Background: Reduction in functional beta cells in pancreas is the major obstacle in diabetes.
Results: Mice deficient in FXYD2 subunit of Na,K-ATPase possess a metabolic phenotype of low blood glucose along with hyperplastic pancreatic islets and hyperinsulinemia.
Conclusion: The phenotype observed in Fxyd2−/− mice results from an increase in beta cell mass.
Significance: FXYD2 may be a novel target for development of cell-based interventions in diabetes.
Keywords: Akt; Beta Cell; Diabetes; Insulin; Na,K-ATPase; Pancreatic Islets; Hyperinsulinemia; Hyperplasia; Knockout Mice
Abstract
Restoration of the functional potency of pancreatic islets either through enhanced proliferation (hyperplasia) or increase in size (hypertrophy) of beta cells is a major objective for intervention in diabetes. We have obtained experimental evidence that global knock-out of a small, single-span regulatory subunit of Na,K-ATPase, FXYD2, alters glucose control. Adult Fxyd2−/− mice showed significantly lower blood glucose levels, no signs of peripheral insulin resistance, and improved glucose tolerance compared with their littermate controls. Strikingly, there was a substantial hyperplasia in pancreatic beta cells from the Fxyd2−/− mice compared with the wild type littermates, compatible with an observed increase in the level of circulating insulin. No changes were seen in the exocrine compartment of the pancreas, and the mice had only a mild, well-adapted renal phenotype. Morphometric analysis revealed an increase in beta cell mass in KO compared with WT mice. This appears to explain a phenotype of hyperinsulinemia. By RT-PCR, Western blot, and immunocytochemistry we showed the FXYD2b splice variant in pancreatic beta cells from wild type mice. Phosphorylation of Akt kinase was significantly higher under basal conditions in freshly isolated islets from Fxyd2−/− mice compared with their WT littermates. Inducible expression of FXYD2 in INS 832/13 cells produced a reduction in the phosphorylation level of Akt, and phosphorylation was restored in parallel with degradation of FXYD2. Thus we suggest that in pancreatic beta cells FXYD2 plays a role in Akt signaling pathways associated with cell growth and proliferation.
Introduction
FXYD2 is the γ subunit of Na,K-ATPase, and it belongs to the FXYD family of single-span membrane proteins involved in regulation of Na,K-ATPase (1). Expression of FXYD proteins is tissue- and cell-specific, and it is responsive to physiological and pathological stimuli. FXYD proteins affect the kinetic properties of the sodium pump (for review, see Ref. 2). FXYD2 was recently proposed as a selective beta cell biomarker suitable for non-invasive BCM (beta cell mass) imaging (3). By utilizing massively parallel signature sequencing and differential microarray data, FXYD2 was detected in beta cells from human pancreatic islets and purified rat beta cells. Here we investigated its pancreatic role in globally deficient Fxyd2−/− mice.
FXYD2 is not expressed exclusively in beta cells. There is evidence from other tissues and cells of molecular and cellular regulation of its properties and distribution. FXYD2 is the most abundant FXYD protein in kidney (4). There it exists in two splice variants, FXYD2a and FXYD2b, which differ only in the first exon coding for the extracellular N terminus of the molecule (1, 5, 6). The splice variants are selectively localized at the plasma membrane of specific nephron tubules, implying a difference in modulation of Na,K-ATPase activity in those segments (5, 7). Stable renal cell lines lack FXYD2, however, and both FXYD2a and FXYD2b were shown to reduce ATPase activity when expressed in renal cells either by transfection (8–11) or by induction with stress (12). Importantly, with both splice variants there was also a negative effect on the rate of cell growth (11, 12) in cultured cells. Moreover, we showed recently that mRNA for FXYD2b may be modified in renal cells by a single nucleotide substitution at the position 172C→T, presumably by RNA editing. Such modification introduced a premature stop codon, and as result, biosynthesis of a truncated version of the FXYD2b variant, ΔFXYD2b (13). The modified protein exposed an endoplasmic reticulum retrieval signal and was retained intracellularly, and this abrogated the negative pressure of FXYD2b on proliferation of cells in culture.
Besides kidney, FXYD2 was identified in several other tissues, such as salivary and mammary glands (14), dorsal root ganglia (15), and pancreas (3, 14) where the level of FXYD2 expression was the highest after kidney. Although FXYD2 was initially implicated in blastocoel formation during preimplantation development (16), in FXYD2 knock-out mice embryo genotype did not influence blastocyst development in vivo (17).
Characterization of kidney membranes from the FXYD2 knock-out mice confirmed that FXYD2 reduces the affinity of Na,K-ATPase for Na+ (17) as we had demonstrated in transfected cells (9). Its absence could impair renal Na+ reabsorption in principle. Here we report that, instead of an anticipated renal phenotype, FXYD2-deficient mice exhibit a pancreatic phenotype. The absence of FXYD2 made those animals more tolerant of glucose. Hyperplasia observed in pancreatic islets from the knock-out mice appears to underlie this phenomenon.
EXPERIMENTAL PROCEDURES
Animals
All procedures involving mice were carried out using protocols approved by the Massachusetts General Hospital Subcommittee on Research Animal Care in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Fxyd2−/− mice (Fxyd2tm1Kdr) were used from the 9th to the 16th backcross to the C57BL/6 mouse strain. In keeping with good breeding practice, each generation of mice for experiments was produced from heterozygote parents that resulted from back-crosses to fresh wild types obtained from Charles River Laboratories, Wilmington, MA. Offspring were genotyped by PCR amplification of ear punch DNA taken at weaning as described in Ref. 17. Mice were given regular diet with free access to water on a 12-h dark/light cycle.
Metabolic Cage
Twenty-four-hour urine samples were collected from wild type and Fxyd2−/− male littermates, 9–18 weeks of age, housed in metabolic cages that were maintained in a temperature-controlled room with a 12-h light-dark cycle. All animals were fed ground pellets with free access to water. Mice were on standard diet (ProLab IsoPro RMH 3000) with 25% kcal of protein, 14% kcal of fat, and 60% kcal of fiber. Urine output and water and food intake were monitored daily. Urine and blood osmolalities were measured using an osmometer (Wescor, Logan, UT). Urine electrolytes were analyzed by the MGH Center Comparative Medicine Veterinary Clinical Pathology Lab. The data were assessed for statistical significance by Student's t test.
Laboratory Tests
Plasma electrolytes (Na+, K+, and Cl−), blood glucose, and BUN were measured with an Instat system blood analyzer (Abbott). Na+ in urine was measured at IDEXX Preclinical Research Laboratories with a DX Chemistry Analyzer.
Glucose and Insulin Tolerance Tests
Age- and sex-matched control and knock-out mice were fasted for 16 h. Before the test, animals were weighed, tails were nicked, and the base-line blood was drawn (∼50 μl). Glucose was injected intraperitoneally (1g/kg), and blood was sampled at 15, 30, 60, and 120 min. Blood glucose was measured with an Accu-Check Glucometer (Roche Diagnostics). For insulin tolerance test, fasted mice (3 h) were injected with human insulin 0.75 units/kg, and blood was drawn at 15, 30, 60, and 90 min post-injection. Plasma insulin concentration was measured by immunosorbent assay with the Mouse Ultrasensitive ELISA kit (ALPCO Diagnostics, Salem, NH) using mouse insulin as a standard.
