Background: CHIP is a U-box E3 ubiquitin ligase that facilitates the proteasomal degradation of many client proteins.
Results: Ca2+/S100 proteins directly interact with CHIP and suppress the ubiquitination and degradation of the client proteins.
Conclusion: We have identified S100 proteins as novel Ca2+-dependent regulators of the CHIP-proteasome pathway.
Significance: This is the first indication that S100 proteins form a link between Ca2+ signal transduction and the CHIP-proteasome pathway.
Keywords: Calcium-binding Proteins, Protein Degradation, S100 Proteins, Ubiquitin Ligase, Ubiquitination, CHIP, TPR Domain
Abstract
The U-box E3 ubiquitin ligase CHIP (C terminus of Hsc70-interacting protein) binds Hsp90 and/or Hsp70 via its tetratricopeptide repeat (TPR), facilitating ubiquitination of the chaperone-bound client proteins. Mechanisms that regulate the activity of CHIP are, at present, poorly understood. We previously reported that Ca2+/S100 proteins directly associate with the TPR proteins, such as Hsp70/Hsp90-organizing protein (Hop), kinesin light chain, Tom70, FKBP52, CyP40, and protein phosphatase 5 (PP5), leading to the dissociation of the interactions of the TPR proteins with their target proteins. Therefore, we have hypothesized that Ca2+/S100 proteins can interact with CHIP and regulate its function. GST pulldown assays indicated that Ca2+/S100A2 and S100P bind to the TPR domain and lead to interference with the interactions of CHIP with Hsp70, Hsp90, HSF1, and Smad1. In vitro ubiquitination assays indicated that Ca2+/S100A2 and S100P are efficient and specific inhibitors of CHIP-mediated ubiquitination of Hsp70, Hsp90, HSF1, and Smad1. Overexpression of S100A2 and S100P suppressed CHIP-chaperone complex-dependent mutant p53 ubiquitination and degradation in Hep3B cells. The association of the S100 proteins with CHIP provides a Ca2+-dependent regulatory mechanism for the ubiquitination and degradation of intracellular proteins by the CHIP-proteasome pathway.
Introduction
Ca2+ signaling plays a pivotal role in regulating various cellular responses, including cell metabolism, cytoskeletal dynamics, the cell cycle, gene expression, neurotransmission, and intracellular signal transduction processes (1, 2). The signal-induced change in the intracellular free Ca2+ concentration has been portrayed as a switch through a class of Ca2+ sensor proteins containing a specific Ca2+ binding motif called the EF-hand (3). Among such proteins, it is well known that calmodulin (CaM),2 as the prototypical Ca2+ sensor, is involved in many aspects of Ca2+ regulation systems in various cell types (4). The S100 proteins, as evolutionary latecomers, occur only in vertebrates. The S100 protein family is composed of at least 25 members that share two EF-hand motifs and 25–65% amino acid sequence identity with a molecular mass of 10–12 kDa (5). S100 proteins are proposed to have intracellular and extracellular roles in the regulation of many cellular processes such as cell motility, cell cycle progression, transcription, protein phosphorylation, and tumor progression or suppression (5–10). However, the precise intracellular roles of the S100 proteins are not fully understood because the target proteins of individual S100 proteins have not been completely identified.
We have recently reported that S100A2 and S100A6 interact with the tetratricopeptide repeat (TPR) domains of Hsp70/Hsp90-organizing protein (Hop), kinesin light chain (KLC), and Tom70 in a Ca2+-dependent manner, leading to the dissociation of the Hsp90-Hop-Hsp70, KLC-JIP1, and Tom70-Hsps interactions both in vitro and in vivo (11). Ca2+/S100A1 and S100A2 bind to the TPR domains of FK506-binding protein 52 (FKBP52) and cyclophilin 40 (CyP40) and lead to the inhibition of the FKBP52-Hsp90 and CyP40-Hsp90 interactions (12). In addition, Ca2+/S100A1, S100A2, S100A6, and S100B bind to the TPR domains of protein phosphatase 5 (PP5) and lead to the inhibition of the PP5-Hsp90 interaction (13). TPRs are loosely conserved, 34-amino acid helix-turn-helix sequence motifs that have been shown to mediate protein-protein interactions. This property enables TPR-containing proteins to work as scaffold proteins and allows them to be involved in a variety of cellular functions (14–17).
The C terminus of Hsc70-interacting protein (CHIP) was originally identified as a novel TPR-containing protein by means of screening a human heart cDNA library with a fragment coding for three TPR domains of CyP40 (18). CHIP also contains a U-box domain for accepting an E2 ubiquitin-conjugating enzyme (e.g. UbcH5) and functions as a multisubunit E3 ubiquitin ligase complex (19–21). Thus, CHIP functions as both a co-chaperone and an E3 ubiquitin ligase and serves as a molecular link between cellular protein folding and degradation. For selective ubiquitination, the proper selection of target proteins by E3 ligase is essential (22, 23). The TPR domain of CHIP plays a central role in the selection of target proteins. In the case of chaperone-dependent ubiquitination, CHIP associates with the molecular chaperones Hsp70/Hsc70 and Hsp90 through the TPR domain and ubiquitinates chaperone-bound target proteins. For instance, GR (24), tumor suppresser p53 (25), ErbB-2 (26), CYP2E1 (27), and CYP3A4 (28) are ubiquitinated by CHIP-Hsp90 and CHIP-Hsc70 complexes, leading to proteasomal degradation. Alternatively, the TPR domain of CHIP directly interacts with some target proteins and conjugates of ubiquitin. It has been reported that HSF1 (29) and Smad1 (30, 31) are ubiquitinated by CHIP independent of chaperones.
Because S100 proteins interact with TPR motifs (11–13), we explored the potential for S100 proteins to regulate CHIP functions. We found that S100A2 and S100P interact with CHIP and inhibit the ubiquitination of Hsp70, Hsp90, HSF1, and Smad1. In addition, we found that S100A2 and S100P suppress ubiquitination and degradation of mutant p53 in intact cells in a Ca2+-dependent manner; thus, free intracellular Ca2+ transiently regulates the ubiquitination and proteasomal degradation of intracellular proteins.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were used in this study: anti-Smad1 (R&D Systems), anti-S100A2 (R&D Systems), anti-S100P (R&D Systems), anti-S100A12 (R&D Systems), anti-HSF1 (StressGen Biotechnologies), anti-Hsp70 (StressGen Biotechnologies), anti-Hsp90 (StressGen Biotechnologies), anti-β-actin (Santa Cruz Biotechnology), anti-CHIP (Santa Cruz Biotechnology), anti-CHIP (clone EPR4448, Epitomics Inc.), anti-S100A6 (Epitomics Inc.), anti-UbcH5 (Boston Biochem), anti-FLAG (Sigma Aldrich), anti-p53 (clone DO-1, Santa Cruz Biotechnology), and anti-ubiquitin (clone FK2, BIOMOL International).
Plasmids
pET16b-Hsp70, pET16b-Hsp90, pET11a-CaM, pET11a-S100s, and pME18S-S100s plasmids were previously described (11, 12). Human CHIP cDNA was purchased from Open Biosystems and subcloned into pGEX4T2, pQE80L, and pME18S. CHIP deletion mutants (TPR, residues 1–197; U-box, residues 198–303) and point mutants (K30A, H260Q, and P269A) were cloned into pGEX4T2. Smad1 and UbcH5a were amplified by PCR from a human cDNA library and cloned into pQE80L. pcDNA3.1-HSF1 was kindly provided by Dr. Hideaki Itoh (Akita University) and subcloned into pPROExHTb. Human p53 from a cDNA library was cloned into pME18S-FLAG, and FLAG-p53R175H was generated by inverse PCR.
Preparation of Recombinant Proteins
All recombinant proteins were produced in Escherichia coli strain BL21 (DE3) or BL21 (DE3) CodonPlus-RIL (Novagen). S100 proteins (S100A1, S100A2, S100A4, S100A6, S100A10, S100A11, S100A12, S100B, and S100P) were expressed in a tag-free fashion and prepared as described previously (32, 33). CaM was prepared as described by N. Hayashi et al. (34). Glutathione S-transferase (GST) fusions (CHIP and its derivatives) were purified using glutathione-Sepharose 4B (GE Healthcare) according to the manufacturer's instructions. His-tagged proteins (Hsp90, Hsp70, HSF1, Smad1, UbcH5a, and CHIP) were purified by nickel-nitrilotriacetic acid-agarose (Qiagen) according to the protocols of manufacturer.
GST Pulldown Assay
To assess the binding of S100 proteins to CHIP, the Ca2+-binding proteins (S100 proteins or CaM: 20 μg each), GST-CHIP (20 μg), and glutathione-Sepharose 4B (30 μl) were mixed in Buffer A (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.02% Tween 20) in the presence of 1 mm CaCl2 or EGTA. The reaction mixtures (200 μl) were incubated for 60 min at 25 °C. After the resin was washed three times with 1.0 ml of Buffer A, the resin was boiled in SDS-sample buffer (30 μl). The eluted samples were subjected to Tricine-SDS-PAGE and visualized by Coomassie Blue staining.
