Background: FAF1, which has multiple ubiquitin-like domains, interacts with various proteins (VCP, Hsp70, and polyubiquitinated proteins).
Results: Association of FAF1 UBX with VCP-Npl4-Ufd1 complex regulates ubiquitin binding to FAF1 UBA domain and promotes CD3δ degradation in ERAD.
Conclusion: FAF1 is a ubiquitin receptor that promotes ERAD by delivering polyubiquitinated proteins from UBX domain to UBA domain.
Significance: FAF1 plays a role in ERAD by modulating domain-domain interaction.
Keywords: ER-associated Degradation, Fas, Protein Domains, Ubiquitin, X-ray Crystallography, Fas-associated Factor 1, UBA and UBX, VCP-Npl4-Ufd1, Ubiquitin Receptor, Ubiquitin-related Domain
Abstract
Fas-associated factor 1 (FAF1) is a ubiquitin receptor containing multiple ubiquitin-related domains including ubiquitin-associated (UBA), ubiquitin-like (UBL) 1, UBL2, and ubiquitin regulatory X (UBX). We previously showed that N-terminal UBA domain recognizes Lys48-ubiquitin linkage to recruit polyubiquitinated proteins and that a C-terminal UBX domain interacts with valosin-containing protein (VCP). This study shows that FAF1 interacts only with VCP complexed with Npl4-Ufd1 heterodimer, a requirement for the recruitment of polyubiquitinated proteins to UBA domain. Intriguingly, VCP association to C-terminal UBX domain regulates ubiquitin binding to N-terminal UBA domain without direct interaction between UBA and UBX domains. These interactions are well characterized by structural and biochemical analysis. VCP-Npl4-Ufd1 complex is known as the machinery required for endoplasmic reticulum-associated degradation. We demonstrate here that FAF1 binds to VCP-Npl4-Ufd1 complex via UBX domain and polyubiquitinated proteins via UBA domain to promote endoplasmic reticulum-associated degradation.
Introduction
FAF15 is a ubiquitin receptor containing a ubiquitin-associated (UBA) domain and three domains with ubiquitin-like folds, UBL1, UBL2, and ubiquitin regulatory X (UBX) domains. N-terminal UBA domain (47 amino acids long) recruits polyubiquitinated proteins crucial for FAF1-mediated apoptosis and stress response (1, 2). UBL1 binds to Hsp70 and regulates its chaperone activity by promoting Hsp70 degradation (3, 4). C-terminal UBX domain of FAF1 binds to valosin-containing protein (VCP), a molecular chaperone in the ubiquitin-proteasome system. VCP, the mammalian homologue of the multifunctional Cdc48p in yeast and p97 in Xenopus, is a member of the AAA (ATPase associated with a variety of cellular activities) family and acts as a molecular chaperone in diverse functions such as cell cycle regulation, apoptosis, transcription activation, organelle biogenesis, vesicular transport, and endoplasmic reticulum-associated degradation (ERAD) (5–8). In the performance of these functions, VCP uses two classes of cofactors, substrate-recruiting cofactors and substrate-processing cofactors. FAF1 is a substrate-recruiting cofactor, whereas E3 ligases and deubiquitinating enzymes are substrate-processing cofactors (9).
The ER is the organelle that produces secretory proteins to be properly folded before they are delivered to their functional destination. ERAD as a quality control for proteins eliminates misfolded proteins and prevents their accumulation. The ERAD process includes retrotranslocation of misfolded proteins from the ER membrane into the cytosol, polyubiquitination by E3 ligases, and delivery to the proteasome for final degradation (10, 11). It is known that VCP uses nuclear protein localization protein 4 (Npl4)-Ufd1 heterodimer as the major ubiquitin-recruiting cofactor (12, 13), and furthermore, VCP-Npl4-Ufd1 complex plays an essential role in retrotranslocation as well as delivery to the proteasome (13).
We previously demonstrated that UBX interacts with VCP in a stress-dependent manner and that FAF1 plays a key role as a ubiquitin receptor (1). However, how FAF1 functions as a ubiquitin receptor is not well understood. In this study, we examined the domain-domain interactions possibly involved in the regulation of FAF1-VCP interaction. We constructed various mutants in ubiquitin-related domains of FAF1 and examined their interactions with VCP. We found that recruitment of polyubiquitinated proteins to N-terminal UBA domain in FAF1 is regulated by the interaction of C-terminal UBX domain with VCP-Npl4-Ufd1 complex. Furthermore, because VCP-Npl4-Ufd1 complex is known to play a key role in ERAD, we investigated whether FAF1 participates in the ERAD process. We report here that FAF1 promotes the degradation of ERAD substrate CD3δ in a VCP-Npl4-Ufd1-dependent manner.
EXPERIMENTAL PROCEDURES
Antibodies, Reagents, and Plasmids
The sources of antibodies used in this study are as follows: monoclonal anti-FLAG antibody from Sigma; anti-VCP, anti-Npl4, and anti-Ufd1 antibodies from Abcam (Cambridge, UK); anti-ubiquitin antibody from Chemicon International; anti-HA antibody, anti-FAF1, and anti-GAPDH antibodies from AbFrontier (Seoul, Korea); and anti-Hsp70 antibody from Stressgene (British Columbia, Canada). Doxycycline was purchased from Sigma. Human FAF1 was cloned into pFLAG-CMV2 vector to generate pFLAG-CMV2-FAF1. pFLAG-CMV2-FAF1(82–650) and pFLAG-CMV2-FAF1 I41N were prepared as described previously (1, 2). FLAG-CMV2-FAF1 constructs used in this study are listed in Fig. 1A.
FIGURE 1.
Constructs of FAF1 and VCP mutants studied. A, FAF1 WT; FAF1(TFPR → AG), VCP-interacting loop (618TFPR621) of FAF1 UBX domain was shortened to AG; FAF1(82–650), FAF1 UBA domain (1–47) was deleted; FAF1 ΔUBX, FAF1 UBX domain (569–650) was deleted; FAF1 ΔUBL1, FAF1 UBL1 domain (100–174) was deleted; FAF1 ΔUBL1–2, FAF1 UBL1 domain (100–174) and UBL2 domain (195–270) were deleted (Δ100–270); FAF1(TFPR → AG) ΔUBL1, UBL1 domain was deleted in FAF1(TFPR → AG) construct; FAF1(TFPR → AG) ΔUBL1–2, UBL1,2 domains were deleted in FAF1(TFPR → AG) construct; FAF1 I41N, point mutation of UBA domain at Ile41, which is crucial for ubiquitin interaction with N-terminal UBA domain (2). B, constructs of FAF1, VCP, and Npl4-Ufd1 complex used for structural and biochemical studies. Constructs used for the crystal structure are marked with a star.
FAF1(TFPR → AG), which shortened 618TFPR621 to AG; FAF1(82–650) (aa 82–650); FAF1ΔUBX (aa 1–568); FAF1ΔUBL1 (aa Δ100–174); FAF1ΔUBL1–2 (aa Δ100–270); FAF1(TFPR → AG)ΔUBL1, FAF1(TFPR → AG) (aa Δ100–174); and FAF1(TFPR → AG)ΔUBL1–2, FAF1(TFPR → AG) (aa Δ100–270) constructs were generated based on structural studies (14). CD3δ expression plasmid, pYR-CD3δ-FLAG, was constructed by introducing a PCR-generated BglII-XbaI fragment containing human CD3δ ORF into the same sites in pYR-cFLAG as described before (15). Because pYR-CD3δ-FLAG carries a tetracycline-regulated promoter, cells were co-transfected with pYR-CD3δ-FLAG and the plasmid encoding tTS (pTet-Off, Clontech) to express CD3δ-FLAG.
Cell Extracts and Immunoprecipitation
HEK293T and HeLa cells were grown and maintained in DMEM (Eagle's minimal essential medium) supplemented with 10% fetal bovine serum (FBS) at 37 °C in 5% CO2. Transfections were carried out with Effectene (Qiagen) transfection reagent in a 1:10 ratio as instructed by the manufacturer. For immunoprecipitation, cells (2–10 × 106) were lysed with a hypotonic lysis buffer (10 mm HEPES, pH 7.4, 1.5 mm MgCl2, 0.5% Nonidet P-40) containing protease inhibitors (10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin, 100 μg/ml phenylmethylsulfonyl fluoride) and phosphatase inhibitors (10 mm NaF, 10 mm Na3VO4) for 30 min on ice and centrifuged at 16,000 × g for 15 min. The supernatant was incubated for 3 h at 4 °C with anti-FLAG-agarose affinity beads. The beads were washed five times with 1 ml of lysis buffer containing 0.5% Nonidet P-40 to remove nonspecific binding, and the immune complex was solubilized in SDS gel sample buffer, separated by 10% SDS-PAGE, and detected with silver staining or Western analysis.