Pancreatic Islets
Pancreata from mice were perfused with 0.2% collagenase, and 0.05% DNase solution in RPMI 1640 medium and digested for 20 min at 37 °C. Enzymes were washed out by four centrifugations (1000 rpm, 1 min each) in Hanks' buffer containing 0.5% bovine serum albumin. The final pellet was resuspended in the same buffer, and islets were picked up manually under a microscope. Human islets were obtained from The Integrated Islet Distribution Program (IIDP) City of Hope, Duarte, CA.
Glucose-stimulated insulin secretion was performed in vitro on islets from WT and KO mice (4 animals for each genotype, 10 islets per group). Freshly isolated islets were cultured overnight in RPMI 1640 medium with 10% calf serum. The next morning, the medium was replaced with KRBH solution: 115 mm NaCl, 5 mm KCl, 24 mm NaHCO3, 2.5 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 2% bovine serum albumin (essentially fatty acid free), pH 7.4, supplemented with 2.8 mm glucose. Islets were preincubated for 1 h at 37 °C. Stimulation was with 16.7 mm glucose for 1 h at 37 °C. Insulin in the supernatants was measured with the ALPCO Mouse Ultrasensitive ELISA assay.
Membrane Preparations and Gel Analysis
Crude membrane preparations (used for gel electrophoresis) were obtained from pancreatic islets by homogenization and centrifugation at 3000 × g, 15 min, 4 °C (Sorvall, SS-34) (to remove unbroken cells and nuclear fraction) followed by another centrifugation at 100,000 × g, 30 min, 4 °C (Beckman, Ti 70.5). The final pellet (crude membranes) was resuspended in a buffer containing 250 mm sucrose, 1 mm EDTA, and 10 mm Tris, pH 7.4. Alternatively, mouse islet cell lysates were obtained in a buffer containing 50 mm Tris-Cl, pH 8.0, 5 mm EDTA, 1% Nonidet P-40, and protein phosphatase inhibitor mixture I for Ser/Thr phosphatases (Sigma) (1:100, v/v). Islet cells were triturated, and insoluble material was removed by centrifugation at 3000 × g for 10 min (Sorvall, SS-34). Isolation of membranes from mouse and monkey (Macaca mulatta) kidney outer medulla was performed as described elsewhere (17). Proteins were resolved on 4–12% NuPage MES-SDS gels (Invitrogen), transferred to nitrocellulose, and incubated with specific antibodies. Detection was with chemiluminescence using a digital imaging system, ImageQuant LAS4000 (GE Healthcare). Quantification was with ImageQuant TL image analysis software (GE Healthcare).
Antibodies
Monoclonal antibodies 6F (Developmental Studies Hybridoma Bank, Iowa City, IA) and 9A7 (a kind gift from Dr. M. McEnery, Case Western Reserve University, Cleveland, OH) were employed for detection of the α1-subunit of Na,K-ATPase on blots. The RCT-G1 polyclonal antibody raised against the shared FXYD2 COOH-terminal peptide and the RNGB FXYD2b N-terminal-specific antibody were described elsewhere (5, 9). Antibody 969 was a kind gift from Dr. R. Mercer (Washington University, St. Louis, MO), and it was used to detect both splice variants of human FXYD2. Anti-insulin and anti-glucagon antibodies were from Cell Signaling Technology (Beverly, MA) and Sigma, respectively. Antibodies for pAkt (Ser-473) as well for total Akt were from Cell Signaling Technology. Anti-actin HRP-conjugated antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence
Detection of FXYD2 and islet markers was performed as described elsewhere in more detail (5). Cryostat sections (5-μm thickness) of paraformaldehyde/lysine/periodate-fixed pancreata were post-fixed with 4% paraformaldehyde in phosphate-buffered saline, pH 7.4, treated with SDS for antigen retrieval (18), and then stained with rabbit antibodies RNGB against the FXYD2b splice variant or with mouse monoclonal antibody against α1 subunit of Na,K-ATPase. Beta cells were identified by staining with the anti-insulin antibody. Detection was with Alexa-Fluor (Invitrogen)- or fluorescein isothiocyanate (Jackson ImmunoResearch)-conjugated secondary antibodies. Images were collected on a Zeiss LSM Pascal 5 scanning laser confocal system. For morphometric analysis (3–4-month-old mice, 5 for each genotype, at least 5 sections for each animal), images of islets and total pancreatic area surveyed were traced manually and analyzed with Image J software (National Institutes of Health). The beta cell mass was estimated by multiplying beta cell proportion by pancreas weight. Results were compared with independent Student's t tests.
RT-PCR Analysis
Total RNA from cells was prepared with the RNeasy system (Qiagen). The cDNA was obtained using 1 μg of total RNA from islets, oligo(dT) as the priming oligonucleotide, and avian myeloblastosis virus reverse transcriptase (Invitrogen). The PCR was performed with TaqPCR Master Mix Kit or Multiplex PCR kit (Qiagen). The PCR reaction was for 30 cycles. PCR products were separated by electrophoresis in 1.2% agarose gels. Primers were chosen according to sequences of FXYD genes from mouse and human using software provided by Invitrogen. PCR products were purified with QIAquick Gel Extraction kit (Qiagen) and sequenced at the Massachusetts General Hospital DNA Core facility.
Generation of Stable Cell Lines
Clone INS 832/13 derived from INS-1 cells was obtained from Dr. Cris Newgard (Duke University Medical Center) (19). Cells were co-transfected with a regulatory plasmid pcDNA/6TR (a tet repressor) and the pcDNA4/TO plasmid containing mouse FXYD2 cDNA. The latter was cloned into BamHI-XhoI sites in the MCS of pDNA4/TO. Both vectors are under control of human CMV promoter (T-Rex system; Invitrogen). Transfection was with Lipofectamine 2000 (Invitrogen). Clones stably expressing FXYD2 (FXYD2-INS) were selected with blasticidin S (Invitrogen) and zeocin (InvivoGen, San Diego, CA). Induction of FXYD2 was achieved by adding tetracycline (Sigma) (∼1 μg/ml) to the growth media.
RESULTS
FXYD2 Knock-out Mice Have Impaired Viability
As we reported earlier, Fxyd2−/− mice are viable and fertile (17) and do not have different weight from their wild type littermates. However, a more thorough analysis revealed significant problems in reproduction. First, as can be seen from Table 1, the conception rate was approximately half in pairs with Fxyd2−/− or Fxyd2+/− dams compared with WT females; the average occurrence of visible pregnancies per month was 0.56 ± 0.05 versus 1.02 ± 0.06 for mutant and WT mice, respectively. Similar to WT dams in this colony, a rate of about 1 pregnancy/month was observed in a colony of C57BL/6 mice with an unrelated mutation of Na,K-ATPase that were housed in the same rack (data not shown).
