Background: Within activated T-cells, the binding of IL-2 to its receptor initiates the Jak3/Stat5 cascade culminating in proliferation.
Results: Elevated levels of cAMPi within activated T-cells suppresses proliferation by targeting multiple levels of the IL-2R cascade.
Conclusion: Cross-talk occurs between cAMP/PKA and the IL-2R/Jak3/Stat5 cascade in human T-cells.
Significance: Therapeutic potential exists in manipulating the described cross-talk for the treatment of various immune diseases.
Keywords: Cyclic AMP (cAMP), Cytokine, Phosphodiesterases, Proliferation, Signal Transduction
Abstract
Cytokine-mediated regulation of T-cell activity involves a complex interplay between key signal transduction pathways. Determining how these signaling pathways cross-talk is essential to understanding T-cell function and dysfunction. In this work, we provide evidence that cross-talk exists between at least two signaling pathways: the Jak3/Stat5 and cAMP-mediated cascades. The adenylate cyclase activator forskolin (Fsk) significantly increased intracellular cAMP levels and reduced proliferation of the human T-cells via inhibition of cell cycle regulatory genes but did not induce apoptosis. To determine this inhibitory mechanism, effects of Fsk on IL-2 signaling was investigated. Fsk treatment of MT-2 and Kit 225 T-cells inhibited IL-2-induced Stat5a/b tyrosine and serine phosphorylation, nuclear translocation, and DNA binding activity. Fsk treatment also uncoupled IL-2 induced association of the IL-2Rβ and γc chain, consequently blocking Jak3 activation. Interestingly, phosphoamino acid analysis revealed that Fsk-treated cells resulted in elevated serine phosphorylation of Jak3 but not Stat5, suggesting that Fsk can negatively regulate Jak3 activity possibly mediated through PKA. Indeed, in vitro kinase assays and small molecule inhibition studies indicated that PKA can directly serine phosphorylate and functionally inactivate Jak3. Taken together, these findings suggest that Fsk activation of adenylate cyclase and PKA can negatively regulate IL-2 signaling at multiple levels that include IL-2R complex formation and Jak3/Stat5 activation.
Introduction
Interleukin-2 receptor (IL-2R)2 signaling in T-cells provides a necessary third signal for driving cellular proliferation during an immune response. The transmission of a proliferative signal by IL-2 requires a heterotrimeric receptor complex comprised of α, β, and common γ (γc) chains. Subsequently, Janus tyrosine kinase 1 (Jak1) and Jak3 are recruited to the β and γc chains, respectively, allowing for autoactivation of both proteins. Activated Jaks then phosphorylate tyrosine residues on the IL-2Rβ chain, creating docking sites for downstream effectors, including signal transducer and activator of transcription 5a/b (Stat5a/b), which associate via their Src homology 2 domains (1). Stat5a/b become phosphorylated on Tyr694/Ser726/Ser780 and Ser193/Tyr699/Tyr725/Ser731/Tyr740/Tyr743, respectively, dissociate from the receptor, form hetero- or homodimers, and translocate to the nucleus where they mediate transcription of genes important for cell growth and differentiation (2–8). Conversely, this signaling pathway is negatively regulated by three families of proteins, suppressor of cytokine signaling, protein tyrosine phosphatases, and protein inhibitors of activated Stats (PIAS), which target different levels within the Jak/Stat signaling cascade (reviewed in Ref. 9).
T-cell activity is also tightly controlled by other effectors, including Mapk and PI3K pathways, as well as non-protein signaling molecules such as cAMP (10). cAMP is produced by adenylate cyclase (AC), a 12-transmembrane-spanning enzyme that catalyzes the conversion of ATP to 3′,5′-cAMP and pyrophosphate (11). Cellular phosphodiesterases then degrade newly synthesized cAMP by hydrolyzing the phosphodiester bonds to produce 5′AMP (12). While cAMP has multiple functions including activation of cyclic nucleotide-gated (CNG) ion channels (13) and exchange protein activated by cAMP (EPAC) (14), in T-cells it predominately exerts its effects through Protein kinase A (PKA) (15). PKA is a ubiquitous serine/threonine kinase comprised of two catalytic and two regulatory subunits. Interestingly, cAMPi levels can be elevated via addition of forskolin (Fsk), phosphodiesterase inhibitors, prostaglandin E2, or adenosine, which results in suppression of T-cell proliferation (16–18). Although the antiproliferative effects of cAMP within cells of the immune system are well documented (19–23), the mechanism of action is less clear. It is tempting to speculate that cAMP-mediated effectors can suppress a T-cell proliferative signaling pathway such as Jaks and Stats. Nearly a decade earlier, Zhang et al. (16) demonstrated that adenosine, a cAMP activator, can suppress T-cell proliferation that may involve Stat5 but not Jak3.
Similarly, previous studies have reported that high intensity activation of the TCR can inhibit IL-2, IL-4, IL-6, and IFN-α signaling in murine T-cells by uncoupling Erk and Mek (24). Ivashkiv and co-workers (25) have also shown that inhibition of cytokine signaling was independent of new protein synthesis not dependent on suppressor of cytokine signaling, but implicated Stat5, Jak3, and Akt as possible targets. His work suggests that PKC/Mek/Erk signaling may uncouple this pathway (25). However, a role for PKA was not investigated or linked to negative regulation of these cytokine-dependent pathways.
The objective of this study was to determine the role cAMP-PKA plays in regulating IL-2R signaling, how it affects T-cell activity, and consequently immune function. Evidence is provided that Fsk activation of AC/cAMP/PKA disrupts IL-2R complex formation, Jak3 catalytic activity, Stat5 signaling, and expression of cell cycle-dependent genes. This work suggests that a novel cross-talk pathway dependent on cAMPi can negatively affect T-cell proliferation and Jak/Stat signaling.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
The human leukemic cell line MT-2 (26) and human embryonic kidney cells 293 (Hek293) were maintained as described previously (27). Human IL-2 dependent leukemic Kit 225 cells (28) were similarly maintained but supplemented with 100 IU/ml human recombinant IL-2 (NCI Preclinical Repository). Prior to cell treatment with Fsk or 3-isobutyl-1-methylxanthine (IBMX), MT-2 cells were made quiescent by growing them to exhaustion or culturing them in 1% FBS overnight at 37 °C. Kit 225 cells were made quiescent by CO2 washes and subsequently culturing in 10% FBS overnight at 37 °C. Quiescent cells were subsequently treated with the indicated concentrations of Fsk (Calbiochem), IBMX (Sigma-Aldrich), or anti-CD3 monoclonal antibody (Santa Cruz Biotechnology) and cultured at 37 °C for different amounts of time as indicated. Control cells were treated with 1% dimethyl sulfoxide (DMSO, vehicle) and cultured as treated cells. Following pre-treatment, cells were stimulated with 1 × 104 IU IL-2 at 37 °C for the times shown. MT-2 cells exposed to ultraviolet (UV) light were subjected to 120,000 mJ/cm2/min for 1 min prior to the final 5 h of incubation.
Proliferation Assays
Quiescent Kit 225 or MT-2 cells were seeded into 96-well plates at 5 × 104 cells per well. Cells were then pretreated for 1 h with 1% DMSO (vehicle) or Fsk at 1, 5, 10, 25, 50, and 100 μm concentrations. The cells were stimulated with IL-2 as above and cultured for an additional 20 h at 37 °C. Control cells were treated with 1% DMSO for 20 h. During the final 4 h of incubation, the cells were pulsed with [3H]thymidine (PerkinElmer Life Sciences) at a concentration of 0.5 μCi/200 μl. Cells were harvested onto fiberglass filters and analyzed using liquid scintillation counting.
cAMP Production Assay
Kit 225 or MT-2 cells were treated with 1, 5, 10, 20, 50, or 100 μm Fsk for 20 min at 37 °C. Cells were lysed and clarified by centrifugation, and concentration of cAMP was detected by direct cAMP ELISA (Assay Designs). Optical density was measured at 405 nm, and the concentration of intracellular cAMP was calculated using a weighted four parameter logistic curve according to the manufactures instructions.
