Background: Phosphatase of regenerating liver (PRL) is a metastasis-associated protein that is susceptible to inactivation by oxidation.
Results: Thioredoxin-related protein 32 (TRP32) specifically binds and reduces oxidized PRL.
Conclusion: The redox state of PRL is regulated by this specific member of the thioredoxin (TRX) family proteins.
Significance: TRP32 may be the key regulator of PRL function and contribute to cancer metastasis.
Keywords: Disulfide, Hydrogen Peroxide, Oxidation-Reduction, Oxidative Stress, Thioredoxin
Abstract
PRL family constitutes a unique class of phosphatases associated with metastasis. The phosphatase activity of PRL has been reported to be important for promoting metastasis, and it is inactivated by reversible oxidation of its catalytic cysteine. Here, we show that TRP32 specifically reduces PRL. Reduction of oxidized PRL in cells is inhibited by 2,4-dinitro-1-chlorobenzene, an inhibitor of TRX reductase. In vitro assays for the reduction of PRL show that only TRP32 can potently reduce oxidized PRL, whereas other TRX-related proteins linked to TRX reductase show little or no reducing activity. Indeed, TRP32 knockdown significantly prolongs the H2O2-induced oxidation of PRL. Binding analyses reveal that the unique C-terminal domain of TRP32 is required and sufficient for its direct interaction with PRL. These results suggest that TRP32 maintains the reduced state of PRL and thus regulates the biological function of PRL.
Introduction
The PRL2 family is a unique subfamily of protein-tyrosine phosphatases; it consists of three homologous members (PRL1–PRL3), which have a high degree (>75%) of amino acid sequence identity. PRLs are the only protein-tyrosine phosphatases known to have a C-terminal CAAX motif, which anchors PRLs to the plasma membrane by prenylation (1). By exhaustive gene expression profiling of human colorectal cancers, PRL3 was identified as the only gene overexpressed in all metastases but not in primary tumors or normal epithelia (2). Indeed, it has been reported that overexpression of PRLs, especially of PRL3, frequently occurs in various types of metastatic human cancers and is associated with patient mortality (3). These results implicate the close relationship between PRL overexpression and cancer malignancy. In addition, the artificial expression of PRL3 in culture cells augments the number of metastatic tumors in experimental metastatic analyses on mice (4), suggesting its causative role in metastasis.
PRL overexpression in culture cells potently activates various signaling pathways involved in the regulation of cell proliferation, invasion, and motility, such as MAP kinase, PI3K/Akt, Src, and Rho signaling (5). How PRL affects these signaling pathways remains unknown; however, the phosphatase activity of PRL is generally considered essential for its function. Indeed, the C104S mutant form of PRL, which lacks the phosphatase activity, neither activates those signaling pathways nor promotes cancer metastasis (6). However, little is known about the mechanism regulating the phosphatase activity of PRL.
One cysteine residue is conserved in the catalytic center of all protein-tyrosine phosphatases, and the side chain of this cysteine tends to be deprotonated to form a thiolate anion (−S−). This renders protein-tyrosine phosphatases highly susceptible to oxidation (7). Indeed, the active site cysteine residues in phosphatase with sequence homology to tensin (PTEN) and protein-tyrosine phosphatase 1B (PTP1B) are oxidized to form a disulfide bond and a sulfenyl amide bond, respectively, in response to physiological stimuli (8, 9). Such reversible oxidation of protein-tyrosine phosphatases generally results in the inhibition of their phosphatase activity (10). Like PTEN, oxidation stimulates the catalytic cysteine residue (Cys-104) of PRL to form an intramolecular disulfide bond with the spatially proximal cysteine residue (Cys-49) (11), suggesting that the phosphatase activity of PRL is also regulated by oxidation. Within cells, oxidized proteins, including protein-tyrosine phosphatases, are generally reduced by various enzymes, such as TRX and TRX-related proteins. These enzymes utilize electrons donated mainly by either TRX reductase (TrxR) or glutathione (GSH) to reduce substrate proteins. TRX is functionally coupled to TrxR, which uses electrons supplied by NADPH (12). Apart from TRX, TRX-related protein 14 (TRP14) and TRP32, among several other TRX-related proteins in the cytosol, are also linked to TrxR/NADPH (13, 14).
The cDNA encoding TRP32 was originally cloned in 1998 (15), and then the protein was identified in the same year as the binding partner for the catalytic fragment of MST (mammalian Ste20-like kinase), which is a caspase-activated kinase involved in apoptosis (16). The same protein is also known as thioredoxin-like 1 (TXNL1) (17). TRP32 is composed of an N-terminal TRX domain and a C-terminal domain of unknown function 1000 (DUF1000) (18, 19). The N-terminal TRX domain includes a conserved pair of cysteine residues, which participates in redox reaction by forming an intramolecular disulfide bond by reversible oxidation (20). Depending on the redox state of its TRX domain, TRP32 binds to Rab5, a key regulator of endocytic membrane trafficking, and inhibits the association between Rab5 and its guanine nucleotide dissociation inhibitor, which transports GDP-bound Rab5 from the early endosomal membrane to the plasma membrane (21). The C-terminal DUF1000 domain binds to Rpn11, a subunit of 19 S and 26 S proteasome, and thus affects proteasomal degradation (20, 22). In addition, TRP32 has been reported to have a protective effect on cells against glucose deprivation-induced cytotoxicity (14). Collectively, these studies have revealed several biochemical functions of TRP32; however, its substrates and significance as an oxidoreductase remain unknown.