Preparation of FAF1, VCP, and Npl4-Ufd1 Heterodimer
Full-length FAF1 (aa 1–650), UBL1-UBL2 domain (aa 100–283), UAS-UBX domain (aa 308–650), UBX domain (aa 571–650), and UBX domain mutant TFPR → AG of FAF1 and full-length VCP (aa 2–806), VCP ND1 domain (aa 2–458), and VCP N-domain (aa 2–208) were cloned into pET-28a (Novagen). Ubiquitin fold domain of Npl4 (aa 1–80) was cloned into pET-15b (Novagen). Full-length Ufd1 (aa 1–307) and Npl4 (aa 1–608) were cloned into pET-28a and pET-22b (Novagen). GST-fused FAF1 UBA domain (aa 1–81), full-length VCP, and Ufd1-Npl4 complex were purified as described previously (2, 33). The full-length FAF1 was overexpressed in Escherichia coli Rosetta-gami (DE3) cells (Novagen), whereas the others were overexpressed in E. coli Rosetta (DE3) cells. The N-terminal histidine-tagged proteins were purified using an Ni-NTA column (GE Healthcare) followed by thrombin treatment (Sigma) and gel filtration on a Superdex-200S 26/60 column (GE Healthcare). Purified FAF1 proteins were dissolved in 50 mm HEPES buffer, pH 7.5 containing 150 mm NaCl, whereas VCP and Npl4-Ufd1 were dissolved in 50 mm HEPES buffer, pH 7.5 containing 150 mm NaCl, 5 mm MgCl2, and 1 mm DTT.
Silencing RNAs
ON-TARGETplus SMARTpool siRNAs and siControl duplex siRNA (Dharmacon) were used to knock down Npl4 and FAF1, respectively, at a final concentration of 100 nm. Silencing was achieved using Dharma FECT1 transfection reagent (Dharmacon) according to the manufacturer's instructions. Changes in the expression of FAF1 in HEK293T and HeLa cells were analyzed 72 h after siRNA transfection.
Protein Identification Using UPLC-ESI-q-TOF Tandem MS
To identify the proteins and modifications, the gel bands were destained and digested with trypsin, and the resulting peptides were extracted as described previously (16). The peptide extracts were evaporated to dryness in a SpeedVac and dissolved in 10% acetonitrile solution containing 1.0% formic acid. The dissolved samples were desalted on line prior to separation using a trap column (5-μm particle size; NanoEaseTM dC18, Waters) cartridge. Peptides were separated using a C18 reversed-phase 75-μm-inner diameter × 150-mm analytical column (3-μm particle size; AtlantisTM dC18, Waters) with an integrated electrospray ionization SilicaTipTM (10-μm inner diameter; New Objective). Chromatography was performed on line to a mass spectrometer (Q-Tof UltimaTM Global, Waters). Raw data obtained from the mass spectrometer were converted to .pkl files using ProteinLynx Global ServerTM 2.3 data processing software (Waters). MS/MS spectra were matched against amino acid sequences in Swiss-Prot. Large numbers and types of potential post-translational modifications were considered. All reported assignments were verified by automatic and manual interpretation of spectra from Mascot and MODi (17) in a blind mode.
Isothermal Titration Calorimetry (ITC)
ITC experiments were performed using VP-ITC and ITC200 instruments (MicroCal, Northampton, MA) at 298 K, and the data were analyzed using the program Origin 7.0. All samples (in 50 mm HEPES, pH 7.5, 150 mm NaCl) were centrifuged and degassed prior to the measurements at 298 K. The injectants were added at 150-s intervals to the sample solution in the cell.
Electron Microscopy (EM)
For EM, purified N-terminal His-tagged full-length FAF1 at 50 μg/ml in 50 mm HEPES buffer, pH 7.5 containing 150 mm NaCl was incubated with a 10-fold molar excess of 1.5-nm Ni-NTA-Nanogold (Nanoprobes Inc.) for 30 min, and the excess Ni-NTA-Nanogold was removed using a Superdex-200GL column (GE Healthcare). For generating FAF1 and VCP-Ufd1-Npl4 complex, gold-labeled FAF1 was incubated with purified VCP-Ufd1-Npl4 complex at a 1:1 molar ratio for 1 h at 4 °C, and the product was further purified on a Superdex-200S column to ensure removal of unbound FAF1 in 50 mm HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 1 mm AMP-PNP. The purified complex was diluted to 0.3 mg/ml concentration, then applied to glow-discharged carbon-coated grids, rinsed, and stained with 2% uranyl acetate. Images were recorded on a 2000 × 2000 charge-coupled device camera using a Tecnai F20 field emission gun electron microscope operated at 200 kV (FEI Co.). For imaging Nanogold (∼1.5 nm in diameter) on the complex, a Tecnai Titan microscope was used at 300 kV without staining the sample (FEI Co.). To observe both gold particles and protein complex, we used a cryotransmission EM method. For cryoexperiments, we diluted purified samples to a concentration of ∼0.02–0.1 mg/ml for VCP-Npl4-Ufd1 with Nanogold-labeled FAF1 complex and loaded samples onto holey carbon film-supported grids and plunge froze them. We recorded images on a charge-coupled device camera (2000 × 2000 charge-coupled device camera, Gatan) using a Tecnai F20 field emission gun electron microscope operated at 200 kV. Particles were selected from the individual digital micrographs.
X-ray Crystallography of FAF1 UBX·VCP N-domain Complex
Diffraction quality crystals of FAF1 UBX domain and VCP N-terminal domain complex (see supplemental Fig. S1) were obtained from 100 mm sodium acetate, 1 m LiCl2, 30% PEG 4000. FAF1 UBX·VCP N-domain complex was methylated and crystallized following the procedure described earlier (18). The crystal structure was determined by the molecular replacement method (MOLREP from the CCP4 suite) (19) using the N-domain of the VCP ND1 crystal structure (Protein Data Bank code 1E32) (20) as a search model. The resulting electron density was good enough to orient the FAF1 UBX using CAPRA. Manual building using Coot (21) and refinement using CNS (22) gave the final models, which were assessed with PROCHECK (23). Crystallographic data collected and the refinement statistics are summarized in supplemental Table 1. The atomic coordinates and structure factors of FAF1 UBX·VCP N-domain complex have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ under accession code 3QWZ.
