Background: ITK-SYK is an oncogenic fusion protein in patients with peripheral T cell lymphomas carrying a unique translocation.
Results: Mutations in critical tyrosines disable the constitutive activity of ITK-SYK. Intracellular signaling of ITK-SYK requires both SLP-76 and the adapter function of SYK/ZAP-70 kinases.
Conclusion: The ITK-SYK fusion protein is dependent on adapters.
Significance: This study provides insight into the activation and signaling of ITK-SYK.
Keywords: Cell Signaling, Immunology, Lymphoma, Molecular Cell Biology, Phosphotyrosine Signaling, ITK-SYK, SLP-76, SYK
Abstract
The inducible T cell kinase-spleen tyrosine kinase (ITK-SYK) oncogene consists of the Tec homology-pleckstrin homology domain of ITK and the kinase domain of SYK, and it is believed to be the cause of peripheral T cell lymphoma. We and others have recently demonstrated that this fusion protein is constitutively tyrosine-phosphorylated and is transforming both in vitro and in vivo. To gain a deeper insight into the molecular mechanism(s) underlying its activation and signaling, we mutated a total of eight tyrosines located in the SYK portion of the chimera into either phenylalanine or to the negatively charged glutamic acid. Although mutations in the interdomain-B region affected ITK-SYK kinase activity, they only modestly altered downstream signaling events. In contrast, mutations that were introduced in the kinase domain triggered severe impairment of downstream signaling. Moreover, we show here that SLP-76 is critical for ITK-SYK activation and is particularly required for the ITK-SYK-dependent phosphorylation of SYK activation loop tyrosines. In Jurkat cell lines, we demonstrate that expression of ITK-SYK fusion requires an intact SLP-76 function and significantly induces IL-2 secretion and CD69 expression. Furthermore, the SLP-76-mediated induction of IL-2 and CD69 could be further enhanced by SYK or ZAP-70, but it was independent of their kinase activity. Notably, ITK-SYK expression in SYF cells phosphorylates SLP-76 in the absence of SRC family kinases. Altogether, our data suggest that ITK-SYK exists in the active conformation state and is therefore capable of signaling without SRC family kinases or stimulation of the T cell receptor.
Introduction
The fusion kinase, interleukin 2-inducible T cell kinase-spleen tyrosine kinase (ITK5-SYK), has recently been identified in a subset of patients with nonspecific peripheral T cell lymphomas (1, 2). Previous studies from two independent groups demonstrated that ITK-SYK localizes to the plasma membrane and harbors constitutive kinase activity (3, 4). Moreover, transgenic mice expressing ITK-SYK display chronic proximal TCR signaling culminating in T cell but not B cell lymphomas (5, 6). To date, the structure-function relationship of the regulatory tyrosines, as well as detailed functional analysis of this molecule in particular, during TCR signaling has not been presented.
ITK-SYK is the only reported chimeric oncogene involving a TEC family kinase (TFK) (7, 8). A chromosomal translocation event t(5;9)(q33;q22) fuses the PH-TH domain of ITK, with most of the interdomain-B and the kinase domain of SYK (1). The PH-TH domain plays key roles in the activation of TEC family members by anchoring the kinases on the inner leaflet of the cell membrane through their phosphatidylinositol (3,4,5)-triphosphate binding capacity (9–12). Phosphatidylinositol 3-kinase catalyzes phosphatidylinositol (3,4,5)-triphosphate formation, and phosphatidylinositol 3-kinase inhibition represses TFK activation. Hence, like TFKs, plasma membrane localization and potent activation of ITK-SYK require intact phosphatidylinositol 3-kinase signaling (3). In Bruton's tyrosine kinase and other TFKs, a missense mutation (R28C in Bruton's tyrosine kinase) in the PH domain abolishes phosphatidylinositol (3,4,5)-triphosphate binding leading to loss-of-function and aberrant signaling (10, 13). Accordingly, the corresponding PH domain missense mutation (R29C) compromises plasma membrane translocation of the ITK-SYK fusion (4). Surprisingly, overexpression of the ITK-SYK-R29C mutant in mice led to a possibly more severe lymphoproliferative disorder as compared with wild-type ITK-SYK (6). These observations suggest that along with ITK-SYK membrane translocation capacity, other parameters contribute to its oncogenic potential. The molecular mechanism(s) underlying this phenomenon, however, remain to be established.