In this study, we explored the molecular mechanism of the redox regulation of PRL and found that TRP32 specifically reduces PRL. We also found that this specific interaction is mediated through the DUF1000 domain of TRP32.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
Complementary DNAs (cDNAs) for human PRL1, PRL3, PTEN, and TRX were generated by RT-PCR. Mouse TRX, PRL1, PRL3 (wild type (WT), C104S, and C49S), human PRL2 (amino acid sequence is identical to mouse PRL2), TRP14, and TRP32 cDNAs were prepared as described previously (23).3 Amino acid-substituted mutant of human TRP32 (C34S/C37S) was generated by using the QuikChange site-directed mutagenesis kit (Agilent). Human TRP32 constructs lacking the C terminus (TRP32-N, 1–114) and N terminus (TRP32-C, 110–289) were generated by PCR. The cDNA fragments were inserted into pEF-BOS, pGEX-6p1 (GE Healthcare), and pQE30 (Qiagen) plasmid vectors.
Antibodies and Reagents
Anti-PRL rabbit polyclonal antibody was prepared as described previously.3 We also used the following commercially available antibodies: rabbit anti-Myc (Cell Signaling), mouse anti-PTEN (Santa Cruz Biotechnology), rabbit anti-TRX (Redox Bio Science), mouse anti-β-tubulin (Sigma), and chicken anti-TRP32 (Abcam). 2,4-dinitro-1-chlorobenzene (DNCB) and buthionine sulfoximine (BSO) were purchased from Sigma and Wako, respectively.
Cell Culture and Transfection
HEK293, COS7, and U2OS cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. For the transfection of plasmid DNA, HEK293 and COS7 cells were plated on a 35-mm diameter dish (for HEK293 cells) or 24-well plate (for COS7 cells) on the day before transfection. Then, 2 μg (for HEK293 cells) or 0.5 μg (for COS7 cells) of pEF-BOS-based expression plasmids were mixed with 2 μl (for HEK293 cells) or 0.5 μl (for COS7 cells) of Lipofectamine 2000 (Invitrogen) and used for transfection, according to the manufacturer's instructions. The cells were incubated for 4 h, and then the medium was replaced with growth medium. After the additional culture for 20 h, the cells were harvested. The transfection efficiencies were estimated to be 76% (for HEK293 cells) and 83% (for COS7 cells), respectively.
Preparation of Recombinant Proteins
His-PRL3 was expressed in Escherichia coli and purified with nickel-nitrilotriacetic acid beads (Qiagen). Recombinant TRX, TRP14, and TRP32 proteins (WT, C34S/C37S, N, and C) were expressed as GST fusion forms in E. coli. These were purified using glutathione-Sepharose beads (GE Healthcare). Where indicated, the GST tag was cleaved off by digestion with PreScission protease (GE Healthcare).
Pulldown Assay
GST fusion proteins (7.5–17 μg) were immobilized on 10 μl of glutathione-Sepharose beads and then incubated with 2.5 μg of His-PRL3 in a lysis buffer containing 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 2 mm EDTA, and 0.5% Triton X-100 for 1 h at 4 °C. The beads were washed seven times in lysis buffer and then suspended in 40 μl of Laemmli sample buffer. Proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue R-250.
Nonreducing SDS-PAGE
To detect protein oxidation, SDS-PAGE was performed under nonreducing conditions as described previously (23). After treatment with H2O2, cells were washed twice with ice-cold PBS and then lysed with lysis buffer supplemented with 40 mm N-ethylmaleimide (Wako). The lysates were mixed with Laemmli sample buffer without 2-mercaptoethanol and were then subjected to SDS-PAGE.
Reduction of Insulin
The activity of the indicated GST-cleaved proteins to reduce disulfide bonds in insulin were compared as described previously (13) with slight modifications. The incubation mixture contained 100 mm HEPES-NaOH (pH 7.6), 1 mm EDTA, 340 μm human insulin (Wako), 400 nm rat TrxR (Sigma), and each of the GST-cleaved proteins. The reaction was initiated by adding NADPH (Wako) to a final concentration of 0.8 mm. After incubation for the indicated time intervals at 30 °C, 8 μl of the reaction mixture were removed and mixed with 72 μl of a solution containing 6 m guanidinium chloride (Wako), 100 mm sodium phosphate (pH 7.3), 1 mm EDTA, and 0.1 mm 5,5′-dithiobis-2-nitrobenzoic acid (Wako) for 5 min at room temperature. The absorbance at 405 nm (A405) of each sample was determined using an LD 400 plate reader (Beckman Coulter).
Reduction of Recombinant PRL3
The reduction assays were performed as described previously (8) with slight modifications. The assay mixture contained 50 mm HEPES-NaOH (pH 7.2), 2 mm EDTA, 0.1 μm rat TrxR, 5 μm His-PRL3, and each of the GST-cleaved proteins. The reaction was initiated by adding NADPH to a final concentration of 0.2 mm. After incubation for the indicated time intervals at 25 °C, small aliquots of the reaction mixture were removed and subjected to nonreducing SDS-PAGE or conventional reducing SDS-PAGE followed by immunoblot analysis with the antibody against PRL. The intensity of the PRL3 bands was measured by optical densitometry using the ImageJ software.
Quantitative Estimation of Intracellular TRX and TRP32
Lysates (10 μg) from HEK293 cells and indicated amounts of purified recombinant human TRX or human TRP32 were subjected to immunoblot analyses with the antibodies against TRX or TRP32 and quantified by optical densitometry. Protein concentration of the lysates was determined using the BCA protein assay kit (Pierce).
Quantitation of Intracellular GSH
The intracellular GSH level was measured as described previously (24), with slight modifications. Cells were suspended with 200 μl of 10 mm HCl and subjected to three freeze-thaw cycles. Then the samples were stirred vigorously on a vortex mixer for 1 min, and 100 μl of 10% trichloroacetic acid were added. After centrifugation at 10,000 × g for 10 min, the supernatants (300 μl) were mixed with 4.7 ml of a solution containing 0.1 m potassium phosphate buffer (pH 8.0) and 0.1 mm 5,5′-dithiobis-2-nitrobenzoic acid. The absorbance at 405 nm (A405) was measured after 5 min using an LD 400 plate reader.