RESULTS
Binding of VCP to C-terminal UBX Domain of FAF1 Is Necessary for the Recruitment of Polyubiquitinated Proteins to N-terminal UBA Domain
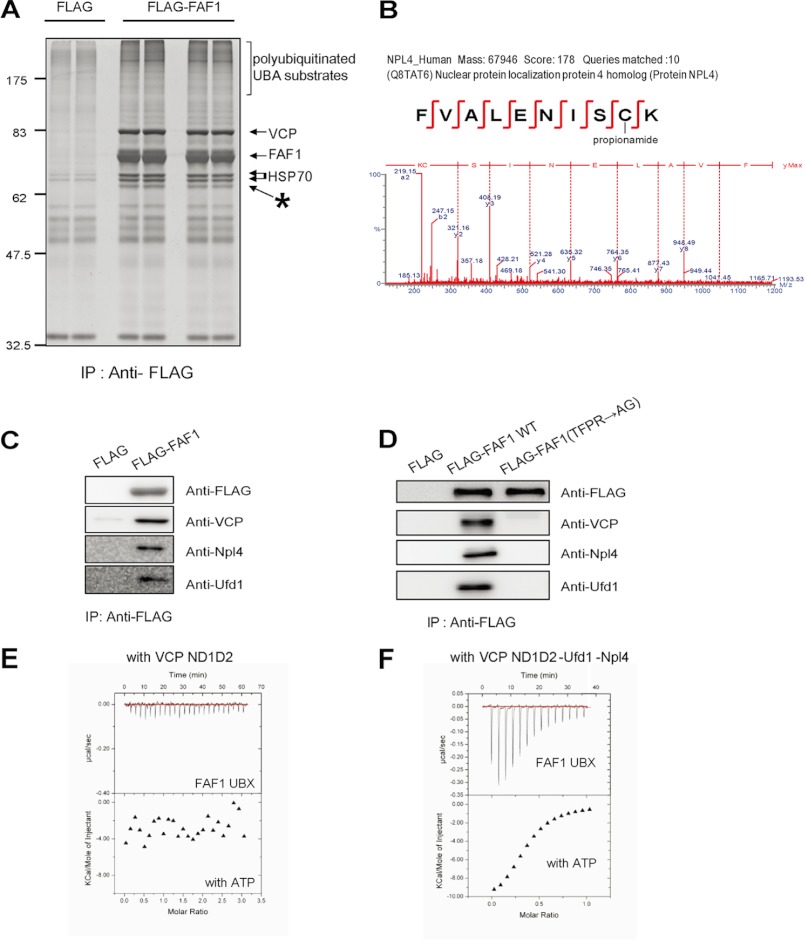
To investigate the effect of VCP binding on the biological function of FAF1, we designed a mutant of FAF1 defective in VCP binding. A previous structural study showed that UBX has a well conserved FPR motif in the S3/S4 loop that is a VCP binding motif (14, 24) and that FAF1 also has an S3/S4 loop in UBX domain, 618TFPR621, that is a possible site for VCP binding of FAF1 UBX domain. We produced a mutant of UBX domain, FAF1(TFPR → AG), that has a shorter S3/S4 loop (618TFPR621 mutated to AG). HEK293T cells were transfected with control FLAG, FLAG-FAF1, and FLAG-FAF1(TFPR → AG), and the cell lysates were immunoprecipitated with anti-FLAG antibody, respectively. Immune complexes were detected with Western analysis using anti-VCP antibody. As shown in Fig. 2A, FLAG-FAF1(TFPR → AG) with the deletion in the S3/S4 loop of UBX abrogated the interaction of FAF1 with VCP. Using the mutant defective in VCP binding, we investigated whether VCP binding to UBX domain affects the scaffolding ability of FAF1 to interact with Hsp70 through UBL1 domain and to recruit polyubiquitinated proteins through UBA domain. HEK293T cells overexpressing FLAG-FAF1 wild type (WT) and FLAG-FAF1(TFPR → AG) were lysed, and the cell lysates were immunoprecipitated with anti-FLAG antibody. The immune complexes were separated by 10% SDS-PAGE and detected with anti-Hsp70 and anti-ubiquitin antibodies. As shown in Fig. 2A, there were no discernible interaction changes of Hsp70 between FAF1 WT and FLAG-FAF1(TFPR → AG). However, FAF1(TFPR → AG) could not recruit polyubiquitinated substrates to N-terminal UBA domain of FAF1. These results indicate that VCP binding to FAF1 is required to recruit polyubiquitinated proteins to UBA domain of FAF1. We therefore hypothesize that VCP binding to FAF1-UBX domain is necessary for the biological function of FAF1.
FIGURE 2.
FAF1 UBX interaction with VCP regulates the recruitment of polyubiquitinated substrates to UBA domain without direct interaction with UBX or UBL domain(s). A, HEK293T cells were transfected with FLAG, FLAG-FAF1 WT, or FLAG-FAF1(TFPR → AG). At 24 h after transfection, lysates were immunoprecipitated (IP) with anti-FLAG antibody. Immune complexes were separated by 10% SDS-PAGE and detected by Western analysis using anti-FLAG, anti-VCP, anti-Hsp70, and anti-ubiquitin antibodies. Whole cell lysates were probed as input. B, immune complexes purified with anti-FLAG tag antibodies in HEK293T cells overexpressing FLAG, FLAG-FAF1 WT, FLAG-FAF1(TFPR → AG), or FLAG-FAF1 ΔUBX constructs were separated by SDS-PAGE, and proteins were detected by Western analysis using anti-FLAG, anti-VCP, and anti-ubiquitin antibodies. Whole cell lysates were probed as input. C, immune complexes purified with anti-FLAG antibodies in HEK293T cells overexpressing FLAG, FLAG-FAF1 WT, or the indicated constructs were separated by SDS-PAGE, and proteins were detected by Western analysis using anti-FLAG, anti-VCP, and anti-ubiquitin antibodies. D, HEK293T cells were transfected with various mutants including FLAG, FLAG-FAF1, FLAG-FAF1(82–650), FLAG-FAF1(TFPR → AG), and FLAG-FAF1 I41N. At 24 h after transfection, cell lysates were immunoprecipitated with anti-FLAG antibodies. Immune complexes were separated by 10% SDS-PAGE, and proteins were detected by Western analysis using anti-FLAG, anti-VCP, anti-Hsp70, and anti-ubiquitin antibodies.
UBX Domain Regulates UBA Domain through VCP Binding without Direct Interaction
It was previously reported that ubiquitin-like folds mimic ubiquitin to interact with ubiquitin binding motifs such as UBA, ubiquitin-interacting motif (UIM), and proteasome subunits (25–27). To elucidate how UBX domain regulates UBA domain in recruiting polyubiquitinated proteins, we examined whether there is a direct interaction between UBLs and UBX with UBA domain, similar to ubiquitin-UBA domain interaction, because they both contain ubiquitin-like folds. We constructed various deletion mutants of UBX, UBL1, and UBL2 domains of FLAG-FAF1 WT and FLAG-FAF1(TFPR → AG) (Fig. 1) and examined whether ubiquitin-like folds inactivate UBA domain by direct interaction.
Because FLAG-FAF1(TFPR → AG) failed to recruit polyubiquitinated proteins, we examined whether ubiquitin-like folds of UBX domain directly inactivate UBA domain by assessing whether the FLAG-FAF1 UBX domain deletion mutant (FAF1ΔUBX) possesses ubiquitin recruiting function of UBA domain. HEK293T cells were transfected with FLAG-FAF1 WT, FLAG-FAF1(TFPR → AG), or FLAG-FAF1ΔUBX; cell lysates were immunoprecipitated with anti-FLAG antibody; and the immune complexes were detected by Western analysis using anti-ubiquitin, anti-FLAG, and anti-VCP antibodies. As shown in Fig. 2B, the recruitment of polyubiquitinated substrates through UBA domain disappeared in cells overexpressing FLAG-FAF1ΔUBX as well as FLAG-FAF1(TFPR → AG). This suggests that UBA domain is regulated by UBX domain through VCP binding rather than via direct UBX-UBA interaction mediated by ubiquitin-like folds of UBX domain.
FAF1 has two UBL domains (UBL1 (aa 100–176) and UBL2 (aa 195–270)) located next to UBA domain (aa 1–47). We examined whether these UBL domains directly interact with UBA domain. Such an interaction is possible based on structural considerations, similar to Rad23 and Dsk2 (26, 28). We constructed UBL domain deletion mutants of FAF1 WT and FAF1(TFPR → AG) (Fig. 1A) and examined the interaction of UBA domain with polyubiquitinated proteins. HEK293 cells were transiently transfected with FLAG-FAF1 WT, FLAG-FAF1(TFPR → AG), and UBL domain deletion constructs ΔUBL1 and ΔUBL1–2. The cell lysates were immunoprecipitated with anti-FLAG antibody, and the immune complexes were separated by 10% SDS-PAGE and detected by Western analysis using anti-FLAG, anti-VCP, and anti-ubiquitin antibodies. As shown in Fig. 2C, recruitment of polyubiquitinated proteins to FAF1 WT and FAF1(TFPR → AG) was not affected by deletion of UBL domains. This indicates that UBL domains do not play any role in this interaction. We also confirmed domain-domain interaction using various domain proteins: FAF1 WT, FAF1(82–650) as a UBA deletion mutant, FAF1 I41N as a mutant defective in UBA domain, and FAF1(TFPR → AG) as a mutant defective in UBX domain (Fig. 2D). These results indicate that the mutant FAF1(TFPR → AG), which does not interact with VCP, also failed to recruit polyubiquitinated proteins like UBA deletion mutants.
Next, we determined the dissociation constants (KD) between FAF1 UBA (aa 1–81) and UBX (aa 571–650) and between UBA and UBL1–2 (aa 100–280) using ITC to examine direct domain interactions (Table 1). FAF1 UBA domain did not interact with UBX domain or UBL1–2 domain, whereas UBA interacted with Lys48-linked diubiquitin with a KD value of 3.5 μm as we reported previously (2). Thus, it appears that UBX domain regulates UBA domain through VCP binding without direct domain interaction mediated by ubiquitin-like folds of UBX or UBL domains.
TABLE 1.