Cell Solubilization, Protein Immunoprecipitation, and Western Blotting
Cell pellets were lysed, clarified by centrifugation, and subjected to immunoprecipitation and Western blot analysis as reported previously (27). Briefly, immunoprecipitation reactions were performed by addition of 2 μg of antisera against Jak3 (29), Stat5b (30), IL-2Rβ (Santa Cruz Biotechnology, C-20), or IL-2Rγ (Santa Cruz Biotechnology, M-20). Immunoblotting was performed using antibodies to Jak3, Stat5b, anti-pY (4G10, Millipore), pY939 Jak3 (31), glutathione S-transferase (GST) (Amersham Biosciences), IL-2Rβ, IL-2Rγ, poly(ADP-ribose) polymerase (PARP) (Cell Signaling), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Research Diagnostics, Inc.), or β-actin (Santa Cruz Biotechnology, AC-40). Secondary antibodies and Western blot conditions were performed as described previously (32).
In Vitro Kinase Assays
For Jak3 kinase assays, Fsk-treated MT-2 cells were lysed, clarified, and immunoprecipitated using Jak3 antibody as described above. Kinase reactions were carried out as described previously (31) at 30 °C for 20 min. For PKA kinase assays, untreated MT-2 cells were lysed, and Jak3 was immunoprecipitated and bound to PAS beads as described previously (31). Immunoprecipitated Jak3 was washed with kinase buffer (50 mm Hepes-NaOH (pH 7.4), 10 mm MgCl2, 0.5 mm EGTA, 0.5 mm DTT, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin A) and incubated with 200 μm ATP and purified protein kinase A catalytic subunit (PKAc) (Invitrogen) as indicated in the figure legends. Kinase reactions were carried out at 32 °C for 30 min followed by vigorous washing of the beads with cold kinase wash buffer as described previously (31). For [γ-32P]ATP radiolabeled kinase assays using recombinant Jak3, Hek293 cells were transfected with wild type (WT) Jak3 or kinase-dead Jak3 K855A (31) using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Cells were lysed and immunoprecipitated with Jak3 antibody (described above). Jak3-bound PAS beads were washed three times in cold lysis buffer (described above) followed by kinase buffer. Kinase reactions were initiated by adding 10 μCi [γ-32P]ATP, 10 μm unlabeled ATP, and 1 μg of purified PKAc (Invitrogen) to Jak3-bound PAS bead reaction mixtures. Kinase reactions were performed at 32 °C for 30 min. Jak3-bound PAS beads were washed three times in radioimmunoassay buffer (10 mm Tris-HCl, pH 7.4, 75 mm NaCl, 20 mm EDTA, 10 mm EGTA, 20 mm Na4P2O7, 50 mm NaF, 20 mm 2-glycerolphosphate, 1 mm p-nitrophenylphosphate, 0.1% Triton X-100) and one time in kinase wash buffer (described above). The reactions were stopped by adding 2× SDS-PAGE sample buffer followed by SDS-PAGE. Coomassie stainable Jak3 bands were excised from the PVDF membrane and subjected to phosphoamino acid analysis.
[32P]Orthophosphate Labeling and Phosphoamino Acid Analysis
Jak3 and Stat5b obtained from MT-2, Kit 225, or Hek293 (transfected with Jak3) were metabolically labeled, separated by SDS-PAGE, transferred to PVDF membrane, and visualized using autoradiography. Coomassie blue staining or Western blotting was used to determine total protein; bands corresponding to Jak3 and Stat5b were excised from the membranes and subjected to two-dimensional phosphoamino acid analysis as described previously (33).
Nuclear Extract and Electrophoretic Mobility Shift Assays
Nuclear extracts were prepared from MT-2 cell pellets as reported previously (34). Oligonucleotide sequences corresponding to the β-casein promoter (5′-AGATTTCTAGGAATTCAATCC-3′) were purchased from Santa Cruz Biotechnology.
RT2 Profiler PCR Array
Kit 225 cells were treated with 1% DMSO (vehicle) or 100 μm Fsk for 1 h prior to stimulation with IL-2 as above for 30 h at 37 °C. Total RNA was isolated from ∼4 × 106 cells using the RNeasy kit (Qiagen) and then were DNase-treated (Qiagen RNase-free DNase set). Complementary DNA was synthesized using RT2 first strand kit (SA Biosciences) as recommended by the manufacturer from 2 μg of total RNA/each sample/plate. SA Biosciences' human cell cycle PCR array (PAHS-020) was used according to the manufacturer's instructions. Quantification based on real-time monitoring of amplification was determined using a Bio-Rad iQ5 machine and 2× SYBR Green Mastermix (SA Biosciences). Samples were run in 25-μl reaction volumes. Treatments were performed in duplicate, and data were analyzed using the ΔΔCt method normalized to the average of four housekeeping genes (B2M, RPL13A, GAPDH, and ACTB) using SA Biosciences' data analysis web tool. Genes with Ct > 32 were ignored in the analysis.
RESULTS
Forskolin-induced cAMPi Inhibits T-cell Proliferation and Disrupts Expression of Cell Cycle Progression Genes
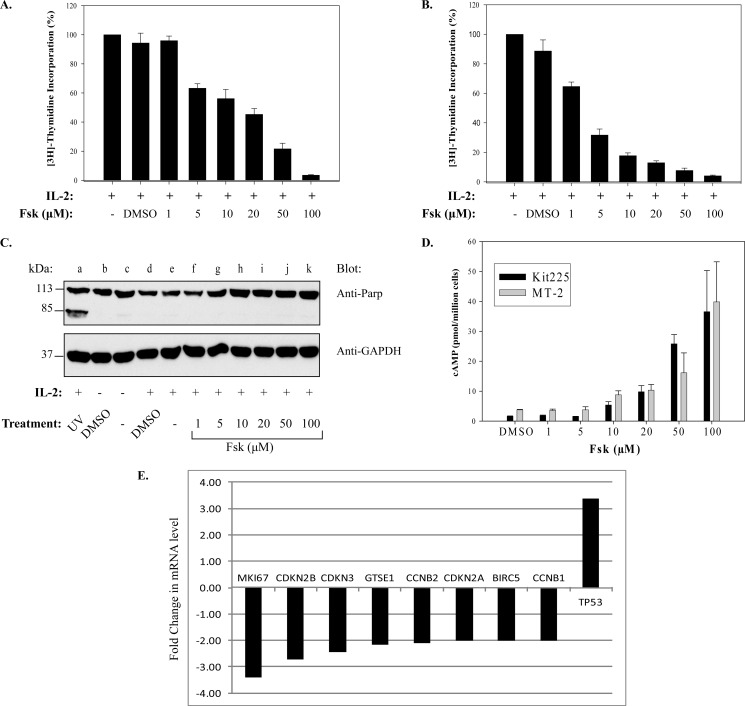
Published reports have extensively documented the inhibitory effects of adenylate cyclase linked G protein-coupled receptor agonists on lymphocyte proliferation (16, 19, 21, 35). To identify the molecular mechanism responsible for this effect, we first sought to determine whether Fsk, a potent adenylate cyclase activator, affects the proliferation of the human T-cell lines such as Kit 225 (Fig. 1A) and MT-2 (Fig. 1B). For this investigation, cells were pretreated with increasing concentrations of Fsk (0–100 μm) or vehicle prior to stimulation with IL-2. Cells were subsequently pulsed with [3H]thymidine to detect DNA synthesis in dividing cells. As shown in Fig. 1, Fsk treatment inhibited the proliferation of both Kit 225 (Fig. 1A) and MT-2 (Fig. 1B) cells in a dose-dependent manner with an IC50 equal to ∼5 μm Fsk. To verify that Fsk concentrations were not causing a nonspecific decrease in cell viability, cleavage of the apoptotic marker PARP was examined (36). PARP cleavage (Fig. 1C) was not detected in negative control or cells treated with increasing concentrations of Fsk compared with the positive apoptotic control (UV light, lane a). Similar results were observed for Kit 225 cells (data not shown). To further support the notion that Fsk-mediated inhibition of MT-2 and Kit 225 cell proliferation was due to an increase in cAMPi, cAMPi generation was measured using an ELISA. Indeed, Fsk treatment (10–100 μm) increased cAMPi levels ∼5- to 20-fold above basal levels, which reached maximum levels between 50–100 μm Fsk (Fig. 1D).
FIGURE 1.