To examine the effects of the S100 proteins on the interactions of CHIP with its client proteins (Hsp90, Hsp70, Smad1, HSF1, and UbcH5a), GST-CHIP (20 μg), S100 proteins (20 μg), appropriate client proteins (20–40 μg), and glutathione-Sepharose 4B (30 μl) were mixed in Buffer A with 1 mm CaCl2 or EGTA. The eluted samples were subjected to SDS-PAGE, and bound client proteins were visualized by Western blotting using appropriate antibodies.
In Vitro Ubiquitination Assay
In vitro ubiquitination assays were performed according to the method of Murata et al. (35). The reaction mixture (40 μl) containing 0.1 μm E1 (BIOMOL International), 2.4 μm UbcH5a, 4 μm His6-tagged CHIP, and 25 μm ubiquitin (BIOMOL International) in a reaction buffer (20 mm Tris-HCl, pH 7.5, 20 mm KCl, 5 mm dithiothreitol, 5 mm MgCl2, and 5 mm ATP) with 1 mm CaCl2 or EGTA was incubated for 2 h at 30 °C. The additional proteins used are described in the figure legends. The reaction was terminated by the addition of SDS sample buffer (40 μl) and boiled for 5 min at 95 °C. The samples were analyzed by Western blotting with appropriate antibodies.
Cell Culture and Transfection
Huh-7 and Hep3B cells were incubated in Dulbecco's modified Eagle's medium (Sigma), and MKN-45 cells were incubated in RPMI 1640 medium (Sigma). Both media were supplemented with 10% fetal bovine serum (Invitrogen). Cells were cultured at 37 °C under a 5% CO2 humidified atmosphere. Transient transfections were performed using Lipofectamine LTX and Plus reagent (Invitrogen) according to the manufacturer's instructions.
Immunoprecipitation and Western Blotting
Cells were washed once with PBS and lysed in a buffer consisting of 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, and 0.5% Nonidet P-40 with protease inhibitor mixture (Roche Applied Science). Lysates were then sonicated and centrifuged at 14,000 rpm for 10 min. For immunoprecipitation, 200 μg of cell extract was incubated with 5 μg of antibody and 30 μl of protein G-Sepharose beads in the presence of 1 mm CaCl2 or EGTA for 1 h at room temperature. The beads were washed three times in buffer A with 1 mm CaCl2 or EGTA. The samples were resolved by SDS-PAGE, generally using Tris-Tricine gels, and were detected by Western blotting.
In Vivo Ubiquitination Assay
In vivo ubiquitination assays were performed as described by Urushitani et al. (36). Hep3B cells (35-mm dishes) were transiently transfected with FLAG-p53R175H (0.3 μg), CHIP (0.1 μg), and S100s (S100A2 or S100P or S100A12, 0.2 μg). The total amount of DNA was kept constant at 0.6 μg/dish by the addition of pME18S. After 2 days, the transfected cells were treated with or without 1 μm A23187 for 6 h. After the incubation, the cells were lysed with 0.5 ml of radioimmune precipitation buffer (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 2 mm EDTA, 1% Nonidet-P40, 0.1% SDS, 0.5% sodium deoxycholate). Cell lysates were incubated with anti-FLAG antibody and protein G-Sepharose (GE Healthcare) overnight at 4 °C. The beads were washed four times in radioimmune precipitation buffer and eluted in SDS sample buffer by boiling for 5 min. Ubiquitination of FLAG-p53R175H was evaluated by Western blotting with an anti-ubiquitin antibody.
In Vivo Degradation Assay
In vivo degradation assays were performed as described by Esser et al. (25). Hep3B cells (35-mm dish) were transiently transfected with FLAG-p53R175H (0.3 μg), CHIP (0.1 μg), and S100A2 or S100P or S100A12 (0.2 μg each). The total amount of DNA was kept constant at 0.6 μg/dish by the addition of pME18S. After 24 h, the cells were treated with cycloheximide (60 μg/ml), with or without 1 μm A23187. Cell lysates were prepared at time points 0, 3, and 6 h, and p53R175H levels were determined by Western blotting.
RESULTS
Interaction of CHIP with S100 Proteins
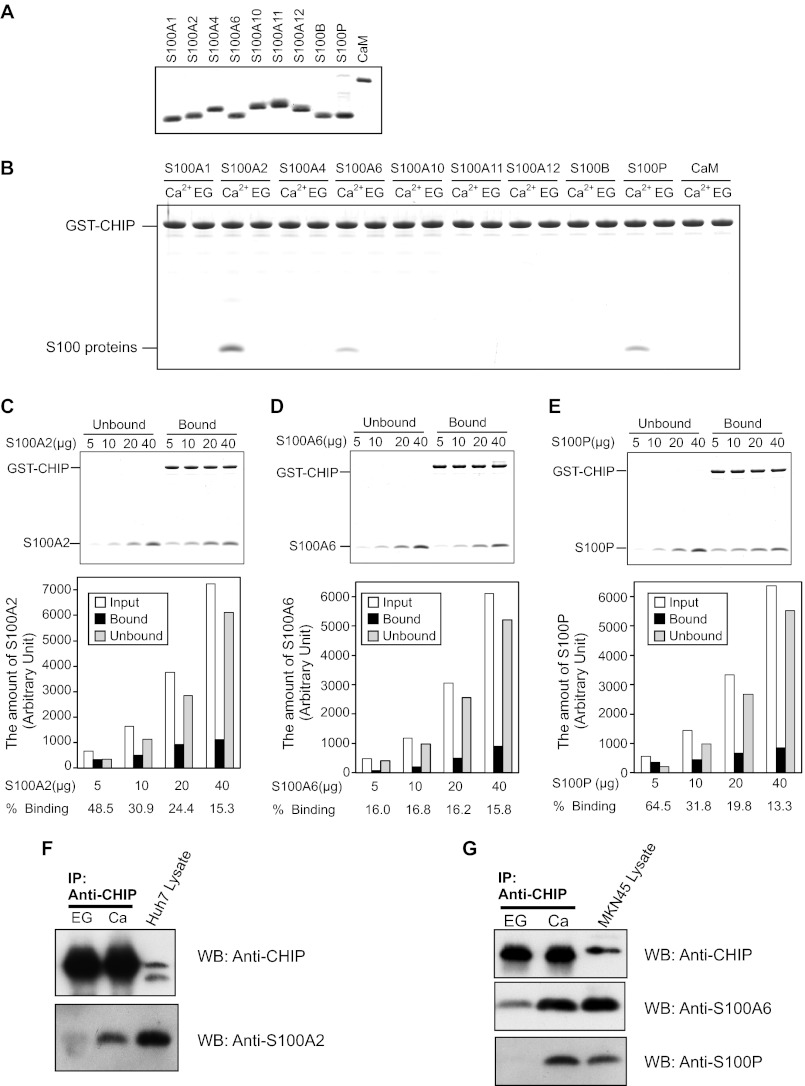
Previously, we demonstrated that S100A1, S100A2, and S100A6 interact with the TPR domains of Hop, Tom70, KLC, CyP40, FKBP52, and PP5 in a Ca2+-dependent manner (11–13). To examine the hypothesis that S100 proteins interact with CHIP, we tested whether CHIP directly binds S100 proteins in vitro by GST pulldown assays in the presence of 1 mm CaCl2 or EGTA. Both GST-CHIP and S100 proteins were expressed in bacteria and purified to near homogeneity (Fig. 1A). As shown in Fig. 1B, S100A2, S100A6, and S100P strongly bound to immobilized GST-CHIP in a Ca2+-dependent manner. In contrast, S100A1, S100A4, S100A10, S100A11, S100A12, S100B, and CaM did not bind to GST-CHIP. For semiquantitative evaluation of S100 protein binding to GST-CHIP, a fixed amount of GST-CHIP (20 μg) was mixed with increasing amounts of S100 proteins (5, 10, 20, and 40 μg), and then pulldown was carried out (Fig. 1, C–E). The percentage of binding of S100A2 (24.4%), S100A6 (16.2%), and S100P (19.8%) has shown that S100 proteins adequately bound to GST-CHIP under the same condition as the experiment in Fig. 1B.
FIGURE 1.
Interaction of CHIP and S100 proteins. A, SDS-PAGE of S100 proteins and CaM used in the binding assays (2.5 μg/lane) are shown. B, GST pulldown assay was performed using wild-type GST-CHIP and the S100 proteins. The S100 proteins (20 μg) and GST-CHIP (20 μg) were incubated with glutathione-Sepharose 4B in Buffer A with 1 mm CaCl2 (Ca2+) or EGTA (EG). After agitation at 25 °C for 60 min, the beads were washed and then eluted with SDS-sample buffer. The eluted samples were analyzed by 10% Tricine-SDS-PAGE. The gel was stained with Coomassie Brilliant Blue. C–E, increasing amounts of S100A2 (C), S100A6 (D), and S100P (E) were mixed with GST-CHIP (20 μg), and pulldown assays were performed in the presence of 1 mm CaCl2. Unbound and bound proteins were analyzed by 10% Tricine-SDS-PAGE (upper panel). The gels were scanned, and arbitrary densitometric values (in pixels) were obtained. The amounts of S100 proteins (Input, Unbound, and Bound) are plotted. Percentages of bindings of the S100 proteins to GST-CHIP are listed below each bar (lower panel). F and G, Huh-7 lysates (F) and MKN-45 lysates (G) were immunoprecipitated with anti-CHIP antibody in the presence of 2 mm CaCl2 (Ca) or EGTA (EG). Immunoprecipitates were analyzed by Western blotting (WB) with the indicated antibodies.