ITC data
ND, no binding detected.
| Cell | Injectant | n | KD | ΔH |
|---|---|---|---|---|
| μm | kcal/mol | |||
| Lys48-diubiquitin | FAF1 UBA | 0.79 ± 0.01 | 3.5 ± 0.2 | −7.7 ± 0.64 |
| UBL1-UBL2 | FAF1 UBA | ND | ||
| UBX | FAF1 UBA | ND | ||
| VCP N | FAF1 UBX | 1.13 ± 0.03 | 25.6 ± 2.1 | −4.3 ± 0.20 |
| VCP N | FAF1 (TFPR → AG) | ND | ||
| VCP N | Npl4 UBD | 1.04 ± 0.02 | 17.8 ± 1.4 | −5.1 ± 0.20 |
| VCP ND1 | FAF1 UBX | 0.72 ± 0.05 | 22.7 ± 1.1 | −5.0 ± 0.51 |
| VCP ND1 + ATP | FAF1 UBX | 0.61 ± 0.02 | 20.1 ± 1.7 | −5.1 ± 0.33 |
| VCP ND1D2 | FAF1 UBX | ND | ||
| VCP ND1D2 | FAF1 UAS-UBX | ND | ||
| VCP ND1D2 + Ufd1-Npl4 | FAF1 UBX | 0.23 ± 0.11 | 08.4 ± 0.1 | −23.4 ± 13.83 |
| VCP ND1D2 + Ufd1-Npl4 + ATP | FAF1 UBX | 0.27 ± 0.08 | 09.6 ± 0.2 | −18.3 ± 07.03 |
| VCP ND1D2 + Ufd1-Npl4 + ATP | FAF1 UAS-UBX | 0.25 ± 0.09 | 19.5 ± 4.2 | −28.0 ± 16.47 |
FAF1 UBX Domain Interacts with VCP N-domain at a 1:1 Molar Ratio Using the S3/S4 Loop
The crystal structure of FAF1 UBX domain complexed to N-terminal domain of VCP at 2.0-Å resolution shows that FAF1 UBX domain binds at the interface between the two barrels of VCP N-domain (Fig. 3A). The β1 and β5 as well as the loop connecting β3 and β4 of FAF1 UBX domain (S3/S4 loop) are involved in both hydrophobic and hydrophilic interactions (Fig. 3B). Hydrophobic interactions involve Phe585, Phe619, Pro620, Pro640, and Phe645 of FAF1 UBX domain and Val38, Phe52, Ile70, Leu72, Pro106, Tyr110, and Tyr143 of p97 N-domain. Hydrogen bonds are formed between Gln641 of FAF1 UBX domain and Gln43 and Asp47 of p97 N-domain, FAF1 UBX domain Arg577 and Thr643 and p97 N-domain Asp55, FAF1 UBX domain Arg579 and p97 N-domain Tyr110, and FAF1 UBX domain Arg621 and Glu141 of p97 N-domain. Thr618-Phe619-Pro620-Arg621 forms a type VI β-turn (Fig. 3B) with Pro620, which is in the cis configuration, and it appears that this cis-Pro620-centered β-turn is important for UBX and VCP N-domain interaction. Recently, two independent studies also showed that the TFPR motif of FAF1 UBX domain adopts a cis-Pro620-centered β-turn (29) and touch-turn structures (which have a tighter turn than a β-turn) (30).
FIGURE 3.
Interaction between FAF1 UBX domain and VCP N-domain. A, crystal structure of FAF1 UBX domain (in pink) bound to p97 N-domain (in cyan). B, interactions between FAF1 UBX domain and p97 N-domain. There are salt bridges between Arg577 and Arg621 of FAF1 UBX domain and Asp55 and Glu141 of VCP N-domain. Hydrophobic interactions involve Phe585, Phe619, Pro620, Pro640, and Phe645 of FAF1 UBX domain and Val38, Phe52, Ile70, Leu72, Pro106, Tyr110, and Tyr143 of VCP N-domain. Water molecules are shown by red spheres. Hydrogen bonds are indicated by dotted black lines. Electron density (2Fo − Fc map) contoured at 1σ level around 618TFPR621 is shown in the box. C, ITC results of VCP N-domain + FAF1 UBX domain (left) and VCP N-domain + FAF1 UBX domain with the residues 618TFPR621 replaced by AG (right). The top panels show the associated heat effects, and the bottom panels show the fit of the experimental data with a one-site binding model.
ITC studies showed that the FAF1 UBX domain binds the VCP N-domain at a 1:1 molar ratio with an apparent KD of 25.6 μm (Fig. 3C). This is comparable with the KD value of 17.8 μm obtained for Npl4 ubiquitin fold domain and VCP N-domain (supplemental Fig. S2A). On the other hand, TFPR → AG mutant showed no detectable binding (Fig. 3C). Therefore, these findings together with crystallographic results clearly show that the highly conserved motif 618TFPR621 in the S3/S4 loop of FAF1 UBX domain is crucial for binding to the N-domain of VCP.
FAF1 Interacts with the Substrate-recruiting Cofactor Npl4-Ufd1 Heterodimer via VCP
To characterize further the proteins that interact with FAF1, we performed immunoprecipitation on a large scale using anti-FLAG affinity beads from lysates of HEK293T cells overexpressing FLAG or FLAG-FAF1. Immune complexes were separated by 10% SDS-PAGE, and the FAF1-interacting protein was detected after silver staining (Fig. 4A). Using peptide sequencing analysis with UPLC-ESI-q-TOF tandem MS, we identified the FAF1-interacting protein as Npl4 (Fig. 4B). Because it is well known that Npl4 forms heterodimers with Ufd1 and binds to VCP through the ubiquitin fold domain (31), we confirmed the binding of Npl4-Ufd1 complex to FAF1 by Western analysis of FLAG-FAF1 immune complex with anti-Npl4, anti-Ufd1, and anti-VCP antibodies as reported previously (32). Interaction of FAF1 with Npl4-Ufd1 heterocomplex is shown in Fig. 4C. To further investigate whether FAF1 interacts directly with Npl4-Ufd1 heterodimer or through VCP, we performed immunoprecipitation of HEK293T cells overexpressing FLAG-FAF1 WT and FLAG-FAF1(TFPR → AG) with anti-FLAG antibody. As shown in Fig. 4D, FAF1(TFPR → AG), a VCP binding-deficient mutant did not interact with Npl4-Ufd1 heterodimer, suggesting that Npl4-Ufd1 heterodimer interacts with FAF1 through VCP.
FIGURE 4.
Npl4 and Ufd1 complex with FAF1 through VCP. A, HEK293T cells were transfected with plasmids expressing FLAG or FLAG-FAF1. At 24 h after transfection, cell lysates were immunoprecipitated (IP) with anti-FLAG antibodies. Immune complexes were separated by 10% SDS-PAGE and detected by silver staining. B, MS/MS spectrum of Npl4 detected as FAF1-binding protein. An unexpected band marked with an asterisk in the silver-stained gel was analyzed by peptide sequencing using UPLC-ESI-q-TOF tandem MS. C, FAF1-interacting proteins were confirmed by Western analysis. Immune complexes purified with anti-FLAG antibodies in HEK293T cells overexpressing FLAG-FAF1 were separated by 10% SDS-PAGE and detected by Western analysis using anti-FLAG, anti-VCP, anti-Ufd1, and anti-Npl4 antibodies. D, HEK293T cells were transfected with plasmids expressing FLAG, FLAG-FAF1, or FLAG-FAF1(TFPR → AG). At 24 h after transfection, lysates were immunoprecipitated with anti-FLAG antibodies. Immune complexes were separated by 10% SDS-PAGE and detected by anti-FLAG, anti-VCP, anti-Ufd1, and anti-Npl4 antibodies. E, ITC result of VCP ND1D2 in the presence of ATP + FAF1 UBX domain. F, VCP ND1D2 complexed with Ufd1-Npl4 in the presence of ATP + FAF1 UBX domain.
We further examined the Ufd1-Npl4 dependence. The binding between full-length as well as various fragments of VCP (see Fig. 1B) and FAF1 was examined using ITC, and the results are summarized in Table 1. When VCP was presented as ND1, UBX domain bounds with an apparent KD of 20.1 μm in the presence of ATP and 22.7 μm in the absence of ATP, but the stoichiometry was no longer 1:1 (supplemental Fig. S2, B and C). These values are in good agreement with value of 30 μm reported previously (9). On the other hand, when the full-length VCP (denoted as VCP ND1D2) was titrated with FAF1 UBX domain, there was no detectable binding whether ATP was present or not (Fig. 4E). However, if VCP ND1D2 was complexed with Npl4-Ufd1 heterodimer, FAF1 UBX domain bound with a slightly increased binding affinity with a KD value of 9.6 μm (Fig. 4F) with one or two FAF1 UBX domains bound to one VCP-Npl4-Ufd1 complex. We tested this further by extending the C-terminal end of FAF1 to include the UAS domain (i.e. FAF1 UAS-UBX) and found that its binding pattern with VCP ND1D2 was quite similar. That is, FAF1 UAS-UBX did not show any binding to full-length VCP alone but did bind to the VCP-Npl4-Ufd1 complex with a corresponding KD value of 19.5 μm (supplemental Fig. S2, D and E). These results clearly suggest that prior binding of Npl4-Ufd1 to VCP is necessary for the interaction of FAF1 UBX domain with VCP.