RNAi Knockdown
For transient knockdown experiments, U2OS cells were plated on a 35-mm diameter dish on the day before transfection, and then 16 pmol of siRNA were mixed with 0.8 μl of Lipofectamine RNAiMAX (Invitrogen) and used for transfection, according to the manufacturer's instructions. The duplex small interfering RNA (siRNA) against human TRP32, which targets the following sequences: 5′-GUGAUGAACAGCUGCUUAU-3′, was purchased from Sigma. MISSION siRNA universal negative control was used as negative control. The transfected cells were cultured for 72 h before harvest.
RESULTS
Sensitive Oxidation of PRL by H2O2
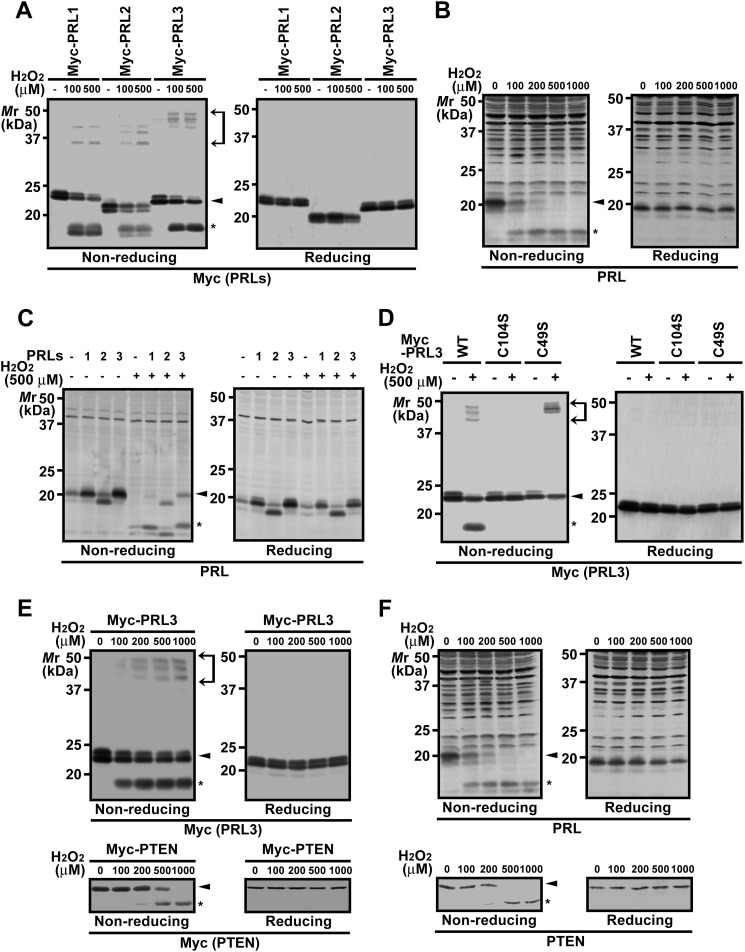
To investigate the intrinsic susceptibility of the PRL family proteins to oxidation, COS7 cells were transfected with expression constructs of Myc-PRL1–3 and then treated with H2O2. The cell lysates were first subjected to nonreducing SDS-PAGE, and then an immunoblot analysis was carried out. The results showed that Myc-PRL1–3 are all similarly oxidized by H2O2 to generate not only the higher mobility forms, which correspond to the previously reported oxidized PRL containing intramolecular disulfide bonds (11), but also the lower mobility forms (between 35 and 50 kDa), which probably represent homo-oligomers of Myc-PRL1–3 or hetero-oligomers with other proteins linked by intermolecular disulfide bonds (Fig. 1A). We also performed similar analysis on endogenous PRL. HEK293 cells treated with H2O2 were subjected to immunoblot analysis with the antibody against PRL, which can recognize all three PRL isoforms.3 We confirmed that the main signal around the 20-kDa region was PRL1 because the signal disappeared when cells were treated with siRNA against PRL1.3 As shown in Fig. 1B, H2O2 stimulation caused the band shift of endogenous PRL1 to the higher mobility form. Because of the multiple nonspecific signals in the higher molecular mass area, it was impossible to detect the presence of oxidized PRL in the lower mobility group. We noticed the presence of a weak signal at a slightly lower molecular weight than PRL1, which similarly disappeared after H2O2 stimulation. We speculated that this signal represents endogenous PRL2 because the molecular mass of PRL2 is slightly smaller than that of PRL1 (2). Indeed, the mobility of ectopically expressed PRL2 (without any tags) was identical to the detected signal (Fig. 1C).
FIGURE 1.
Sensitive oxidation of PRL by H2O2. A, COS7 cells were transfected with the indicated expression constructs and then treated with the indicated concentrations of H2O2 for 10 min. Cell lysates were subjected to nonreducing or conventional (reducing) SDS-PAGE followed by immunoblot analysis with anti-Myc antibody. Area between arrows, oxidized proteins containing intermolecular disulfide bonds; arrowhead, reduced proteins; asterisk, oxidized proteins containing intramolecular disulfide bonds. B, HEK293 cells were treated with the indicated concentrations of H2O2 for 10 min, and cell lysates were subjected to anti-PRL immunoblot analysis. C, HEK293 cells were transfected with the indicated constructs (without any tags) and then treated with 500 μm H2O2 for 10 min. Cell lysates were subjected to anti-PRL immunoblot analysis. D, COS7 cells were transfected with the indicated constructs and then treated with 500 μm H2O2 for 10 min. Cell lysates were subjected to anti-Myc immunoblot analysis. E, COS7 cells were transfected with Myc-PRL3 (upper panels) or Myc-PTEN (lower panels) and then treated with the indicated concentrations of H2O2 for 10 min. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. F, HEK293 cells were treated with the indicated concentrations of H2O2 for 10 min, and cell lysates were subjected to immunoblot analysis with the indicated antibodies.