Fsk-cAMPi inhibits cellular proliferation of human T-cell lines and cell cycle genes. Kit 225 (A) or MT-2 cells (B) were untreated or pretreated with vehicle (1% DMSO) or increasing concentrations of Fsk (1, 5, 10, 25, 50, or 100 μm) prior to stimulation with IL-2 for 20 h. Cells were pulsed with [3H]thymidine for an additional 4 h, and radiolabeled DNA was counted as described under “Experimental Procedures.” Data represent the mean ± S.D. (n = 4). C, MT-2 cells were treated as described above with Fsk (lanes f–k) and IL-2 (lanes d–k). Control cells received either no treatment (lane c), 1% DMSO without IL-2 (lane b) or with IL-2 (lane d). MT-2 cells treated with IL-2 and then exposed to UV light were used as a positive control for apoptosis (lane a). PARP cleavage and GAPDH were detected by Western blot analysis. A representative blot from three independent experiments is shown. D, Kit 225 and MT-2 cells were treated with 1% DMSO or increasing concentrations of Fsk (1–100 μm) for 20 min. cAMP concentrations were determined in triplicates by ELISA. Data represent the mean ± S.D. Each experiment was repeated three times, and representative data from one experiment are shown. E, Kit 225 cells were treated with vehicle (1% DMSO) or 100 μm Fsk for 1 h prior to stimulation with IL-2 for 30 h at 37 °C. cDNA obtained from treated cells was amplified using a human cell cycle PCR array. Quantification based on real-time monitoring of gene amplification was determined. Treatments were performed in duplicate, and data were analyzed using the ΔΔCt method normalized to an average of 4 housekeeping genes (B2M, RPL13A, GAPDH, and ACTB). Genes with Ct > 32 were excluded in the analysis.
Several reports have indicated that cAMPi can block expression of cell cycle genes (37, 38). To determine whether the reduction in T-cell proliferation was due to Fsk similarly affecting cell cycle regulators, these genes were analyzed (Fig. 1E) by quantitative RT-PCR. Indeed, cell cycle-dependent genes, CCNB1 and CCNB2, as well as the cell cycle checkpoint genes CDKN2A, CDKN2B, and CDKN3, were found to be down-regulated following Fsk treatment of Kit 225 cells. Expression of the G2/M transition cell cycle regulatory genes GTSE1 and BIRC5 were similarly down-regulated. Interestingly, the p53 tumor suppressor gene, TP53, was shown to be up-regulated. Similar results were obtained for MT-2 cells (data not shown). These data suggest that cAMP can inhibit cell cycle gene expression, which may serve as a possible inhibitory mechanism of cell proliferation.
Forskolin-cAMPi Inhibits IL-2-induced Stat5 Activation, Translocation, and DNA Binding
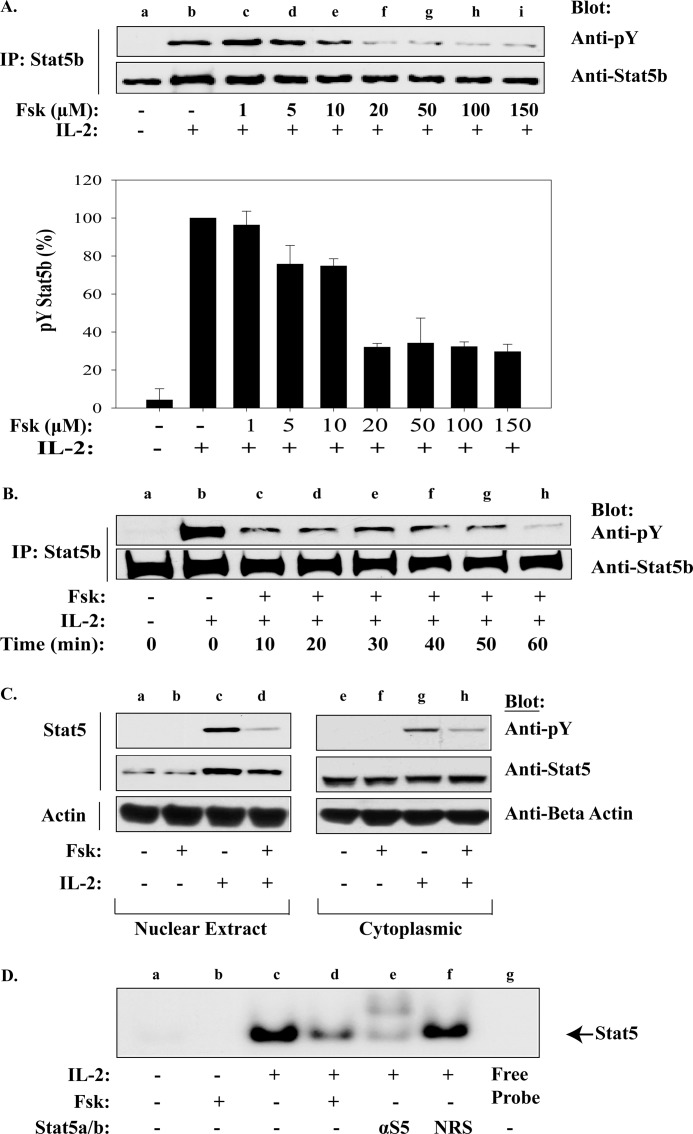
One possibility for the reduced expression of these critical cell cycle genes could be due to uncoupled cytokine signals. Stat5 has been shown to be critical for T-cell expansion and survival in this signaling cascade by targeting G1/S transition and anti-apoptotic genes (34, 39, 40). To determine whether Fsk-cAMPi-activated pathways disrupted IL-2 activation of Stat5, its tyrosine phosphorylation status was examined by Western blot. For this analysis, MT-2 cells were treated with increasing concentrations of Fsk and Stat5b tyrosine phosphorylation monitored (Fig. 2A, upper panel). Indeed, Fsk inhibited Stat5b tyrosine phosphorylation with an IC50 ∼10 μm Fsk without effecting Stat5b expression (Fig. 2A). This experiment was repeated in triplicate, and the densitometry of tyrosine phosphorylated Stat5 normalized to total Stat5 was plotted (Fig. 2A, lower panel). Similar results were observed for Stat5a (data not shown). To determine the kinetics of Fsk-mediated Stat5b inactivation, MT-2 cells were pretreated with 100 μm Fsk for various time points prior to IL-2 stimulation (10 min). As shown in Fig. 2B, 80% inhibition of Stat5b tyrosine phosphorylation occurred by 10 min, whereas maximal inhibition occurred after 60 min (Fig. 2B, lane h). Tyrosine phosphorylation of Stat5 is required for optimal dimerization, nuclear translocation, and DNA binding (3, 4, 41). To determine whether Fsk-cAMPi activated pathways disrupt this event, Western blot analysis of cytoplasmic and nuclear protein fractions from MT-2 cells before and after IL-2 stimulation were investigated (Fig. 2C). Both nuclear and cytoplasmic tyrosine phosphorylated Stat5 and nuclear Stat5 protein levels were reduced following Fsk treatment compared with vehicle-treated cells (Fig. 2C, lanes d and h). Similar results were confirmed by Stat5 EMSA analysis (Fig. 2D), where 100 μm Fsk inhibited IL-2 induced Stat5 DNA binding to a radiolabeled β-casein promoter by ∼60% (Fig. 2D, lane d). These data support the notion that Fsk-induced cAMPi can disrupt IL-2-mediated Stat5 tyrosine phosphorylation, nuclear translocation, and DNA binding activity.
FIGURE 2.
Fsk-cAMPi inhibits Stat5b activation, nuclear translocation, and DNA binding activity. A, MT-2 cells were untreated (lane a) or pretreated with the indicated concentrations of Fsk for 40 min prior to stimulation with IL-2 for 10 min at 37 °C (lanes b–i). Immunoprecipitated Stat5b was Western blotted with anti-phosphotyrosine (pY; upper panel) or anti-Stat5b (lower panel). Representative data from three independent experiments are shown. Densitometric analysis of phosphotyrosine Stat5b was normalized to total Stat5b and graphed (percentage of phosphotyrosine Stat5b) (lane b). Data represent the mean ± S.D. from three independent experiments. B, MT-2 cells were treated as above with vehicle (lanes a and b) or pretreated with 100 μm Fsk for the times indicated before IL-2 stimulation for 10 min at 37 °C (lanes c–h). Immunoprecipitated Stat5b was Western blotted with anti-phosphotyrosine (upper panel) or anti-Stat5b (lower panel). C, MT-2 cells were either untreated or pretreated with 100 μm Fsk for 40 min prior to IL-2 stimulation. Immunoprecipitated nuclear (lanes a–d) and cytoplasmic (lanes e–h) localized Stat5b proteins were separated by SDS-PAGE and Western blotted using antibodies for phosphotyrosine, Stat5, or β-actin (internal standard). D, EMSA analysis was performed on MT-2 cells treated as in C. Nuclear proteins were isolated and incubated with [γ-32P]ATP-labeled β-casein promoter alone (lanes a–d), with anti-Stat5 antibody (lane e) or normal rabbit serum (NRS; lane f) or free probe without cell lysate (lane g). Stat5 migration is indicated by the arrow.