Visualization of FAF1 Binding to VCP-Npl4-Ufd1 Complex by EM
The complex formation of VCP-Npl4-Ufd1 and FAF1 was initially established by size exclusion chromatography and SDS-PAGE studies (supplemental Fig. S3). To confirm the stoichiometry of the VCP-Npl4-Ufd1-FAF1 complex observed in ITC data, we tried EM imaging after gold labeling on the purified FAF1. Examination of VCP alone revealed a closed form with a hole in the center, consistent with a top view of the hexameric ring (33). In contrast to the relatively uniform fields observed for VCP, the VCP-Npl4-Ufd1-FAF1 complex revealed more irregular particles and additional density at the periphery of the VCP ring (Fig. 5A). The irregularity of the complex likely stems from the flexible, elongated structure of FAF1, which may also promote a more variable orientation of the complex on the grid (Fig. 5B). For stoichiometry, we labeled FAF1 with Nanogold and examined the reconstituted complex by high resolution and cryoelectron microscopy. Nanogold particles were ready visualized in association with the complex at 300 kV, revealing a single gold particle and thus a single molecule of FAF1 per complex (Fig. 5C). We also used the cryomethod to confirm both densities from the gold particle and protein complex because the sample used for high resolution EM was not stained to show enough protein density (Fig. 5D). 1.4–1.8-nm Nanogold particles were difficult to recognize in the conventional negatively stained EM but could be seen in the unstained complex with a ratio of one particle per complex when we used high resolution EM and cryotransmission EM. Arrows in Fig. 5 indicate the Nanogold-labeled FAF1 on the VCP-Npl4-Ufd1 complex. We tried the labeling with larger gold particles of 5-nm diameter, but the complex was not stable enough to go through the gel filtration step to get rid of excess gold particles.
FIGURE 5.
Electron micrograph of VCP-Ufd1-Npl4-FAF1 quaternary complex. A, micrographs of negatively stained VCP alone (left) and VCP-Npl4-Ufd1-FAF1 complex (right). Scale bars, 50 nm. B, montage of negatively stained VCP-Npl4-Ufd1 and FAF1 complex. Additional densities of Npl4-Ufd1-FAF1 bound to VCP are indicated by black arrowheads. C, montage of VCP-Npl4-Ufd1-FAF1 complex showing the position of the Nanogold particle by high resolution EM. Scale bar, 5 nm. D, montage of Nanogold-labeled VCP-Npl4-Ufd1-FAF1 complex by cryo-EM. Scale bar, 10 nm. White arrowheads indicate the position of the nanogold particle of the nanogold-labeled VCP-Npl4-Ufd1-FAF1 complex.
Npl4-Ufd1 Heterodimer Is Required for VCP-FAF1 Interaction
VCP functions as a molecular chaperone by interacting with diverse cofactors (9). We showed that VCP binding is crucial for the scaffolding ability of FAF1 and that prior binding of Npl4-Ufd1 heterodimer to VCP is crucial for FAF1 binding to VCP. To confirm this, we examined FAF1-VCP interaction in HeLa cells in which Npl4 was knocked down with its specific siRNA. These cells were transfected with FLAG or FLAG-FAF1, the cell lysates were immunoprecipitated with anti-FLAG antibody, and the immune complexes were analyzed by Western analysis using anti-FLAG, anti-VCP, anti-Npl4, anti-Ufd1, and anti-ubiquitin antibodies. We found that VCP could not bind to FAF1 in HeLa cells in which Npl4 was knocked down and that polyubiquitin binding to UBA domain was abolished as happened with mutant FAF1(TFPR → AG) (Fig. 6). This suggests that Npl4-Ufd1 heterodimer is crucial for the biological function of FAF1 conjugated to VCP.
FIGURE 6.
FAF1 interacts with VCP in an Npl4-Ufd1 dependent manner. HeLa cells were transfected with non-targeting (control) or Npl4 siRNA. At 48 h after siRNA transfection, cells were transfected with FLAG- or FLAG-FAF1-expressing vector for 24 h. At 72 h after siRNA transfection (24 h after expression vector transfection), immunoprecipitation (IP) was performed with anti-FLAG antibody. Immune complexes were separated by 10% SDS-PAGE and detected by anti-FLAG, anti-VCP, anti-Npl4, anti-Ufd1, and anti-ubiquitin antibodies. Whole cell lysates were probed as input.
FAF1 Promotes ER-associated Degradation via Ubiquitin Receptor Function
It is known that VCP-Npl4-Ufd1 complex plays a key role in ERAD. When ubiquitinated misfolded proteins are translocated through the ER membrane, VCP-Npl4-Ufd1 complex delivers them to the proteasome (12, 13, 34). Because FAF1 strongly binds to VCP and this binding is regulated by various stresses including heat shock (1) and by complex formation with Npl4-Ufd1, we examined the role of FAF1 in the ERAD pathway. Using the CD3δ Tet-Off system (pYR-CD3δ-FLAG co-transfected with pTet-Off) as a classical ERAD substrate, we monitored the degradation rates of CD3δ in HeLa cells overexpressing FLAG or FLAG-FAF1. We blocked the synthesis of CD3δ by treating the cells with 50 μg/ml doxycycline and then measured the degradation rates of CD3δ through ERAD. To confirm that the Tet-Off system could be used to monitor CD3δ degradation, we assessed CD3δ degradation by treating the cells with 50 μg/ml doxycycline alone or together with 10 μg/ml cycloheximide. Supplemental Fig. S4 shows that the pattern of CD3δ degradation promoted by FAF1 was the same whether the cells were treated with 50 μg/ml doxycycline alone or with doxycycline plus 10 μg/ml cycloheximide. Fig. 7A shows that FAF1 promoted CD3δ degradation and is thus a component of the ERAD machinery. To further validate the function of FAF1 in ERAD, we examined ERAD in cells in which FAF1 was knocked down. Supplemental Fig. 5A shows that knocking down FAF1 did not affect protein levels of Npl4 and Ufd1. When HeLa cells in which FAF1 was knocked down or control cells treated with non-targeting siRNA were co-transfected with pYR-CD3δ-FLAG and pTet-Off and treated with doxycycline 24 h after transfection, the degradation of CD3δ was attenuated in cells in which FAF1 was knocked down (Fig. 7B). Degradation of CD3δ, which was reduced when FAF1 was knocked down, was restored in cells overexpressing FLAG-FAF1, confirming that FAF1 plays a role in ERAD (Fig. 7C). We further investigated the role of FAF1 in ERAD as a ubiquitin receptor by examining the degradation rates of CD3δ in HeLa cells respectively overexpressing the following mutants of FAF1: FLAG-FAF1 WT, FLAG-FAF1(82–650) (UBA-deleted mutant), or FLAG-FAF1(TFPR → AG) (VCP binding-defective mutant). As shown in Fig. 7D, neither FLAG-FAF1(82–650) nor FLAG-FAF1(TFPR → AG) promoted CD3δ degradation like FAF1 WT. FLAG-FAF1(82–650), the UBA deletion mutant having intact UBX to bind to VCP, and FAF1(TFPR → AG), the mutant without the ability to bind to VCP and consequently its UBA function, did not affect the rate of ERAD. This suggests that FAF1 as a ubiquitin receptor plays a role in ERAD and that both UBA and UBX domains are required for promoting ERAD.
FIGURE 7.