To confirm that the higher mobility group represents proteins containing the intramolecular disulfide bond, we generated PRL3 mutants, in which Cys-104 (catalytically essential) or Cys-49 was replaced with a Ser residue. Unlike Myc-PRL3-WT, neither Myc-PRL3-C104S nor Myc-PRL3-C49S formed the higher mobility group, suggesting that this group was formed by an intramolecular disulfide bond between Cys-49 and Cys-104. Interestingly, Myc-PRL3-C104S also did not generate the lower mobility forms; in contrast, Myc-PRL3-C49S generated a higher number of lower mobility forms than Myc-PRL3 WT (Fig. 1D). Taken together, oxidation-sensitive Cys-104 is essential for disulfide bond formation in PRL. Normally, oxidized Cys-104 preferentially forms a disulfide bond with Cys-49 in the same molecule, but in the absence of Cys-49, it forms intermolecular disulfide bonds instead.
We examined the oxidative sensitivity of PRL in comparison with PTEN, a phosphatase for tyrosine-phosphorylated proteins and phosphatidylinositol-3,4,5-trisphosphates, that regulates cellular signaling in response to physiological oxidative stress (8). COS7 cells expressing Myc-PRL3 or Myc-PTEN were treated with increased concentrations of H2O2. A clear oxidation of Myc-PRL3 was observed when cells were exposed to 100 μm H2O2; however, a very small amount of Myc-PTEN was oxidized at 200 μm H2O2 (Fig. 1E). Furthermore, similar results were obtained when we examined the oxidative state of endogenous PRL1 and PTEN in HEK293 cells (Fig. 1F). These results indicate that PRL is very sensitive to oxidation and suggest that PRL, as well as PTEN, has a potential role in regulating cellular signaling in response to oxidative stress.
DNCB Inhibits the Reduction of Oxidized PRL
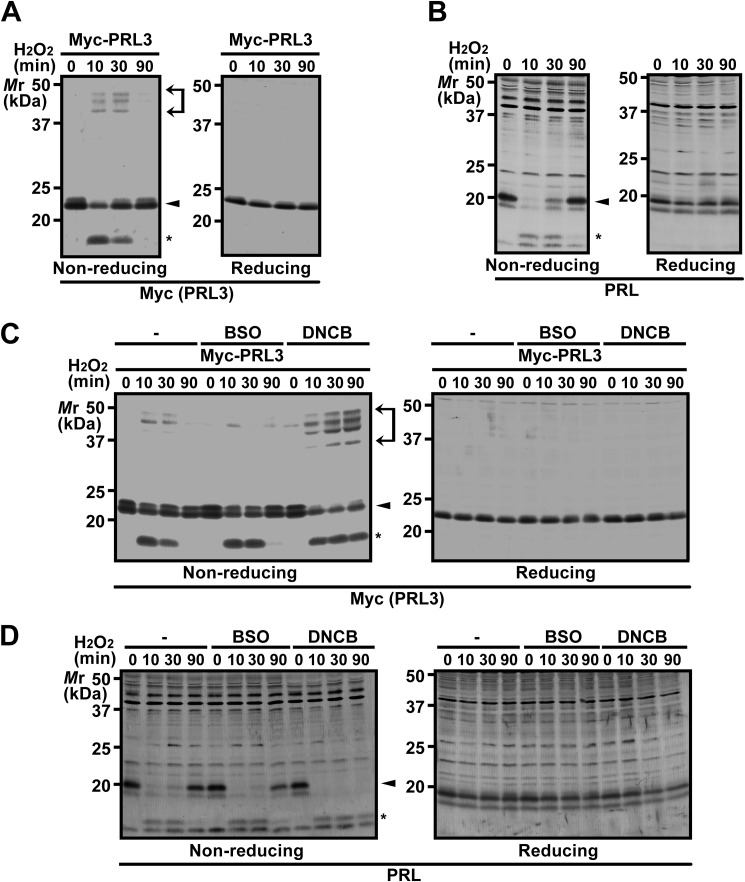
We next performed a time-course analysis of the oxidative state of PRL. COS7 cells expressing Myc-PRL3 and HEK293 cells were treated with H2O2 and subjected to analysis at the indicated time intervals after stimulation. As shown in Fig. 2, A and B, both Myc-PRL3 and endogenous PRL1 were oxidized at 10 min, but both were returned to the reduced state at 90 min. Collectively, these results indicate that PRL is transiently oxidized to form intra- and intermolecular disulfide bonds by H2O2 stimulation, which are reversibly reduced under the normal cytosolic conditions.
FIGURE 2.
DNCB inhibits the reduction of oxidized PRL. A and B, COS7 cells expressing Myc-PRL3 (A) or HEK293 cells (B) were treated with 200 μm H2O2 for the indicated time intervals, and cell lysates were subjected to nonreducing or conventional (reducing) SDS-PAGE followed by immunoblot analysis with the indicated antibodies. Area between arrows, oxidized proteins containing intermolecular disulfide bonds; arrowhead, reduced proteins; asterisk, oxidized proteins containing intramolecular disulfide bonds. C and D, COS7 cells expressing Myc-PRL3 (C) or HEK293 cells (D) were treated with 50 μm DNCB for 30 min or 100 μm BSO for 16 h and then treated with 200 μm H2O2 for the indicated time intervals. Cell lysates were subjected to immunoblot analysis with the indicated antibodies.