Forskolin-cAMPi Inhibits IL-2 Activation of Jak3
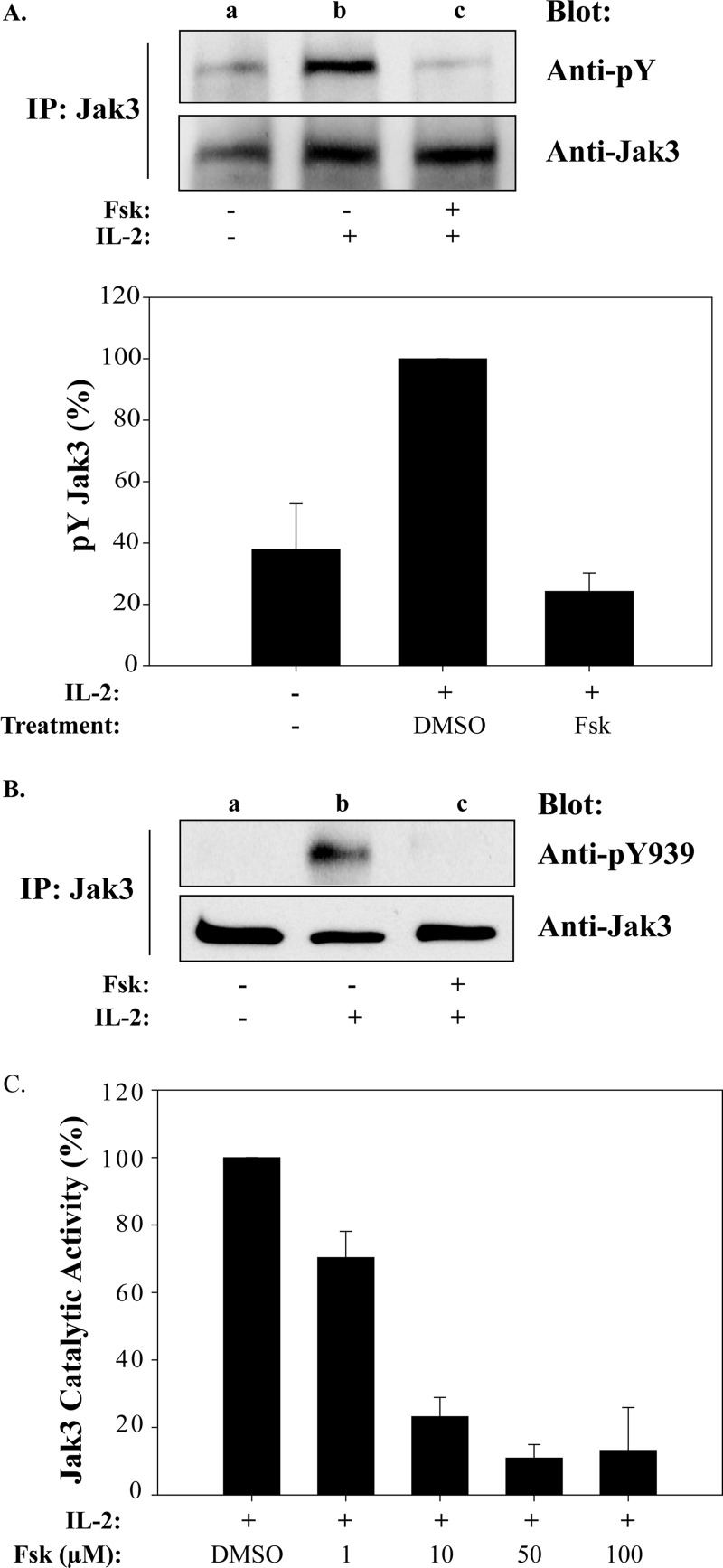
Following IL-2 binding to its cognate receptor, Jak3 is activated and competent to tyrosine phosphorylate substrates such as the IL-2Rβ, γc, and Stat5. Because Jak3 is critical for Stat5 activation, cAMPi may exert its effects by blocking Jak3 activity. To test this model, MT-2 cells were pretreated with Fsk and Jak3 tyrosine phosphorylation status examined. Anti-phosphotyrosine Western blot revealed that Jak3 tyrosine phosphorylation was inhibited by 80% after Fsk pretreatment (Fig. 3A, lane c) when replicated, and data were normalized from three separate experiments (Fig. 3A, graph). Phosphorylation of Tyr980, Tyr981, and Tyr939 have been shown to regulate Jak3 enzymatic activity (31, 42). Given the importance of Tyr939 for Stat5 association, we examined Fsk treatment on Jak3 Tyr939 phosphorylation (Fig. 3B). Indeed, Fsk treatment completely abrogated Jak3 Tyr939 phosphorylation (Fig. 3B, lane c) as compared with control (Fig. 3B, lane b). Because Tyr939 phosphorylation is also critical for Jak3 activity, its enzymatic status was tested using the GST-tagged cytoplasmic portion of γc as a substrate (31). As shown in Fig. 3C, Fsk treatment inhibited Jak3 tyrosine phosphorylation of γc in a dose-dependent manner with an IC50 between 1 and 10 μm. These results closely parallel those Fsk concentrations required to reduce T-cell proliferation (Fig. 1B).
FIGURE 3.
Fsk-cAMPi inhibits IL-2-induced Jak3 activation and catalytic activity. A, MT-2 cells were left untreated (lane a) or treated with vehicle (1% DMSO) (lane b) or 100 μm Fsk for 40 min (lane c) prior to stimulation with 100 nm IL-2 for 10 min (lanes b and c) at 37 °C. Immunoprecipitated Jak3 protein was Western blotted using anti-phosphotyrosine (pY) antibody (upper panel) or total Jak3 (lower panel). Densitometric analyses were carried out (n = 3), and tyrosine phosphorylation levels of Jak3 were normalized to total Jak3 protein and plotted (lower panel). B, cells were treated as described in A and Western blotted with anti-phosphotyrosine 939 (pY939). C, MT-2 cells were treated with increasing concentrations of Fsk for 40 min prior to stimulation with 100 nm IL-2 for 10 min. Immunocaptured Jak3 was used for in vitro kinase assays with GST-γc as a substrate and Western blotted with anti-phosphotyrosine or anti-GST antibodies. Densitometric analysis was performed, and tyrosine phosphorylation of GST-γc was normalized to total GST expression. The results are presented as percentage of IL-2 activated Jak3 activity in the absence or presence of Fsk.
