Background: MyD88 is a critical element for host resistance to L. major.
Results: UNC93B1 mutant and triple TLR3/7/9 knock-out mice are highly susceptible to infection with L. major.
Conclusion: Nucleic acid-sensing TLRs are key sensors for Leishmania parasites.
Significance: We disclose the mechanism by which L. major initiates IL-12 production and mediates development of Th1 lymphocytes and host resistance to infection.
Keywords: Cytokines/Interferon, Innate Immunity, Leishmania, MyD88, Toll-like Receptors (TLR), UNC93B1
Abstract
The mammalian homolog B1 of Unc-93 Caenorhabditis elegans known as UNC93B1 is a chaperone protein that mediates translocation of the nucleic acid-sensing Toll-like receptors (TLRs) from the endoplasmic reticulum to the endolysosomes. The triple deficient (UNC93B1 mutant) mice have a functional single point mutation in the UNC93B1 that results in non-functional TLR3, TLR7, and TLR9. Herein, we demonstrate that UNC93B1 mutant mice, in the C57BL/6 (resistant) genetic background, are highly susceptible to Leishmania major infection. Enhanced swelling of the footpad was associated with high levels of interleukin 10, decreased levels of interferon γ, and increased parasitism. None of the single TLR3, TLR7, and TLR9 knock-out (KO) mice resemble the UNC93B1 mutant phenotype upon infection with L. major. Whereas the double TLR7/TLR9 KO showed a partial phenotype, the triple TLR3/TLR7/TLR9 KO mice were as susceptible as the UNC93B1 mutant mice, when infected with Leishmania parasites. Finally, we demonstrate that treatment with either anti-interleukin 10 receptor monoclonal antibody or recombinant interleukin 12 restored a robust anti-parasite TH1 response and reverted the susceptible phenotype of UNC93B1 mutant mice. Altogether, our results indicate the redundant and essential role of nucleic acid-sensing TLR3, TLR7 and TLR9 in inducing interleukin 12, development of a TH1 response, and resistance to L. major infection in resistant C57BL/6 mice.
Introduction
Leishmaniasis is a devastating disease that leads to the development of localized ulcerative cutaneous lesions in mammals, including humans and mice. Much of the information about the pathogenicity involved in this disease was obtained from studies using murine strains infected with Leishmania major, where development of protective immunity and severity of disease depends on the host genetic background, parasite isolate, and infective dose (1). The current cellular model behind the resistance and susceptibility to L. major infection indicates a dependence of a polarized CD4+ TH1 and TH2 response, respectively (2, 3). In this model, differentiation of CD4+ T cell precursors into IFN-γ-producing TH1 cells is triggered early after infection by IL-12 (4–6). On the other hand, IL-4 and IL-10 are believed to induce the expansion of a TH2 cell that counteract the development of protective TH1 lymphocytes and are unable to promote healing (7–9).
The signaling events that are necessary for the early immune response against Leishmania are largely unknown. Nevertheless, Toll-like receptors (TLRs)4 are thought to trigger effector mechanisms that will operate to eliminate parasites. TLRs are membrane glycoproteins that can recognize pathogen-associated molecular patterns (10). The indication of the involvement of TLRs in immunity against L. major came from studies where disruption of myeloid differentiation factor 88 (MyD88), a universal TLR adaptor molecule (except for TLR3), abolished the ability of naturally resistant C57BL/6 mice to control L. major infection (11–14). Supposedly, TLR engagement triggers host defense responses against L. major promoting production of IL-12 by antigen-presenting cells such as dendritic cells (DCs) (15, 16) and macrophages that will induce naive T cells to differentiate into IFN-γ-secreting TH1 cells (1, 6, 17).
A restricted number of publications have documented the participation of a specific TLR in Leishmania recognition. TLR2, TLR4, and TLR9 appear to be involved in host resistance to L. major. However, in mice deficient in TLR2 and TLR4, the enhanced susceptibility is rather mild and not comparable with the phenotype presented by the MyD88 knock-out (KO) mice (11, 18–20). TLR9 KO mice display a greater susceptibility but eventually resolve their lesions (21–23). Related TLRs can be grouped in subfamilies that sense related pathogen-associated molecular patterns. For instance, TLRs found exclusively in endosomal compartments such as TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids (10). In this context, we hypothesize that a combined effort of different TLRs, in particular those located in the endosomal compartment, account for the MyD88 role in host resistance to infection with L. major. To test this hypothesis, we followed the course of infection in the triple deficient (UNC93B1 mutant) mice carrying a missense mutation in the gene coding for UNC93B1 protein (24), which abolishes the function of TLR3, TLR7, and TLR9 by preventing their translocation to the endolysosomes (25). Our experiments revealed that the UNC93B1 mutant mice are highly susceptible to L. major. We also present evidence that the enhanced susceptibility of UNC93B1 mutant mice is due to the simultaneous deficiency of TLR3, TLR7, and TLR9 functions, as the triple KO mice, but not single KOs, were highly susceptible to L. major infection. Importantly, the UNC93B1 mutant and triple KO mice showed a skewed cytokine expression pattern toward up-regulation of IL-10 and down-regulation of IFN-γ, which is characteristic of a non-protective T cell response in cutaneous leishmaniasis. Thus, our results indicate a critical role of nucleic acid sensing TLRs in eliciting IL-12 production, development of TH1 lymphocytes, and host resistance to L. major.
EXPERIMENTAL PROCEDURES
Mice
All animals used in this work were 6- to 8-week-old female mice. C57BL/6 mice were obtained from The Jackson Laboratory. The UNC93B1 mutant mice were generated by Dr. Bruce Beutler at The Scripps Research Institute, La Jolla, California (24). TLR3-, TLR7-, TLR9-, and MyD88-deficient mice were provided by Dr. Shizuo Akira (Department of Host Defense, Osaka University, Osaka, Japan). TLR3, TLR7, and TLR9 triple KO as well as TLR7 and TLR9 double KO mice were obtained by crossing TLR3, TLR7, or TLR9 mice. All mice used were backcrossed to C57BL/6 background at least for eight generations and housed under specific pathogen-free conditions at the University of Massachusetts Medical School animal facilities. All experiments were conducted following the guidelines of the American Association for Laboratory Animal Science and approved by the Institutional Animal Care and Use Committee (A-1332) at the University of Massachusetts Medical School.
Leishmania Parasites, Infections, and Lesion Monitoring
L. major parasites (strain MHOM/IL/80/Friedlin) were grown to stationary phase in Grace's insect cell culture medium (Invitrogen) supplemented with 20% FBS and 2 mm glutamine. Freeze-thaw leishmanial Ag (FTAg) was obtained from stationary phase promastigotes washed four times in PBS and adjusted to a concentration of 109 cells/ml. Parasite suspensions were then submitted to 10 freeze-thaw cycles of −70 and 37 °C and then stored at −20 °C. For experimental infections, the metacyclic promastigotes were purified using a Ficoll gradient, washed, and resuspended in PBS, and mice were inoculated with 1 × 106 parasites in 40 μl of PBS 1× in the right hind footpad. Lesion size was monitored with a metric caliper once a week during 10 weeks. Individual lesion sizes were expressed as the difference in footpad thickness between the uninfected foot and the infected foot. The parasite burden of infected footpads was determined using a limiting dilution assay described elsewhere (26) and expressed as the negative log parasite titer. A p < 0.05 was considered statistically significant in a two-way ANOVA with Bonferroni's post-test.