TABLE 1.
Fxyd2−/− mouse colony breeding history
Each generation (i.e. each row) comprises all of the pairs or harems bred in a backcross or intercross generation. The breeding success data are broken down into three categories: how many visible pregnancies were observed per month, how many of the litters that were born had any surviving pups, and of successful litters, what percentage of pups survived to weaning. The numbers for the mutant mice represent the most optimistic values, because the % survival of pups did not take into account any pups that died and were eaten before they were detected and counted.
| Breeding partners, genotype | Pregnancies/month. The average for each generation is listed separately. n = number of pregnancies | % Successful litters where any pups survived | % Survival of pups, in litters where any pups survived |
|---|---|---|---|
| Back-cross, | 1.0 (n = 2) | 50 | 87 |
| Fxyd2−/− sire, and | 1.0 (n = 7) | 100 | 87 |
| WT dam | 0.84 (n = 8) | 100 | 89 |
| Back-cross, | 1.0 (n = 12) | 100 | 85 |
| Fxyd2+/− sire, and WT dam | 1.3 (n = 6) | 83 | 85 |
| Back-cross, | 0.4 (n = 4) | 100 | 100 |
| WT sire, and | 0.5 (n = 12) | 42 | 74 |
| Fxyd2−/− dam | |||
| Fxyd2+/− sire | 0.43 (n = 37) | 65 | 82 |
| Fxyd2+/− dam | 0.57 (n = 33) | 66 | 70 |
| 0.3 (n = 16) | 75 | 82 | |
| 0.4 (n = 12) | 75 | 100 | |
| 0.56 (n = 13) | 77 | 98 | |
| 0.92 (n = 23) | 96 | 82 | |
| 0.64 (n = 12) | 75 | 64 | |
| Fxyd2−/− sire | 0.54 (n = 4) | 50a | 50a |
| Fxyd2−/− dam | 0.83 (n = 3) | 33 | 6 |
| 0.46 (n = 2) | 50 | 50 | |
| 0.87 (n = 3) | 66a | 54a |
a Some newborn pups were fostered with WT dams (synchronized pregnancies).
Besides the reduced apparent conception rate and in most cases smaller surviving litter size, the percentage of successful litters (defined as litters with even one pup surviving to weaning) was also significantly lower in pairs with Fxyd2−/− or Fxyd2+/− dams (average 57.9 ± 6.4 versus 89 ± 5.9% with WT females), suggesting high perinatal mortality with the first 3 days being especially crucial. However, beyond this time point, the percentage of pup survival was not much different (72.0 ± 6.3 versus 87.3 ± 0.96%, mutant versus WT, respectively). The estimate may be somewhat high for mutants as in a few cases we used foster WT dams to nurse newborn pups from the FXYD2 colony. The Mendelian genotype ratio of surviving pups was very close to the expected, confirming that reduction in the Fxyd2 gene did not disrupt blastocyst development or implantation.
If the animal survived, it appeared to live a normal lifespan but remained very susceptible to stress, including sudden death. Several Fxyd2−/− mice died during normally routine handling such as blood drawing, blood pressure measurement, time in the metabolic cage, and transferring between containers. Mostly males (mixed population of co-housed littermates) were affected by unexplained death in their home cage with no visible sign of fighting: 21 of172 (knockouts and heterozygotes) versus 5 of 82 (wild types). We did not see much difference in the growth curves for surviving WT and knock-out littermates of the same gender (not shown). The data suggest that deletion or even reduction in the dose of the Fxyd2 gene made them stress-sensitive and was unfavorable for normal female reproduction. The cumulative effect of reductions in pregnancy rate, litter failure, and reduced pup survival resulted in a colony breeding efficiency of 25–30% that of other colonies of C57BL/6 strain mice.
FXYD2 Knock-out Mice Have a Metabolic Phenotype of Low Blood Glucose
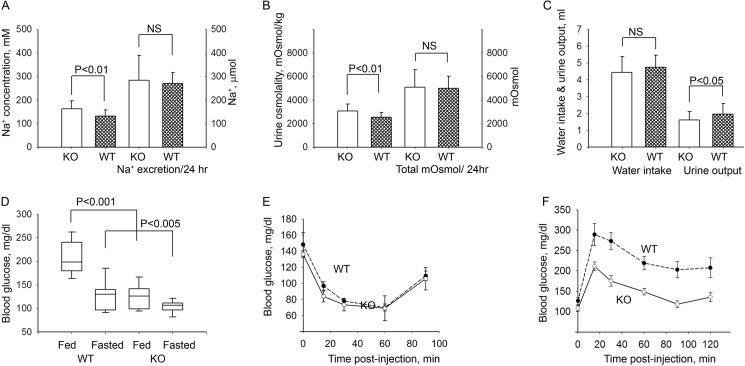
Because the γ subunit of Na,K-ATPase (FXYD2) is abundant in a majority of nephron segments (5, 20) and it was shown to reduce activity of Na,K-ATPase in in vitro assays of cultured cells (9, 11, 12), a renal phenotype was predicted for the knock-out animal. However, under basal conditions with mid-day sampling, no significant differences between genotypes were found in plasma concentration for Na+ or in plasma osmolality (Ref. 17 and data not shown). We found a higher concentration for Na+ (Fig. 1A) and, subsequently, higher osmolality (Fig. 1B) in urine from the knock-out mice with 24-h collection in metabolic cages. However, knock-out mice exhibited a slight but statistically significant reduction in urine output (p < 0.05, n = 19) along with a non-significant difference in water intake (Fig. 1C) thus compensating the total excretion of Na+ (Fig. 1, A and B). Such a mild renal phenotype is very unlikely to cause the problems in breeding of the Fxyd2−/− mice described above. No significant difference in food intake was observed between two groups of animals kept on a standard diet (see “Experimental Procedures”): 101 ± 8% in knock-out mice compared with wild type littermates.
FIGURE 1.
Complex phenotype of Fxyd2−/− mice under basal conditions. Fxyd2−/− mice exhibited a mild subclinical renal phenotype along with improved control of blood glucose. A–C, analysis of urine (24 h collection, metabolic cages) revealed a higher concentration of Na+ (A, left) and higher osmolality (B, left) in urine from KO mice. However, reduced urine output with practically the same water intake (C) compensated absolute excretion of Na+ (A, right) as well as total amount of mosmol per day (B, right). D, shown are blood glucose measurements in males of wild type (WT) and Fxyd2−/− mice (KO) postprandial (n = 11 and n = 10 for WT and KO, respectively) and after a 16-h fast (n = 10 and n = 18, for WT and KO, respectively). E, shown is an insulin tolerance test in 3-h-fasted wild type and Fxyd2−/− mice (n = 8 for each genotype). F, shown is a glucose tolerance test in fasted WT (n = 11) and KO (n = 15) mice. The basal blood glucose level was different in the fasted WT (126.2 ± 9.0 mg/dl) and KO (108.1 ± 6.7 mg/dl) animals. The data are shown as the mean ± S.E. NS, not significant.