SYK includes a number of putative regulatory tyrosines, including the following: Tyr-131 in interdomain-A; Tyr-323, Tyr-348, and Tyr-352 in interdomain-B; Tyr-525/Tyr-526 in the activation loop, and Tyr-629/Tyr-630/Tyr-631 in the C terminus (14, 15). With the exception of Tyr-131, ITK-SYK retains the rest of these regulatory tyrosine sites. In SYK, these tyrosines are subjected to auto-/trans-phosphorylation by SRC family kinases (SFKs) and play key roles in the release from auto-inhibitory “closed” to active “open” conformation. They may also serve as docking sites for various SH2 domain proteins (14–16). To investigate the role of individual tyrosines for activity and regulation, a loss-of-function mutation strategy is frequently employed using phenylalanine replacement (15, 17–19). However, tyrosine substitution with glutamic acid or aspartic acid that gives a permanent negative charge to mimic tyrosine phosphorylation has also been frequently employed (15, 18, 20). Although these substitutions may not faithfully mimic phosphorylations in all respects, the negative charge can induce conformational changes, which likely resemble those of the native phosphorylated kinase. Even protein-protein interactions involving SH2 domains following such substitutions have been reported (20, 21), although this presumably represents a more rare phenomenon.
SYK and ZAP-70 (ζ-chain-associated protein kinase 70 kDa) are recruited to phosphorylated immunoreceptor tyrosine-based activation motifs through their tandem SH2 domains. Tyr-131, which lies between the tandem SH2 domains of SYK, negatively regulates this receptor kinase association (20). Moreover, SYK harboring loss-of-function mutations (Y131F) demonstrated enhanced receptor kinase binding and downstream phosphorylation. Accordingly, substitution with the negatively charged residue glutamic acid (Y131E) resulted in diminished receptor binding and highly compromised downstream phosphorylation (20). Tyr-323, located in interdomain-B, negatively controls SYK function through its phosphorylation-dependent interaction with the E3 ligase c-CBL (22). Substitution of Tyr-323 with phenylalanine (Y323F) results in highly enhanced B cell receptor coupling and phosphorylation of downstream targets like phospholipase Cγ1 and BLNK (23). Tyr-292 on ZAP-70 performs analogous functions where its depletion results in enhanced TCR signaling (24). Tyrosines in the interdomain-B of SYK family kinases are not only involved in auto-inhibitory conformation but also provide docking sites for various interacting partners (14, 15, 25). SYK with aspartic acid substitution Y352D shows activation loop and PLCγ2, c-CBL, and AKT phosphorylations, compared with wild-type SYK, which does not show detectable phosphorylations under the same conditions in BAF3 cells (18). A similar substitution (Y319E) has also been shown sufficient to activate ZAP-70 (26).
SYK mutants bearing phenylalanine replacements of Tyr-348 and/or Tyr-352 have shown that these tyrosines play redundant and specific roles in the activation and signaling (14, 15, 17). Phosphorylation of these tyrosines releases SYK from auto-inhibitory conformation, and elimination of both tyrosines results in substantial reduction of the kinase function and reduced downstream phosphorylation (14, 15, 17). A SYK kinase with a glutamic acid substitution of these tyrosines (Y348E/Y352E) renders the molecule constitutively active, and pre-B cells expressing this mutant fail to differentiate upon IL-7 withdrawal and continue to proliferate (15). This is unlike the SYK wild-type or loss of function mutant Y348F/Y352F that results in differentiation of cells (15). The roles of SYK family activation loop tyrosines remain intriguingly obscure as they adopt an active “loop-out” conformation in the nonphosphorylated state, a configuration that other kinases acquire following phosphorylation (27, 28). SYK mutants, where both activation loop tyrosines were replaced with phenylalanine, showed phosphorylation of downstream substrates. Interestingly, aspartic acid replacement of both activation loop tyrosines rather rendered the molecule catalytically inactive. This mutant has been suggested to obtain inhibitory activation loop conformation (18). However, the tyrosines on the C terminus/tail of SYK are known to stabilize an auto-inhibitory conformation and also to interact with the adapter protein BLNK/SLP-65 (29). Phenylalanine replacement of tail tyrosines has been reported to result in gain-of-function in SYK family kinases (19). Another recent study showed that loss of C-terminal/tail tyrosines results in enhanced phosphorylation of SYK activation loop tyrosines 525/526, whereas downstream signaling events, such as nuclear factor of activated T cells and NFκB activation, were severely hampered (30).