Treatment of C57BL/6 and UNC93B1 Mutant Mice with rIL-12p70 and anti-IL-10R
We administered recombinant IL-12p70 (eBioscience) directly into the infected hind footpad of UNC93B1 mutant mice on the day of infection and every 3 days. We also adopted a protocol for treating UNC93B1 mutant mice with an antibody against IL-10 receptor (BioXCell), in which inoculations were done intraperitoneally 1 day prior to footpad infection and every 3 days. Differences between values were considered significant with a p < 0.05 in a two-way ANOVA with Bonferroni's post-test.
Lymphocyte Cultures and Cytokine Detection in Supernatants
Spleens were homogenized, lysed using red blood cell lysis buffer (Sigma), and resuspended in complete RPMI medium. Cells were cultured for 48 h, 37 °C, 5% CO2 at a density of 4 × 106 cells/well in 24-well tissue culture plates in absence of exogenous stimuli, 1 × 106 FTAg or (10 μg/ml) Concanavalin A (Sigma). Cells were spun down in a plaque centrifuge at 1500 × g for 5 min, and the collected supernatants were assayed for IFN-γ, IL-10, and IL-12 cytokines using DuoSet ELISA kits (R&D Systems) according to the manufacturer's instructions.
Titration of IgG1 ad IgG2c by ELISA
Sera obtained after 10-week infections with L. major were assayed for IgG1 and IgG2c antibodies by means of FTAg-coated immunoassay plates. Serum was diluted 1:400 and incubated in duplicate for 2 h. Bound biotinylated mouse anti-mouse IgG2a and anti-mouse IgG2C were developed with streptavidin-peroxidase conjugate (BD Biosciences) diluted 1:200 in PBS 1× BSA 1%, SureBlue, and the reaction was stopped with sulfuric acid 10 volumes. Optical density was determined at 450 nm for each sample. A p < 0.05 was considered statistically significant between samples after two-way ANOVA with Bonferroni's post-test.
Evaluation of Immune Response at the Beginning of L. major Infection
C57BL/6 were infected in the right footpad with 106 metacyclic forms of L. major. Non-infected C57BL/6 mice were used as controls. At 3, 7, and 12 days after inoculation, animals were sacrificed, and the draining lymph node of the infected leg were obtained. Cells were cultivated in absence (medium) or presence of a total lysate of L. major metacyclic parasites (FTAg) for 48 h. Stimulation was performed in 96-well plates with 2 × 106 cells per well and FTAg at a multiplicity of five parasites per cell, in a final volume of 250 μl. Cells were stimulated for 48 h at 37 °C and 5% CO2. Cytokines were measured in cell-free supernatants in ELISA assays in samples from four non-infected and four infected mice, each one consisting of a pool of three lymph nodes.
Stimulation of Dendritic Cells with L. major
DCs (total CD11c+ cell population) were isolated from spleens of non-infected wild type (C57BL/6) and mice deficient in TLR3/7/9, using the EasySepTM CD11c-positive selection kit (StemCell). Purified DCs were then plated at 150,000 cells per well in 96-well plates and cultivated in absence of stimulation (medium) or in presence of metacyclic forms of L. major at a multiplicity of one parasite (1:1) or five parasites (1:5) per DC, for 24 or 48 h. As controls, we used a CpG containing oligonucleotide (oligonucleotide 1826, 1 μm), LPS (100 ng/ml), and R848 (20 μm). All stimulations were tested in duplicates. Cytokines were measured in cell free supernatants in ELISA assays.
Quantification of RNA Expression by Nanostring
Total cell lysates from C57BL/6 and UNC93B1 mutant mice infected with L. major metacyclic promastigotes in the hind footpad and mice from both groups treated with anti-IL-10R and rIL-12p70 were analyzed by NanoString methodology (27). Briefly, 10,000 cells were resuspended in 1 μl of Qiagen RLT lysis buffer and hybridized to the target specific code set ON at 65 °C. The code set contained probes against a panel of 53 genes encoding relevant innate immunity proteins. After incubation, samples (three animals per group) were loaded onto the NanoString Prep station for excess reporter removal, binding to cartridge surface, and probe scanning. After scanning and data collection onto a digital analyzer, data normalization was performed against positive and negative control oligonucletides and three housekeeping genes (Hprt1, GAPDH, and Gus1). Normalized results are represented as the relative mRNA level, and statistical significance was calculated using Student's t test with p < 0.01.
RESULTS
C57BL/6 Mice Lacking Functional UNC93B1 Develop a TH2 Response and Are Highly Susceptible to L. major Infection
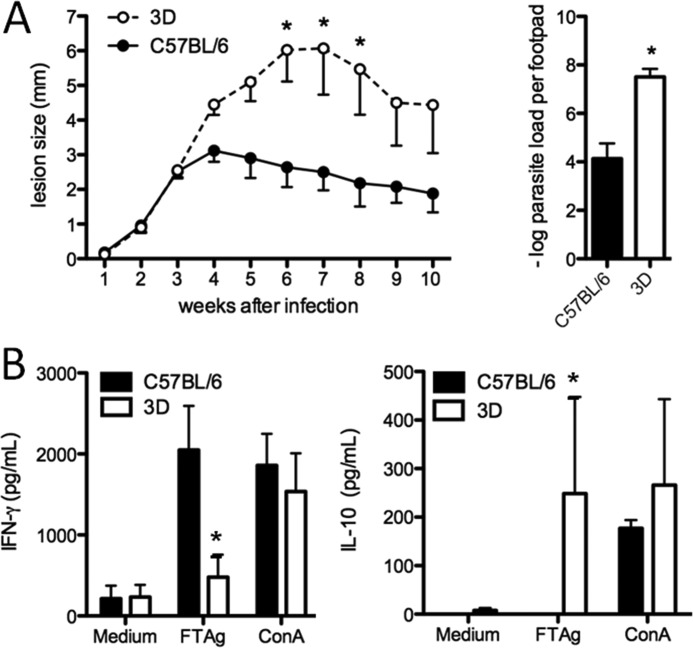
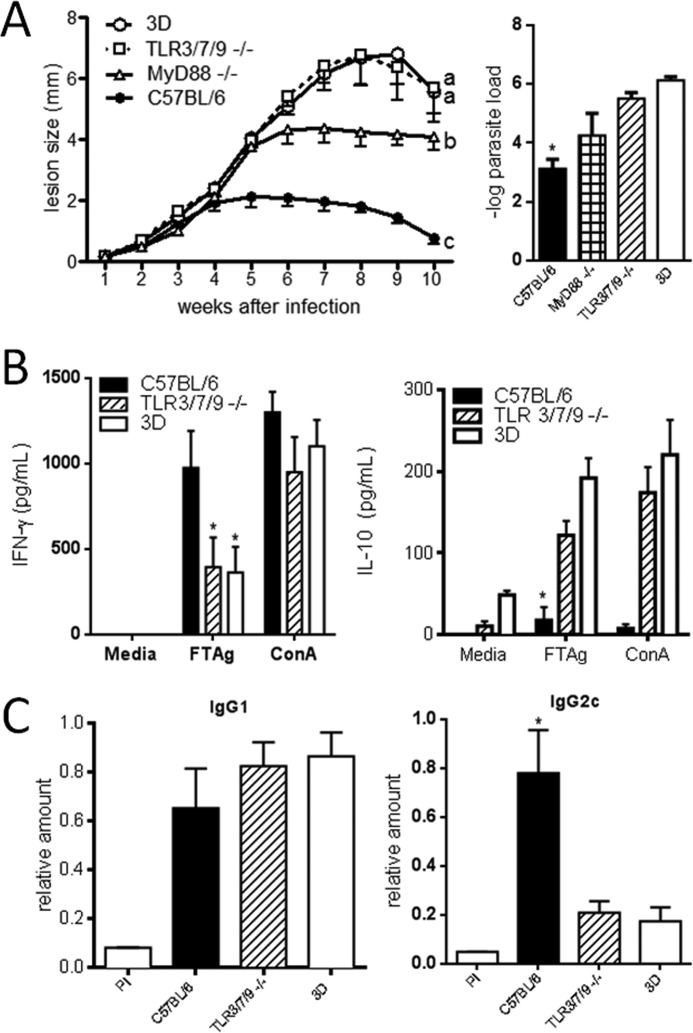
The triple deficient (UNC93B1 mutant) mice present a point mutation and altered function of UNC93B1, which results in impaired responses of TLR3, TLR7, and TLR9 ligands (19, 20). To assess the role of UNC93B1 in protective immunity to L. major, UNC93B1 mutant and C57BL/6 wild type (WT) mice were inoculated with promastigote parasites into the hind footpad and progression of lesion swelling was monitored during 10 weeks. The UNC93B1 mutant mice showed significantly increased lesion size when compared with WT mice (Fig. 1A). In addition, the number of viable parasites in the lesions showed a significantly higher parasite burden for UNC93B1 mutant mice compared with WT mice (a difference of 3–4 log) (Fig. 1A). After the 4th week, C57BL/6 started to gradually control the infection. This control of parasite replication was not observed in UNC93B1 mutant mice, which presented a progressive increase in the swelling of the infected footpad.