Surprisingly, a reduced blood glucose level was revealed in adult Fxyd2−/− mice (both fed and fasted) compared with their wild type controls (Fig. 1D). No difference in insulin sensitivity was detected between the two genotypes in both young (6 weeks) (not shown) and mature animals (up to 13 months, Fig. 1E). On the other hand, glucose tolerance in the knock-out animals was dramatically improved (Fig. 1F) as compared with WT. All of the above observations suggest that FXYD2 knock-out mice have a metabolic phenotype of low blood glucose.
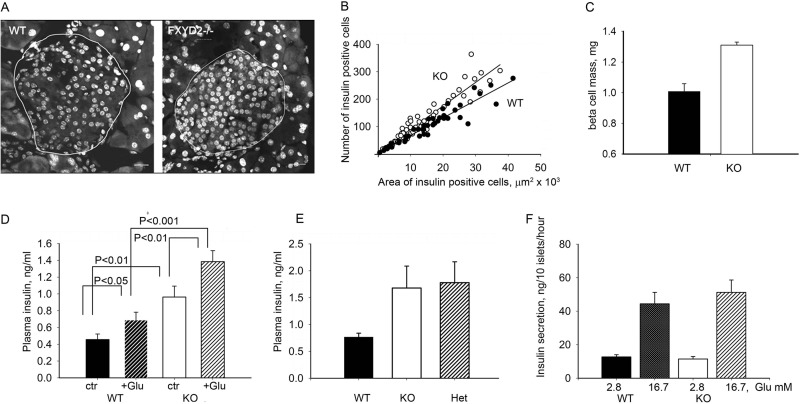
Islet structure and composition was investigated by immunofluorescence microscopy. There was a substantial hyperplasia in pancreatic beta cells from the Fxyd2−/− mice compared with the wild type littermates suggesting an abnormally elevated rate of cell division (an example is shown in Fig. 2A). To quantify it, the number of insulin-containing beta cells was plotted against islet area sampled (measured in at least 5 sections from every mouse, n = 8 mice for each genotype), and the data were analyzed by linear regression. Fig. 2B demonstrates a difference in slope obtained from the two genotypes; the coefficient was higher in the knock-out animals compared with the wild type controls with R2 values close to 1.0 (0.97 and 0.92 for WT and KO, respectively). These hyperplastic islets were primarily made of beta cells, as they were positive for insulin (not shown). No morphological difference was seen in acinar cells from Fxyd2−/− and wild type mice, implying preferential hyperplasia of the endocrine islet compartment (not shown). Immunodetection of glucagon showed α cells present as a typically minor component (not shown). Morphometric analysis revealed a significant increase in beta cells mass in KO mice compared with WT controls (Fig. 2C). All together the data suggest that removal of FXYD2 is beneficial for beta cells in pancreatic islets.
FIGURE 2.
Hyperplasia in pancreatic islets from Fxyd2−/− mice correlated with a higher level of insulin in blood. A, representative pancreatic sections from WT and Fxyd2−/− mice were counterstained for nuclei (To-Pro, Invitrogen). Islets are outlined, and images were analyzed with the LSM Image Browser (Zeiss). Bar, 20 μm. B, shown is a correlation plot between average counts of beta cells (y axis, insulin-positive cells) and the area surveyed (x axis). At least 5–6 sections from each animal (n = 8 mice for each genotype) were analyzed. Correlation coefficients, R2, were 0.97 and 0.92, for WT and KO, respectively. C, quantification of beta cells was performed with Image J software (National Institutes of Health) with insulin-stained sections. The ratio of area occupied by beta cells to total area surveyed was multiplied by pancreatic wet weight from WT and Fxyd2−/− mice (n = 5 for each genotype). The data are shown as the mean ± S.E. D, plasma insulin was measured in WT and KO mice (n = 8 for each genotype) with and without stimulation with glucose, 1 g/kg for 30 min. Statistical analysis was with Student's t test. Notably, the level of plasma insulin was higher in the KO animals under either condition. E, blood from the tail vein was collected from pregnant dams close to delivery (E18–20). Plasma insulin was significantly higher in both Fxyd2−/− (KO, n = 4) and Fxyd2+/− (Het, n = 3) mice compared with WT females (n = 4). F, isolated pancreatic islets from WT and Fxyd2−/− mice (4 animals for each genotype) were assayed for insulin secretion in vitro by stimulation with 16.7 mm glucose. No statistically significant difference was observed either under basal or stimulated conditions. The data are shown as the mean ± S.E.
Such an increase in beta cells may result in elevation of insulin production that in turn may underlie the observed phenotype of low blood glucose. Indeed, Fig. 2D demonstrates that the plasma insulin level in KO animals was significantly higher under both control and glucose-stimulated conditions.
Interestingly, there was also greater than 2-fold elevation in plasma insulin concentration in pregnant KO and heterozygote females (at E18-E20) compared with the WT dams (Fig. 2E). No hypoglycemia was seen in surviving newborn pups (P1-P3); blood glucose was 77.8 ± 8.2 (WT) versus 82.3 ± 9.6 (KO) (5 pups for each genotype). It is notable that the high perinatal mortality affected all genotypes equally: i.e. knock-out, heterozygote, and wild type pups present in 1:2:1 Mendelian ratios in the offspring of heterozygote x heterozygote pairings. This is consistent with maternal pathophysiology as the reason for the fragility and low survival rate of newborn pups and not consistent with pathophysiology intrinsic to the pups. Maternal hyperinsulinemia or depressed blood glucose could be speculated to affect either milk production or nurturing behavior.
To evaluate whether the absence of FXYD2 affects the rate of insulin release, glucose-stimulated insulin secretion was performed in vitro on freshly isolated pancreatic islets from WT and KO mice. As shown in Fig. 2F, no significant difference was seen between these two groups either under basal (2.8 mm glucose) or glucose- stimulated conditions (16.7 mm glucose). The data suggest that the higher level of insulin in plasma from knock-out mice is due to the increase in insulin-producing cell mass.