cAMPi Elevators Induce Jak3 Serine Phosphorylation but Disrupt IL-2-inducible Stat5 Serine Phosphorylation
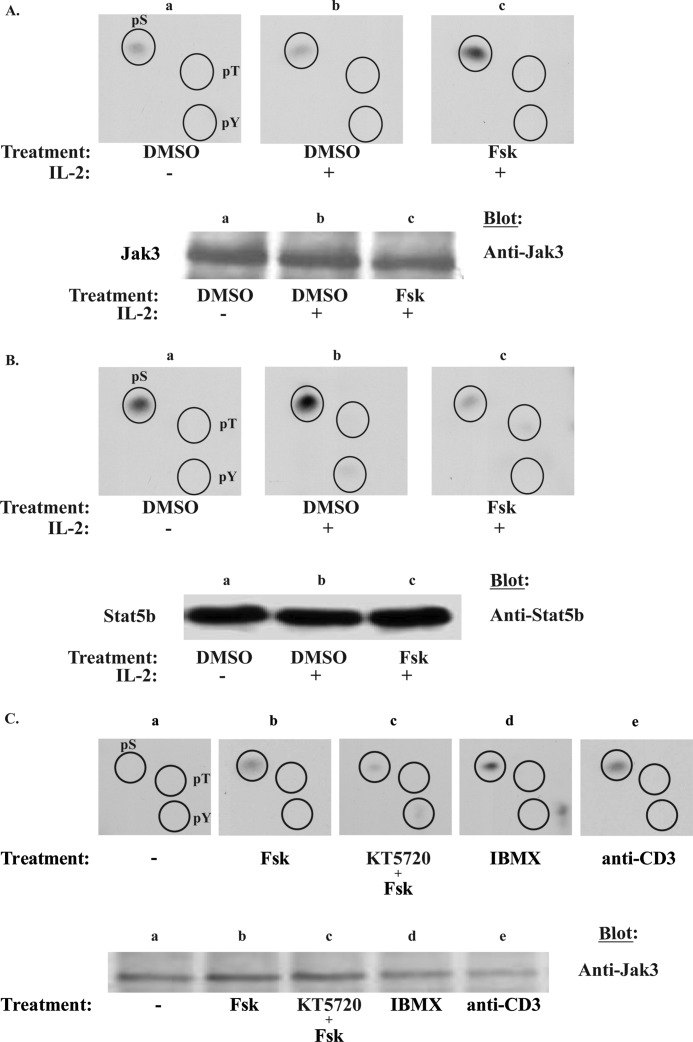
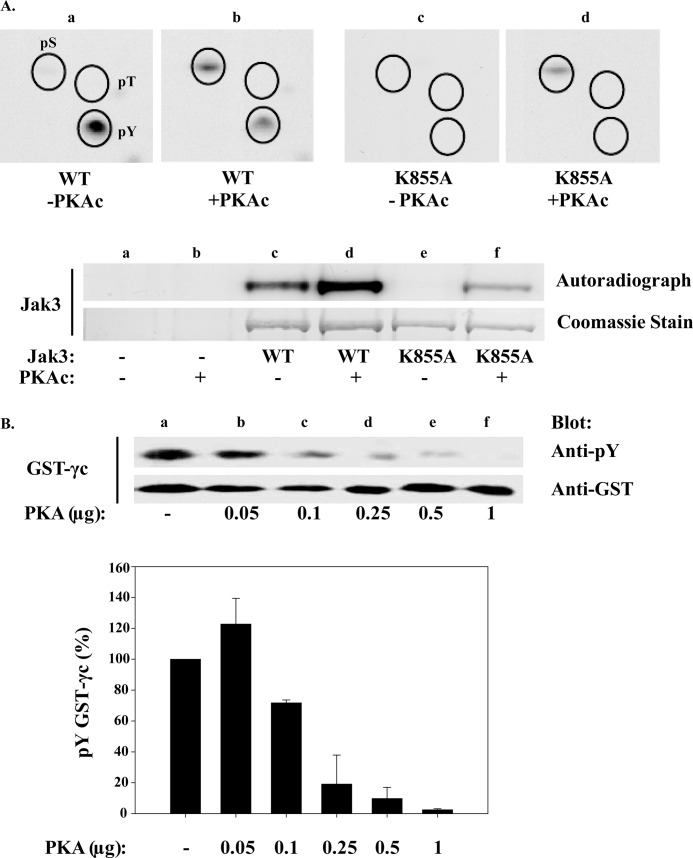
The positive regulatory role of tyrosine phosphorylation of IL-2R signaling proteins has been well documented (3, 42, 43), and many also undergo serine phosphorylation to positively or negatively regulate their activity (8, 44–46). The role of serine phosphorylation within IL-2R signaling, however, is not fully established. Because cAMPi most notably activates the serine-threonine protein kinase PKA (10), we sought to determine the effects of Fsk treatment on Jak3 and Stat5b serine phosphorylation by phosphoamino acid analysis (Fig. 4). Parallel mass spectrometry analysis was used to confirm the purity of Jak3 and Stat5b proteins (data not shown). Indeed, Fsk-cAMPi induced Jak3 serine phosphorylation (Fig. 4A, panel c) as compared with control (Fig. 4A, panel b). In contrast, IL-2-inducible Stat5b serine phosphorylation was inhibited by Fsk treatment (Fig. 4B, panel c) but not untreated Stat5b (Fig. 4B, panel b). It should be noted that under the hydrolysis conditions used to maximize the detection of phosphoserine, the more labile tyrosine and threonine phosphorylation is lost. Nevertheless, one possible mechanism by which cAMPi disrupts Jak3 catalytic activity may occur via serine phosphorylation. To determine whether Fsk-induced Jak3 serine phosphorylation was confined to the human lymphotropic virus-1 transformed MT-2 cells, we next examined non-virally transformed T-cells, Kit 225. As was the case in MT-2 cells, Fsk induced Jak3 serine phosphorylation (Fig. 4C, upper panel, panel b), which could be uncoupled with the PKA inhibitor KT5720 prior to Fsk treatment (Fig. 4C, upper panel, panel c). These data suggest that PKA is an important mediator within the Fsk-cAMPi cascade to promote Jak3 serine phosphorylation. To further substantiate this notion, cells were treated with the nonspecific phosphodiesterase inhibitor, IBMX, which can elevate cAMPi by preventing its degradation. As shown in Fig. 4C (upper panel, panel d) IBMX-pretreated MT-2 cells in the absence of Fsk also induced Jak3 serine phosphorylation (representative data from two independent experiments). Interestingly, treatment of Kit 225 cells with anti-CD3 monoclonal antibody also increased Jak3 serine phosphorylation (Fig. 4C, upper panel, panel e).
FIGURE 4.
Fsk and IBMX induce Jak3 serine phosphorylation but blocks IL-2 inducible Stat5b serine phosphorylation. A, MT-2 cells were metabolically labeled with [32P]orthophosphate and treated with 1% DMSO (panel a), IL-2 for 10 min (panel b), or 100 μm Fsk followed by 10 min of IL-2 stimulation (panel c). Jak3 or Stat5b (B) were immunoprecipitated and Western blotted for protein. Bands corresponding to Jak3 or Stat5b were excised and subjected to phosphoamino acid analysis (n = 3 and n = 2, respectively) to determine the global phosphorylation status of serine (pS), threonine (pT), and tyrosine (pT) residues within these residues. Comparable amounts of Jak3 (A, lower panel) and Stat5b (B, lower panel) were determined using Western blot analysis to ensure equal protein loading. C, Kit 225 cells were similarly labeled and treated with 1% DMSO (panel a), 100 μm Fsk for 1 h (panel b), Fsk and 10 μm KT5720 (KT) (panel c), 1 mm IBMX for 15 min (panel d), or 5 μg of anti-CD3 for 10 min (panel e). Phosphoamino acid analysis was assessed for Jak3 phosphorylation status (upper panel, n = 2). The lower panel indicates the separation of Jak3 immunoprecipitates prior to phosphoamino acid analysis. The immunoprecipitates were electrophorized and autoradiographed (upper bands) or Western blotted using anti-Jak3 antibody (lower bands).
To determine whether PKA is capable of directly phosphorylating serine residues on Jak3, a radiolabeled in vitro kinase assay using purified PKAc and wild type (WT) Jak3 or catalytically inactive Jak3 (K855A) was performed in conjunction with phosphoamino acid analysis. Under these conditions, WT Jak3 showed an increase in total phosphorylation in the presence of PKAc (Fig. 5A, lower panel, lane d). Subsequent phosphoamino acid analysis revealed a 5.8-fold increase in serine and a 2-fold decrease in tyrosine autophosphorylation in the presence of PKAc (Fig. 5A, upper panel, panel b). Interestingly, threonine phosphorylation was not detected on WT Jak3 (lane c). To confirm that Jak3 tyrosine and serine phosphorylation were due to WT Jak3 and PKAc, respectively, catalytically inactive Jak3, K855A, was tested. For this analysis, autoradiography revealed that PKAc increased serine phosphorylation of catalytically inactive Jak3 5-fold (Fig. 5A, lower panel, lane f), whereas threonine and tyrosine phosphorylation were not detected (Fig. 5A, upper panel, lane d). These data suggest that Jak3 can be inhibited by a Fsk-cAMPi-PKA pathway via serine phosphorylation. For this analysis, GST-γc was used as a substrate, and Jak3 inactivation by increasing concentrations of PKAc was monitored. As shown in Fig. 5B, as little as 0.1 μg of PKAc disrupted the ability of Jak3 to tyrosine phosphorylate the GST-γc substrate. Multiple lots of PKAc were tested and revealed a similar dose response in disrupting Jak3 catalytic activity. Normalized data from three separate experiments are shown in Fig. 5B, lower panel. These results suggest that PKA can phosphorylate Jak3 serine residues and negatively regulate its catalytic activity.
FIGURE 5.
PKA directly phosphorylates Jak3 serine residues and disrupts its kinase activity. A, Hek 293 cells were transfected with wild type (WT) or catalytically inactive (K855A) Jak3. Cells were then lysed, and Jak3 immunoprecipitates used for a [γ-32P]ATP in vitro kinase assay in the presence or absence of purified PKAc and subjected to phosphoamino acid analysis (upper panels a–d). The lower panel indicates the Coomassie Blue stain and autoradiograph of reactions separated by SDS-PAGE; Hek293 cells carrying an empty vector unexposed (lane a) or exposed to PKAc (lane b), WT Jak3 in the absence (lane c) and presence (lane d) of PKAc, and kinase dead K855A Jak3 with (lane e) or without (lane f) PKAc. B, Jak3 was immunopurified from MT-2 cells and subsequently incubated without (lane a) or with increasing amounts of purified PKAc for 30 min (lanes b–f). Jak3-bound beads were then washed free of PKAc and used in a second in vitro kinase assay with GST-γc substrate. The upper panel shows Western blots of the latter in vitro kinase reaction using anti-phosphotyrosine antibody to assess γc tyrosine phosphorylation and reblotted for total GST (lower panel). Each error bar represents the mean ± S.D. of three independent experiments. γc tyrosine phosphorylation normalized to GST is plotted below.