To further confirm the interaction of CHIP with S100A2 in cultured cells, co-immunoprecipitation experiments were performed with Huh-7 lysates, which contained endogenous CHIP and S100A2. For the detection of the interaction of CHIP with S100A6 and S100P, MKN-45 cells were used. When the cell lysates were subjected to co-immunoprecipitation with anti-CHIP monoclonal antibody, endogenous CHIP was precipitated in the presence of either Ca2+ or EGTA (Fig. 1, F and G). In the presence of Ca2+, S100A2, S100A6, and S100P were co-precipitated with endogenous CHIP. Small amounts of S100A2 and S100A6 were precipitated with CHIP in the presence of EGTA. These results demonstrate that S100A2, S100A6, and S100P bind to CHIP in a Ca2+-dependent manner in cultured cells.
Interaction Sites for S100 Proteins Are Located in Both the TPR and the U-box Domains of CHIP
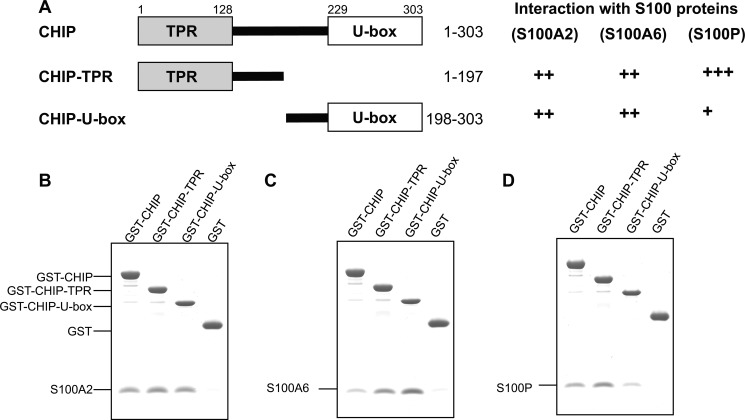
CHIP consists of two structural and functional domains, an N-terminal TPR domain and a C-terminal U-box domain for E2 enzyme binding. The domain structure of CHIP is shown in Fig. 2A. To determine the S100 binding domain in CHIP, we prepared truncation mutants (i.e. TPR and U-box) and assessed them for their interaction with the S100 proteins. As shown in Fig. 2, B and C, S100A2 and S100A6 equally bound to the TPR and U-box domains of CHIP. In contrast, S100P preferentially interacted with TPR and slightly bound to U-box (Fig. 2D). These S100 proteins did not bind to immobilized GST. The results suggest that S100A2, S100A6, and S100P bound to both TPR and U-box domains with different affinities.
FIGURE 2.
Interactions of S100 proteins with CHIP and its deletion mutants. A, schematic diagrams depict a series of CHIP deletion mutants, and a summary of their interaction with S100 proteins is also listed on the right. The numbering refers to amino acid positions in CHIP. B–D, GST-CHIP, -TPR, -U-box, and control GST were assayed for S100A2 (B), S100A6 (C), and S100P (D) binding with 1 mm CaCl2. The assay was performed as described in the legend for Fig. 1.
CHIP Lys-30 and Pro-269 Are Involved in the CHIP-S100 Protein Interactions
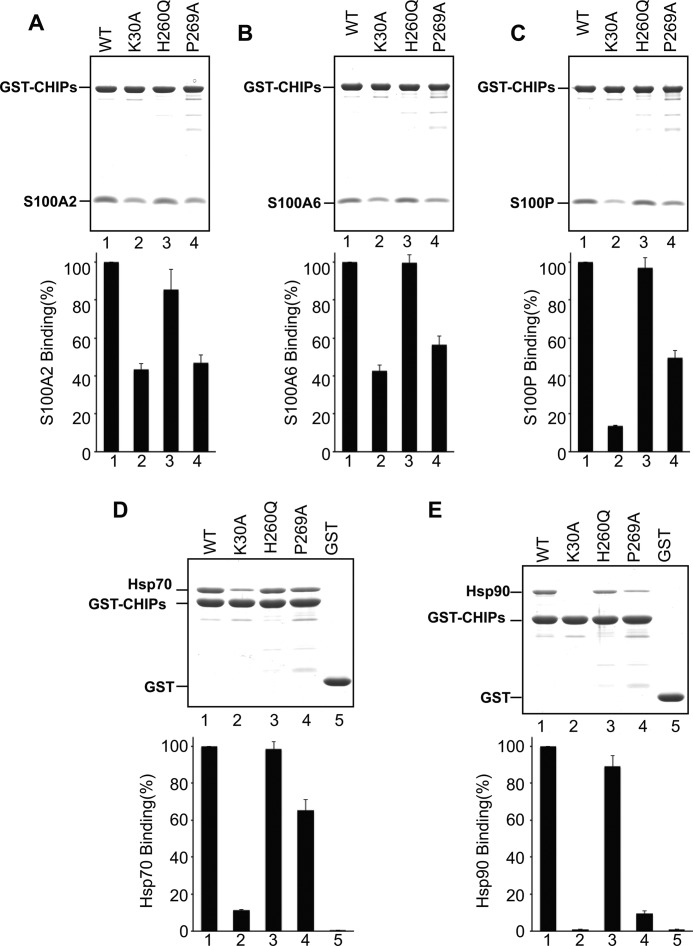
To further explore the interaction between CHIP and S100 proteins, we investigated whether S100 proteins bind to the same Hsp70/Hsp90 and E2 ligase acceptor sites on CHIP. Introducing a K30A mutation into the CHIP TPR domain renders CHIP unable to bind Hsp70 and Hsp90 (26). H260Q mutation and P269A mutation in the U-box render CHIP unable to bind its cognate E2 ligase and therefore to be inactive as an E3 ligase (19, 26).
We assessed the interaction between CHIP-K30A, -H260Q, and -P269A mutants and S100 proteins (S100A2, S100A6, and S100P) by GST pulldown assays. As shown in Fig. 3, A–C, the binding of the S100 proteins was decreased in CHIP-K30A and CHIP-P269A. In contrast, the binding of the S100 proteins to CHIP-H260Q mutant was unchanged. These results suggest that Lys-30 and Pro-269 in CHIP are involved in the interaction with the S100 proteins. To confirm the binding of Hsp70 and Hsp90 to the CHIP mutants, similar GST pulldown assays were performed using bacterial expressed Hsp90 and Hsp70. As expected, K30A failed to interact with Hsp70 and Hsp90 (Fig. 3, D and E). Because CHIP-P269A slightly decreased binding to Hsp70 and Hsp90, we presumed that Pro-269 contributes to Hsps binding. Based on these results, we speculated that S100 proteins bind to CHIP through the same sites for Hsps binding.
FIGURE 3.
Interactions of CHIP point mutants with S100 proteins, Hsp70 and Hsp90. A–E, upper panel, GST pulldown assays were performed using GST-CHIP (WT) and its point mutants (K30A, H260Q, and P269A). GST-CHIPs or GST (20 μg each) were mixed with 20 μg of S100A2 (A), S100A6 (B), S100P (C), Hsp70 (D), and 40 μg Hsp90 (E). The experiments were performed as described in the legend for Fig. 1. The samples were subjected to SDS-PAGE and visualized by Coomassie Brilliant Blue staining. Lower panel, the gels were scanned, and arbitrary densitometric values were obtained for S100 proteins and Hsps. The binding level of these proteins to GST-CHIP (lane 1) was designated as 100% (control), and the relative binding levels (percentage of control) were plotted. The error bars represent the S.E. with n = 3.