FXYD2 in Pancreas; Splice Form and Cell Type Specificity of Expression
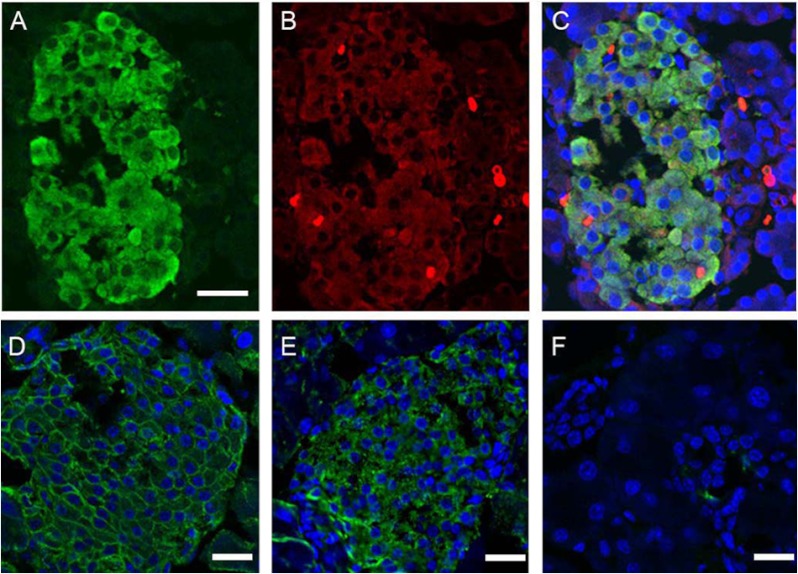
FXYD2-expressing cells were investigated by immunofluorescence on cryosections from rodent pancreata. Both splice variants FXYD2a and FXYD2b were tested with splice-specific antibodies. As shown in Fig. 3B, we identified FXYD2b in beta cells from rat pancreas co-localized with insulin (panel A, merged images panel C). Fig. 3, D–F, demonstrates mouse pancreatic islet stained for α1 subunit of Na,K-ATPase (panel D, green) and FXYD2b (panel E, green), both with rabbit antibodies. Panel F shows the negative secondary antibody control. No FXYD2b was seen in acinar cells, although Na,K-ATPase α1 was present (not shown). A double-label experiment with anti-glucagon antibodies ruled out FXYD2b expression in alpha pancreatic cells (not shown).
FIGURE 3.
Immunocytochemical localization of FXYD2 in rodent pancreas. Representative confocal images of rat pancreatic islet double-labeled for insulin (A) and FXYD2b (B) are shown. Secondary antibodies were FITC-conjugated anti-guinea pig (A) and Alexa-555 anti-rabbit (B) antibodies. Co-localization is seen in the merged image (C). Counterstaining for nuclei (blue, C) was with To-Pro. Bar, 20 μm. D–F, shown is immunostaining of mouse pancreatic islet with polyclonal rabbit antibodies against α1 subunit of Na,K-ATPase (green, D) and FXYD2b (green, E). Secondary antibodies were donkey-anti-rabbit Alexa 488. F, shown is the negative control for secondary antibodies alone. Nuclei (D–F) were counterstained with To-Pro. Bars, 20 μm.
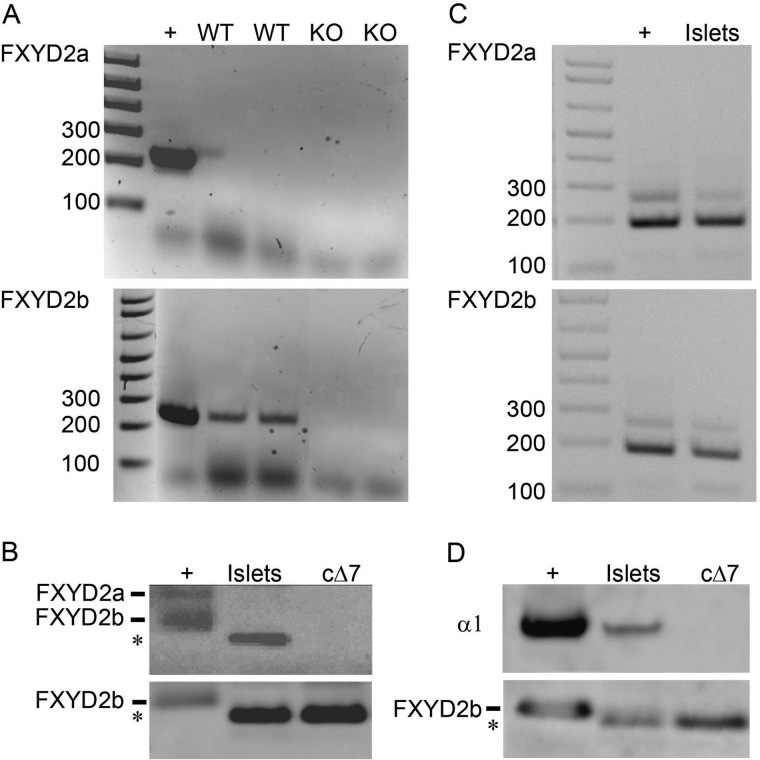
Staining with specific anti-FXYD2a antibodies was negative on islets and pancreas for both rat and mouse sections. However, this antibody has a lower affinity for FXYD2a when used on sections, thus making negative results unconvincing. Confirmation of a lack of FXYD2a expression in mouse islets came from PCR and Western blot experiments. RT-PCR with two different sets of splice-specific primers, one set covering only coding sequence and the other covering some 5′-untranslated sequence (see the supplemental table), was performed on RNA from pancreatic islets isolated from WT and KO mice. The knock-out was used as a negative control for specificity of the reaction, whereas cDNA from mouse kidney was used as a positive control. Data using the primers that included some 5′-untranslated sequence are shown in Fig. 4A. No FXYD2a PCR product was seen in islet samples with either set of primers, whereas products covering both coding and 5′-untranslated regions for FXYD2b were recovered from WT islets but not the KO samples. On Western blots, membrane fractions (or lysates, not shown) from mouse islets were positive with specific antibodies recognizing either the N terminus of FXYD2b or the shared C terminus (Fig. 4B). No staining for FXYD2a was observed (not shown). We also tested human islets to compare with the report of the presence of FXYD2a by Flamez et al. (3). Both splice variants, FXYD2a and FXYD2b, were seen by PCR using cDNA from human islets (Fig. 4C). However, only FXYD2b protein was reliably detected on Western blot (Fig. 4D), whereas detection of FXYD2a was not convincing (not shown). Whether there is translational control of FXYD2a expression in human or mouse islets requires further investigation.
FIGURE 4.
FXYD2 splice variants in pancreatic islets. A, RT-PCR was performed with RNA isolated from mouse pancreatic islets. The primers and PCR conditions were the same as in Ref. 3. RNA from mouse kidney was used as positive control, whereas RNA isolated from pancreatic islets from Fxyd2−/− mice served as negative control. Only the FXYD2b splice variant was detected. +, positive control, cDNA from mouse kidney. B, shown is a Western blot analysis of crude membranes isolated from pancreatic islets from WT mice (Islets). Membranes from mouse kidney (+) were the positive control. cΔ7 is a membrane fraction obtained from NRK-52E (normal rat kidney epithelial) cells stably transfected with the truncated form of FXYD2b (13). Detection was with the RCT-G1 antibody recognizing the C terminus from both FXYD2a and FXYD2b (upper panel) or with RNGB antibody specific for the N terminus of FXYD2b (lower panel). RCT-G1 staining of FXYD2a was seen in membranes from mouse kidney (+, upper band in the doublet) but not in the membranes from mouse islets (Islets). A blot representative of three independent experiments is shown. C, shown is RT-PCR with human FXYD2a- and FXYD2b-specific primers on human pancreatic islets. +, positive control, cDNA from human intestinal epithelial Caco2 cells (38). D, shown is a Western blot analysis of membranes (islets) isolated from frozen human pancreatic islets. Monkey kidney was a positive control (+). cΔ7 was the truncated form of rat FXYD2b (see above). Detection was with RNGB antibody specific for the FXYD2b splice variant and monoclonal antibody 6F specific for α1 of Na,K-ATPase.