In this study, we first set out to investigate the structure-function relationship of the ITK-SYK fusion kinase to delineate the molecular mechanism(s) responsible for its constitutive activation and signaling. Second, we sought to determine the roles of key proximal TCR components that could be critical in ITK-SYK-mediated chronic signaling. To address the structure-function relationship of ITK-SYK, we created various mutants of the putative phosphorylatable tyrosines in the interdomain-B, the activation loop, and the C-terminal tail. To create a nonphosphorylatable residue to block negative charge induction or to induce a permanent negative charge, these tyrosines were replaced with either phenylalanine or glutamic acid, respectively. We systematically analyzed the phosphorylation status of the selected tyrosines on ITK-SYK mutants as well as their kinase activity using SYK and SLP-76 (SH2 domain-containing leukocyte protein of 76 kDa) as substrates. Phosphorylation levels served as surrogate markers for the kinase activity. Phosphorylation of the different tyrosines on SYK in the presence or absence of SLP-76 was also determined. To further determine the significance of SLP-76, ZAP-70, and SYK in ITK-SYK-mediated activation of T cells, the Jurkat cell lines J14 (SLP-76KO), P116 (SYK/ZAP-70KO), and E6-1 (SYKKO) were used. Finally, IL-2 secretion and CD69 expression were also determined as a functional readout of the ITK-SYK mutants in these T cell lymphoma cell lines. SYF cells were used to delineate the phosphorylation status and kinase activity of ITK-SYK in the absence of SFKs.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
ITK-SYK mutants were created by site-directed mutagenesis, and all constructs were verified by sequencing. The following constructs were used in this study (Table 1): ITK-SYK-Y183F; ITK-SYK-Y208F; ITK-SYK-Y208E; ITK-SYK-Y212F; ITK-SYK-Y212E; ITK-SYK-Y385F; ITK-SYK-Y386F; ITK-SYK-Y385F/Y386F; ITK-SYK-Y385E; ITK-SYK-Y386E; ITK-SYK-Y385E/Y386E; ITK-SYK-Y489F; ITK-SYK-Y490F; ITK-SYK-Y491F; ITK-SYK-Y489F/Y490F/Y491F, and ITK-SYK-Y489E/Y490E/Y491E. SYK-Y525E, SYK-Y526E, ZAP-70-Y492E, ZAP-70-Y493E were also used. GFP-SLP-76 and GFP-SLP-76–3YF were kindly provided by Dr. Stephen C. Bunnell, Tufts University. pCDNA3-SYK and pMSCV-SLP-76 plasmids have been described earlier (3).
TABLE 1.
Constructs used in this study. Names of the plasmids as well as mutated region/residues are described
| Name | Construct | Mutated region or residue |
|---|---|---|
| Mock/vehicle | pCDNA3 | |
| WT | pCDNA3-ITK-SYK | |
| KD | pCDNA3-ITK-SYK-K262R | Invariant catalytic lysine |
| Y183F | pCDNA3-ITK-SYK-Y183F | Interdomain-B |
| Y208F | pCDNA3-ITK-SYK-Y208F | Interdomain-B |
| Y212F | pCDNA3-ITK-SYK-Y212F | Interdomain-B |
| Y208E | pCDNA3-ITK-SYK-Y208E | Interdomain-B |
| Y212E | pCDNA3-ITK-SYK-Y212E | Interdomain-B |
| Y385F | pCDNA3-ITK-SYK-Y385F | Activation loop |