S100A2 and S100P Interfere with TPR-mediated CHIP-Client Protein Interactions
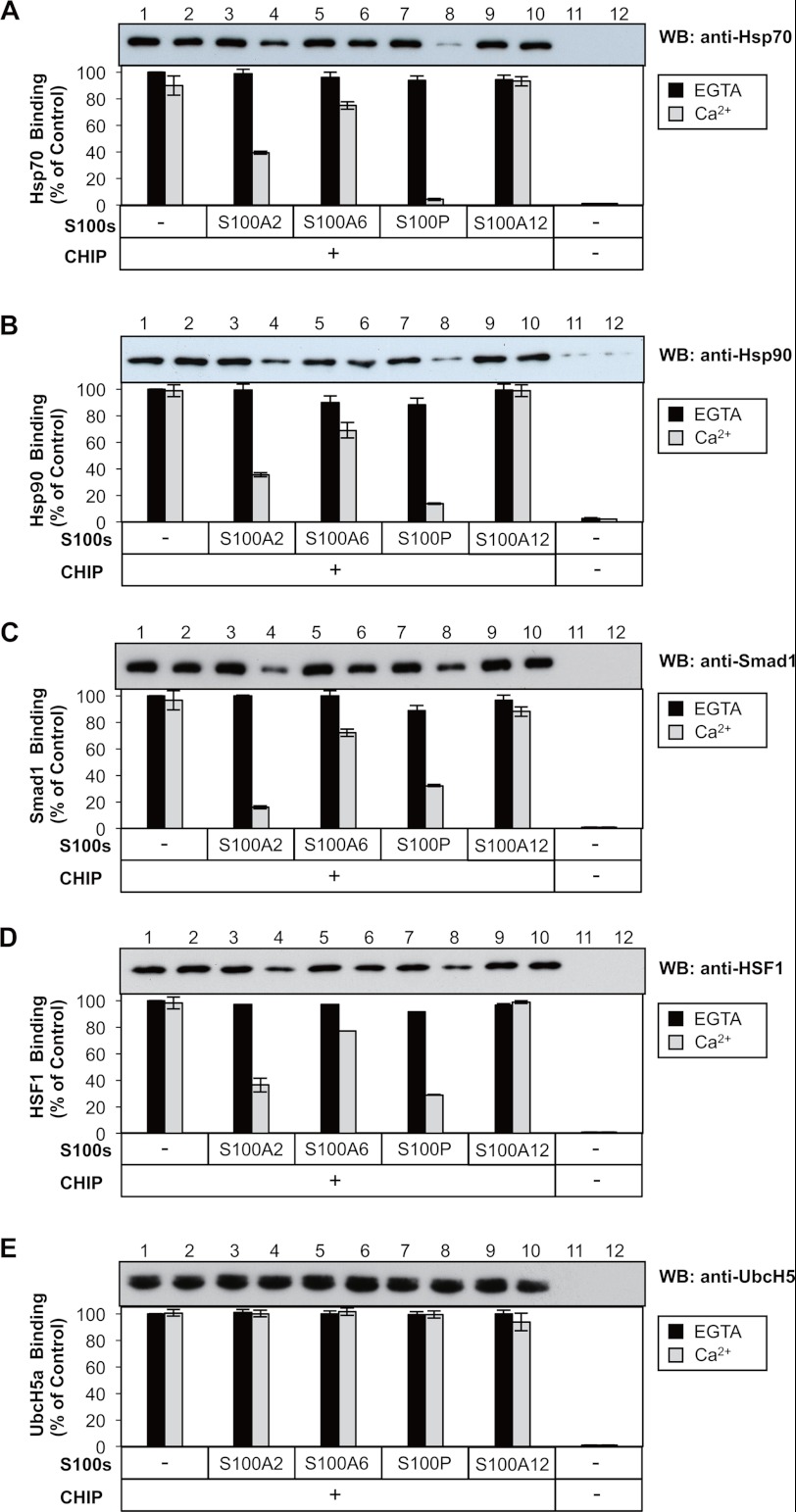
Because the S100 proteins and Hsp70/Hsp90 bind to the same domain of CHIP, we investigated whether the S100 proteins and Hsp70/Hsp90 compete in binding to CHIP. GST-CHIP was immobilized on an affinity resin and incubated with purified S100 proteins and Hsp70/Hsp90 in the presence of 1 mm CaCl2 or EGTA. Western blots were used to visually assess the displacement of the Hsp70/Hsp90 bindings by the S100 proteins (Fig. 4, A and B). In the presence of EGTA, the addition of purified S100 proteins to the binding reactions did not affect the amount of Hsp70 retained on immobilized GST-CHIP (Fig. 4A, lanes 1, 3, 5, 7, and 9). In the presence of CaCl2, the addition of S100A2 and S100P strongly reduced the amount of Hsp70 retained on immobilized GST-CHIP (Fig. 4A, lanes 4 and 8), whereas S100A6 exerted slight inhibitory effects (Fig. 4A, lane 6). No clear competition was observed with S100A12 as a negative control (Fig. 4A, lane 12). Similarly, S100A2 and S100P strongly interfered with the binding of Hsp90 to CHIP in a Ca2+-dependent manner, and S100A6 exerted slight inhibitory effects (Fig. 4B).
FIGURE 4.
Effects of S100 proteins on the CHIP-client protein interactions in vitro. A–E, upper panel, GST-CHIP (20 μg), S100 proteins (20 μg each) and appropriate client proteins were mixed with glutathione-Sepharose 4B in the presence of CaCl2 or EGTA. Control assay (CHIP−) was conducted using GST (20 μg). S100 proteins used were as follows: S100A2, S100A6, S100P, S100A12, and control (−). The client proteins were as follows: purified 20 μg of Hsp70 (A), 40 μg of Hsp90 (B), 20 μg of Smad1 (C), 20 μg of HSF1 (D), and 20 μg of UbcH5a (E). Details of the GST pulldown assay are described under “Experimental Procedures.” The resulting samples were subjected to SDS-PAGE and visualized by Western blotting (WB) using the indicated antibodies. Lower panel, the binding levels of these client proteins to CHIP without S100 proteins (lane 1) were designated as 100% (Control), and the relative binding levels (% of Control) were plotted. The error bars represent the S.E. with n = 3.
To ascertain whether the S100 proteins generally inhibit CHIP-client protein interactions or specifically compete with Hsp70/Hsp90 binding to CHIP, we examined the effects of S100 proteins on the binding of HSF1 and Smad1 to CHIP. It has been reported that these client proteins directly bind to the TPR domain of CHIP. As shown in Fig. 4, C and D, S100A2 and S100P effectively disrupted the CHIP-Smad1 and CHIP-HSF1 interactions in a Ca2+-dependent manner. No clear competition was observed with S100A6 and S100A12. Because the S100 proteins also bound to the U-box domain (Figs. 2 and 3), we tested whether S100 proteins interfere with the CHIP-UbcH5a interaction. As shown in Fig. 4E, none of the four S100 proteins influenced the CHIP-UbcH5a interaction. These results suggest that S100A2 and S100P bind to the TPR domain of CHIP and inhibit the interactions of CHIP with Hsp70, Hsp90, HSF1, and Smad1 in a Ca2+-dependent manner.
S100A2 and S100P Suppress CHIP-mediated Ubiquitination
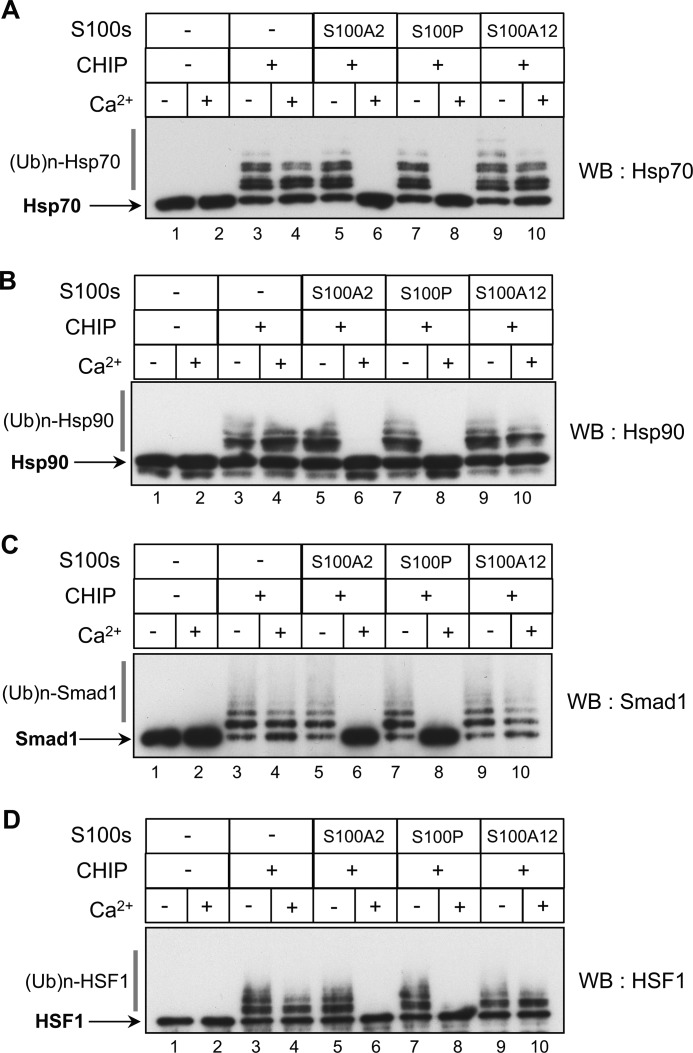
Next, we asked whether the association of S100 proteins with CHIP affected ubiquitination using a previously established in vitro assay (35). Because S100A6 only weakly inhibited the interactions of CHIP with its client proteins (Fig. 4), we focused on the effect of S100A2 and S100P on the CHIP-mediated ubiquitination. In this assay, CHIP mediated the ubiquitination of Hsp70 and Hsp90 in the presence of the ubiquitin-activating enzyme E1 and the ubiquitin-conjugating E2 enzyme UbcH5a. At first, we tested whether Ca2+ itself affected the CHIP-dependent ubiquitination of Hsp70 and Hsp90. As shown in Fig. 5, the levels of CHIP-mediated ubiquitination of Hsp70 (Fig. 5A, lanes 1–4) and Hsp90 (Fig. 5B, lanes 1–4) did not change in the presence or absence of Ca2+. The addition of purified S100A2 and S100P effectively inhibited the CHIP-mediated ubiquitination of Hsp70 (Fig. 5A, lanes 5–8) and Hsp90 (Fig. 5B, lanes 5–8) in a Ca2+-dependent fashion. S100A12, as a negative control, did not affect the ubiquitination (Fig. 5, A and B, lanes 9 and 10). Similarly, we conducted in vitro ubiquitination assays with Smad1 and HSF1 as client proteins. As is shown in Fig. 5, C and D, the CHIP-mediated ubiquitination of Smad1 and HSF1 was strongly suppressed by S100A2 and S100P in a Ca2+-dependent manner. These results suggest that S100 proteins interfere with the interaction of CHIP with Hsp70, Hsp90, HSF1, and Smad1 in a Ca2+-dependent manner and lead to inhibition of the ubiquitination of these client proteins.