Unexpected Features; Cytoplasmic Distribution and Gel Mobility
Although there was clear characteristic plasma membrane localization of Na,K-ATPase α1 (Fig. 3D), only some of the FXYD2b signal appeared to be located at the plasma membrane, and FXYD2b was also observed in the cytoplasm (Fig. 3, panels B and E). Whether the apparent separation of these two subunits is an indication of a novel role for FXYD2 in pancreatic beta cells other than as a regulator of Na,K-ATPase activity is unknown. In prior work (13) we reported that single nucleotide substitution at the position 172C→T (generated presumably by RNA editing) introduced a premature stop codon in FXYD2b, producing a truncated version, ΔFXYD2b. It was retained intracellularly because the truncation exposed an endoplasmic reticulum retention signal. To evaluate whether RNA editing occurs in pancreatic islets, FXYD2b PCR products from both mouse and human were isolated and sequenced. We did not see much heterogeneity at position 172 in the FXYD2b RNA, however, thus ruling out extensive RNA editing in this tissue under basal conditions. Whether modification of RNA can be triggered by physiological or pathological stimuli is under active investigation.
Although RNA editing may not explain the cytoplasmic distribution of FXYD2b in Fig. 3, nonetheless the SDS gel electrophoretic mobility of FXYD2b in preparations from mouse islets was faster than in samples from mouse kidney (Fig. 4B, lower panel). It coincided very well with electrophoretic mobility of the truncated construct. However, reactivity with both N- and C-terminal-specific antibodies suggests that both termini are intact, and therefore, FXYD2b is expressed as the full-length protein in islets. The differences in mobility were puzzling, but they may be caused by differences in post-translational modification of FXYD2b. It should be noted that FXYD proteins are highly regulated by post-translational modifications (11, 21, 22) and, therefore, present challenges for gel mobility analysis.
Modulation of FXYD2 Expression Alters Phosphorylation of Akt Kinase
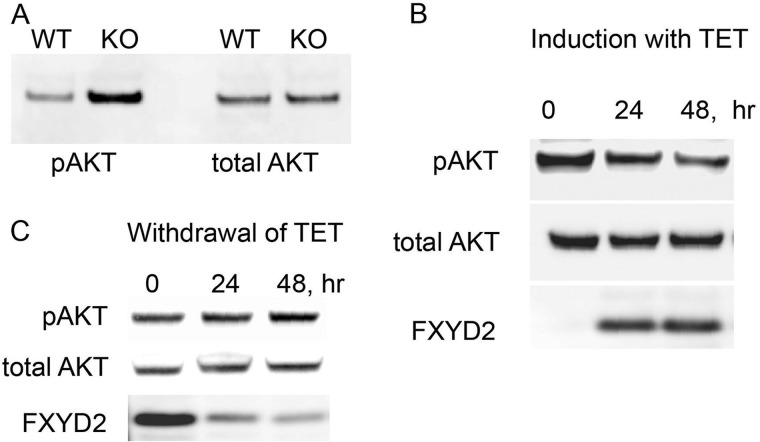
Akt kinase has been strongly implicated in regulation of beta cell mass and function (23), and so it was examined for evidence of alteration. Islets from wild type and knock-out mice were assayed for Akt phosphorylation. Fig. 5A demonstrates gel analysis of protein lysates obtained from freshly isolated islets (a representative of three independent experiments is shown). The data suggest that indeed the phosphorylation level of Akt is significantly higher (168 ± 22% based on quantitation normalized to staining for actin) in samples obtained from FXYD2-deficient islets with almost no distinguishable difference in the level of total Akt. Such an increase in phosphorylation of Akt may underlie the islet hyperplasia observed in knock-out animals and reflect ongoing signaling in adults.
FIGURE 5.
Expression of FXYD2 alters phosphorylation of Akt in pancreatic beta cells. A, cell lysates from freshly isolated islets from WT and Fxyd2−/− mice were tested on Western blot with antibodies against phospho-Akt (Ser-473) and total Akt. The blot is representative of three independent experiments. Gel loading was controlled by staining with HRP-conjugated anti-actin antibodies (not shown). B, induction of FXYD2 in stable transfectants, FXYD2-INS is shown. Cells were grown to 50% confluence followed by the addition of tetracycline and were incubated for another 24 and 48 h. Lysates were resolved on a 4–12% gel and transferred to nitrocellulose, and the blot was stained for phospho-Akt (Ser-473), total Akt, and FXYD2. Induction of FXYD2 negatively correlated with a reduction in the phosphorylation level of Akt. C, degradation of FXYD2 shows a reverse relationship with phosphorylation of Akt. FXYD2-INS cells were grown in the presence of tetracycline for 2 days followed by withdrawal of antibiotic from the medium for 24 and 48 h. Cell lysates were analyzed by Western blot as in B.
To test whether this phenomenon is intrinsic to beta cells, stable cell lines (FXYD2-INS) with inducible expression of FXYD2 were generated in rat INS 832/13 cells (19). These cells have no FXYD2 at base line either by PCR or immunostain. Fig. 5B shows that the addition of tetracycline to the medium caused an induction of FXYD2 paralleled by a reduction in phosphorylation of Akt (29.5 ± 4.8% in 48 h, 4 independent experiments), whereas the total level of Akt remained stable. Conversely, removal of tetracycline from the medium (Fig. 5C) resulted in time-dependent degradation of FXYD2 along with a gradual elevation in the level of Akt phosphorylation (25.5 ± 9.5% by 48 h, three independent experiments). Thus the data suggest that FXYD2 in beta cells is engaged in Akt signaling.
DISCUSSION
The principal discovery is a new candidate, FXYD2, for the control of beta cell mass and of the level of circulating insulin. This grew out of an unexpected phenotype in a FXYD2 global knock-out mouse first described in Ref. 17. Although viable and fertile, these mice have significant physiological problems including a reduced pregnancy rate when the dam carries at least one null allele; mutant allele-carrying sires appear to be reproductively unaffected. In addition, there is high perinatal mortality, again associated with the mother carrying the null allele. Adults of both sexes exhibit high susceptibility to stress. Adult KO mice showed significantly lower blood glucose level and improved glucose tolerance, which is consistent with an overall increase in insulin secretion. The attainment of increased mass of functional beta cells offers obvious therapeutic potential for ameliorating conditions with beta cell attrition.