In general, protein reduction is catalyzed by various reducing enzymes, such as TRX and TRX-related proteins (12). To investigate the reduction mechanism of PRL, we tested the effects of the inhibitors of two major cellular reducing systems, the TRX system (using DNCB, an inhibitor of TrxR) and the GSH system (using BSO, an inhibitor of GSH biosynthesis). COS7 cells expressing Myc-PRL3 were pretreated with either DNCB or BSO and then stimulated with H2O2. In control cells, Myc-PRL3 was oxidized at 10 min but returned to the reduced state at 90 min (Fig. 2C). DNCB treatment suppressed the reduction of oxidized Myc-PRL3 at 90 min, whereas no significant differences were observed by BSO treatment. We also observed similar effects of DNCB and BSO on endogenous PRL1 (Fig. 2D). We confirmed that treatment of cells with 100 μm BSO for 16 h sufficiently depleted endogenous GSH (86.6% depletion). These results suggest that PRL is primarily reduced by a reductase that acts downstream of TrxR.
It should be mentioned that overexpressed Myc-PRL3 proteins were significantly abundant when compared with endogenous PRL (roughly estimated to be about eight times more than the sum of PRL1 and PRL2). Nonetheless, we confirmed that overexpressed Myc-PRL3 localized at the plasma membrane as endogenous PRL (25) and recovered from H2O2-induced oxidation in a similar time course (Fig. 3A). Thus, our overexpression experiments are considered to properly reflect the redox status of endogenous PRL.
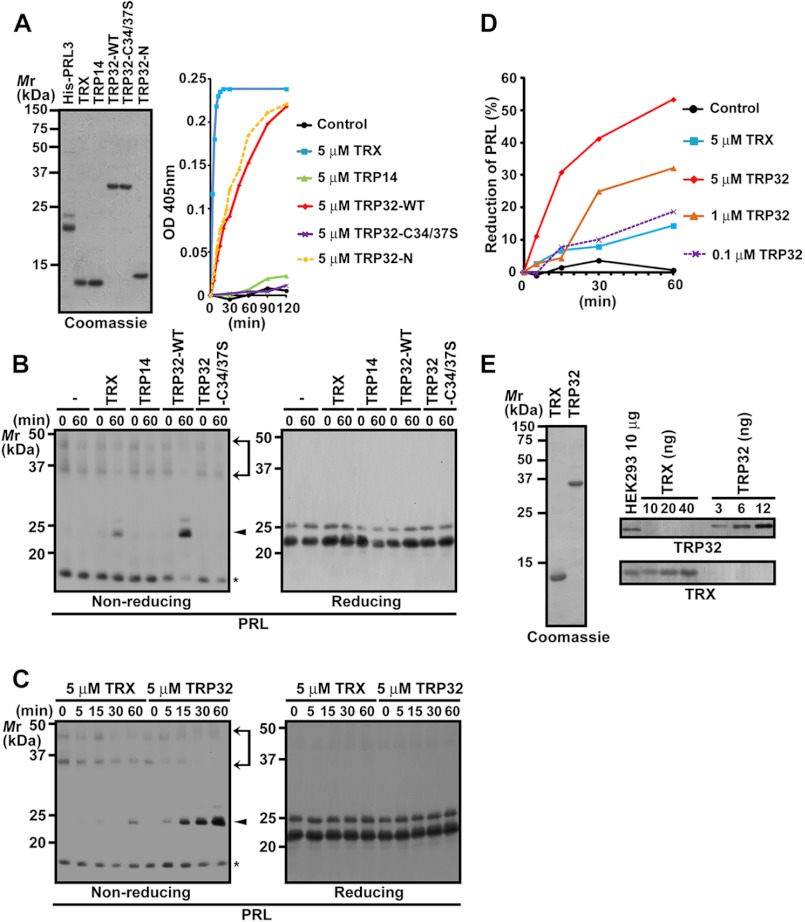
FIGURE 3.
TRP32 reduces oxidized PRL. A, purified His-PRL3 and various GST-cleaved proteins were subjected to SDS-PAGE and Coomassie Brilliant Blue R-250 staining (left). The GST-cleaved proteins were subjected to a reduction assay by using insulin as the substrate. The assay mixture lacking GST-cleaved proteins served as the control. Insulin reduction was monitored by changes in absorbance at 405 nm (right). OD, optical density. B–D, His-PRL3 was incubated in the assay mixture containing the indicated GST-cleaved proteins at a final concentration of 5 μm (B and C) or 0.1–5 μm (D) and then subjected to nonreducing or conventional (reducing) SDS-PAGE followed by immunoblot analysis. The amounts of reduced PRL3 were quantified and are indicated as a percentage of those of total PRL3 (D). Area between arrows, oxidized proteins containing intermolecular disulfide bonds; arrowhead, reduced proteins; asterisk, oxidized proteins containing intramolecular disulfide bonds. E, lysates of HEK293 cells (10 μg) and the indicated amounts of recombinant GST-cleaved TRX or TRP32 proteins of human origin were subjected to immunoblot analysis with the indicated antibodies (right). The purity of the recombinant proteins is also shown (left).