FIGURE 5.
Effects of S100 proteins on CHIP-mediated ubiquitination in vitro. To test the effects of S100 proteins (S100s) on CHIP-mediated ubiquitination, in vitro ubiquitination assays were performed in the presence of 1 mm CaCl2 (+) or EGTA (−). The assay details are as described under “Experimental Procedures.” S100 proteins (12.5 μm) used were as follows: S100A2, S100P, S100A12, and control (−). The client proteins were as follows: A–D, purified 2 μm Hsp70 (A), 2 μm Hsp90 (B), 1.2 μm Smad1 (C), and 2 μm HSF1 (D). The samples were analyzed by Western blotting (WB) with the indicated antibodies. An arrowhead indicates unmodified Hsp70, Hsp90, Smad1, and HSF1. Ubiquitylated client proteins with a high molecular mass are shown as (Ub)n-Hsp70, (Ub)n-Hsp90, (Ub)n-Smad1, and (Ub)n-HSF1. The data shown in each panel are representative of three independent experiments.
S100A2 and S100P Are Substrates for the CHIP E3 Ligase
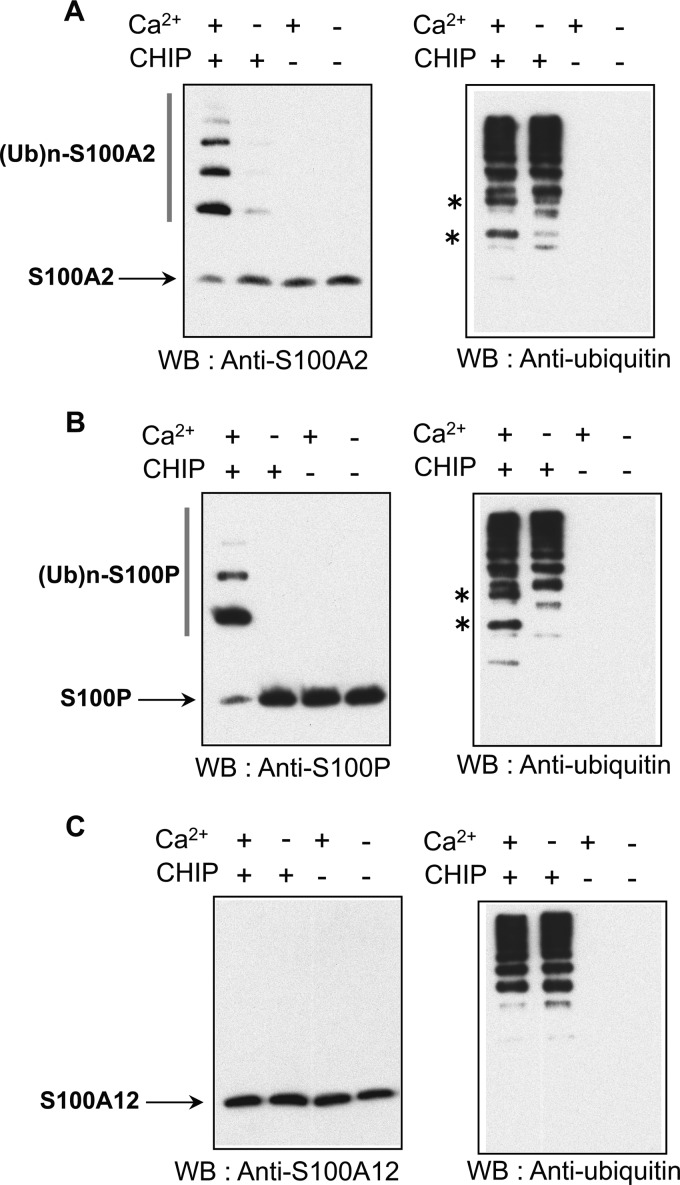
Because S100 proteins bind to the TPR domain of CHIP (Fig. 2) in a Ca2+-dependent manner, we tested whether S100 proteins are substrates of CHIP (Fig. 6). Purified S100A2, S100P, and S100A12 were subjected to in vitro ubiquitination assays with or without CHIP and CaCl2. The ubiquitinated proteins were analyzed by Western blotting with appropriate anti-S100 antibodies (left panels) and anti-ubiquitin antibodies (right panels). As shown in Fig. 6, A and B (left panels), ubiquitination of S100A2 and S100P was detected as high molecular mass bands by Western blotting in the presence of both CHIP and CaCl2. To further confirm the ubiquitination of the S100 proteins, we analyzed the same samples with anti-ubiquitin antibody. Ubiquitin-conjugated S100A2 and S100P were detected in the presence of both CHIP and CaCl2 as indicated with asterisks (Fig. 6, A and B, right panels). For S100A12, which did not associate with CHIP, ubiquitin conjugates were not detected (Fig. 6C, left and right panels). These data suggest that CHIP induces the ubiquitination of S100A2 and S100P in a Ca2+-dependent fashion.
FIGURE 6.
S100 protein itself is ubiquitinated by CHIP in a Ca2+-dependent manner. A–C, purified 10 μm S100A2 (A), S100P (B), and S100A12 (C) were subjected to in vitro ubiquitination assays with (+) or without (−) 1 mm CaCl2 (Ca2+) and CHIP. The details are described under “Experimental Procedures.” The samples were analyzed by Western blotting (WB) with anti-specific S100 antibodies (left panel) and anti-ubiquitin antibody (right panel) as indicated. Asterisks indicate significant ubiquitin conjugates of S100 proteins. Unmodified S100A2, S100P, and S100A12 are indicated by arrows. Ubiquitylated S100 proteins are shown as (Ub)n-S100A2 and (Ub)n-S100P. The data shown in each panel are representative of three independent experiments. (Ub)n-S100A2, ubiquitylated S100A2; (Ub)n-S100P, ubiquitylated S100P.
S100A2 and S100P Suppress the Chaperone-assisted Ubiquitination of Mutant p53 in Vivo
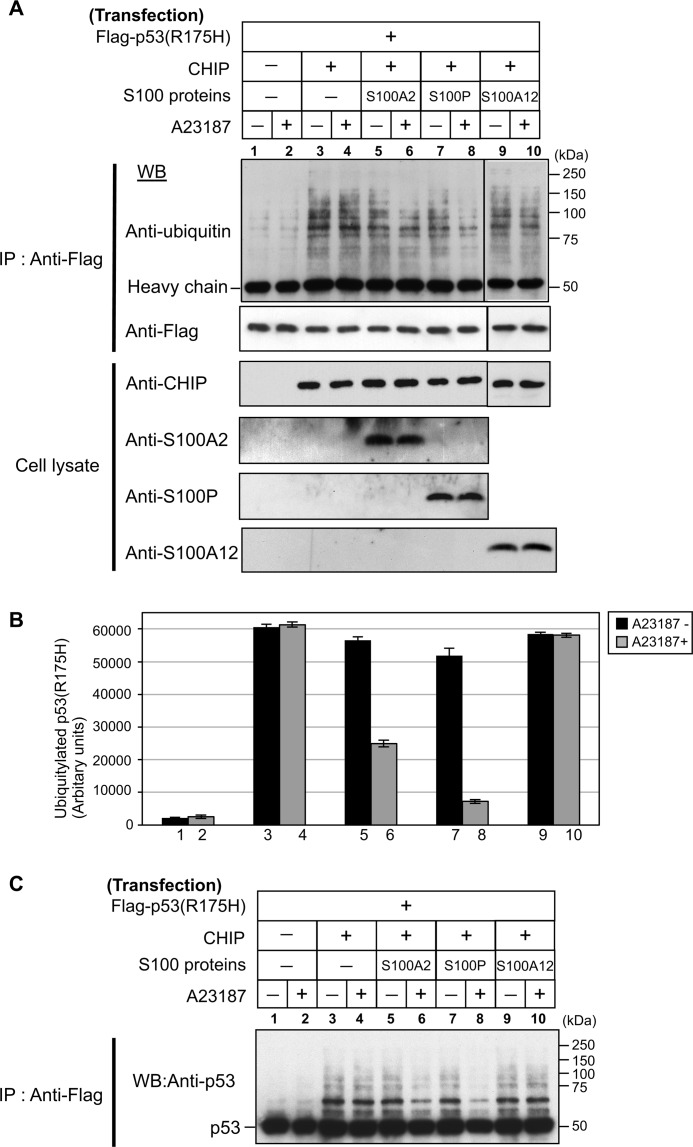
Through binding to the molecular chaperones, CHIP mediates the ubiquitination of chaperone-bound client proteins. For instance, mutant p53 (p53R175H) can have ubiquitination induced by the CHIP-chaperone complex (25). To study the cellular role of S100 proteins in the CHIP-mediated ubiquitination of client proteins, we performed in vivo ubiquitination experiments (Fig. 7). We co-transfected Hep3B cells (a p53-deficient cell line) with FLAG-p53R175H and CHIP with or without S100 proteins. The transfected cells were treated with or without ionophore A23187 (1 μm) for 6 h and immunoprecipitated with an anti-FLAG antibody from the total cell extracts. Western blotting showed that FLAG-p53R175H was equally precipitated in all samples (Fig. 7A, upper panel, lanes 1–10). To assess the levels of ubiquitin conjugations, the FLAG-p53R175H precipitates were analyzed by Western blotting with anti-ubiquitin antibody (Fig. 7A, uppermost panel) and quantified by densitometry (Fig. 7B). As shown in Fig. 7, A and B (lanes 1–4), transfected CHIP indeed induced p53R175H ubiquitination in Hep3B cells. The levels of the CHIP-mediated p53R175H ubiquitination did not change with A23187 treatment (Fig. 7, A and B, lanes 3 and 4). Overexpression of S100A2 itself had no clear effect on the ubiquitination level of p53R175H (Fig. 7, A and B, lane 5); however, A23187 treatment caused a significant decrease in the ubiquitination level (Fig. 7, A and B, lane 6). Similarly, elevation of S100P levels in Hep3B cells had no clear effect on p53R175H ubiquitination (Fig. 7, A and B, lane 7), whereas A23187 treatment markedly decreased p53R175H ubiquitination (Fig. 7, A and B, lane 8). In S100A12-overexpressing cells, A23187 treatment did not affect the p53R175H ubiquitination (Fig. 7, A and B, lanes 9 and 10). To ensure the validity of the above experiments, we further analyzed the FLAG-p53R175H precipitates by Western blotting with anti-p53 antibody. As shown in Fig. 7C, S100A2 and S100P indeed suppressed the CHIP-mediated ubiquitination of p53R175H in a Ca2+-dependent manner. These results indicate that S100A2 and S100P suppress the CHIP-mediated ubiquitination of p53R175H in a Ca2+-dependent manner.