Fsk-cAMPi Disrupt IL-2 Receptor Subunit Association
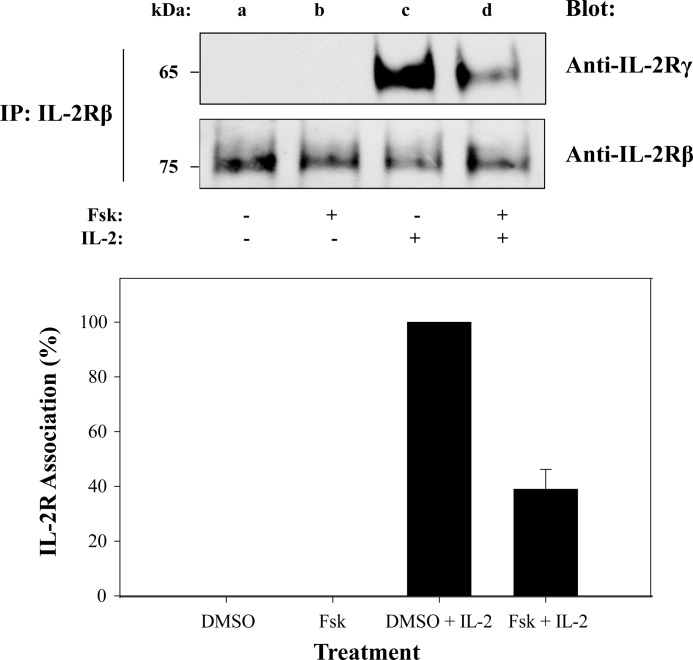
IL-2 binds to the IL-2Rα (55 kDa, Kd of 10−8 m), which allows for IL-2Rβ (75 kDa, Kd of 10−9 m) to bind and finally for the recruitment of the γc (65 kDa, Kd of 10−11 m) (4, 47). Cytoplasmically localized Jak1 and Jak3 bind to the IL-2Rβ and γc, respectively, allowing for Jak autophosphorylation (9) and subsequent tyrosine phosphorylation of the receptor subunits. This in turn promotes Stat5 binding and subsequent tyrosine phosphorylation. To determine whether the loss of Stat5b tyrosine phosphorylation mediated by Fsk-cAMPi (Fig. 2) was due to inactivated IL-2R complex, the following experiment was performed. MT-2 cells were either untreated or treated with Fsk (100 μm for 1 h) prior to stimulation with IL-2, and receptor association was analyzed. The IL-2Rβ chain was immunoprecipitated and subjected to SDS-PAGE and Western blotted with antibodies directed to IL-2Rβ or γc (Fig. 6). Western blot analysis revealed that Fsk-cAMPi blocked IL-2Rβ and γc association by ∼60% (Fig. 6, lane d), which was confirmed in triplicate with densitometry (Fig. 6, lower panel). Reverse co-immunoprecipitation by first capturing the γc subunit to detect IL-2Rβ by Western blot yielded similar results (data not shown). These data indicate Fsk-cAMPi can disrupt IL-2Rβ/γc association in the presence of IL-2 as another mechanism to uncouple cytokine activation of Jak/Stat signaling.
FIGURE 6.
cAMP disrupts IL-2 receptor β and γ chain association. MT-2 cells were treated with 1% DMSO (lanes a and c) or 100 μm Fsk for 1 h (lanes b and d) followed by IL-2 stimulation for 15 min (lanes c and d). Cells were lysed, immunoprecipitated for IL-2Rβ, separated by SDS-PAGE, and Western blotted using antibodies against γc or IL-2Rβ (upper panel). IL-2Rγ band intensities were normalized to IL-2Rβ levels using densitometric analysis and presented as percentage of IL-2R association (lower panel). Representative data from three independent experiments are shown.
DISCUSSION
Immune system function is dependent on positive and negative regulation of T-cell signaling pathways, including the Jak3/Stat5 cascade. These key signaling proteins have been shown to be hyperactivated in various hematopoietic cancers, including certain lymphomas and leukemias (48–50), and therefore represent therapeutic targets for treating various diseases. In this study, IL-2 activation of Stat5 (Fig. 2) and Jak3 (Fig. 3) was shown to be inhibited by elevated cAMP levels induced by Fsk in a dose-dependent manner, which resulted in a loss of T-cell proliferation (Fig. 1). Multiple cell cycle genes were identified as possible targets (Fig. 1E), including the gene for KD-67, a protein present during all phases of the cell cycle except G0 (51). It is likely that cAMPi accumulation does not allow T-cells to exit the G0 phase. This hypothesis is supported by a similarly identified and reported case where cAMP induced an anergic-like state in Th1 cells (52).
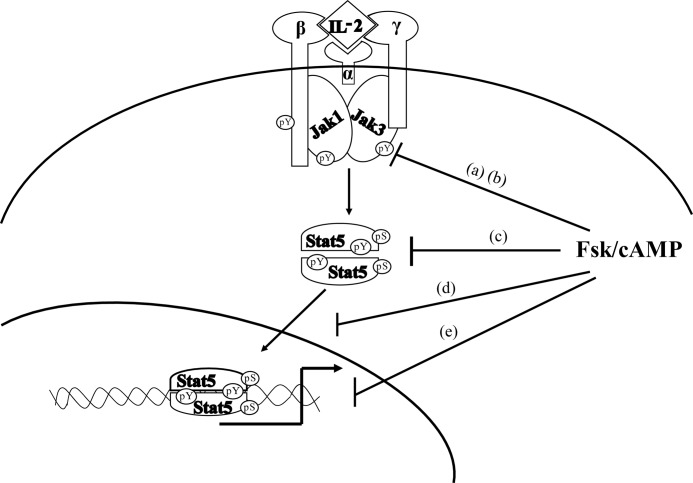
The reason for the loss of Jak3/Stat5 activity appears to be the result of uncoupling of the IL-2R complex and disruption of IL-2-mediated association of the IL-2Rβ and γc (Fig. 6). Although the mechanism by which this occurs is currently under investigation by our lab, it is tempting to speculate that cAMP activates a kinase that phosphorylates the β or γc chains, which disrupts their functional association. This may represent the first level of IL-2R uncoupling (Fig. 7a). Within this same level, cAMP results in Jak3 serine phosphorylation that suppresses its catalytic activity (Fig. 7b) and ability to phosphorylate substrates such as Stat5 (Fig. 7c) and GST-γc (Fig. 3C). It is important to note that Jak3 becomes stabilized as part of the IL-2R complex over time (53), and thus, a loss of Jak3-mediated stabilization of the receptor complex may account for the reduced IL-2Rβ/γc association following Fsk treatment in this study. PKA may represent a key kinase in this cascade as it was activated by Fsk or IBMX and the event inhibited by KT5720 (Fig. 4). Indeed, PKA could directly serine phosphorylate Jak3 and disrupt its catalytic activity (Fig. 5). The identification and validation of putative PKA consensus sites within Jak3 are currently under investigation. As such, PKA or a PKA-dependent pathway may act to negatively regulate the IL-2R complex (Fig. 6), Jak3 catalytic activity (Fig. 5), and Stat5 (Figs. 2 and 4). Attempts to deplete PKA via shRNA and other approaches did not reveal significant protein knockdown, making confirmation difficult.
FIGURE 7.
Model for the putative mechanism by which Fsk-cAMPi negatively regulates IL-2R signaling in human T-cells. Multiple targets for Fsk-cAMPi regulation are shown. Fsk prevents the association of IL-2Rγ and β chain (a) as well as Jak3 activation and kinase activity (b). Stat5 activation (c), nuclear translocation (d), and DNA binding (e) are prevented, resulting in severe reduction in IL-2R signaling and T lymphocyte proliferation.