| Y386F | pCDNA3-ITK-SYK-Y386F | Activation loop |
| Y385F/Y386F | pCDNA3-ITK-SYK-Y385F/Y386F | Activation loop |
| Y385E | pCDNA3-ITK-SYK-Y385E | Activation loop |
| Y386E | pCDNA3-ITK-SYK-Y386E | Activation loop |
| Y385E/Y385E | pCDNA3-ITK-SYK-Y385E/Y385E | Activation loop |
| Y489F | pCDNA3-ITK-SYK-Y489F | C terminus |
| Y490F | pCDNA3-ITK-SYK-Y490F | C terminus |
| Y491F | pCDNA3-ITK-SYK-Y491F | C terminus |
| Y489F/Y490F/Y491F | pCDNA3-ITK-SYK-Y489F/Y490F/Y491F | C terminus |
| Y489E/Y490E/Y491E | pCDNA3-ITK-SYK-Y489E/Y490E/Y491E | C terminus |
| SYK | pCDNA3-SYK | |
| SYK-KD | pCDNA3-SYK-K402R | Invariant catalytic lysine |
| Y525E | pCDNA3-SYK-Y525E | Activation loop |
| Y526E | pCDNA3-SYK-Y526E | Activation loop |
| ZAP-70 | pCDNA3-ZAP-70 | |
| ZAP-70-KD | pCDNA3-ZAP-70-K369A | Invariant catalytic lysine |
| Y492E | pCDNA3-ZAP-70-Y492E | Activation loop |
| Y493E | pCDNA3-ZAP-70-Y493E | Activation loop |
| SLP-76-myc | pMSCV-SLP-76-myc | C-terminal Myc tag |
| GFP-SLP-76 | pEGFP-SLP-76 | N-terminal GFP tag |
| GFP-SLP-76-Y3F | pEGFP-SLP-76-Y3F | N-terminal tag and Y112F, Y128F, and Y145F |
Cell Lines and Transfections
COS7, 293T, Jurkat, SYKKO E6-1, and SYF cell lines were from the ATCC. SLP-76KO J14 and SYK/ZAP-70KO P116 were kind gifts of Dr. Arthur Weiss (University of California) and were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen). 293T, COS7, and SYF cells were transfected as described earlier (3). All Jurkat cell types were transfected using the Neon transfection system (Invitrogen). Briefly, 2 million cells were transfected with 2 μg of plasmid DNA.
FACS Analysis, IL-2 ELISA, and Western Blot
CD69 expression was analyzed on FACSCalibur, using phycoerythrin-tagged mouse anti-human CD69 antibody (BD Biosciences), 14 h post-transfection. The arithmetic mean from 50,000 live gated cells for CD69 expression intensity was taken, and results from four/six experiments were used for statistical analyses. IL-2 was measured 48 h post-transfection, using ELISA OPtEIA (BD Biosciences) as described by the manufacturer. Because of the multiple comparisons made, we have only indicated the statistical differences with p values <0.01. For clarity, in most figures, only the nonsignificant differences are indicated together with the least significant difference at p < 0.01. At 48 h post-transfection, cells were lysed with modified RIPA buffer containing protease and phosphatase inhibitors. Proteins were resolved on 4–12% SDS gels and transferred onto nitrocellulose membranes using Iblot (Invitrogen). The phospho-SYK antibodies Tyr-323, Tyr-352, and Tyr-525/526 were purchased from Cell Signaling Technology. SYK, MYC, p-Y128(SLP-76), pY (4G10), and actin antibodies have been mentioned earlier (3). Secondary antibodies goat anti-mouse-800CW, goat anti-rabbit-800CW, goat anti-mouse-680LT, and goat anti-rabbit-680 were from LI-COR Biosciences. Membranes were scanned on Odyssey Imager (LI-COR Biosciences).