FIGURE 7.
Ca2+/S100 proteins suppress the CHIP-mediated ubiquitination of mutant p53 in vivo. A, Hep3B cells were transiently transfected with (+) or without (−) FLAG-p53R175H, CHIP, S100A2, S100P, and S100A12. The transfected components of each dish are indicated on the top panels. Transfected Hep3B cells were treated with (+) or without (−) 1 μm A23187 for 6 h. Cell lysates were immunoprecipitated (IP) with anti-FLAG antibody. Details are described under “Experimental Procedures.” Lysates and immunoprecipitated proteins were detected by Western blotting (WB) with the indicated antibodies. Molecular mass markers are indicated on the right. B, the ubiquitination levels of p53R175H with A23187 (gray) or without A23187 (black) are plotted. The error bars represent the S.E. with n = 3. C, immunoprecipitated proteins were detected by Western blotting (WB) with anti-p53 antibody. Molecular mass markers are indicated on the right.
S100A2 and S100A6 Suppress the Chaperone-assisted Degradation of Mutant p53
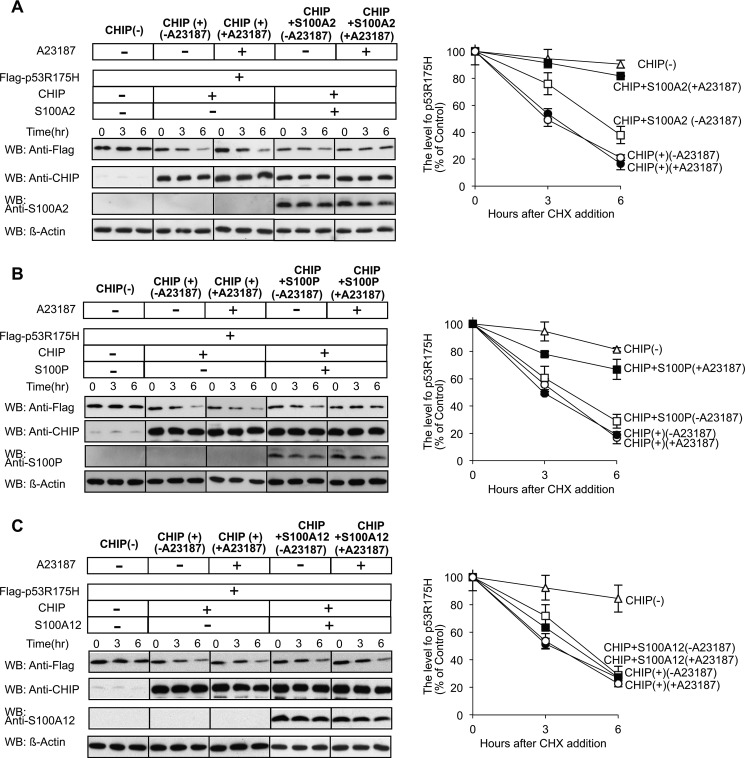
Next, we investigated the effect of the S100 proteins on p53R175H degradation (Fig. 8). We transiently co-transfected Hep3B cells with FLAG-p53R175H and CHIP with or without the S100 proteins and treated them with or without A23187 (1 μm). To estimate p53R175H levels, cell lysates were analyzed by Western blotting using anti-FLAG antibody (left panels) and quantified by a densitometric analysis (right panels). With elevation of the cellular levels of CHIP in Hep3B cells, a significant decrease of p53R175H levels was observed according to incubation time. In S100A2- (Fig. 8A) and S100P- (Fig. 8B) overexpressing cells without A23187 treatment, p53R175H levels were unchanged. However, the A23187 treatment suppressed the CHIP-mediated degradation of p53R175H. Co-transfection with S100A12 did not affect the rate of p53R175H degradation (Fig. 8C). These results suggest that S100A2 and S100P inhibit the CHIP-mediated ubiquitination and proteasomal degradation of p53R175H in Hep3B cells in a Ca2+-dependent manner.
FIGURE 8.
Ca2+/S100 proteins suppress the degradation of mutant p53 by the CHIP-chaperone complex. A–C, left panel, Hep3B cells were transiently transfected with FLAG-p53R175H, CHIP, S100A2 (A), S100P (B), and S100A12 (C) as indicated. The transfected components of each dish are indicated on the top panels. The cells were treated with cycloheximide (CHX), with (+) or without (−) A23187. Lysates were prepared at time points 0, 3, and 6 h. The amount of p53R175H, CHIP, and S100s was analyzed by Western blotting (WB) with the indicated antibodies. Equal amounts of protein were loaded for each time point, and β-actin served as a loading control. A–C, right panel, the level of p53R175H was quantified. The relative amount of each protein present at t = 0 is expressed as 100% of the control. The error bars represent the S.E. with n = 3.
DISCUSSION
CHIP has been shown to regulate Hsp70/Hsp90 function in part by regulating the ubiquitin-proteasome pathway and determining whether proteins enter the folding pathway or the degradation pathway. CHIP is implicated in various neurodegenerative diseases and cancers and is characterized by playing a crucial role in the protein quality control system (37–39). For example, CHIP can facilitate the chaperone-dependent degradation of a number of neurodegenerative proteins (36, 40–43) and oncogenic proteins (44–47). To date, a linkage between intracellular signal transduction pathways and the CHIP-proteasome pathway has not been demonstrated. Here, we report the identification of S100 proteins, a class of intracellular Ca2+ signaling proteins, as CHIP-interacting proteins and provide evidence that they can regulate the ubiquitination and proteasome-dependent degradation of CHIP client proteins.
TPR proteins are involved in many protein-protein interactions (14–17); in particular, several co-chaperones, including Hop (48), Tom70 (49), CyP40 (50), FKBP52 (51), and PP5 (52), interact with Hsp70 or Hsp90 through TPR domains. Based on recent work on co-chaperones, we have postulated the existence of a S100-TPR pathway in which S100 proteins are Ca2+-dependent regulators of the chaperone/co-chaperone interaction (11–13). To further explore the relationship between S100 proteins and TPR proteins, we have focused on CHIP. In vitro binding studies and in vivo co-immunoprecipitation experiments showed that S100A2, S100A6, and S100P directly interact with CHIP in a Ca2+-dependent manner (Fig. 1). The attachments of polyubiquitin chains to S100A2 and S100P further indicated a direct interaction between CHIP and the S100 proteins (Fig. 6). In vitro binding studies using truncation mutants demonstrated that S100A2, S100A6, and S100P bound to both TPR and U-box domains with different affinities (Fig. 2). We studied the effect of S100 proteins on the interaction of CHIP with TPR-mediated client proteins (Hsp70, Hsp90, Smad1, and HSF1) and a U-box-mediated protein (UbcH5a). In vitro competition assays showed that S100A2 and S100P Ca2+-dependently inhibited the interaction of CHIP with Hsp70, Hsp90, HSF1, and Smad1; however, Ca2+/S100A6 slightly affected the interaction of these client proteins with CHIP (Fig. 4). We tested the effect of the S100 protein on the CHIP-UbcH5a interaction; however, no S100 proteins (including S100A6) had an influence on the interactions. The functional role of S100A6 in the CHIP machinery is unclear at present. From these results, we conclude that S100A2 and S100P interfere with the interaction of CHIP with the TPR-mediated client proteins in a Ca2+-dependent manner.