Previous studies have identified a mechanism by which AC activity modulates T-cell responses. Activation of the TCR complex and Ca2+ mobilization are known to activate AC (54). Early events in T-cell activation require the intracellular accumulation of calcium to activate key signaling pathways. Human T-cells and lymphoid cell lines, including MT-2 and Kit 225 cells, express AC III3 known to be activated by calcium (55). Similarly, other groups have shown that high avidity TCR engagement inhibits T-cell proliferation by blocking activation of Stat5, Jak1, and Jak3 mediated by IL-2 or IL-4 (25). Similar to our results, TCR ligation blocked activation of cell cycle regulatory proteins. Inhibition of IL-4 signaling following TCR ligation was shown to be dependent on PKC, Erk, and Ca2+ signaling through calcineurin in murine T-cells (24, 56). We found that activation of the TCR complex by anti-CD3 cross-linking induced serine phosphorylation of Jak3 (Fig. 4C) perhaps mimicking the Fsk effect. In addition to Mek/Erk and Ca2+ signaling, PKA may represent a possible cross-talk between these pathways and the Jak/Stat cascade within T-cells.
Physiologically, inhibition of T-cell proliferation is needed at the culmination of a successful immune response. Because Jak3 is not expressed in naïve or resting T-cells, the inhibitory effects of cAMP on IL-2R signaling may be a mechanism for down-regulating T-cell activity. Indeed, for primed T-cells, TCR signaling/Ca2+ mobilization (signal 1 and 2) would inhibit IL-2R signaling (signal 3) and possibly initiate activation-induced cell death via Ca2+ mobilization/activated AC/PKA. This is possible because the Jak3/Stat5 pathway is critical for delivering T-cell survival signals. The finding that TCR activation induces cAMP production in T-cells (57) may support this hypothesis. A second possible physiological mechanism is that T regulatory cells would suppress autoreactive T-cells by inducing activation-induced cell death or anergy. Indeed, it has been reported that T regulatory cells maintain high levels of intracellular cAMP and can transfer it through gap junctions into CD4+ T-cells (58), whereas others have reported that T-cells enter an anergic state in response to cAMP (52).
The findings presented herein provide new insights into the mechanism underlying immunosuppression in disease, including human immunodeficiency virus (HIV) infection and severe combined immunodeficiency (SCID) disorder. Indeed, it has been reported that replication of HIV requires cAMP and increases intracellular cAMP concentrations (59, 60). Thus, uncoupling the Jak3/Stat5 pathway may be one possible mechanism by which HIV may inhibit T-cell function. Although SCID can result from mutations in Jak3 or the γc subunit, this phenotype can also be manifested in individuals with an adenosine deaminase deficiency. Because adenosine binds an AC-linked GPCR that can prevent IL-2 induced phosphorylation of Stat5 (16) and lymphocyte proliferation (61), it is tempting to speculate that cAMP mediates this process. Further identification and characterization of putative serine residues targeted by PKA in the IL-2R complex remain to be identified and possibly hold new strategies to control T-cell-mediated diseases. In summary, this work demonstrates that elevated cAMPi can disrupt IL-2R complex formation, Jak3 catalytic activity, and its ability to phosphorylate Stat5 and transcriptionally responsive Stat5 genes (Fig. 7), resulting in a loss of T-cell proliferation.
This work was supported by grants from the Lizanell and Colbert Coldwell Foundation, the Edward N. and Margaret G. Marsh Foundation, and Grant G12MD007592 from the National Institutes on Minority Health and Health Disparities, National Institutes of Health.
R. A. Kirken and G. Rodriguez, unpublished data.
- IL-2
- interleukin-2
- Fsk
- forskolin
- γc
- common γ chain
- Jak
- Janus tyrosine kinase
- Stat
- signal transducer and activator of transcription
- AC
- adenylate cyclase
- PKA
- protein kinase A
- IBMX
- 3-isobutyl-1-methyl-xanthine
- DSMO
- dimethyl sulfoxide
- PARP
- poly (ADP-Ribose) polymerase
- PKAc
- protein kinase A catalytic subunit.
REFERENCES
- 1. Leonard W. J. (1996) STATs and cytokine specificity. Nat. Med. 2, 968–969 [DOI] [PubMed] [Google Scholar]
- 2. Weaver A. M., Silva C. M. (2006) Modulation of signal transducer and activator of transcription 5b activity in breast cancer cells by mutation of tyrosines within the transactivation domain. Mol. Endocrinol. 20, 2392–2405 [DOI] [PubMed] [Google Scholar]
- 3. Lin J. X., Mietz J., Modi W. S., John S., Leonard W. J. (1996) Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J. Biol. Chem. 271, 10738–10744 [PubMed] [Google Scholar]
- 4. Lin J. X., Leonard W. J. (2000) The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 19, 2566–2576 [DOI] [PubMed] [Google Scholar]
- 5. Park S. H., Yamashita H., Rui H., Waxman D. J. (2001) Serine phosphorylation of GH-activated signal transducer and activator of transcription 5a (STAT5a) and STAT5b: impact on STAT5 transcriptional activity. Mol. Endocrinol. 15, 2157–2171 [DOI] [PubMed] [Google Scholar]
- 6. Xue H. H., Fink D. W., Jr., Zhang X., Qin J., Turck C. W., Leonard W. J. (2002) Serine phosphorylation of Stat5 proteins in lymphocytes stimulated with IL-2. Int. Immunol. 14, 1263–1271 [DOI] [PubMed] [Google Scholar]
- 7. Weaver A. M., Silva C. M. (2007) S731 in the transactivation domain modulates STAT5b activity. Biochem. Biophys. Res. Commun. 362, 1026–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitra A., Ross J. A., Rodriguez G., Nagy Z. S., Wilson H. L., Kirken R. A. (2012) Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phospho-regulatory site that is constitutively activated in primary hematopoietic malignancies. J. Biol. Chem. 287, 16596–16608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross J. A., Nagy Z. S., Cheng H., Stepkowski S. M., Kirken R. A. (2007) Regulation of T cell homeostasis by JAKs and STATs. Arch. Immunol. Ther. Exp. 55, 231–245 [DOI] [PubMed] [Google Scholar]
- 10. Taskén K., Aandahl E. M. (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 [DOI] [PubMed] [Google Scholar]
- 11. Vandamme J., Castermans D., Thevelein J. M. (2012) Molecular mechanisms of feedback inhibition of protein kinase A on intracellular cAMP accumulation. Cell Signal. 24, 1610–1618 [DOI] [PubMed] [Google Scholar]
- 12. Houslay M. D. (2010) Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 35, 91–100 [DOI] [PubMed] [Google Scholar]
- 13. Biel M., Michalakis S. (2009) Cyclic nucleotide-gated channels. Handb. Exp. Pharmacol. 191, 111–136 [DOI] [PubMed] [Google Scholar]
- 14. Grandoch M., Roscioni S. S., Schmidt M. (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br. J. Pharmacol. 159, 265–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosenden R., Taskén K. (2011) Cyclic AMP-mediated immune regulation–overview of mechanisms of action in T cells. Cell Signal. 23, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 16. Zhang H., Conrad D. M., Butler J. J., Zhao C., Blay J., Hoskin D. W. (2004) Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: role of cyclic adenosine 3′,5′-monophosphate and phosphatases. J. Immunol. 173, 932–944 [DOI] [PubMed] [Google Scholar]
- 17. Elliott L., Brooks W., Roszman T. (1992) Inhibition of anti-CD3 monoclonal antibody-induced T-cell proliferation by dexamethasone, isoproterenol, or prostaglandin E2 either alone or in combination. Cell Mol. Neurobiol. 12, 411–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekholm D., Mulloy J. C., Gao G., Degerman E., Franchini G., Manganiello V. C. (1999) Cyclic nucleotide phosphodiesterases (PDE) 3 and 4 in normal, malignant, and HTLV-I transformed human lymphocytes. Biochem. Pharmacol 58, 935–950 [DOI] [PubMed] [Google Scholar]
- 19. Bury T. B., Corhay J. L., Radermecker M. F. (1992) Histamine-induced inhibition of neutrophil chemotaxis and T-lymphocyte proliferation in man. Allergy 47, 624–629 [DOI] [PubMed] [Google Scholar]
- 20. Essayan D. M., Huang S. K., Undem B. J., Kagey-Sobotka A., Lichtenstein L. M. (1994) Modulation of antigen- and mitogen-induced proliferative responses of peripheral blood mononuclear cells by nonselective and isozyme selective cyclic nucleotide phosphodiesterase inhibitors. J. Immunol. 153, 3408–3416 [PubMed] [Google Scholar]