FIGURE 1.
UNC93B1 mutant mice show larger footpad lesions and higher parasite load after infection with L. major when compared with C57BL/6 wild type mice. A, mice were infected with 1 × 106 promastigotes subcutaneously in the right hind footpad. The course of infection was monitored weekly and the diameter of the primary footpad lesions was measured. The mean size of lesions and S.E. are shown (five mice per group). Bar graphs correspond to the parasite load quantitation in lesions by limiting dilution analysis at week 10 post-infection. B, spleens were harvested from infected mice, and cells were cultured with no stimulation, under the stimuli of 1 × 106 FTAg or Concanavalin A (10 μg/ml). Accumulation of IFN-γ and IL-10 in supernatants of cells cultured for 24 h was evaluated by ELISA assay as described under “Experimental Procedures.” Results are expressed as mean and S.E. of duplicate measurements of four animals per group. Asterisks indicate statistically significance (p < 0.05), when comparing triple deficient (3D) and WT mice by two-way ANOVA with Bonferroni's post-test. The data shown are representative of three independent experiments.
The resistance of C57BL/6 mice to L. major is dependent on the development of a CD4+ TH1 response, characterized by the production of high levels of IL-12 and IFN-γ (1). In contrast, susceptible mice, such as the BALB/c strain, produce high levels of IL-4 and IL-10, but low levels of IFN-γ, which is characteristic of a non-protective Th2 response. To evaluate the cytokine production, spleen cells harvested from WT and UNC93B1 mutant mice infected with L. major for 10 weeks, were cultured in the presence of total parasite lysate (FTAg), and cytokine accumulation in culture supernatant was assessed after 48 h. Our data show that spleen cells from UNC93B1 mutant mice produced lower levels of IFN-γ and higher levels of IL-10 when compared with C57BL/6 mice (Fig. 1B). This observation is in accordance with the high susceptibility of UNC93B1 mutant mice to L. major infection and implies a failure in the development of TH1 lymphocytes in mice lacking functional endosomal TLRs.
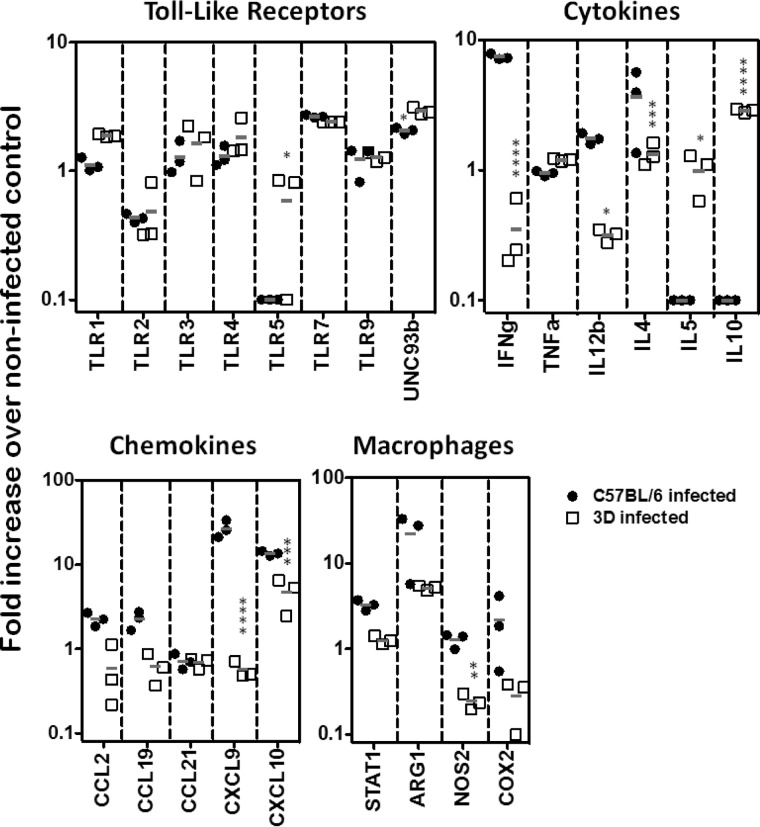
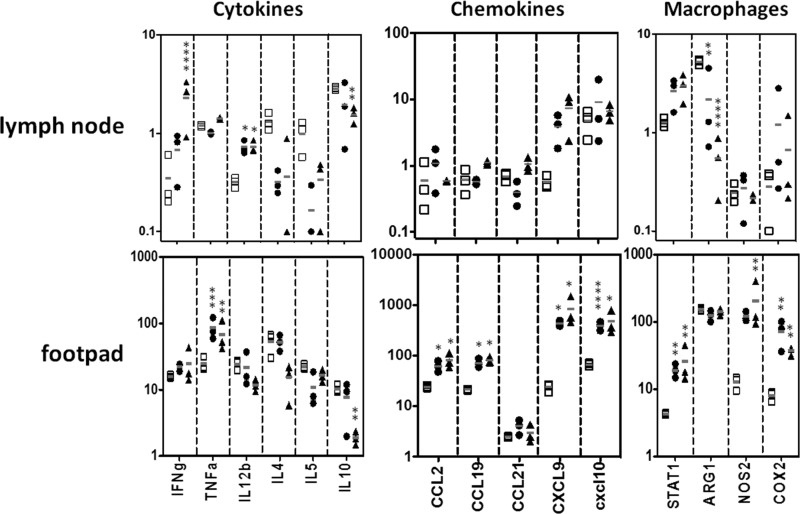
We also evaluated gene expression in the lymph nodes of WT and UNC93B1 mutant mice at 4 weeks post-infection. We observed no difference when comparing expression of genes encoding different TLRs or UNC93B1. When comparing genes that encode cytokines, expression of IL-12 and IFN-γ genes was associated with resistance, whereas the IL-5 and IL-10 transcripts clearly correlated with susceptibility to infection. We also observed enhanced expression of CXCL9, CXCL10, and NOS2 genes in cells from lymph nodes derived from resistant C57BL/6 mice infected with L. major (Fig. 2).
FIGURE 2.