TRP32 Specifically Reduces Oxidized PRL
Previous studies have shown that three TRX-related proteins in the cytosol, TRX, TRP14, and TRP32, use electrons supplied by TrxR (13, 14). To investigate which of these proteins is responsible for reducing PRL, we expressed and purified the recombinant proteins of TRX, TRP14, and TRP32 and then performed a reduction assay by using insulin as the substrate. The most rapid reduction of insulin was achieved by TRX, whereas TRP32 also showed a moderate level of insulin reduction (Fig. 3A). Consistent with the previous study (13), the reductase activity of TRP14 was much lesser than that of TRX or TRP32. Next, we performed a reduction assay by using purified recombinant PRL3 as the substrate. We noticed that the purified recombinant PRL3 proteins were mostly oxidized, and thus, we directly subjected them to the assay. Purified PRL3 was incubated with each recombinant TRX-related protein, and the reduction of PRL3 was monitored by the mobility shift in nonreducing SDS-PAGE. As shown in Fig. 3, B and C, TRP32 exhibited the strongest activity, whereas TRX was much less active than TRP32, and TRP14 showed no reducing activity. TRP32-C34S/C37S, wherein the 2 cysteine residues (Cys-34 and Cys-37) corresponding to the catalytically conserved pair of cysteine residues in the TRX active site were substituted by serine residues, could not reduce insulin or recombinant PRL3 (Fig. 3, A and B). To further compare the PRL3 reduction activity, recombinant PRL3 was incubated with various concentrations of recombinant TRP32. The activity of TRX at 5 μm was similar to that of TRP32 at 0.1 μm (Fig. 3D). By quantitative immunoblot analysis, the amount of endogenous TRP32 and TRX in HEK293 cells was found to be 0.5 and 1.5 ng/μg of cell lysate, respectively (Fig. 3E). The molecular masses of TRX and TRP32 were 12 and 32 kDa, respectively; hence, the molar ratio of TRX to TRP32 was ∼8 in HEK293 cells. Taken together with the results of the reduction assays, TRP32 appears to play the dominant role in the reduction of oxidized PRL in cells.
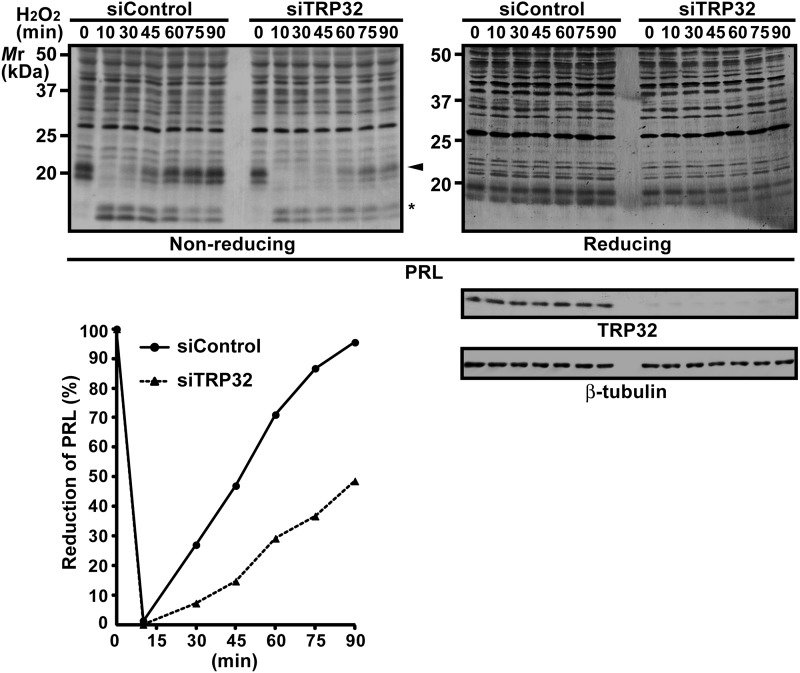
To confirm the importance of endogenous TRP32, we also performed TRP32 knockdown experiments and examined the effect on the redox status of endogenous PRL. For this purpose, we used U2OS cells because they endogenously expressed both TRP32 and PRL, and the treatment with TRP32 siRNA could successfully decrease the protein level of endogenous TRP32 (Fig. 4). As shown in Fig. 4, H2O2-induced oxidation of PRL was significantly prolonged in TRP32 knockdown cells in comparison with control cells. These results further substantiate the importance of TRP32 in the redox regulation of PRL.
FIGURE 4.
Prolonged oxidation of PRL by TRP32 knockdown. U2OS cells were transfected with siRNA against TRP32 (siTRP32) and then treated with 200 μm H2O2 for the indicated time intervals. Cell lysates were subjected to nonreducing or conventional (reducing) SDS-PAGE followed by immunoblot analysis with the indicated antibodies (upper panels). The amounts of reduced PRL3 were quantified and indicated as a percentage of those of total PRL3 (lower panel). Arrowhead, reduced proteins; asterisk, oxidized proteins containing intramolecular disulfide bonds. siControl, siRNA negative control.
PRL Specifically Binds to TRP32
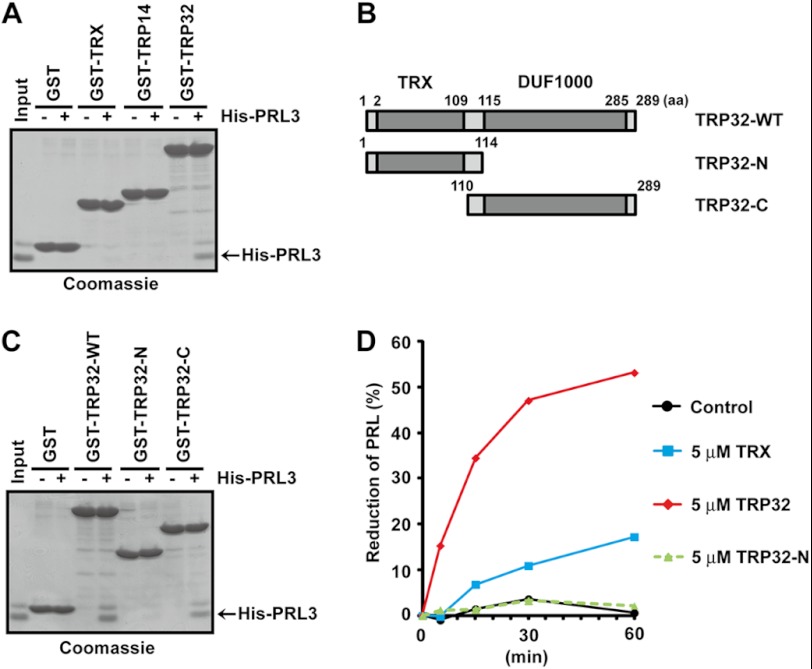
We assumed that the reduction of PRL3 by TRP32 is due to the specific interaction between these proteins. To test this hypothesis, purified PRL3 was subjected to pulldown assays with GST-TRX, GST-TRP14, and GST-TRP32, and the bound proteins were detected by Coomassie Brilliant Blue R-250 staining. As shown in Fig. 5A, only GST-TRP32 was found to be associated with His-PRL3, indicating their direct and specific interaction.