RESULTS
ITK-SYK Induces Phosphorylation of SYK at Interdomain-B but Not at the Activation Loop Tyrosines
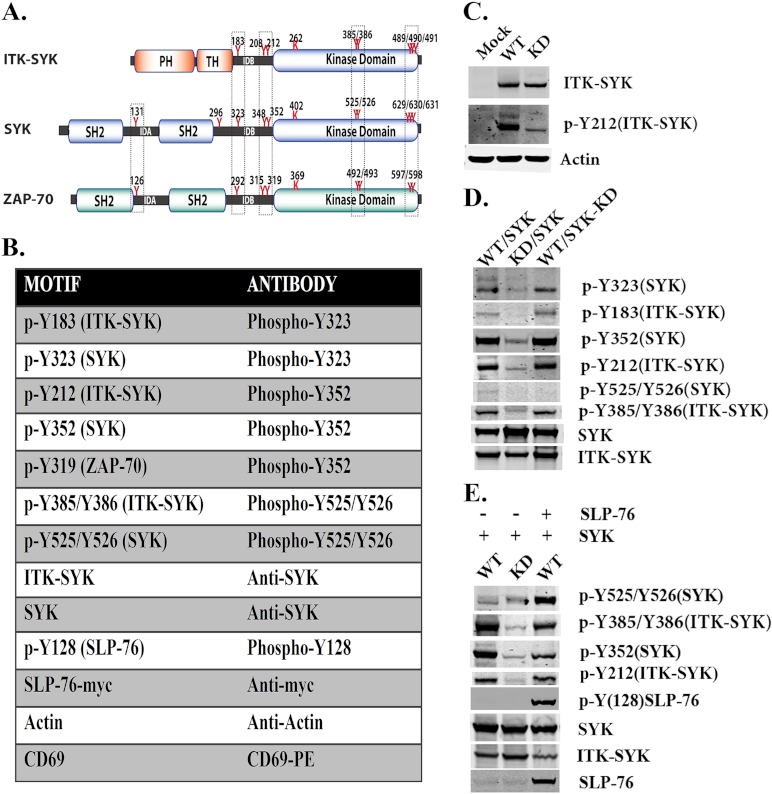
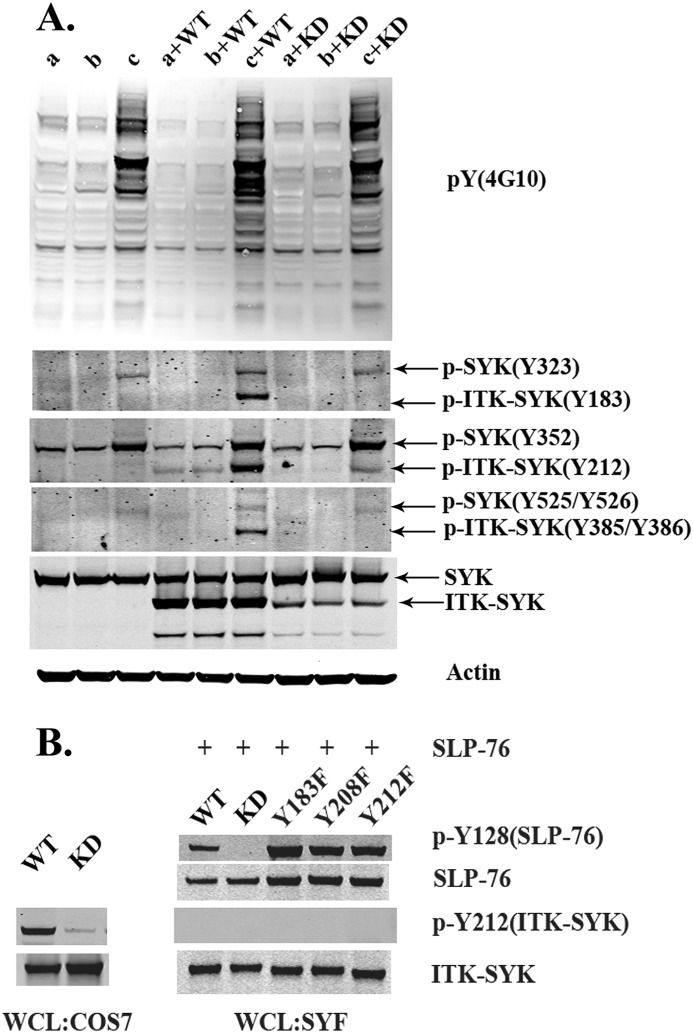
Interdomain-B Tyr-183, Tyr-208, and Tyr-212 in ITK-SYK correspond to Tyr-323, Tyr-348, and Tyr-352 in SYK and to Tyr-292, Tyr-315, and Tyr-319 in ZAP-70, respectively (Fig. 1A). We therefore set out to investigate the role of some of these residues in SYK and ITK-SYK. To detect the corresponding phosphorylated proteins, the same site-specific antibodies have been utilized (Fig. 1B). Previously, we have shown in steady state that ITK-SYK is phosphorylated at the interdomain-B tyrosine (Tyr-212) and at activation loop Tyr-385/Tyr-386, an observation that has now been reconfirmed (Fig. 1, C and D). In stark contrast, SYK displayed residual phosphorylation at the corresponding tyrosine (Tyr-352) in the interdomain-B but not at activation loop Tyr-525/Tyr-526 (3). In the same report, we have also demonstrated that ITK-SYK, but not SYK, potently phosphorylates the adapter proteins BLNK/SLP-65 and SLP-76 (3). To determine whether ITK-SYK is also involved in the activation of other members of the TCR signaling pathways, we transiently co-transfected constructs encoding ITK-SYK and SYK in the COS7 cell line (Fig. 1D). In the presence of ITK-SYK, substantial phosphorylation of SYK occurred at interdomain-B Tyr-323 and Tyr-352 (Fig. 1D). Notably, phosphorylation of Tyr-352 is known to positively regulate SYK kinase activity and is a target of both auto- and trans-phosphorylation events (31, 32). To further verify that these phosphorylations are not due to autocatalytic activity, a kinase-inactive mutant of SYK (SYK-KD) was co-expressed with ITK-SYK. Interestingly, ITK-SYK was found to phosphorylate the same residues (Tyr-323 and Tyr-352) in kinase-deficient SYK, ruling out the possibility of autocatalysis (Fig. 1D). Although SYK and SYK-KD were readily phosphorylated by ITK-SYK at Tyr-323 and Tyr-352, there was no subsequent phosphorylation in the activation loop Tyr-525/Tyr-526 (Fig. 1D).