- 21. Huang S., Apasov S., Koshiba M., Sitkovsky M. (1997) Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90, 1600–1610 [PubMed] [Google Scholar]
- 22. Lingk D. S., Chan M. A., Gelfand E. W. (1990) Increased cyclic adenosine monophosphate levels block progression but not initiation of human T cell proliferation. J. Immunol. 145, 449–455 [PubMed] [Google Scholar]
- 23. Maca R. D. (1984) The effects of cyclic nucleotides on the proliferation of cultured human T-lymphocytes. Immunopharmacology 8, 53–60 [DOI] [PubMed] [Google Scholar]
- 24. Zhu J., Huang H., Guo L., Stonehouse T., Watson C. J., Hu-Li J., Paul W. E. (2000) Transient inhibition of interleukin 4 signaling by T cell receptor ligation. J. Exp. Med. 192, 1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee I. H., Li W. P., Hisert K. B., Ivashkiv L. B. (1999) Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J. Exp. Med. 190, 1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyoshi I., Kubonishi I., Yoshimoto S., Shiraishi Y. (1981) A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gann 72, 978–981 [PubMed] [Google Scholar]
- 27. Kirken R. A., Erwin R. A., Taub D., Murphy W. J., Behbod F., Wang L., Pericle F., Farrar W. L. (1999) Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J. Leukoc Biol. 65, 891–899 [DOI] [PubMed] [Google Scholar]
- 28. Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. (1987) Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood 70, 1069–1072 [PubMed] [Google Scholar]
- 29. Malabarba M. G., Rui H., Deutsch H. H., Chung J., Kalthoff F. S., Farrar W. L., Kirken R. A. (1996) Biochem. J. 319 (Pt 3), 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Behbod F., Nagy Z. S., Stepkowski S. M., Karras J., Johnson C. R., Jarvis W. D., Kirken R. A. (2003) Specific inhibition of Stat5a/b promotes apoptosis of IL-2-responsive primary and tumor-derived lymphoid cells. J. Immunol. 171, 3919–3927 [DOI] [PubMed] [Google Scholar]
- 31. Cheng H., Ross J. A., Frost J. A., Kirken R. A. (2008) Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its activity. Mol. Cell Biol. 28, 2271–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross J. A., Cheng H., Nagy Z. S., Frost J. A., Kirken R. A. (2010) Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J. Biol. Chem. 285, 3582–3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross J. A., Nagy Z. S., Kirken R. A. (2008) The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J. Biol. Chem. 283, 4699–4713 [DOI] [PubMed] [Google Scholar]
- 34. Nagy Z. S., Rui H., Stepkowski S. M., Karras J., Kirken R. A. (2006) A preferential role for STAT5, not constitutively active STAT3, in promoting survival of a human lymphoid tumor. J. Immunol. 177, 5032–5040 [DOI] [PubMed] [Google Scholar]
- 35. Bartik M. M., Brooks W. H., Roszman T. L. (1993) Modulation of T cell proliferation by stimulation of the β-adrenergic receptor: lack of correlation between inhibition of T cell proliferation and cAMP accumulation. Cell Immunol. 148, 408–421 [DOI] [PubMed] [Google Scholar]
- 36. Mullen P. (2004) PARP cleavage as a means of assessing apoptosis. Methods Mol. Med. 88, 171–181 [DOI] [PubMed] [Google Scholar]
- 37. Zambon A. C., Zhang L., Minovitsky S., Kanter J. R., Prabhakar S., Salomonis N., Vranizan K., Dubchak I., Conklin B. R., Insel P. A. (2005) Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 102, 8561–8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stork P. J., Schmitt J. M. (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12, 258–266 [DOI] [PubMed] [Google Scholar]
- 39. Moriggl R., Topham D. J., Teglund S., Sexl V., McKay C., Wang D., Hoffmeyer A., van Deursen J., Sangster M. Y., Bunting K. D., Grosveld G. C., Ihle J. N. (1999) Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10, 249–259 [DOI] [PubMed] [Google Scholar]
- 40. Nagy Z. S., LeBaron M. J., Ross J. A., Mitra A., Rui H., Kirken R. A. (2009) STAT5 regulation of BCL10 parallels constitutive NFκB activation in lymphoid tumor cells. Mol. Cancer 8, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gouilleux F., Wakao H., Mundt M., Groner B. (1994) Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 13, 4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou Y. J., Hanson E. P., Chen Y. Q., Magnuson K., Chen M., Swann P. G., Wange R. L., Changelian P. S., O'Shea J. J. (1997) Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. U.S.A. 94, 13850–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ellery J. M., Nicholls P. J. (2002) Alternate signalling pathways from the interleukin-2 receptor. Cytokine Growth Factor Rev. 13, 27–40 [DOI] [PubMed] [Google Scholar]
- 44. Kirken R. A., Malabarba M. G., Xu J., DaSilva L., Erwin R. A., Liu X., Hennighausen L., Rui H., Farrar W. L. (1997) Two discrete regions of interleukin-2 (IL2) receptor β independently mediate IL2 activation of a PD98059/rapamycin/wortmannin-insensitive Stat5a/b serine kinase. J. Biol. Chem. 272, 15459–15465 [DOI] [PubMed] [Google Scholar]
- 45. Nagy Z. S., Wang Y., Erwin-Cohen R. A., Aradi J., Monia B., Wang L. H., Stepkowski S. M., Rui H., Kirken R. A. (2002) Interleukin-2 family cytokines stimulate phosphorylation of the Pro-Ser-Pro motif of Stat5 transcription factors in human T cells: resistance to suppression of multiple serine kinase pathways. J. Leukoc. Biol. 72, 819–828 [PubMed] [Google Scholar]
- 46. Decker T., Kovarik P. (2000) Serine phosphorylation of STATs. Oncogene 19, 2628–2637 [DOI] [PubMed] [Google Scholar]
- 47. Boyman O., Sprent J. (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 [DOI] [PubMed] [Google Scholar]
- 48. Chai S. K., Nichols G. L., Rothman P. (1997) Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J. Immunol. 159, 4720–4728 [PubMed] [Google Scholar]
- 49. Weber-Nordt R. M., Egen C., Wehinger J., Ludwig W., Gouilleux-Gruart V., Mertelsmann R., Finke J. (1996) Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood 88, 809–816 [PubMed] [Google Scholar]
- 50. Takemoto S., Mulloy J. C., Cereseto A., Migone T. S., Patel B. K., Matsuoka M., Yamaguchi K., Takatsuki K., Kamihira S., White J. D., Leonard W. J., Waldmann T., Franchini G. (1997) Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. U.S.A. 94, 13897–13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gerdes J., Schwab U., Lemke H., Stein H. (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20 [DOI] [PubMed] [Google Scholar]
- 52. Cone R. E., Cochrane R., Lingenheld E. G., Clark R. B. (1996) Elevation of intracellular cyclic AMP induces an anergic-like state in Th1 clones. Cell Immunol. 173, 246–251 [DOI] [PubMed] [Google Scholar]
- 53. Kirken R. A., Rui H., Malabarba M. G., Howard O. M., Kawamura M., O'Shea J. J., Farrar W. L. (1995) Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor β-chain. Cytokine 7, 689–700 [DOI] [PubMed] [Google Scholar]
- 54. Taskén K., Stokka A. J. (2006) The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem. Soc. Trans. 34, 476–479 [DOI] [PubMed] [Google Scholar]
- 55. Sadana R., Dessauer C. W. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17, 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ivashkiv L. B. (2008) A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Rev. Immunol. 8, 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kammer G. M., Boehm C. A., Rudolph S. A., Schultz L. A. (1988) Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc. Natl. Acad. Sci. U.S.A. 85, 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bopp T., Becker C., Klein M., Klein-Hessling S., Palmetshofer A., Serfling E., Heib V., Becker M., Kubach J., Schmitt S., Stoll S., Schild H., Staege M. S., Stassen M., Jonuleit H., Schmitt E. (2007) Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 204, 1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nokta M. A., Pollard R. B. (1992) Human immunodeficiency virus replication: modulation by cellular levels of cAMP. AIDS Res. Hum. Retroviruses 8, 1255–1261 [DOI] [PubMed] [Google Scholar]
- 60. Nokta M., Pollard R. (1991) Human immunodeficiency virus infection: association with altered intracellular levels of cAMP and cGMP in MT-4 cells. Virology 181, 211–217 [DOI] [PubMed] [Google Scholar]
- 61. Antonysamy M. A., Moticka E. J., Ramkumar V. (1995) Adenosine acts as an endogenous modulator of IL-2-dependent proliferation of cytotoxic T lymphocytes. J. Immunol. 155, 2813–2821 [PubMed] [Google Scholar]