Although a role for FXYD2 in pancreas was previously unknown, the protein is well studied in kidney. We pioneered the characterization of the splice variants of FXYD2 and discovered that association with Na,K-ATPase results in modulation of Na,K-ATPase activity via reduction of the affinity for Na+ and decrease of the Vmax (5, 9, 11). We also showed a direct link between FXYD2 and growth control; that is, that expression of FXYD2 in renal cells either by transfection or induction by cellular stress, such as hypertonicity, heat shock, or oxidative stress, was accompanied by a reduction in the rate of cell division (12). Selective silencing of FXYD2 induction with FXYD2-specific siRNA duplexes resulted in escape from cell growth arrest even under non-permissive conditions of cellular stress. The data imply that expression of FXYD2 is negatively correlated with the proliferative status of the cell and may have adaptive value, being a part of a general mechanism of regulation of cell growth. The mechanism of growth suppression is not identified yet, but it is consistent with a cell-autonomous basis for the beta cell hyperplasia seen in the knock-out islets.
It is well accepted now that besides being a major regulator of ionic homeostasis, Na,K-ATPase participates directly in signal transduction via activation of signaling pathways associated with cell survival, proliferation, and hypertrophy (for review, see Ref. 24). Binding of extracellular ligands (cardiotonic steroids for instance) stimulates tyrosine phosphorylation of downstream effectors (such as Src-tyrosine kinase), which in turn recruit additional kinases and adaptor proteins followed by activation of protein kinase cascades and generation of second messengers in signaling pathways associated with cell proliferation or hypertrophy (25). One of the pathways identified in association with transducing properties of Na,K-ATPase entails PI3K-dependent activation of Akt protein kinase (26), the most promising molecular target critical for regulation of beta cell mass and function (27, 28). Our results suggest that in beta cells expression of FXYD2 is negatively correlated with the phosphorylation status of Akt protein kinase either ex vivo (freshly isolated islets) or in vitro (inducible transfectants, FXYD2-INS). FXYD proteins are associated with Na,K-ATPase in crystal structures from pig kidney (29) and shark rectal gland (30). Thus the hypothesis here is that the absence of FXYD2 from the complex might disturb the assembly of a signaling module, with a cell-intrinsic effect on beta cell hyperplasia, such as via PI3K/Akt-dependent signaling.
FXYD2 was recently proposed as a novel pancreatic beta cell-specific biomarker (3). By PCR and on tissue microarray, both splice variants FXYD2a and FXYD2b were found in human islets. A close correlation between loss of FXYD2a and loss of insulin-positive cells in three patients with type I diabetes and in streptozotocin-treated macaques emphasized the potential value of FXYD2 as a biomarker (3).
Here we confirmed expression of FXYD2 in pancreatic beta cells; however, in our hands, this was the FXYD2b splice variant in both mouse and human islets, whereas FXYD2a was identified in human islets only. One should keep in mind, however, that FXYD2a is a stress-responsive protein that can be induced under certain conditions (12). Whether this could explain the discrepancy in results requires further investigation.
We also confirmed the data by Flamez et al. (3) on an unusual intracellular location of FXYD2 within pancreatic beta cells. That stain for FXYD2 does not colocalize completely with Na,K-ATPase α1 subunit suggests at least partial dissociation. Whether or not this indicates intracellular trafficking of FXYD2 within beta cells in response to yet an unidentified physiological stimulus and the molecular mechanism of intracellular retention is for future investigation.
As the only source of tightly controlled insulin secretion for metabolic homeostasis (31), particular attention has been drawn to the proliferation or regeneration of beta cells (32, 33). Recent work convincingly demonstrated that pancreas homeostasis relies on the replication of differentiated insulin-expressing beta cells rather than stem cells (34). Thus, it is crucial to characterize any unique factors contributing to proliferative capacity. FXYD2 does not belong to the universally expressed regulators of cell cycle (35, 36) or signal transduction pathways through established receptor-tyrosine kinases, JAK-binding proteins, G-protein-coupled receptors and other signaling pathways (for review, see Ref. 37). This makes it a potentially practical target for disruption, one that will not have a plethora of unwanted side effects.
To summarize, we demonstrated that global deletion of the Fxyd2 gene correlates with hyperplasia in pancreatic islets and low blood glucose in Fxyd2−/− adult mice. An initial dissection of molecular mechanisms suggests that Akt signaling is associated with this phenomenon. There is hope that further experiments will lead to novel regulators of beta cell mass that could be used to enhance insulin secretion for therapeutic purposes.
Acknowledgments
We thank Alanna Kelly for excellent technical assistance, Dr. Richard Bouley for providing expertise with metabolic cages, and Dr. Chris Newgard (Duke University Medical Center) for providing Ins 832/13 cells.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grants HL036271, NS045083, and NS050696. This work was also supported by Pilot Feasibility Grant 5P30DK057521-12 (Boston Area Diabetes Endocrinology Research Center) and American Recovery and Reinvestment Act supplement to Grant NS050696.

This article contains a supplemental table.
REFERENCES
- 1. Sweadner K. J., Rael E. (2000) The FXYD gene family of small ion transport regulators or channels. cDNA sequence, protein signature sequence, and expression. Genomics 68, 41–56 [DOI] [PubMed] [Google Scholar]
- 2. Geering K. (2006) FXYD proteins. New regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 290, F241–F250 [DOI] [PubMed] [Google Scholar]
- 3. Flamez D., Roland I., Berton A., Kutlu B., Dufrane D., Beckers M. C., De Waele E., Rooman I., Bouwens L., Clark A., Lonneux M., Jamar J. F., Goldman S., Maréchal D., Goodman N., Gianello P., Van Huffel C., Salmon I., Eizirik D. L. (2010) A genomic-based approach identifies FXYD domain containing ion transport regulator 2 (FXYD2)γa as a pancreatic beta cell-specific biomarker. Diabetologia 53, 1372–1383 [DOI] [PubMed] [Google Scholar]
- 4. Mercer R. W., Biemesderfer D., Bliss D. P., Jr., Collins J. H., Forbush B., 3rd (1993) Molecular cloning and immunological characterization of the γ polypeptide, a small protein associated with the Na,K-ATPase. J. Cell Biol. 121, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arystarkhova E., Wetzel R. K., Sweadner K. J. (2002) Distribution and oligomeric association of splice forms of the Na,K-ATPase regulatory γ subunit in rat kidney. Am. J. Physiol. Renal Physiol. 282, F393–F407 [DOI] [PubMed] [Google Scholar]