FIGURE 1.
ITK-SYK phosphorylates SYK at the interdomain-B tyrosines 323 and 352. A, schematic representation of ITK-SYK, SYK, and ZAP-70. Critical tyrosines in each molecule are indicated. In ITK-SYK, the N terminus is derived from ITK, whereas the SYK fusion partner is devoid of SH2 domains together with the interdomain-A (IDA) as well as additional 43 residues of the interdomain-B (IDB). The interdomain-B Tyr-183, Tyr-208, and Tyr-212 of ITK-SYK correspond to interdomain-B Tyr-323, Tyr-348, and Tyr-352 of SYK and interdomain-B Tyr-292, Tyr-315, and Tyr-319 of ZAP-70, although activation loop tyrosines 385/386 of ITK-SYK correspond to the activation loop tyrosines 525/526 of SYK and 492/493 of ZAP-70. Similarly, C-terminal triplet tyrosines of ITK-SYK Tyr-489 to Tyr-491 correspond to the C-terminal triplet 629–631 of SYK and doublet 597/598 of ZAP-70. B, toolbox for antibodies used in this study (for details, see “Experimental Procedures”). C, Western blot analysis of COS7 cells transfected with plasmids encoding WT, KD, or plasmid pCDNA3. WT = ITK-SYK; KD = ITK-SYK kinase-inactive; Mock = pCDNA3. Native and phosphorylated proteins were detected with the corresponding antibodies described in B. D, Western blot analysis of COS7 cells transfected with plasmids encoding SYK or SYK-KD and with ITK-SYK or ITK-SYK-KD. E, Western blot analysis of COS7 cells co-expressing ITK-SYK and SYK, in the absence or presence of Myc-tagged SLP-76. (For details regarding constructs, see Table 1.)
SLP-76 Is Required for the ITK-SYK-mediated Phosphorylation of SYK at the Activation Loop Tyrosines
As shown above, ITK-SYK was found to robustly phosphorylate interdomain-B Tyr-323 and Tyr-352 but not those in the activation loop (Tyr-525/Tyr-526) (Fig. 1D). We anticipated that along with phosphorylation at interdomain-B Tyr-323 and Tyr-352, either SYK would be membrane-tethered or interact with some other protein facilitating the membrane tethering/open conformation, enabling its activation loop phosphorylation. To get a deeper insight into this, we decided to investigate the role of other proteins that are critical in TCR signaling. Because SLP-76 functions as a scaffold protein, its presence could potentially be required for the phosphorylation events at the activation loop. To investigate this possibility experimentally, SLP-76 was co-expressed with ITK-SYK and SYK. In the presence of SLP-76, ITK-SYK caused robust phosphorylation of the activation loop of SYK at Tyr-525/Tyr-526 (Fig. 1E).