Quantification of RNA expression after L. major infection. C57BL/6 (black circles) and UNC93B1 mutant (white squares) mice were infected with 1 × 106 promastigotes and had their popliteal lymph nodes harvested after 4 weeks. Gene expression was quantified in total RNA by nCounter Analysis System. Data represent the mean ± S.E. (n = 3). Differences considered statistically significant between WT and UNC93B1 mutant mice (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001) after two-way ANOVA with Bonferroni's post-test, are indicated.
The Single TLR3, TLR7, or TLR9 KO Mice Develop TH1 Response and Are Resistant to L. major Infection
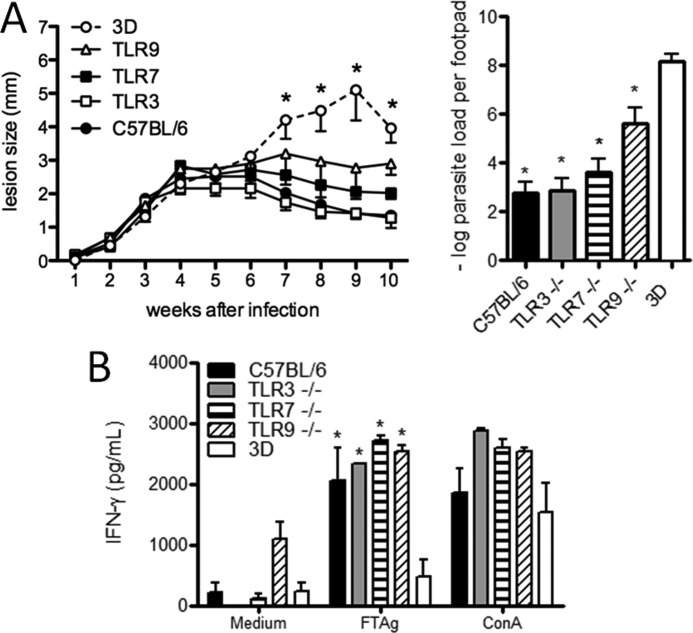
UNC93B1 is a chaperone protein that mediates the translocation of TLR receptors, including TLR3, TLR7, and TLR9 from the endoplasmic reticulum to endosomal compartment, where they are activated by microbial DNA or RNA (25). Because UNC93B1 mutant mice are highly susceptible to L. major infection, we considered the possibility that single TLRs could be determinants of host resistance to infection. Thus, we decided to evaluate the development of IFN-γ-mediated immune responses and lesion development in single TLR3, TLR7, or TLR9 KO mice infected with L. major. We found that TLR3 KO mice were as resistant as C57BL/6 mice, and both groups showed similar levels of parasitism in the footpad lesion. Although TLR7 and TLR9 single KO mice showed intermediate levels in the severity of lesion swelling between WT and UNC93B1 mutant mice (Fig. 3A), these differences were not statistically significant. The parasite burden estimated in the lesions of infected footpads showed a significant difference between UNC93B1 mutant mice and those from the other groups (p < 0.01). Spleen cells from WT, TLR single KO, and UNC93B1 mutant mice infected for 10 weeks with L. major were cultured under FTAg stimulation and IFN-γ levels measured in culture supernatants. We observed that cultured splenocytes from the UNC93B1 mutant group produced lower levels of IFN-γ as compared with WT, TLR3, TLR7, and TLR9 single KO mice, which produced similar levels of this cytokine (Fig. 3B). Taken together, these results indicate that individual TLRs are not responsible for the severe phenotype observed in UNC93B1 mutant mice.
FIGURE 3.
UNC93B1 mutant mice show larger footpad lesions and higher parasite load when compared with TLR3, TLR7 and TLR9 single KOs infected with L. major. A, WT, UNC93B1 mutant, and single KO mice were infected subcutaneously in the right hind footpad with 1 × 106 promastigotes. The course of infection was monitored weekly and the diameter of the primary footpad lesions was measured. The mean size of lesions and S.E. are shown (five mice per group). Bar graphs correspond to the parasite load quantification in lesions by limiting dilution analysis at week 10 post-infection. The mean counts of four footpads per group are shown. B, accumulation of IFN-γ in supernatants of splenocytes from infected WT, 3d, and single KO mice was measured by ELISA, 24 h after antigen stimulation, as described under “Experimental Procedures.” Data shown are mean ± S.E. and are representative of three independent experiments. Asterisks indicate statistically significant differences, after two-way ANOVA with Bonferroni's post-test (p < 0.01), when comparing infected 3d mice with infected WT (C57BL/6), TLR3 KO, TLR7 KO, and TLR9 KO mice. No significant difference was observed when comparing different single KOs and WT mice.
As we did not observe a substantial increase in susceptibility in mice lacking a single TLR (TLR3, TLR7, or TLR9), we verified whether a combined TLR deficiency would result in increased susceptibility to L. major infection. To achieve this goal, we generated double TLR3/7, TLR7/9, and TLR3/9 KO mice, which were infected with L. major promastigotes in the hind footpad and the course of infection followed for 10 weeks. As seen in Fig. 4A, all double KO mice tested showed increased footpad swelling when compared with control mice but statistically lower when compared with UNC93B1 mutant mice. Data regarding the estimated footpad parasitemia agreed with the lesion curves obtained, and parasitemia from TLR3/7 and TLR7/9 double KO mice showed intermediate values between WT and UNC93B1 mutant mice. We also observed that cultured splenocytes from TLR-KO mice produced lower amounts of IFN-γ and higher amounts of IL-10 when compared with control mice (Fig. 4B).
FIGURE 4.
UNC93B1 mutant mice are more susceptible to infection with L. major than TLR3/7, TLR7/9, and TLR3/9 double KO mice. A, animals were infected subcutaneously in the right hind footpad with 1 × 106 promastigotes. The course of infection was monitored weekly, and the diameter of the primary footpad lesions was measured. The mean size of lesions and S.E. are shown (five mice per group). Bar graphs correspond to the parasite load quantification in lesions by limiting dilution analysis at week 10 post-infection. Data shown are mean ± S.E. and are representative of three independent experiments. B, spleens were harvested from infected mice, and cells were cultured with no stimulation, under the stimuli of 1 × 106 FTAg or Concanavalin A. Accumulation of IFN-γ and IL-10 in culture supernatants, 48 h after antigen stimulation, was evaluated by ELISA assay as described under “Experimental Procedures.” Results are expressed as mean and S.E. of duplicate measurements of four animals per group. The presented data are representative of three independent experiments.