FAF1 promotes ERAD by acting as a ubiquitin receptor. A, HeLa cells were co-transfected with FLAG vector or FLAG-FAF1 WT and Tet-Off CD3δ (pYR-CD3δ-FLAG and pTet-Off). At 24 h after transfection, cells were treated with 50 μg/ml doxycycline for the indicated times to monitor CD3δ degradation. Proteins were detected by Western analysis using anti-FLAG and anti-GAPDH antibodies. The amounts of CD3δ remaining after doxycycline (Dox) treatment were determined by MultiGauge V3.0 (Fujifilm). Bars show means ± S.D. from three independent experiments. *, p < 0.01. B, CD3δ degradation in HeLa cells in which FAF1 was knocked down was monitored the same way as in A. *, p < 0.01. C, HeLa cells were transfected with non-targeting (control) or FAF1 siRNA. At 48 h after siRNA transfection, cells were co-transfected with control FLAG vector or FLAG-FAF1 and Tet-Off CD3δ (pYR-CD3δ-FLAG and pTet-Off) for 24 h. At 72 h after siRNA transfection (24 h after expression vector transfection), cells were treated with 50 μg/ml doxycycline for the indicated times (0, 1, 2, and 3 h). Proteins were detected by Western analysis using anti-FLAG and anti-GAPDH antibodies. The amounts of CD3δ remaining after doxycycline treatment were determined by MultiGauge V3.0 (Fujifilm). Bars show means ± S.D. from three independent experiments. *, p < 0.05 (FAF1 siRNA/FLAG versus FAF1 siRNA/FAF1). D, HeLa cells were co-transfected with FLAG vector, FLAG-FAF1 WT, FLAG-FAF1(82–650), or FLAG-FAF1(TFPR → AG) and Tet-Off CD3δ (pYR-CD3δ-FLAG and pTet-Off). At 24 h after transfection, cells were treated with 50 μg/ml doxycycline for the indicated times to monitor CD3δ degradation (0, 1, and 2 h). Proteins were detected by Western analysis using anti-FLAG and anti-GAPDH antibodies. The amounts of CD3δ remaining after doxycycline treatment were determined by MultiGauge V3.0 (Fujifilm). Bars show means ± S.D. from three independent experiments. *, p < 0.05 (FLAG-FAF1(TFPR → AG) versus FLAG-FAF1 WT); **, p < 0.05 (FLAG-FAF1(82–650) versus FLAG-FAF1 WT).
FAF1 Promotes ER-associated Degradation in a VCP-Npl4-Ufd1-dependent Manner
We further investigated the VCP-Npl4-Ufd1-related function of FAF1 in the ERAD pathway by examining the connection of CD3δ degradation to VCP-Npl4-Ufd1 complex. We monitored CD3δ degradation in HeLa cells in which Npl4 was knocked down with siRNA. Knocking down Npl4 did not affect the expression level of FAF1 but did decrease the level of Ufd1 (supplemental Fig. 5A) as reported previously (35). HeLa cells treated with non-Npl4-targeting siRNA (control) or Npl4 siRNA were co-transfected with pYR-CD3δ-FLAG, pTet-Off, and FLAG-FAF1 or FLAG-FAF1(82–650) and treated with doxycycline to monitor CD3δ degradation. FAF1-overexpressing cells in which Np14 was knocked down did not recover the ability to promote the degradation rate of CD3δ, whereas FAF1-overexpressing control cells did (Fig. 8). These results provide further evidence that FAF1 plays a role in ERAD in a VCP-Npl4-Ufd1-dependent manner.
FIGURE 8.
FAF1 promotes ERAD in a VCP-Npl4-Ufd1-dependent manner. We monitored CD3δ degradation with the Tet-Off system in cells in which Npl4 was knocked down (middle panel) and in control cells (top panel). HeLa cells were transfected with control siRNA or Npl4 siRNA overnight and then co-transfected with FLAG vector, FLAG-FAF1 WT, or FLAG-FAF1(82–650) and Tet-Off CD3δ (pYR-CD3δ-FLAG and pTet-Off). At 72 h after siRNA transfection (24 h after expression vector transfection), cells were treated with 50 μg/ml doxycycline (Dox) for the indicated times (0, 1, and 2 h). Proteins were detected by Western analysis using anti-FLAG, anti-FAF1, anti-Npl4, and anti-GAPDH antibodies. The amounts of CD3δ remaining after doxycycline treatment were determined by MultiGauge V3.0 (Fujifilm) (bottom panel). Bars show means ± S.D. from three independent experiments. *, p < 0.01 (control siRNA/FLAG-FAF1(82–650) versus control siRNA/FLAG-FAF1 WT); **, p < 0.01 (control siRNA/FLAG versus control siRNA/FLAG-FAF1 WT).
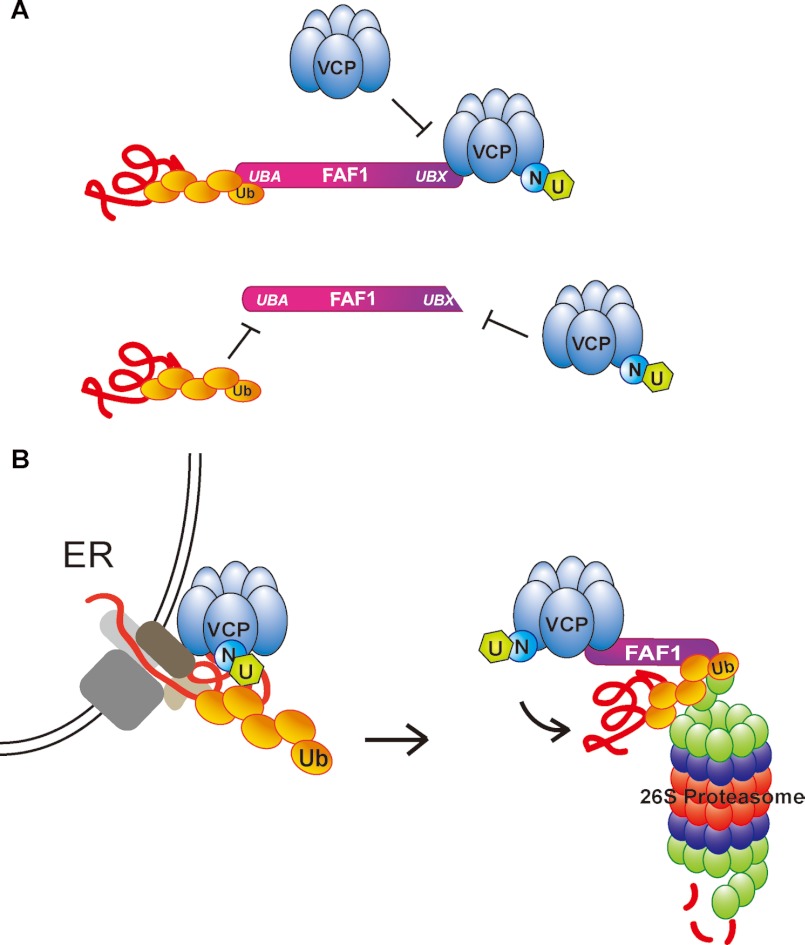
Fig. 9 depicts our model showing how VCP-Npl4-Ufd1 complex selectively interacts with FAF1 through UBX domain to form FAF1-(VCP-Npl4-Ufd1) complex and that this complex formation is required for recruiting polyubiquitinated substrates to FAF1 UBA (Fig. 9A). Only intact FAF1 complex interacting with polyubiquitinated substrates via UBA domain and with VCP-Npl4-Ufd1 via UBX domain can promote ERAD by targeting the proteasome. Interactions between FAF1 and various proteasome subunits were identified (data not shown) (Fig. 9B). This model describes our hypothesis that FAF1 is a ubiquitin receptor that delivers ERAD substrates to the proteasome through a cooperative mechanism between UBX and UBA domains.
FIGURE 9.
A model depicting selective interaction of VCP-Npl4-Ufd1 complex with FAF1 to promote ERAD. A, FAF1 selectively interacts with VCP-Npl4-Ufd1 complex through UBX domain, and this interaction regulates the recruitment of polyubiquitinated substrate proteins to UBA domain. B, FAF1 promotes ERAD by interacting with VCP-Npl4-Ufd1 complex and polyubiquitinated proteins and delivering ERAD substrates to the proteasome. FAF1 is a ubiquitin scaffolding receptor that promotes ERAD by regulating domain interactions through UBX and UBA domains. N, Npl1; U, Ufd1; Ub, ubiquitin.
DISCUSSION
We demonstrated here that binding of FAF1 to VCP-Npl4-Ufd1 complex via UBX domain is a prerequisite to the binding of polyubiquitinated proteins to UBA domain of FAF1 and that only intact FAF1 complex plays a role in ERAD. Our study shows that VCP N-domain interacts with C-terminal UBX domain of FAF1, thereby facilitating the recruitment of polyubiquitinated proteins to N-terminal UBA domain of FAF1. Fig. 3A, which depicts the crystal structure of the FAF1 UBX·VCP N-domain complex, shows that the major interaction between the two involves the S3/S4 loop of the FAF1 UBX domain. ITC results also confirmed that 618TFPR621 is critical for VCP binding because the 618TFPR621 → AG mutation showed no detectable binding between the two. Furthermore, the crystal structure studies revealed that the cis-Pro620-centered β-turn of 618TFPR621 is important in the interaction.