- 6. Kuster B., Shainskaya A., Pu H. X., Goldshleger R., Blostein R., Mann M., Karlish S. J. (2000) A new variant of the γ subunit of renal Na,K-ATPase. Identification by mass spectrometry, antibody binding, and expression in cultured cells. J. Biol. Chem. 275, 18441–18446 [DOI] [PubMed] [Google Scholar]
- 7. Pu H. X., Cluzeaud F., Goldshleger R., Karlish S. J., Farman N., Blostein R. (2001) Functional role and immunocytochemical localization of the γa and γb forms of the Na,K-ATPase γ subunit. J. Biol. Chem. 276, 20370–20378 [DOI] [PubMed] [Google Scholar]
- 8. Therien A. G., Karlish S. J., Blostein R. (1999) Expression and functional role of the γ subunit of the Na,K-ATPase in mammalian cells. J. Biol. Chem. 274, 12252–12256 [DOI] [PubMed] [Google Scholar]
- 9. Arystarkhova E., Wetzel R. K., Asinovski N. K., Sweadner K. J. (1999) The γ subunit modulates Na+ and K+ affinity of the renal Na,K-ATPase. J. Biol. Chem. 274, 33183–33185 [DOI] [PubMed] [Google Scholar]
- 10. Béguin P., Wang X., Firsov D., Puoti A., Claeys D., Horisberger J. D., Geering K. (1997) The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 16, 4250–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arystarkhova E., Donnet C., Asinovski N. K., Sweadner K. J. (2002) Differential regulation of renal Na,K-ATPase by splice variants of the γ subunit. J. Biol. Chem. 277, 10162–10172 [DOI] [PubMed] [Google Scholar]
- 12. Wetzel R. K., Pascoa J. L., Arystarkhova E. (2004) Stress-induced expression of the γ subunit (FXYD2) modulates Na,K-ATPase activity and cell growth. J. Biol. Chem. 279, 41750–41757 [DOI] [PubMed] [Google Scholar]
- 13. Sweadner K. J., Pascoa J. L., Salazar C. A., Arystarkhova E. (2011) Post-transcriptional control of Na,K-ATPase activity and cell growth by a splice variant of FXYD2 with modified mRNA. J. Biol. Chem. 286, 18290–18300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Floyd R. V., Wray S., Martín-Vasallo P., Mobasheri A. (2010) Differential cellular expression of FXYD1(phospholemman) and FXYD2 (γ subunit of Na,K-ATPase) in normal human tissues. A study using high density human tissue microarrays. Ann. Anat. 192, 7–16 [DOI] [PubMed] [Google Scholar]
- 15. Ventéo S., Bourane S., Méchaly I., Sar C., Abdel Samad O., Puech S., Blostein R., Valmier J., Pattyn A., Carroll P. (2012) Regulation of the Na,K-ATPase γ-subunit FXYD2 by Runx1 and Ret signaling in normal and injured non-peptidergic nociceptive sensory neurons. PLoS One 7, e29852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kidder G. M. (2002) Trophectoderm development and function. The roles of Na+/K+-ATPase subunit isoforms. Can. J. Physiol. Pharmacol. 80, 110–115 [DOI] [PubMed] [Google Scholar]
- 17. Jones D. H., Li T. Y., Arystarkhova E., Barr K. J., Wetzel R. K., Peng J., Markham K., Sweadner K. J., Fong G.-H., Kidder G. M. (2005) Na,K-ATPase from mice lacking the γ subunit (FXYD2) exhibits altered Na+ affinity and decreased thermal stability. J. Biol. Chem. 280, 19003–19011 [DOI] [PubMed] [Google Scholar]
- 18. Brown D., Lydon J., McLaughlin M., Stuart-Tilley A., Tyszkowski R., Alper S. (1996) Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS) Histochem. Cell Biol. 105, 261–267 [DOI] [PubMed] [Google Scholar]
- 19. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 20. Wetzel R. K., Sweadner K. J. (2001) Immunocytochemical localization of the Na,K-ATPase α and γ subunits in the rat kidney. Am. J. Physiol. Renal Physiol. 281, F531–F545 [DOI] [PubMed] [Google Scholar]
- 21. Tulloch L. B., Howie J., Wypijewski K. J., Wilson C. R., Bernard W. G., Shattock M. J., Fuller W. (2011) The inhibitory effect of phospholemman on the sodium pump requires its palmitoylation. J. Biol. Chem. 286, 36020–36031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bibert S., Liu C. C., Figtree G. A., Garcia A., Hamilton E. J., Marassi F. M., Sweadner K. J., Cornelius F., Geering K., Rasmussen H. H. (2011) FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its β1 subunit. J. Biol. Chem. 286, 18562–18572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blandino-Rosano M., Chen A. Y., Scheys J. O., Alejandro E. U., Gould A. P., Taranukha T., Elghazi L., Cras-Méneur C., Bernal-Mizrachi E. (2012) mTORC1 signaling and regulation of pancreatic β-cell mass. Cell Cycle 11, 1892–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reinhard L., Tidow H., Clausen M. J., Nissen P. (2013) Na+,K+-ATPase as a docking station. Protein-protein complexes of the Na+,K+-ATPase. Cell. Mol. Life Sci. 70, 205–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., Maksimova E., Huang X.-Y., Xie Z.-J. (2006) Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 17, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu L., Zhao X., Pierre S.V., Askari A. (2007) Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am. J. Physiol. Cell Physiol. 293, C1489–C1497 [DOI] [PubMed] [Google Scholar]
- 27. Elghazi L., Rachdi L., Weiss A.J., Cras-Méneur C., Bernal-Mizrachi E. (2007) Regulation of β-cell mass and function by the Akt/protein kinase B signaling pathway. Diabetes Obes. Metab. 9, 147–157 [DOI] [PubMed] [Google Scholar]
- 28. Tuttle R. L., Gill N. S., Pugh W., Lee J. P., Koeberlein B., Furth E. E., Polonsky K. S., Naji A., Birnbaum M. J. (2001) Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 29. Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. (2007) Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 30. Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Crystal structure of the sodium-potassium pump at 2.4 Å resolution. Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]
- 31. Sachdeva M.M., Stoffers D.A. (2009) Meeting the demand for insulin. Molecular mechanism of adaptive postnatal β-cell mass expansion. Mol. Endocrinol. 23, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ackermann A.M., Gannon M. (2007) Molecular recognition of pancreatic β-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193–206 [DOI] [PubMed] [Google Scholar]
- 33. Georgia S., Bhushan A. (2004) β cell replication is the primary mechanism for maintaining postnatal β cell mass. J. Clin. Invest. 114, 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dor Y., Brown J., Martinez O. I., Melton D.A. (2004) Adult pancreatic beta cells are formed by self-duplication rather than tem-cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- 35. Handschin C., Choi C. S., Chin S., Kim S., Kawamori D., Kurpad A. J., Neubauer N., Hu J., Mootha V. K., Kim Y. B., Kulkarni R. N., Shulman G. I., Spiegelman B. M. (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell cross-talk. J. Clin. Invest. 117, 3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heit J. J., Karnik S. K., Kim S. K. (2006) Intrinsic regulators of pancreatic β-cell proliferation. Annu. Rev. Cell Dev. Biol. 22, 311–338 [DOI] [PubMed] [Google Scholar]
- 37. Yesil P., Lammert E. (2008) Islet dynamics. A glimpse at beta cell proliferation. Histol. Histopathol. 23, 883–895 [DOI] [PubMed] [Google Scholar]
- 38. Arystarkhova E., Donnet C., Muñoz-Matta A., Specht S.C., Sweadner K.J. (2007) Multiplicity of expression of FXYD proteins in mammalian cells. Dynamic exchange of phospholemman and γ subunit in response to stress. Am. J. Physiol. Cell Physiol. 292, C1179–C1191 [DOI] [PubMed] [Google Scholar]