Both UNC93B1 Mutant and Triple TLR3/7/9 KO Mice Are More Susceptible Than MyD88 KO Mice upon Infection with L. major Parasites
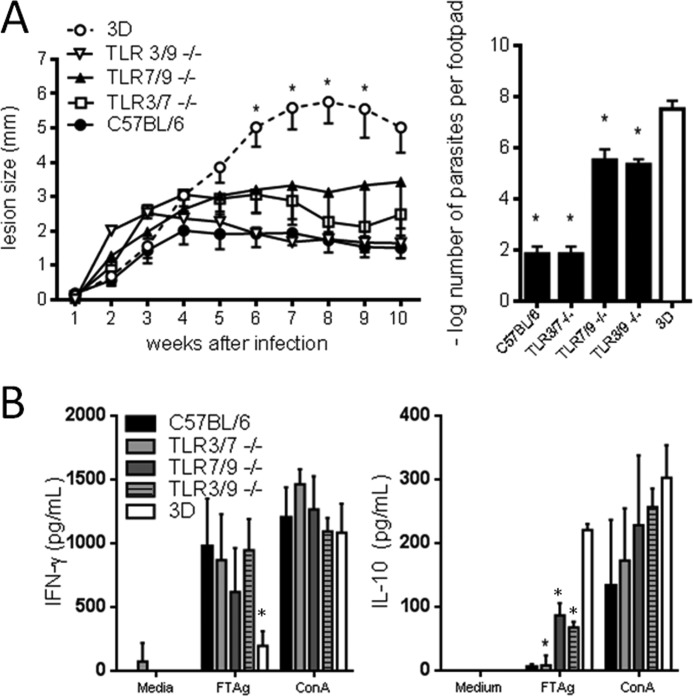
As knocking down a combination of two TLR genes were not sufficient to emulate the UNC93B1 mutant phenotype, we generated triple TLR-KO mice (deficient in TLR3, TLR7 and TLR9 genes) and compared susceptibility to UNC93B1 mutant mice. As expected, the triple TLR3/7/9 KO mice were as susceptible to infection as UNC93B1 mutant mice, showing similar levels of footpad swelling (Fig. 5A). Interestingly, mice deficient in MyD88 (an adaptor protein that mediates cell signaling upon TLR (with exception of TLR3) (14) developed less severe lesions, when compared with the triple TLR3/7/9 KO and UNC93B1 mutant mice. Evaluation of parasite burden in the footpad showed a similar parasitism for TLR3/7/9 KO and UNC93B1 mutant mice, which was significantly higher when compared with WT and MyD88 mice (Fig. 5A, bar graph). In addition, splenocytes from susceptible TLR3/7/9 KO and UNC93B1 mutant mice produced higher levels of IL-10 when compared with C57BL/6-resistant mice, which produced higher levels of IFN-γ (Fig. 5B). These data are in accordance with the higher resistance seen for WT mice as compared with UNC93B1 mutant, double and triple KO mice. Because TLR3 employs TRIF adaptor protein and not MyD88, and we found that triple TLR3/7/9 KO were more susceptible than the double TLR7/9 KO mice, our results suggest that in absence of MyD88 (or TLR7/TLR9) activation, TRIF may contribute to mice resistance to L. major infection.
FIGURE 5.
Severely impaired TH1 responses and susceptibility of TLR3/7/9 triple KO mice to infection with L. major. A, WT, UNC93B1 mutant, MyD88, and TLR3/7/9 triple KO mice were infected with 1 × 106 promastigotes subcutaneously in the right hind footpad. The course of infection was monitored weekly and the diameter of the primary footpad lesions was measured. The mean size of lesions and S.E. are shown (five mice per group). The letters a, b, and c indicate that lesion curve is significantly different when comparing UNC93B1 mutant and TLR3/7/9 KO mice to MyD88 KO and WT mice. Bar graphs correspond to the quantification of parasite load in the footpad lesions by limiting dilution analysis at week 10 post-infection. B, spleens were harvested from infected mice and cells cultured with no stimulation, under the stimuli of 1 × 106 FTAg or Concanavalin A. Accumulation of IFN-γ and IL-10 in culture supernatants, 48 h after antigen stimulation, was evaluated by ELISA assay as described under “Experimental Procedures.” Results are expressed as mean and S.E. of duplicate measurements of four animals per group. The presented data are representative of three independent experiments. C, serum was obtained from five animals per group, and the levels of circulating L. major specific IgG1 and IgG2c determined by ELISA. Data shown are mean ± S.E. and are representative of three independent experiments. Asterisks or different letters (a, b, c) indicate statistically significant differences after two-way ANOVA with Bonferroni's post test (p < 0.01) when comparing results from UNC93B1 mutant and TLR3/7/9 KO mice with WT and TLR7/9 KO mice.
The IgG2c isotype switching associates with the development of TH1 responses. To further characterize the susceptibility presented by UNC93B1 mutant and combined TLR knock-out mice 10 weeks after being inoculated with L. major promastigotes, we measured the relative amount of L. major-specific IgG1 and IgG2c isotypes in sera from infected mice. We found similar levels of IgG1 for all groups tested (Fig. 5C). In contrast, TLR3/7/9 KO and UNC93B1 mutant mice showed a significant 3–4-fold reduction in the levels of IgG2c when compared with WT mice, further indicating a defective development of protective TH1 response in these groups.
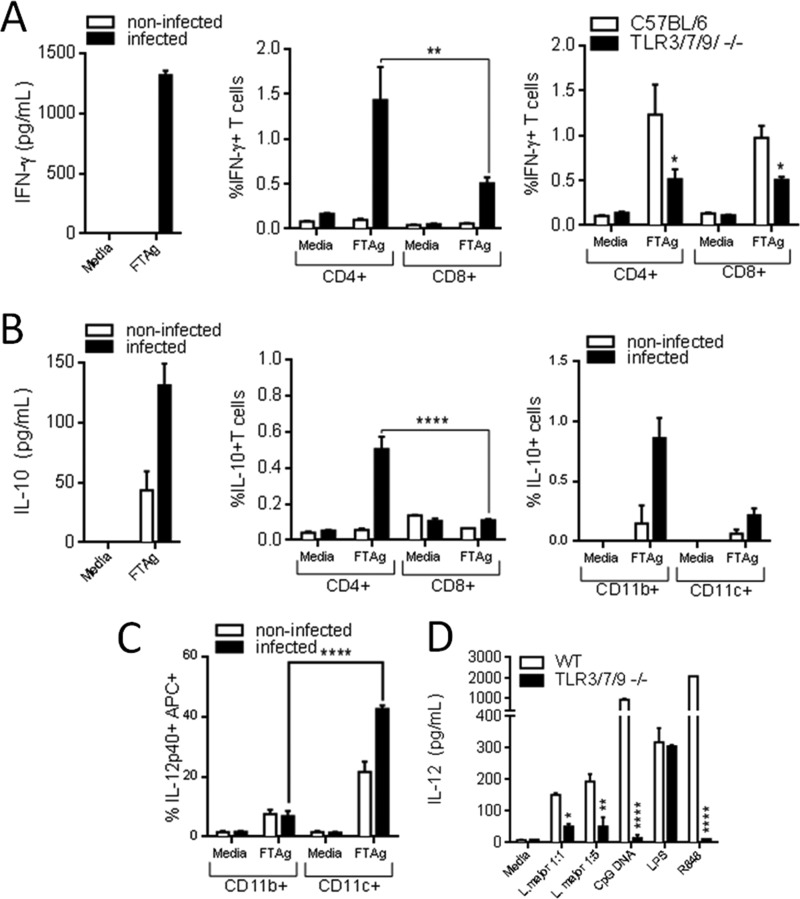
We were also interested in evaluating early cytokine production after infection by L. major. Cultured lymph node cells from the infected leg of C57BL/6 wild type mice 3, 7, and 12 days after infection with L. major were tested for production of IFN-γ, IL-10, and IL-12 48 h after being stimulated with a total lysate of L. major metacyclic parasites. We observed that lymph nodes showed a small increase in size only at 12 days post-infection and was the earliest time point when we could detect production of IFN-γ and IL-10 cytokines by ELISA (Fig. 6, A and B). We also determined whether T cells or antigen-presenting cells mediate IFN-γ and IL-10 production by flow cytometry. We found that CD4+ T cells are the primary source of these cytokines after L. major infection, and CD8+ T cells contribute in a smaller scale also secreting IFN-γ but not IL-10 (Fig. 6, A and B). We also found that CD11b+ cells contribute in IL-10 production (Fig. 6B). In contrast, CD11c+ cells produced significant amounts of IL-12, both ex vivo (Fig. 6C) and in vitro (Fig. 6D). We also observed that CD4+ and CD8+ cells from TLR3/7/9−/− produce small quantities of IFN-γ when compared with WT 12 days after infection (Fig. 6A). These results suggest an impairment of a Th1 response in triple TLR KO mice.