We also showed that the Npl4-Ufd1 heterodimer is essential for VCP-FAF1 interaction. No direct binding of FAF1 UBX domain to full-length VCP was observed in ITC studies. This was true even when UBX domain was extended further to include a UAS domain. However, when VCP and Npl4-Ufd1 complex were used, the FAF1 UBX and UAS-UBX domain titration showed binding at a molar ratio of about 1:6 with KD values of 9.6 and 19.5 μm, respectively. The EM study (Fig. 5) also clearly showed a single gold bead per VCP-Npl4-Ufd1-FAF1 complex, suggesting one FAF1 bound to the VCP-Npl4-Ufd1 complex. These results demonstrate that full-length FAF1 binds at a 1:6 molar ratio with full-length VCP but not at a 1:1 molar ratio, i.e. one FAF1 binding to a hexameric unit of VCP. Furthermore, FAF1 binding to VCP required prior binding of Npl4-Ufd1 to VCP.
Intriguingly, FAF1(TFPR → AG), a mutant deficient in VCP binding via C-terminal UBX, could not recruit polyubiquitinated proteins to N-terminal UBA domain (Fig. 2). We also showed by monitoring direct domain-domain interactions of various FAF1 mutants such as UBL and UBX deletion mutants by ITC that FAF1 UBA domain does not directly interact with UBL1 and UBL2 or UBX domains. Together with our previous findings that UBA domain is crucial for FAF1-mediated apoptosis and proteasomal inhibition (1) and that UBA domain facilitates Lys48-linked polyubiquitinated protein recruitment (2), we can conclude that UBA is the main functional domain of FAF1 and that UBA is regulated by UBX binding to VCP already bound to Npl4-Ufd1 heterodimer. However, there is no direct interaction between UBA and UBX domains.
Because UBX domain is module that binds to VCP, many UBX-containing proteins can promote VCP-mediated processes by changing the cofactors. In yeast, membrane-bound UBA-UBX protein Ubx2 membrane-bound UBA-UBX protein, recruits Cdc48-Npl4-Ufd1 to the ER membrane to perform ERAD (35–37). p47, another UBX protein, acts as a cofactor for p97-mediated membrane fusion by forming a p97-p47 complex (38). A recent study showed that various UBA-UBX proteins bind to ubiquitin ligases and that UBXD7 promotes binding of HIF1α to p97 by selectively interacting with von Hippel-Lindau tumor suppressor (pVHL) (32). How VCP performs its specific functions together with these various cofactors should be further studied. This concept of cooperative regulation between domains (UBX-UBA) in a ubiquitin receptor has not been reported before.
We examined the role of FAF1 in ERAD because FAF1 interacts with VCP-Npl4-Ufd1 complex, which is known to be involved in ERAD. ERAD was measured in cells that either overexpressed FAF1 or in which FAF1 was knocked down by monitoring degradation of CD3δ, an ERAD substrate. Overexpression of FAF1 promoted ERAD, whereas depletion of FAF1 retarded it (Fig. 7, A and B). These effects were examined again in cells in which FAF1 was knocked down. Wild type FAF1, FAF1(82–650) (UBA domain deletion mutant), and FAF1(TFPR → AG) (a mutant deficient in the interaction with VCP and polyubiquitinated proteins) were reintroduced in these cells. Although FAF1 WT accelerated CD3δ degradation, FAF1 UBA domain mutants FAF1(82–650) and FAF1 I41N lost the ability to promote ERAD because they could not recruit polyubiquitinated substrates even though they could interact with VCP. FAF1 UBX domain mutants FAF1(TFPR → AG) and FAF1ΔUBX failed to interact with VCP, resulting in no recruitment of polyubiquitinated substrates to UBA domain, and could not promote ERAD (Figs. 2A and 7D). This suggests that FAF1 UBX-(VCP-Npl4-Ufd1) interaction is required for recruiting polyubiquitinated proteins to FAF1 UBA domain and that UBX domain ensures polyubiquitinated substrate recruitment to UBA domain through VCP-Npl4-Ufd1 complex rather than by inactivating UBA domain through direct interaction. This was confirmed in cells in which Npl4 was knocked down (Fig. 8). Because most cellular Npl4 interacted with FAF1 in immunoprecipitation with anti-FAF1 antibody (data not shown), Npl4 appears to be a major regulator of FAF1. We examined the effect of FAF1 on ERAD in cells in which Npl4 was knocked down. Effects of FAF1 on ERAD were completely abolished in these cells (Fig. 8). This finding implies that FAF1 exerts its biological function in ERAD by first interacting with VCP-Npl4-Ufd1 complex and then recruiting polyubiquitinated proteins to UBA domain. Specific scaffolding characteristics of FAF1, which are necessary for its biological role in ERAD, are finely regulated via changes in protein-protein interactions. The molecular mechanisms underlying this regulation should be studied further.
So far, UBL-UBA proteins Rad23 and Dsk2 have been proposed as proteasome-targeting factors that mediate ERAD (39, 40). It has been shown that UBA domain recruits polyubiquitinated ERAD substrates and that UBL domain interacts with proteasome subunits to deliver substrates. FAF1 also interacts with 26 S proteasome subunits including 20 S core particles (proteasome subunit α-3,4,5,6) and parts of 19 S regulatory particles (Rpn1, -2, -5, -12, and Rpt2,3) (data not shown). The present study proposes that FAF1 is a scaffolding ubiquitin receptor involved in ERAD by regulating protein-protein interaction through UBA and UBX domains and delivering ERAD substrates to the proteasome. However, the proteasome-interacting motif has yet to be identified.
FAF1 was reported to suppress NF-κB activity by interfering with nuclear translocation of the RelA subunit of NF-κB and to inhibit IκB kinase activation by interacting with p65 and IκB kinase (41). A Drosophila homolog of FAF1 called Caspar was also found to inhibit NF-κB. Caspar negatively regulates immune deficiency (Imd) responses by blocking nuclear translocation of NF-κB (42). However, no role for FAF1 as a ubiquitin receptor in NF-κB inactivation has been described, although such a role for FAF1 was demonstrated in other signaling pathways through interaction with various binding partners. Using the artificial ubiquitin-proteasome system substrate ubiquitin-X-GFP, we previously found that FAF1 inhibits proteasomal degradation through its UBA domain (1). In this study, we demonstrated that FAF1 UBA domain specificity is ensured by UBX domain through VCP-Npl4-Ufd1 complexation. FAF1 seems to promote ERAD, whereas nonspecific ubiquitin-proteasome system substrates retard degradation. Nonetheless, the substrate specificity of FAF1 UBA domain is hard to explain because many types of polyubiquitinated proteins including Hsp70 and β-catenin have been shown to interact with FAF1 UBA, and their degradation is regulated by FAF1 (4). One possibility is that the specific UBX-interacting complex provides the substrate specificity of UBA domain by delivering specific polyubiquitinated proteins to FAF1.
In summary, both in vivo and in vitro data suggest that VCP-Npl4-Ufd1 complex selectively interacts with UBX domain of FAF1, which in turn regulates the recruitment of polyubiquitinated substrates to FAF1 UBA domain. We propose that FAF1-UBA domain is a ubiquitin receptor for ERAD regulated by the interaction between UBX and VCP-Npl4-Ufd1 complex. The multiple functions of FAF1 should be investigated in further studies by examining the interaction between UBX and VCP-Npl4-Ufd1 complex and characterizing the various complexes of FAF1 formed inside cells in response to various stresses.
Acknowledgments
We thank Dr. Kyung Eun Lee at Advanced Analysis Center at Korea Institute of Science and Technology for help with electron microscope and the Advanced Analysis Center at Korea Institute of Science and Technology for use of the transmission electron microscope.
This work was supported in part by Center for Cell Signaling Research and Drug Discovery Research Grant R15-2006-020 at Ewha Womans University from the National Core Research Center program, Global Research Laboratory Program Grant 2012045441, Bio and Medical Technology Development Program Grant 2012035580 of the National Research Foundation of Korea, the Functional Proteomics Center, the 21C Frontier Research and Development Program and Global Research Laboratory Program of the Korea Ministry of Science and Technology, and an institutional grant from the Korea Institute of Science and Technology.

This article contains supplemental Table 1 and Figs. S1–S5.