The C-terminal regions of Hsp70 and Hsp90, both of which terminate with the amino acid sequence EEVD, are involved in the interaction with the CHIP-TPR domains. Crystallographic studies (PDB no. 2C2L) have shown that the C terminus of the Hsp90 peptide makes polar interactions with basic amino acid Lys-30 on the surface of the CHIP TPR domain (53) and that the K30A mutation abolishes the CHIP-Hsp70/Hsp90 interactions (26). We showed that the bindings of the S100 proteins and Hsp70/Hsp90 to CHIP K30A were decreased (Fig. 3). Because S100A2 and S100P disrupted the CHIP-Hsp70/Hsp90 interaction, we speculated that these S100 proteins and Hsp70/Hsp90 compete with each other for CHIP interaction. Consistent with this hypothesis, Hsp70/Hsc70 and Smad1 compete for CHIP at Lys-30 (30). In the complex of the CHIP U-box and the E2 enzyme, the motif SPA (residues 94–96 of UbcH5a) makes a hydrogen bond with the carbonyl group of Pro-269 and van der Waals contacts with His-260 (54, 55). We showed that S100A2, S100A6, and S100P also bound to the U-box (Fig. 2), and the binding affinities of the S100 proteins and Hsp70/Hsp90 to CHIP P269A (but not to H260Q) were reduced (Fig. 3). We have assumed that Pro-269 contributes to the interaction of the S100 proteins and Hsps with CHIP-U-box. Consistent with the present results, Ballinger et al. (18) suggested that the TPR domain of CHIP is necessary, but not sufficient, for binding with Hsp70. Collectively, we conclude that the S100 proteins and Hsps compete at identical sites (i.e. Lys-30 and Pro-269).
For selective ubiquitination, the proper selection of target proteins by ubiquitin ligase is essential (22, 23). The TPR domain of CHIP plays a critical role as an adaptor for Hsp70/Hsp90 binding and as a client selector. We studied the effect of S100 proteins on the ubiquitination of Hsp70, Hsp90, HSF1, and Smad1 (Fig. 5). In accordance with in vitro competition assays, S100A2 and S100P strongly inhibited the ubiquitination of these proteins in vitro in a Ca2+-dependent manner. These results indicate that S100 proteins can modulate the ubiquitin-ligase function of CHIP by competing with the binding of CHIP and its client proteins.
HSF1 is a major transactivator of heat shock genes in response to stress and mediates cell protection against various harmful conditions (56). Smad1 is a member of the transforming growth factor β (TGF-β) superfamily signaling pathway that regulates many important biological processes, including cell growth, differentiation, apoptosis, and specification of developmental fate (57). S100 proteins such as S100A2 and S100P could modulate the cellular levels of these client proteins by CHIP and may exert influence on their biological processes.
p53 missense mutant proteins commonly show increased stability when compared with wild-type p53, which is thought to depend on the inability of mutant p53 to induce the ubiquitin ligase MDM2. However, Muller et al. (58) demonstrated a major role for CHIP in the degradation of unfolded p53 mutants, with little or no roles for CHIP in degrading wild-type p53. The CHIP-Hsp70 complex promotes the ubiquitination of Hsp70-bound p53R175H. In cultured cells, overexpression of S100A2 and S100P with A23187 treatment showed a significant decrease of CHIP-mediated p53R175H ubiquitination (Fig. 7). Experiments using the cycloheximide chase assay further confirmed that S100A2 and S100P indeed suppressed CHIP-dependent degradation of p53R175H in vivo by A23187 stimulation (Fig. 8). These observations indicate that increased intracellular Ca2+ stimulated the binding of S100 proteins to CHIP, disrupted the CHIP-Hsp70 interaction, and led to suppression of the ubiquitination and degradation of p53R175H in vivo.
Our results strongly suggest that the S100 proteins can modulate CHIP-mediated ubiquitination and proteasomal degradation through the regulation of CHIP and its client protein interactions in response to intracellular Ca2+ signaling. Considering the potent protein quality control effect of CHIP, it is not surprising that its activity is highly regulated. Several regulators of CHIP activity have already been described. For example, the Hsp70 nucleotide exchange factors BAG-1, BAG-2, and HspBP1 have been shown to regulate the degradation of polypeptides bound by the chaperone. BAG-1 interacts with the proteasome through its ubiquitin-like (UBL) domain and thus mediates the docking of a CHIP-chaperone complex at the proteasome, facilitating proteasomal degradation (59). In contrast, BAG-2 and HspBP1 suppress CHIP-mediated ubiquitination and degradation by distinct mechanisms; BAG-2 abrogates CHIP-E2 cooperation (60, 61), whereas HspBP1 seems to induce a conformational change in CHIP-chaperone complexes and shields Hsc70-bound client proteins from the ubiquitin attachment site of CHIP (62). Thus, S100A2 and S100P present an entirely new mechanism for the regulation of the CHIP-proteasome pathway. In conclusion, we have identified S100A2 and S100P as novel Ca2+-dependent regulators of the CHIP-proteasome pathway.
Acknowledgments
We thank Dr. Hideaki Itoh (Akita University) for kindly providing plasmids. We thank Keiko Tsurumi (Kagawa University) for excellent technical assistance.
This research was supported by the Kagawa University Characteristic Prior Research Fund 2011.
- CaM
- calmodulin
- TPR
- tetratricopeptide repeat
- CHIP
- C terminus of Hsc70-interacting protein
- Hop
- Hsp70/Hsp90-organizing protein
- FKBP52
- FK506-binding protein 52
- CyP40
- cyclophilin 40
- PP5
- protein phosphatase 5
- KLC
- kinesin light chain
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- GR
- glucocorticoid receptor.
REFERENCES
- 1. Clapham D. E. (2007) Calcium signaling. Cell 131, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 2. Bennett M. K. (1997) Ca2+ and the regulation of neurotransmitter secretion. Curr. Opin. Neurobiol. 7, 316–322 [DOI] [PubMed] [Google Scholar]
- 3. Grabarek Z. (2006) Structural basis for diversity of the EF-hand calcium-binding proteins. J. Mol. Biol. 359, 509–525 [DOI] [PubMed] [Google Scholar]
- 4. Chin D., Means A. R. (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10, 322–328 [DOI] [PubMed] [Google Scholar]
- 5. Santamaria-Kisiel L., Rintala-Dempsey A. C., Shaw G. S. (2006) Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 396, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckert R. L., Broome A. M., Ruse M., Robinson N., Ryan D., Lee K. (2004) S100 proteins in the epidermis. J. Invest. Dermatol. 123, 23–33 [DOI] [PubMed] [Google Scholar]
- 7. Schaub M. C., Heizmann C. W. (2008) Calcium, troponin, calmodulin, S100 proteins: from myocardial basics to new therapeutic strategies. Biochem. Biophys. Res. Commun. 369, 247–264 [DOI] [PubMed] [Google Scholar]
- 8. Les̸niak W., Słomnicki Ł. P., Filipek A. (2009) S100A6 – new facts and features. Biochem. Biophys. Res. Commun. 390, 1087–1092 [DOI] [PubMed] [Google Scholar]
- 9. Wolf S., Haase-Kohn C., Pietzsch J. (2011) S100A2 in cancerogenesis: a friend or a foe? Amino Acids 41, 849–861 [DOI] [PubMed] [Google Scholar]
- 10. Arumugam T., Logsdon C. D. (2011) S100P: a novel therapeutic target for cancer. Amino Acids 41, 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimamoto S., Takata M., Tokuda M., Oohira F., Tokumitsu H., Kobayashi R. (2008) Interactions of S100A2 and S100A6 with the tetratricopeptide repeat proteins, Hsp90/Hsp70-organizing protein and kinesin light chain. J. Biol. Chem. 283, 28246–28258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimamoto S., Kubota Y., Tokumitsu H., Kobayashi R. (2010) S100 proteins regulate the interaction of Hsp90 with cyclophilin 40 and FKBP52 through their tetratricopeptide repeats. FEBS Letters 584, 1119–1125 [DOI] [PubMed] [Google Scholar]
- 13. Yamaguchi F., Umeda Y., Shimamoto S., Tsuchiya M., Tokumitsu H., Tokuda M., Kobayashi R. (2012) S100 proteins modulate protein phosphatase 5 function: a link between Ca2+ signal transduction and protein dephosphorylation. J. Biol. Chem. 287, 13787–13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blatch G. L., Lässle M. (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21, 932–939 [DOI] [PubMed] [Google Scholar]
- 15. D'Andrea L. D., Regan L. (2003) TPR proteins: the versatile helix. Trends. Biochem. Sci. 28, 655–662 [DOI] [PubMed] [Google Scholar]
- 16. Smith D. F. (2004) Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones. 9, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allan R. K., Ratajczak T. (2011) Versatile TPR domains accommodate different modes of target protein recognition and function. Cell Stress Chaperones. 16, 353–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballinger C. A., Connell P., Wu Y., Hu Z., Thompson L. J., Yin L. Y., Patterson C. (1999) Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell Biol. 19, 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hatakeyama S., Yada M., Matsumoto M., Ishida N., Nakayama K. I. (2001) U box proteins as a new family of ubiquitin-protein ligases. J. Biol. Chem. 276, 33111–33120 [DOI] [PubMed] [Google Scholar]