FIGURE 6.
Characterization of early cellular immune response upon infection with L. major. Wild type C57BL/6 mice were infected in the right footpad with 106 metacyclic forms of the L. major and, at 12 days after inoculation, animals were sacrificed, and the draining lymph nodes of the infected footpad were obtained. Cells were grown in absence (medium) or presence of a total lysate of L. major metacyclic parasites (FTAg) 48 h, and supernatant was used for quantification of IFN-γ (A), IL-10 (B), and IL-12 (C) by ELISA. Analysis by flow cytometry of CD4+ and CD8+ cells from WT and TLR3/7/9−/− mice 12 days after infection is also shown. B and C, T cells expressing CD4+ and CD8+ surface markers were stained for endogenous IFN-γ and IL-10, and cellular populations were separated by flow cytometry, as well CD11b+ and CD11c+ cells that were tested for IL-10 and IL-12 (please refer to “Experimental Procedures” for all cytokines tested). E, data represent the mean ± S.E. (n = 3). Differences are considered statistically significant (*, p < 0.05; **, p < 0.01; and ****, p < 0.0001) after two-way ANOVA with Bonferroni's post-test, are indicated.
Considering that CD11c+ cells are a main source of IL-12, we decided to compare IL-12 production by DCs derived from WT and TLR3/7/9−/− mice after in vitro stimulation with living L. major metacyclic promastigotes. We found that DCs from triple KO mice display and impaired production of IL-12, when compared with WT mice, at both 1:1 and 1:5 multiplicity of infection (Fig. 6D). As a control, we also found a deficient IL-12 production in DCs from TLR3/7/9−/− stimulated with CpG DNA and R848, which are known activators of TLR9 and TLR7, respectively. We also performed in vitro infections of bone marrow-derived macrophages (CD11b+ cells) from C57BL/6 and TLR3/7/9 KO mice with L. major promastigotes at a multiplicity of infection of 1:1, and we did not observe any difference in the average number of parasites per infected cell or the number of infected cells at 24 h after infection (data not shown). Altogether, these results indicate that the main role of TLR3, TLR7, and TLR9 in host resistance to infection with L. major is the activation and induction of IL-12 production by CD11c+ cells.
Enhanced Susceptibility of UNC93B1 Mutant Mice to L. major Infection Is Reversed by Administration of Recombinant IL-12 or Blockage of IL-10 Receptor
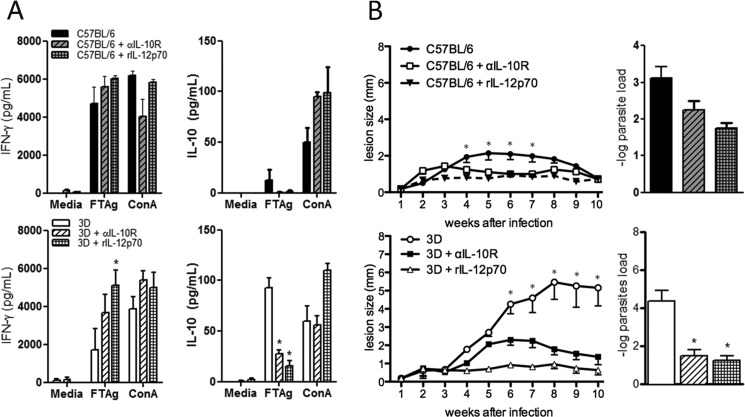
So far, our data suggests that an imbalance of Th1/Th2 differentiation of T cells is involved in the susceptibility of UNC93B1 mutant mice to L. major. As mentioned above, low expression of IL-12/IFN-γ genes and high expression of the IL-10 gene clearly associated with enhanced susceptibility of UNC93B1 mutant mice infected with L. major. In an attempt to restore resistance in UNC93B1 mutant mice, we administered recombinant IL-12p70 or anti-IL-10 receptor antibodies to mice infected with L. major. When we looked for gene expression in the footpad, we found that either treatment resulted in enhanced expression of TNF-α, CCL2, CCL19, CXCL9, CXCL10, STAT1, NOS2, and COX2 genes (Fig. 7). We also found that treatment with rIL-12 efficiently restored the expression of IFN-γ, IL-12b, CXCL9, and lowered expression of IL-10 gene in lymph node cells from UNC93B1 mutant mice (Fig. 7). While showing the same tendency, the effects of treatment with anti-IL-10R on gene expression by spleen cells from infected UNC93B1 mutant mice were less pronounced. Moreover, we found that treatment with either anti-IL-10R or rIL-12 restored the TH1 responses (i.e. high IFN-γ and low IL-10 levels) of spleen cells from infected UNC93B1 mutant mice stimulated with Leishmania antigen (Fig. 8A). Importantly, we observed resolution of lesions in UNC93B1 mutant mice treated with IL-12p70 or anti-IL-10R, indicating that both treatments were effective in promoting a resistant phenotype (smaller lesion size) to the otherwise susceptible UNC93B1 mutant mice (Fig. 8B). We also found a significant reduction in parasite burden (at 10 weeks p.i.) in UNC93B1 mutant mice treated with either IL-12p70 or anti-IL-10R (Fig. 8B).
FIGURE 7.
Quantification of RNA expression in UNC93B1 mutant mice treated with αIL-10R and rIL-12p70 after L. major infection. UNC93B1 mutant mice (white squares), UNC93B1 mutant+αIL-10R (black circles), and UNC93B1 mutant+rIL-12p70 (black triangles) mice were infected with 1 × 106 promastigotes and had their popliteal lymph nodes harvested after 10 weeks (see “Experimental Procedures” for details). Quantification of gene expression was evaluated in total RNA by nCounter Analysis System. Data represent the mean ± S.E. (n = 3). Differences considered statistically significant between WT and UNC93B1 mutant mice (*, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001) after two-way ANOVA with Bonferroni's post-test, are indicated.
FIGURE 8.
Administration of anti-IL-10R and rIL-12p70 restores TH1 responses and reduces the development of footpad lesions in UNC93B1 mutant mice infected with L. major. A, C57BL/6 and UNC93B1 mutant mice were infected with 1 × 106 promastigotes in the footpad and treated with either anti-IL-10R or rIL-12p70 (five mice per group). Splenocytes were cultured for 48 h in the presence or absence of antigen or Concanavalin A (10 μg/ml), and accumulation of IFN-γ and IL-10 in culture supernatants evaluated by ELISA, as described under “Experimental Procedures.” Results are expressed as mean and S.E. of duplicate measurements of four animals per group. The data shown are representative of three independent experiments. B, UNC93B1 mutant mice were infected with 1 × 106 promastigotes promastigotes in the footpad and treated with anti-IL-10R or rIL-12p70 (five mice per group). The course of infection was monitored weekly, and the diameter of the primary footpad lesions was measured. The mean size of lesions and S.E. are shown. Lesion size progression was monitored with a metric caliper. Bar graphs correspond to the parasite load quantification in lesions by limiting dilution analysis. Data represent the mean ± S.E. from two independent experiments. Asterisks indicate statistically significant differences after two-way ANOVA with Bonferroni's post-test when comparing non-treated controls and mice receiving treatment with either anti-IL-10R or rIL-12p70 (p < 0.01 for C57BL/6 and p < 0.0001 for UNC93B1 mutant mice).