FIGURE 5.
PRL specifically binds to TRP32. A, various GST fusion proteins immobilized on beads were mixed with His-PRL3 proteins. The bound proteins were detected by Coomassie Brilliant Blue R-250 staining. B, schematic illustration of TRP32. The TRX domain and DUF1000 domain are indicated with gray color. C, GST fusion WT or deletion mutants (N and C) of TRP32 were subjected to pulldown assays with His-PRL3 proteins as in A. D, His-PRL3 was incubated in the assay mixture containing the indicated GST-cleaved proteins at a final concentration of 5 μm and then subjected to nonreducing or conventional (reducing) SDS-PAGE followed by immunoblot analysis. The amounts of reduced PRL3 were quantified and are indicated as a percentage of those of total PRL3.
TRP32 consists of two domains: the N-terminal TRX domain and the C-terminal DUF1000 domain (Fig. 5B) (16). To determine the binding site to PRL, each domain of TRP32 was prepared as a GST fusion form and was then subjected to pulldown assays with His-PRL3. The DUF1000 domain (TRP32-C) associated with PRL3, as did full-length TRP32, whereas the TRX domain (TRP32-N) did not (Fig. 5C). Therefore, we concluded that the DUF1000 domain of TRP32 is required and sufficient for its interaction with PRL. Next, we examined the reductase activity of the isolated TRX domain (TRP32-N), which lacks the DUF1000 domain. When PRL3 was used as the substrate, TRP32-N showed little activity (Fig. 5D) despite the fact that both TRP32-N and TRP32-WT exhibited similar levels of the reductase activity against insulin (Fig. 3A). These results suggest that the specific interaction with PRL via the DUF1000 domain is crucial for the strong reductase activity of TRP32.
DISCUSSION
It is generally believed that TRX maintains intracellular redox homeostasis by reducing several oxidized proteins (12). In this study, we showed that TRP32, a TRX-related protein of unknown function, specifically binds to and reduces PRL. Similarly, Jeong et al. (26) have reported that TRP14 specifically reduces LC8, the light chain of dynein, which interacts with IκBα and the proapoptotic Bcl-2 relative Bim. These results suggest that each of the poorly characterized TRX-related proteins serve a specific role in redox regulation by reducing particular substrate proteins.
Previous studies have shown that the phosphatase activity of PRL is inactivated by the oxidation of its catalytic cysteine (11) and that the catalytically inactive mutant of PRL lacks the ability to promote metastasis (6). These studies imply that PRL needs to be maintained in the reduced state to promote cancer metastasis. Taken together with the findings of this study, TRP32 may also contribute to cancer metastasis by protecting PRL from oxidative inactivation. It has been reported that TRP32-overexpressing cells are resistant to glucose deprivation-induced cell death (14). PRL is known to activate antiapoptotic Akt/PI3K signaling (27, 28), and thus, the suppression of cell death may be attributed to augmented Akt/PI3K signaling due to TRP32 overexpression. Collectively, TRP32 may regulate cell death and cancer progression by maintaining the activity of PRL through specific reduction of its catalytic cysteine in PRL.
This work was supported by the Funding Program for Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science (JSPS) (to H. M.), Exciting Leading-Edge Research Projects from Osaka University (to H. M.), and grants-in-aid for scientific research from the JSPS and Ministry of Education, Culture, Sports, Science and Technology-Japan (to H. M. and Y. F.).
Y. Funato, K. Furutani, S. Mizukami, T. Sawasaki, Y. Endo, K. Kikuchi, Y. Kurachi, and H. Miki, manuscript in preparation.
- PRL
- phosphatase of regenerating liver
- TRP
- thioredoxin-related protein
- TRX
- thioredoxin
- TrxR
- thioredoxin reductase
- DNCB
- 2,4-dinitro-1-chlorobenzene
- PTEN
- phosphatase with sequence homology to tensin
- DUF1000
- domain of unknown function 1000
- BSO
- buthionine sulphoximine.