- 20. Jiang J., Ballinger C. A., Wu Y., Dai Q., Cyr D. M., Höhfeld J., Patterson C. (2001) CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 276, 42938–42944 [DOI] [PubMed] [Google Scholar]
- 21. Murata S., Minami Y., Minami M., Chiba T., Tanaka K. (2001) CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO. Rep. 2, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pickart C. M. (2004) Back to the future with ubiquitin. Cell 116, 181–190 [DOI] [PubMed] [Google Scholar]
- 23. Hatakeyama S., Nakayama K. I. (2003) U-box proteins as a new family of ubiquitin ligases. Biochem. Biophys. Res. Commun. 302, 635–645 [DOI] [PubMed] [Google Scholar]
- 24. Connell P., Ballinger C. A., Jiang J., Wu Y., Thompson L. J., Höhfeld J., Patterson C. (2001) The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3, 93–96 [DOI] [PubMed] [Google Scholar]
- 25. Esser C., Scheffner M., Höhfeld J. (2005) The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J. Biol. Chem. 280, 27443–27448 [DOI] [PubMed] [Google Scholar]
- 26. Xu W., Marcu M., Yuan X., Mimnaugh E., Patterson C., Neckers L. (2002) Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl. Acad. Sci 99, 12847–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morishima Y., Peng H. M., Lin H. L., Hollenberg P. F., Sunahara R. K., Osawa Y., Pratt W. B. (2005) Regulation of cytochrome P450 2E1 by heat shock protein 90-dependent stabilization and CHIP-dependent proteasomal degradation. Biochemistry. 44, 16333–16340 [DOI] [PubMed] [Google Scholar]
- 28. Pabarcus M. K., Hoe N., Sadeghi S., Patterson C., Wiertz E., Correia M. A. (2009) CYP3A4 ubiquitination by gp78 (the tumor autocrine motility factor receptor, AMFR) and CHIP E3 ligases. Arch. Biochem. Biophys. 483, 66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim S. A., Yoon J. H., Kim D. K., Kim S. G., Ahn S. G. (2005) CHIP interacts with heat shock factor 1 during heat stress. FEBS Letters 579, 6559–6563 [DOI] [PubMed] [Google Scholar]
- 30. Wang L., Liu Y. T., Hao R., Chen L., Chang Z., Wang H. R., Wang Z. X., Wu J. W. (2011) Molecular mechanism of the negative regulation of Smad1/5 protein by carboxyl terminus of Hsc70-interacting protein (CHIP). J. Biol. Chem. 286, 15883–15894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li R. F., Zhang F., Lu Y. J., Sui S. F. (2005) Specific interaction between Smad1 and CHIP: a surface plasmon resonance study. Colloids Surf. B Biointerfaces 40, 133–136 [DOI] [PubMed] [Google Scholar]
- 32. Yamashita K., Oyama Y., Shishibori T., Matsushita O., Okabe A., Kobayashi R. (1999) Purification of bovine S100A12 from recombinant Escherichia coli. Protein Expr. Purif. 16, 47–52 [DOI] [PubMed] [Google Scholar]
- 33. Okada M., Hatakeyama T., Itoh H., Tokuta N., Tokumitsu H., Kobayashi R. (2004) S100A1 is a novel molecular chaperone and a member of the Hsp70/Hsp90 multichaperone complex. J. Biol. Chem. 279, 4221–4233 [DOI] [PubMed] [Google Scholar]
- 34. Hayashi N., Matsubara M., Takasaki A., Titani K., Taniguchi H. (1998) An expression system of rat calmodulin using T7 phage promoter in Escherichia coli. Protein Expr. Purif. 12, 25–28 [DOI] [PubMed] [Google Scholar]
- 35. Murata S., Minami M., Minami Y. (2005) Purification and assay of the chaperone-dependent ubiquitin ligase of the carboxyl terminus of Hsc70-interacting protein. Methods Enzymol. 398, 271–279 [DOI] [PubMed] [Google Scholar]
- 36. Urushitani M., Kurisu J., Tateno M., Hatakeyama S., Nakayama K., Kato S., Takahashi R. (2004) CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J. Neurochem. 90, 231–244 [DOI] [PubMed] [Google Scholar]
- 37. McDonough H., Patterson C. (2003) CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8, 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murata S., Chiba T., Tanaka K. (2003) CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int. J. Biochem. Cell Biol. 35, 572–578 [DOI] [PubMed] [Google Scholar]
- 39. Kajiro M., Hirota R., Nakajima Y., Kawanowa K., So-ma K., Ito I., Yamaguchi Y., Ohie S. H., Kobayashi Y., Seino Y., Kawano M., Kawabe Y., Takei H., Hayashi S., Kurosumi M., Murayama A., Kimura K., Yanagisawa J. (2009) The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat. Cell Biol. 11, 312–319 [DOI] [PubMed] [Google Scholar]
- 40. Tetzlaff J. E., Putcha P., Outeiro T. F., Ivanov A., Berezovska O., Hyman B. T., McLean P. J. (2008) CHIP targets toxic α-synuclein oligomers for degradation. J. Biol. Chem. 283, 17962–17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jana N. R., Dikshit P., Goswami A., Kotliarova S., Murata S., Tanaka K., Nukina N. (2005) Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J. Biol. Chem. 280, 11635–11640 [DOI] [PubMed] [Google Scholar]
- 42. Petrucelli L., Dickson D., Kehoe K., Taylor J., Snyder H., Grover A., De Lucia M., McGowan E., Lewis J., Prihar G., Kim J., Dillmann W. H., Browne S. E., Hall A., Voellmy R., Tsuboi Y., Dawson T. M., Wolozin B., Hardy J., Hutton M. (2004) CHIP and Hsp70 regulate Tau ubiquitination, degradation, and aggregation. Hum. Mol. Genet. 13, 703–714 [DOI] [PubMed] [Google Scholar]
- 43. Imai Y., Soda M., Hatakeyama S., Akagi T., Hashikawa T., Nakayama K. I., Takahashi R. (2002) CHIP is associated with Parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity. Mol. Cell 10, 55–67 [DOI] [PubMed] [Google Scholar]
- 44. Kamynina E., Kauppinen K., Duan F., Muakkassa N., Manor D. (2007) Regulation of proto-oncogenic dbl by chaperone-controlled, ubiquitin-mediated degradation. Mol. Cell Biol. 27, 1809–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tateishi Y., Sonoo R., Sekiya Y., Sunahara N., Kawano M., Wayama M., Hirota R., Kawabe Y., Murayama A., Kato S., Kimura K., Yanagisawa J. (2006) Turning off estrogen receptor β-mediated transcription requires estrogen-dependent receptor proteolysis. Mol. Cell Biol. 26, 7966–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xin H., Xu X., Li L., Ning H., Rong Y., Shang Y., Wang Y., Fu X. Y., Chang Z. (2005) CHIP controls the sensitivity of transforming growth factor-β signaling by modulating the basal level of Smad3 through ubiquitin-mediated degradation. J. Biol. Chem. 280, 20842–20850 [DOI] [PubMed] [Google Scholar]
- 47. Fan M., Park A., Nephew K. P. (2005) CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-α. Mol. Endocrinol. 19, 2901–2914 [DOI] [PubMed] [Google Scholar]
- 48. Smith D. F., Sullivan W. P., Marion T. N., Zaitsu K., Madden B., McCormick D. J., Toft D. O. (1993) Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol. Cell Biol. 13, 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young J. C., Hoogenraad N. J., Hartl F. U. (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112, 41–50 [DOI] [PubMed] [Google Scholar]
- 50. Ratajczak T., Carrello A. (1996) Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J. Biol. Chem. 271, 2961–2965 [DOI] [PubMed] [Google Scholar]
- 51. Radanyi C., Chambraud B., Baulieu E. E. (1994) The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc. Natl. Acad. Sci 91, 11197–11201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Das A. K., Cohen P. W., Barford D. (1998) The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17, 1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang M., Windheim M., Roe S. M., Peggie M, Cohen P., Prodromou C., Pearl L. H. (2005) Chaperoned ubiquitylation–crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell 20, 525–538 [DOI] [PubMed] [Google Scholar]
- 54. Xu Z., Devlin K. I., Ford M. G., Nix J. C., Qin J., Misra S. (2006) Structure and interactions of the helical and U-box domains of CHIP, the C terminus of HSP70 interacting protein. Biochemistry 45, 4749–4759 [DOI] [PubMed] [Google Scholar]
- 55. Xu Z., Kohli E., Devlin K. I., Bold M., Nix J. C., Misra S. (2008) Interactions between the quality control ubiquitin ligase CHIP and ubiquitin conjugating enzymes. BMC Struct. Biol. 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morano K. A., Thiele D. J. (1999) Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 7, 271–282 [PMC free article] [PubMed] [Google Scholar]
- 57. Attisano L., Wrana J. L. (2002) Signal transduction by the TGF-β superfamily. Science 296, 1646–1647 [DOI] [PubMed] [Google Scholar]
- 58. Muller P., Hrstka R., Coomber D., Lane D. P., Vojtesek B. (2008) Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene 27, 3371–3383 [DOI] [PubMed] [Google Scholar]
- 59. Alberti S., Demand J., Esser C., Emmerich N., Schild H., Hohfeld J. (2002) Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J. Biol. Chem. 277, 45920–45927 [DOI] [PubMed] [Google Scholar]
- 60. Dai Q., Qian S. B., Li H. H., McDonough H., Borchers C., Huang D., Takayama S., Younger J. M., Ren H. Y., Cyr D. M., Patterson C. (2005) Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J. Biol. Chem. 280, 38673–38681 [DOI] [PubMed] [Google Scholar]
- 61. Arndt V., Daniel C., Nastainczyk W., Alberti S., Höhfeld J. (2005) BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol. Biol. Cell 16, 5891–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alberti S., Böhse K., Arndt V., Schmitz A., Höhfeld J. (2004) The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15, 4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]