The atomic coordinates and structure factors (code 3QWZ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- FAF1
- Fas-associated factor 1
- VCP
- valosin-containing protein
- ERAD
- endoplasmic reticulum-associated degradation
- UBA
- ubiquitin-associated
- UBL
- ubiquitin-like
- UBX
- ubiquitin regulatory X
- ER
- endoplasmic reticulum
- aa
- amino acids
- Ni-NTA
- nickel-nitrilotriacetic acid
- UPLC-ESI-q-TOF
- ultraperformance LC-electrospray ionization-quadrupole time of flight
- ITC
- isothermal titration calorimetry
- Npl4
- nuclear protein localization protein 4.
REFERENCES
- 1. Song E. J., Yim S. H., Kim E., Kim N. S., Lee K. J. (2005) Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol. Cell. Biol. 25, 2511–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Song J., Park J. K., Lee J. J., Choi Y. S., Ryu K. S., Kim J. H., Kim E., Lee K. J., Jeon Y. H., Kim E. E. (2009) Structure and interaction of ubiquitin-associated domain of human Fas-associated factor 1. Protein Sci. 18, 2265–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim H. J., Song E. J., Lee Y. S., Kim E., Lee K. J. (2005) Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J. Biol. Chem. 280, 8125–8133 [DOI] [PubMed] [Google Scholar]
- 4. Lee J. J., Kim Y. M., Jeong J., Bae D. S., Lee K. J. (2012) Ubiquitin-associated (UBA) domain in human Fas associated factor 1 inhibits tumor formation by promoting hsp70 degradation. PLoS One 7, e40361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rape M., Hoppe T., Gorr I., Kalocay M., Richly H., Jentsch S. (2001) Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48 (UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107, 667–677 [DOI] [PubMed] [Google Scholar]
- 6. Wang Q., Song C., Li C. C. (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 146, 44–57 [DOI] [PubMed] [Google Scholar]
- 7. Woodman P. G. (2003) p97, a protein coping with multiple identities. J. Cell Sci. 116, 4283–4290 [DOI] [PubMed] [Google Scholar]
- 8. Ye Y. (2006) Diverse functions with a common regulator: ubiquitin takes command of an AAA ATPase. J. Struct. Biol. 156, 29–40 [DOI] [PubMed] [Google Scholar]
- 9. Schuberth C., Buchberger A. (2008) UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell. Mol. Life. Sci. 65, 2360–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirsch C., Gauss R., Horn S. C., Neuber O., Sommer T. (2009) The ubiquitylation machinery of the endoplasmic reticulum. Nature 458, 453–460 [DOI] [PubMed] [Google Scholar]
- 11. Vembar S. S., Brodsky J. L. (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye Y., Meyer H. H., Rapoport T. A. (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652–656 [DOI] [PubMed] [Google Scholar]
- 13. Ye Y., Meyer H. H., Rapoport T. A. (2003) Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 162, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dreveny I., Kondo H., Uchiyama K., Shaw A., Zhang X., Freemont P. S. (2004) Structural basis of the interaction between the AAA ATPase p97/VCP and its adaptor protein p47. EMBO J. 23, 1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Min K. W., Hwang J. W., Lee J. S., Park Y., Tamura T. A., Yoon J. B. (2003) TIP120A associates with cullins and modulates ubiquitin ligase activity. J. Biol. Chem. 278, 15905–15910 [DOI] [PubMed] [Google Scholar]
- 16. Seo J., Jeong J., Kim Y. M., Hwang N., Paek E., Lee K. J. (2008) Strategy for comprehensive identification of post-translational modifications in cellular proteins, including low abundant modifications: application to glyceraldehyde-3-phosphate dehydrogenase. J. Proteome. Res. 7, 587–602 [DOI] [PubMed] [Google Scholar]
- 17. Na S., Jeong J., Park H., Lee K. J., Paek E. (2008) Unrestrictive identification of multiple post-translational modifications from tandem mass spectrometry using an error-tolerant algorithm based on an extended sequence tag approach. Mol. Cell. Proteomics 7, 2452–2463 [DOI] [PubMed] [Google Scholar]
- 18. Walter T. S., Meier C., Assenberg R., Au K. F., Ren J., Verma A., Nettleship J. E., Owens R. J., Stuart D. I., Grimes J. M. (2006) Lysine methylation as a routine rescue strategy for protein crystallization. Structure 14, 1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 20. Zhang X., Shaw A., Bates P. A., Newman R. H., Gowen B., Orlova E., Gorman M. A., Kondo H., Dokurno P., Lally J., Leonard G., Meyer H., van Heel M., Freemont P. S. (2000) Structure of the AAA ATPase p97. Mol. Cell 6, 1473–1484 [DOI] [PubMed] [Google Scholar]
- 21. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 22. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 23. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 24. Buchberger A., Howard M. J., Proctor M., Bycroft M. (2001) The UBX domain: a widespread ubiquitin-like module. J. Mol. Biol. 307, 17–24 [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara K., Tenno T., Sugasawa K., Jee J. G., Ohki I., Kojima C., Tochio H., Hiroaki H., Hanaoka F., Shirakawa M. (2004) Structure of the ubiquitin-interacting motif of S5a bound to the ubiquitin-like domain of HR23B. J. Biol. Chem. 279, 4760–4767 [DOI] [PubMed] [Google Scholar]
- 26. Kang Y., Zhang N., Koepp D. M., Walters K. J. (2007) Ubiquitin receptor proteins hHR23a and hPLIC2 interact. J. Mol. Biol. 365, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller T. D., Feigon J. (2003) Structural determinants for the binding of ubiquitin-like domains to the proteasome. EMBO J. 22, 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowe E. D., Hasan N., Trempe J. F., Fonso L., Noble M. E., Endicott J. A., Johnson L. N., Brown N. R. (2006) Structures of the Dsk2 UBL and UBA domains and their complex. Acta Crystallogr. D Biol. Crystallogr. 62, 177–188 [DOI] [PubMed] [Google Scholar]
- 29. Hänzelmann P., Buchberger A., Schindelin H. (2011) Hierarchical binding of cofactors to the AAA ATPase p97. Structure 19, 833–843 [DOI] [PubMed] [Google Scholar]
- 30. Kim K. H., Kang W., Suh S. W., Yang J. K. (2011) Crystal structure of FAF1 UBX domain in complex with p97/VCP N domain reveals a conformational change in the conserved FcisP touch-turn motif of UBX domain. Proteins 79, 2583–2587 [DOI] [PubMed] [Google Scholar]
- 31. Bruderer R. M., Brasseur C., Meyer H. H. (2004) The AAA ATPase p97/VCP interacts with its alternative co-factors, Ufd1-Npl4 and p47, through a common bipartite binding mechanism. J. Biol. Chem. 279, 49609–49616 [DOI] [PubMed] [Google Scholar]
- 32. Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. (2008) UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell 134, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pye V. E., Beuron F., Keetch C. A., McKeown C., Robinson C. V., Meyer H. H., Zhang X., Freemont P. S. (2007) Structural insights into the p97-Ufd1-Npl4 complex. Proc. Natl. Acad. Sci. U.S.A. 104, 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rabinovich E., Kerem A., Fröhlich K. U., Diamant N., Bar-Nun S. (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22, 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowis D., McConnell E., Wójcik C. (2006) Destabilization of the VCP-Ufd1-Npl4 complex is associated with decreased levels of ERAD substrates. Exp. Cell Res. 312, 2921–2932 [DOI] [PubMed] [Google Scholar]
- 36. Schuberth C., Buchberger A. (2005) Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat. Cell Biol. 7, 999–1006 [DOI] [PubMed] [Google Scholar]
- 37. Neuber O., Jarosch E., Volkwein C., Walter J., Sommer T. (2005) Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat. Cell Biol. 7, 993–998 [DOI] [PubMed] [Google Scholar]
- 38. Kondo H., Rabouille C., Newman R., Levine T. P., Pappin D., Freemont P., Warren G. (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature 388, 75–78 [DOI] [PubMed] [Google Scholar]
- 39. Medicherla B., Kostova Z., Schaefer A., Wolf D. H. (2004) A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep. 5, 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richly H., Rape M., Braun S., Rumpf S., Hoege C., Jentsch S. (2005) A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120, 73–84 [DOI] [PubMed] [Google Scholar]
- 41. Park M. Y., Moon J. H., Lee K. S., Choi H. I., Chung J., Hong H. J., Kim E. (2007) FAF1 suppresses IκB kinase (IKK) activation by disrupting the IKK complex assembly. J. Biol. Chem. 282, 27572–27577 [DOI] [PubMed] [Google Scholar]
- 42. Kim M., Lee J. H., Lee S. Y., Kim E., Chung J. (2006) Caspar, a suppressor of antibacterial immunity in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 103, 16358–16363 [DOI] [PMC free article] [PubMed] [Google Scholar]