REFERENCES
- 1. Cates C. A., Michael R. L., Stayrook K. R., Harvey K. A., Burke Y. D., Randall S. K., Crowell P. L., Crowell D. N. (1996) Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 110, 49–55 [DOI] [PubMed] [Google Scholar]
- 2. Rios P., Li X., Köhn M. (2013) Molecular mechanisms of the PRL phosphatases. FEBS J. 280, 505–524 [DOI] [PubMed] [Google Scholar]
- 3. Bessette D. C., Qiu D., Pallen C. J. (2008) PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 27, 231–252 [DOI] [PubMed] [Google Scholar]
- 4. Zeng Q., Dong J. M., Guo K., Li J., Tan H. X., Koh V., Pallen C. J., Manser E., Hong W. (2003) PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 63, 2716–2722 [PubMed] [Google Scholar]
- 5. Al-Aidaroos A. Q., Zeng Q. (2010) PRL-3 phosphatase and cancer metastasis. J. Cell Biochem. 111, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 6. Guo K., Li J., Tang J. P., Koh V., Gan B. Q., Zeng Q. (2004) Catalytic domain of PRL-3 plays an essential role in tumor metastasis: formation of PRL-3 tumors inside the blood vessels. Cancer Biol. Ther. 3, 945–951 [DOI] [PubMed] [Google Scholar]
- 7. Peters G. H., Frimurer T. M., Olsen O. H. (1998) Electrostatic evaluation of the signature motif (H/V)CX5R(S/T) in protein-tyrosine phosphatases. Biochemistry 37, 5383–5393 [DOI] [PubMed] [Google Scholar]
- 8. Kwon J., Lee S. R., Yang K. S., Ahn Y., Kim Y. J., Stadtman E. R., Rhee S. G. (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U.S.A. 101, 16419–16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salmeen A., Andersen J. N., Myers M. P., Meng T. C., Hinks J. A., Tonks N. K., Barford D. (2003) Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 [DOI] [PubMed] [Google Scholar]
- 10. Ostman A., Frijhoff J., Sandin A., Böhmer F. D. (2011) Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 150, 345–356 [DOI] [PubMed] [Google Scholar]
- 11. Yu L., Kelly U., Ebright J. N., Malek G., Saloupis P., Rickman D. W., McKay B. S., Arshavsky V. Y., Bowes Rickman C. (2007) Oxidative stress-induced expression and modulation of phosphatase of regenerating liver-1 (PRL-1) in mammalian retina. Biochim. Biophys. Acta 1773, 1473–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holmgren A. (2000) Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox. Signal. 2, 811–820 [DOI] [PubMed] [Google Scholar]
- 13. Jeong W., Yoon H. W., Lee S. R., Rhee S. G. (2004) Identification and characterization of TRP14, a thioredoxin-related protein of 14 kDa: new insights into the specificity of thioredoxin function. J. Biol. Chem. 279, 3142–3150 [DOI] [PubMed] [Google Scholar]
- 14. Jiménez A., Pelto-Huikko M., Gustafsson J. A., Miranda-Vizuete A. (2006) Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett. 580, 960–967 [DOI] [PubMed] [Google Scholar]
- 15. Miranda-Vizuete A., Gustafsson J. A., Spyrou G. (1998) Molecular cloning and expression of a cDNA encoding a human thioredoxin-like protein. Biochem. Biophys. Res. Commun. 243, 284–288 [DOI] [PubMed] [Google Scholar]
- 16. Lee K. K., Murakawa M., Takahashi S., Tsubuki S., Kawashima S., Sakamaki K., Yonehara S. (1998) Purification, molecular cloning, and characterization of TRP32, a novel thioredoxin-related mammalian protein of 32 kDa. J. Biol. Chem. 273, 19160–19166 [DOI] [PubMed] [Google Scholar]
- 17. Anagnostopoulos A. K., Vougas K., Kolialexi A., Mavrou A., Fountoulakis M., Tsangaris G. (2005) The protein profile of the human immature T-cell line CCRF-CEM. Cancer Genomics Proteomics 2, 271–300 [PubMed] [Google Scholar]
- 18. Jin J., Chen X., Zhou Y., Bartlam M., Guo Q., Liu Y., Sun Y., Gao Y., Ye S., Li G., Rao Z., Qiang B., Yuan J. (2002) Crystal structure of the catalytic domain of a human thioredoxin-like protein. Eur. J. Biochem. 269, 2060–2068 [DOI] [PubMed] [Google Scholar]
- 19. Goroncy A. K., Koshiba S., Tochio N., Tomizawa T., Inoue M., Tanaka A., Sugano S., Kigawa T., Yokoyama S. (2010) Solution structure of the C-terminal DUF1000 domain of the human thioredoxin-like 1 protein. Proteins 78, 2176–2180 [DOI] [PubMed] [Google Scholar]
- 20. Wiseman R. L., Chin K. T., Haynes C. M., Stanhill A., Xu C. F., Roguev A., Krogan N. J., Neubert T. A., Ron D. (2009) Thioredoxin-related protein 32 is an arsenite-regulated thiol reductase of the proteasome 19 S particle. J. Biol. Chem. 284, 15233–15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felberbaum-Corti M., Morel E., Cavalli V., Vilbois F., Gruenberg J. (2007) The redox sensor TXNL1 plays a regulatory role in fluid phase endocytosis. PLoS One 2, e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersen K. M., Madsen L., Prag S., Johnsen A. H., Semple C. A., Hendil K. B., Hartmann-Petersen R. (2009) Thioredoxin Txnl1/TRP32 is a redox-active cofactor of the 26 S proteasome. J. Biol. Chem. 284, 15246–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morinaka A., Yamada M., Itofusa R., Funato Y., Yoshimura Y., Nakamura F., Yoshimura T., Kaibuchi K., Goshima Y., Hoshino M., Kamiguchi H., Miki H. (2011) Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci. Signal. 4, ra26. [DOI] [PubMed] [Google Scholar]
- 24. Cheng J. Z., Sharma R., Yang Y., Singhal S. S., Sharma A., Saini M. K., Singh S. V., Zimniak P., Awasthi S., Awasthi Y. C. (2001) Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J. Biol. Chem. 276, 41213–41223 [DOI] [PubMed] [Google Scholar]
- 25. Basak S., Jacobs S. B. R., Krieg A. J., Pathak N., Zeng Q., Kaldis P., Giaccia A. J., Attardi L. D. (2008) The metastasis-associated gene Prl-3 is a p53 target involved in cell-cycle regulation. Mol. Cell 30, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeong W., Chang T. S., Boja E. S., Fales H. M., Rhee S. G. (2004) Roles of TRP14, a thioredoxin-related protein in tumor necrosis factor-α signaling pathways. J. Biol. Chem. 279, 3151–3159 [DOI] [PubMed] [Google Scholar]
- 27. Wang H., Quah S. Y., Dong J. M., Manser E., Tang J. P., Zeng Q. (2007) PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 67, 2922–2926 [DOI] [PubMed] [Google Scholar]
- 28. Wang H., Vardy L. A., Tan C. P., Loo J. M., Guo K., Li J., Lim S. G., Zhou J., Chng W. J., Ng S. B., Li H. X., Zeng Q. (2010) PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell 18, 52–62 [DOI] [PubMed] [Google Scholar]