DISCUSSION
TLRs are important mediators of host resistance to every known category of microbial pathogens (28), including protozoan parasites (29). The most convincing data suggesting the importance of TLRs in host resistance to protozoan infections are those obtained with MyD88-deficient mice. Increased susceptibility associated with impaired production of the TH1-associated cytokines (e.g. IL-12 and IFN-γ) (1) is observed in MyD88 KO mice infected with different protozoan parasites, including L. major (7, 8, 14, 21, 30). However, the assignment of specific TLRs that account for the role of MyD88 in host resistance to L. major is still unsatisfactory. In this study, we demonstrate that UNC93B1 mutant mice, which lack functional UNC93B1 that mediates translocation and function of nucleic acid-sensing TLRs (24), are highly susceptible to experimental infection with L. major. Thus, we hypothesize that the combined action of TLR3, TLR7, and TLR9 is essential for host resistance to L. major infection. The importance of TLR3 in this process was evident because the triple TLR3/7/9 KOs are more susceptible than the double TLR7/9 KO mice infected with L. major. Consistently, triple TLR3/7/9 KO mice present a phenotype that resemble the UNC93B1 mutant mice, in terms of impaired production of IFN-γ, enhanced production of IL-10, as well as extreme susceptibility to primary infection with Leishmania parasites.
Only a limited number of studies have documented the participation of a specific TLR in the innate immune responses elicited by Leishmania parasites. Lipophosphoglycans from Leishmania was shown to activate TLR2 (11, 18), although upon infection with L. major, resistance of TLR2 KO mice was not altered. In a different study, the deficiency of TLR4 resulted in a rather modest enhancement of susceptibility to L. major, but no specific L. major pathogen-associated molecular pattern was shown to interact directly with this receptor (19). Furthermore, double TLR2/4 KO mice developed a protective TH1 response and normal resistance against L. major (12). Several other studies also support a role of TLR9 in promoting resistance to L. major infection (21–23, 31). However, TLR9 deficiency only transiently prevented the development of the healing TH1 response, and mice showing larger footpad lesions and higher parasitism, when compared with C57BL/6 control mice, were eventually able to control parasite growth and resolve their lesions (21, 22).
The susceptibility of MyD88 KO mice infected with L. major, in contrast to TLR9 KO mice, is very pronounced (14) and could not be explained based on the studies described above. Nevertheless, the importance of other TLRs such as TLR3, TLR5, and TLR7 during L. major infection has not been explored. Furthermore, it is possible that the combined action of different TLRs may account for the role of MyD88 in host infection with L. major. Indeed, in a recent study performed by our group, we observed an extreme susceptibility of UNC93B1 mutant mice to infection with another trypanosomatide, namely Trypanosoma cruzi. Here, we show that the UNC93B1 mutant mice, which are deficient in functional UNC93B1 as a result of a H412R missense mutation (24), do not mount an effective TH1 response and are also highly susceptible to L. major infection. The UNC93B1 mutant mice have a major defect in the translocation of endosomal TLRs and are unresponsive to TLR3 (dsRNA), TLR7 (ssRNA), and TLR9 (unmethylated CpG DNA) agonists (24, 25, 32), whereas the function of cell surface TLRs, such as TLR1, TLR2, TLR4, TLR5, and TLR6, mostly involved with bacterial sensing, are not affected. Thus, our findings support the hypothesis that the combined action of the nucleic acid-sensing TLRs are responsible for activation of MyD88 during L. major infection.
In an attempt to dissect the role of individual endosomal TLRs in L. major recognition, we evaluated the susceptibility of single KO mice for TLR3, TLR7, and TLR9. We observed that whereas the absence of TLR3 by itself did not affect host resistance to infection the single TLR7 or single TLR9 were slightly more susceptible to L. major infection. Hence, our findings suggest a hierarchical relationship between endosomal TLRs in triggering immune responses and host resistance to infection with this intracellular parasite. Importantly, the mice lacking TLR7/9 or TLR3/7/9 were more susceptible to infection than single KOs. The susceptibility of triple TLR3/7/9 KO mice was similar to the UNC93B1 mutant mice infected with L. major. Much to our surprise, in the absence of TLR7 and TLR9, TLR3 was shown to play a role in host resistance to L. major infection, as triple TLR3/7/9 KO mice were consistently more susceptible than the double TLR7/9 KO mice. Furthermore, we also found that MyD88 KO mice develop less severe footpad lesions, when compared with TLR3/7/9 KO or UNC93B1 mutant mice. These findings further imply the involvement of TLR3 and the TRIF adaptor in host resistance to Leishmania infection. Indeed, an earlier study has shown that Leishmania parasites activate TLR3 (33). In a recent study, it was shown that a symbiotic viral infection in Leishmania guyanensis is responsible for activation of TLR3 and that this event mediates host susceptibility to infection through the induction of type I IFN (34). Thus, our results suggest the ability to activate TLR3 may extend to other species of Leishmania, and in some situations, it may promote resistance rather than host susceptibility to infection.
Our results show that the nucleic acid-sensing TLRs have a redundant role in Leishmania infection and act together in inducing an optimal protective TH1 response, which is characterized by IFN-γ-mediated immunity. Hence, the absence of these TLRs results in low levels of IFN-γ and high levels of IL-10 production in response to parasite antigens, which characterizes a non-protective TH2 response observed in susceptible mouse strains. Whereas a noticeable impairment of TH1 was already observed in the double TLR7/9 KO mice, the profound impairment, similar to the UNC93B1 mutant mice, was only observed in the triple TLR3/7/9 KO mice. Indeed, previous publications indicate that IFN-γ production in mice infected with L. major is at least partially independent of MyD88 because mice deficient in MyD88 adaptor still mount an IFN-γ response (13, 14, 35). These authors ascribed the susceptibility to L. major found in MyD88 KO mice to the high levels of Th2 cytokines (i.e. IL-10, IL-4, and IL-13), which are probably suppressing CD4+ Th1 cell development and mounting a non-protective cellular response (14). One explanation for the partial susceptibility seen in MyD88 KO is that TRIF could partially compensate the lack this adaptor molecule and promote partial resistance to Leishmania infection.
The two main defects in UNC93B1 mutant mice is the impairment of IFN-γ production and high levels of the regulatory cytokine (IL-10) and the consequent lack of IFN-γ induced immune responses that are required for control of parasite replication (32). Thus, we asked which of these events was critical in rendering UNC93B1 mutant mice highly susceptible to L. major infection. Administration of either rIL-12p70 (a potent inducer of IFN-γ production by T lymphocytes and NK cells) or anti-IL-10R rescued UNC93B1 mutant mice from high susceptibility to this parasitic infection. Our results showed that mice that received either treatment developed smaller footpad lesions and had lower parasitism at 10 weeks post-infection, with a concomitant increase in IFN-γ production and decrease in IL-10 secretion upon stimulation with parasite antigens.
Finally, UNC93B1 has now been shown to be a key mediator of inflammatory response and host resistance to infection with protozoan parasites (36–38). Different mechanisms, such as altered antigen cross-presentation or autonomous control of parasite replication by host cells, have been proposed to explain the UNC93B1 mediated host resistance to Toxoplasma gondii and Plasmodium falciparum (37, 38). In contrast, the results presented here and elsewhere (31) support the hypothesis that in the case of trypanosomatides, including L. major, the mechanism by which UNC93B1 mediates host resistance to infection, can be solely explained by the defect in the nucleic acid-sensing TLRs.
Acknowledgments
We are grateful to Dr. Helton Santiago for help with gene expression analysis and discussion and Anna Cerny for maintaining the knock-out mouse colonies.
