am. Chem. Soc. 127, 350–356 [DOI] [PubMed] [Google Scholar]
- 12. Xiu X., Puskar N. L., Shanata J. A., Lester H. A., Dougherty D. A. (2009) Nicotine binding to brain receptors requires a strong cation-π interaction. Nature 458, 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong W., Gallivan J. P., Zhang Y., Li L., Lester H. A., Dougherty D. A. (1998) From ab initio quantum mechanics to molecular neurobiology: a cation-π binding site in the nicotinic receptor. Proc. Natl. Acad. Sci. U.S.A. 95, 12088–12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen S. B., Sulzenbacher G., Huxford T., Marchot P., Bourne Y., Taylor P. (2006) Structural characterization of agonist- and antagonist-bound acetylcholine-binding protein from Aplysia californica. J. Mol. Neurosci. 30, 101–102 [DOI] [PubMed] [Google Scholar]
- 15. Hansen S. B., Sulzenbacher G., Huxford T., Marchot P., Taylor P., Bourne Y. (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24, 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blum A. P., Lester H. A., Dougherty D. A. (2010) Nicotinic pharmacophore: the pyridine N of nicotine and carbonyl of acetylcholine hydrogen bond across a subunit interface to a backbone NH. Proc. Natl. Acad. Sci. U.S.A. 107, 13206–13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deechongkit S., Dawson P. E., Kelly J. W. (2004) Toward assessing the position-dependent contributions of backbone hydrogen bonding to β-sheet folding thermodynamics employing amide-to-ester perturbations. J. Am. Chem. Soc. 126, 16762–16771 [DOI] [PubMed] [Google Scholar]
- 18. Deechongkit S., Nguyen H., Powers E. T., Dawson P. E., Gruebele M., Kelly J. W. (2004) Context-dependent contributions of backbone hydrogen bonding to β-sheet folding energetics. Nature 430, 101–105 [DOI] [PubMed] [Google Scholar]
- 19. England P. M., Zhang Y., Dougherty D. A., Lester H. A. (1999) Backbone mutations in transmembrane domains of a ligand-gated ion channel: implications for the mechanism of gating. Cell 96, 89–98 [DOI] [PubMed] [Google Scholar]
- 20. Gleitsman K. R., Kedrowski S. M. A., Lester H. A., Dougherty D. A. (2008) An intersubunit hydrogen bond in the nicotinic acetylcholine receptor that contributes to channel gating. J. Biol. Chem. 283, 35638–35643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koh J. T., Cornish V. W., Schultz P. G. (1997) An experimental approach to evaluating the role of backbone interactions in proteins using unnatural amino acid mutagenesis. Biochemistry 36, 11314–11322 [DOI] [PubMed] [Google Scholar]
- 22. Nowak M. W., Gallivan J. P., Silverman S. K., Labarca C. G., Dougherty D. A., Lester H. A. (1998) In vivo incorporation of unnatural amino acids into ion channels in a Xenopus oocyte expression system. Methods Enzymol. 293, 504–529 [DOI] [PubMed] [Google Scholar]
- 23. Nowak M. W., Kearney P. C., Sampson J. R., Saks M. E., Labarca C. G., Silverman S. K., Zhong W., Thorson J., Abelson J. N., Davidson N., Schultz P. G., Dougherty D. A., Lester H. A. (1995) Nicotinic receptor binding site probed with unnatural amino acid incorporation in intact cells. Science 268, 439–442 [DOI] [PubMed] [Google Scholar]
- 24. Labarca C., Nowak M. W., Zhang H., Tang L., Deshpande P., Lester H. A. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516 [DOI] [PubMed] [Google Scholar]
- 25. Filatov G. N., White M. M. (1995) The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol. Pharmacol. 48, 379–384 [PubMed] [Google Scholar]
- 26. Gleitsman K. R., Shanata J. A. P., Frazier S. J., Lester H. A., Dougherty D. A. (2009) Long-range coupling in an allosteric receptor revealed by mutant cycle analysis. Biophys. J. 96, 3168–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blum A. P. (2012) Structure-Function Studies of Nicotinic Acetylcholine Receptors Using Unnatural Amino Acids and Synthetic Agonist Analogs. Ph.D. thesis, California Institute of Technology, Pasadena, CA [Google Scholar]
- 28. Beers W. H., Reich E. (1970) Structure and activity of acetylcholine. Nature 228, 917–922 [DOI] [PubMed] [Google Scholar]
- 29. Linnell R. H. (1960) Dissociation constants of 2-substituted pyridines. J. Org. Chem. 25, 290 [Google Scholar]
- 30. Da Silva Tavares X., Blum A. P., Nakamura D. T., Puskar N. L., Shanata J. A. P., Lester H. A., Dougherty D. A. (2012) Variations in binding among several agonists at two stoichiometries of the neuronal, α4β2 nicotinic receptor. J. Am. Chem. Soc. 134, 11474–11480 [DOI] [PMC free article] [PubMed] [Google Scholar]