This work was supported in part by the National Institutes of Health Grants R01 AI071319-01 and R01 AI76257 and T32 AI55400-8 and the National Institute of Science and Technology for Vaccines, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
- TLR
- Toll-like receptor
- MyD88
- myeloid differentiation factor 88
- DC
- dendritic cell
- FTAg
- freeze-thaw leishmanial Ag
- ANOVA
- analysis of variance
- IL-10R
- IL-10 receptor.
REFERENCES
- 1. Sacks D., Noben-Trauth N. (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2, 845–858 [DOI] [PubMed] [Google Scholar]
- 2. Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. (1988) Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168, 1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. (1989) Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. (1993) Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177, 1797–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. (1993) Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177, 1505–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scharton-Kersten T., Afonso L. C., Wysocka M., Trinchieri G., Scott P. (1995) IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154, 5320–5330 [PubMed] [Google Scholar]
- 7. Radwanska M., Cutler A. J., Hoving J. C., Magez S., Holscher C., Bohms A., Arendse B., Kirsch R., Hunig T., Alexander J., Kaye P., Brombacher F. (2007) Deletion of IL-4Rα on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 3, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noben-Trauth N., Lira R., Nagase H., Paul W. E., Sacks D. L. (2003) The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. J. Immunol. 170, 5152–5158 [DOI] [PubMed] [Google Scholar]
- 9. Kane M. M., Mosser D. M. (2001) The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166, 1141–1147 [DOI] [PubMed] [Google Scholar]
- 10. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 11. de Veer M. J., Curtis J. M., Baldwin T. M., DiDonato J. A., Sexton A., McConville M. J., Handman E., Schofield L. (2003) MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33, 2822–2831 [DOI] [PubMed] [Google Scholar]
- 12. Debus A., Gläsner J., Röllinghoff M., Gessner A. (2003) High levels of susceptibility and T helper 2 response in MyD88-deficient mice infected with Leishmania major are interleukin-4 dependent. Infect. Immun. 71, 7215–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muraille E., De Trez C., Brait M., De Baetselier P., Leo O., Carlier Y. (2003) Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170, 4237–4241 [DOI] [PubMed] [Google Scholar]
- 14. Revaz-Breton M., Ronet C., Ives A., Torre Y. H., Masina S., Tacchini-Cottier F., Launois P. (2010) The MyD88 protein 88 pathway is differently involved in immune responses induced by distinct substrains of Leishmania major. Eur. J. Immunol. 40, 1697–1707 [DOI] [PubMed] [Google Scholar]
- 15. Berberich C., Ramírez-Pineda J. R., Hambrecht C., Alber G., Skeiky Y. A., Moll H. (2003) Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J. Immunol. 170, 3171–3179 [DOI] [PubMed] [Google Scholar]
- 16. von Stebut E., Belkaid Y., Nguyen B. V., Cushing M., Sacks D. L., Udey M. C. (2000) Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur. J. Immunol. 30, 3498–3506 [DOI] [PubMed] [Google Scholar]
- 17. Iwasaki A., Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 [DOI] [PubMed] [Google Scholar]
- 18. Becker I., Salaiza N., Aguirre M., Delgado J., Carrillo-Carrasco N., Kobeh L. G., Ruiz A., Cervantes R., Torres A. P., Cabrera N., González A., Maldonado C., Isibasi A. (2003) Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 130, 65–74 [DOI] [PubMed] [Google Scholar]
- 19. Kropf P., Freudenberg M. A., Modolell M., Price H. P., Herath S., Antoniazi S., Galanos C., Smith D. F., Müller I. (2004) Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72, 1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siednienko J., Halle A., Nagpal K., Golenbock D. T., Miggin S. M. (2010) TLR3-mediated IFN-β gene induction is negatively regulated by the TLR adaptor MyD88 adaptor-like. Eur. J. Immunol. 40, 3150–3160 [DOI] [PubMed] [Google Scholar]
- 21. Liese J., Schleicher U., Bogdan C. (2007) TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 37, 3424–3434 [DOI] [PubMed] [Google Scholar]
- 22. Abou Fakher F. H., Rachinel N., Klimczak M., Louis J., Doyen N. (2009) TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J. Immunol. 182, 1386–1396 [DOI] [PubMed] [Google Scholar]
- 23. Carvalho L. P., Petritus P. M., Trochtenberg A. L., Zaph C., Hill D. A., Artis D., Scott P. (2012) Lymph node hypertrophy following Leishmania major infection is dependent on TLR9. J. Immunol. 188, 1394–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabeta K., Hoebe K., Janssen E. M., Du X., Georgel P., Crozat K., Mudd S., Mann N., Sovath S., Goode J., Shamel L., Herskovits A. A., Portnoy D. A., Cooke M., Tarantino L. M., Wiltshire T., Steinberg B. E., Grinstein S., Beutler B. (2006) The Unc93b1 mutation UNC93B1 mutant disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7, 156–164 [DOI] [PubMed] [Google Scholar]
- 25. Kim Y. M., Brinkmann M. M., Paquet M. E., Ploegh H. L. (2008) UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 [DOI] [PubMed] [Google Scholar]
- 26. Afonso L. C., Scott P. (1993) Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61, 2952–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geiss G. K., Bumgarner R. E., Birditt B., Dahl T., Dowidar N., Dunaway D. L., Fell H. P., Ferree S., George R. D., Grogan T., James J. J., Maysuria M., Mitton J. D., Oliveri P., Osborn J. L., Peng T., Ratcliffe A. L., Webster P. J., Davidson E. H., Hood L., Dimitrov K. (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 26, 317–325 [DOI] [PubMed] [Google Scholar]
- 28. Hemmi H., Akira S. (2005) TLR signalling and the function of dendritic cells. Chem. Immunol. Allergy 86, 120–135 [DOI] [PubMed] [Google Scholar]
- 29. Gazzinelli R. T., Denkers E. Y. (2006) Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat. Rev. Immunol. 6, 895–906 [DOI] [PubMed] [Google Scholar]
- 30. Stobie L., Gurunathan S., Prussin C., Sacks D. L., Glaichenhaus N., Wu C. Y., Seder R. A. (2000) The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. U.S.A. 97, 8427–8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimmermann S., Egeter O., Hausmann S., Lipford G. B., Röcken M., Wagner H., Heeg K. (1998) CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160, 3627–3630 [PubMed] [Google Scholar]
- 32. Pifer R., Benson A., Sturge C. R., Yarovinsky F. (2011) UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J. Biol. Chem. 286, 3307–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flandin J. F., Chano F., Descoteaux A. (2006) RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-γ-primed macrophages. Eur. J. Immunol. 36, 411–420 [DOI] [PubMed] [Google Scholar]
- 34. Ives A., Ronet C., Prevel F., Ruzzante G., Fuertes-Marraco S., Schutz F., Zangger H., Revaz-Breton M., Lye L. F., Hickerson S. M., Beverley S. M., Acha-Orbea H., Launois P., Fasel N., Masina S. (2011) Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331, 775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaisho T., Akira S. (2001) Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22, 78–83 [DOI] [PubMed] [Google Scholar]
- 36. Caetano B. C., Carmo B. B., Melo M. B., Cerny A., dos Santos S. L., Bartholomeu D. C., Golenbock D. T., Gazzinelli R. T. (2011) Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J. Immunol. 187, 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Melo M. B., Kasperkovitz P., Cerny A., Könen-Waisman S., Kurt-Jones E. A., Lien E., Beutler B., Howard J. C., Golenbock D. T., Gazzinelli R. T. (2010) UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 6, e1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cockburn I. A., Tse S. W., Radtke A. J., Srinivasan P., Chen Y. C., Sinnis P., Zavala F. (2011) Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo. PLoS Pathog. 7, e1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]