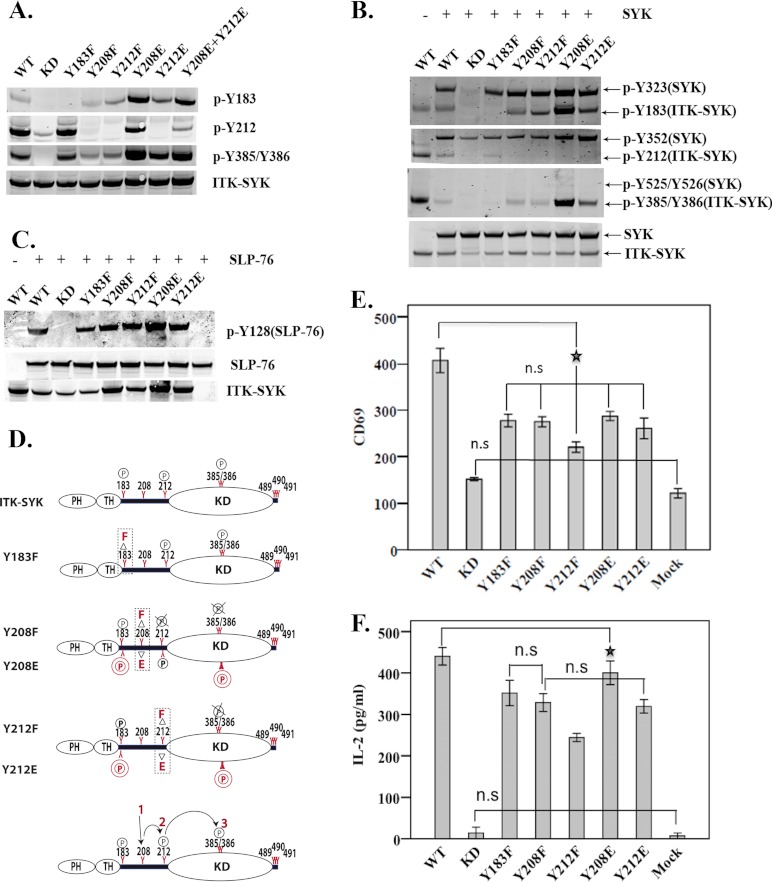
ITK-SYK Interdomain-B Tyrosines Show a Hierarchical Phosphorylation Pattern but Only Modestly Affect Downstream Signaling
We noticed that the ITK-SYK mutation of Tyr-183 to phenylalanine did not affect phosphorylation levels of Tyr-212 or Tyr-385/Tyr-386 (Fig. 2A). In contrast, phosphorylation at Tyr-212 and Tyr-385/Tyr-386 was severely compromised following substitution of Tyr-208. Similar results were obtained for Tyr-212, where Tyr-385/Tyr-386 phosphorylation was highly compromised (Fig. 2A). Furthermore, replacement of Tyr-208 and Tyr-212 with glutamic acid (Y208E and Y212E) resulted in increased phosphorylation at residues Tyr-183 and Tyr-385/Tyr-386. Phosphorylation at these residues in comparison with Y208E was, however, less in the Y212E mutant (Fig. 2A). Interestingly, co-expression of the ITK-SYK interdomain-B mutants with highly compromised phosphorylations together with SYK or SLP-76 resulted in the phosphorylation of SYK at Tyr-323 and Tyr-352 sites, in addition to SLP-76 phosphorylation (Fig. 2, B and C). To further dissect downstream signaling events, constructs encoding various versions of ITK-SYK were expressed in the Jurkat cell line, and expression of the surface receptor CD69 and secretion of IL-2 were monitored as T cell activation markers. Expression of CD69 was augmented in Jurkat cells transfected with ITK-SYK but not in those transfected with kinase-deficient ITK-SYK (Fig. 2E). Expression of the interdomain-B mutants as compared with wild-type ITK-SYK resulted in only slightly reduced CD69 expression and IL-2 secretion, with the most pronounced effects seen with the Y212F mutant (Fig. 2, F and E)
FIGURE 2.
Loss-of-function mutations of the ITK-SYK interdomain-B tyrosines reduce kinase activity and modestly impair downstream signaling. Whole cell lysates of COS7 cells expressing ITK-SYK IDB mutants alone (A) or together with SYK (B) (for details regarding constructs, see Table 1). Detection of native and phosphorylated proteins was as in Fig. 1B. C, Myc-tagged SLP-76 was expressed in COS7 cells alone or together with the corresponding ITK-SYK IDB mutants and processed for Western blot analysis as described in A and B. D, schematic representation showing the effects of mutations of various interdomain-B tyrosines. Phenylalanine (upper panel in each graph) was used as a loss-of-function mutation and glutamic acid (lower panel in each graph) as a potential gain-of-function. Bottom panel depicts the hierarchical phosphorylation pattern observed in IDB mutants. The P in 2 circles represents enhanced phosphorylation; the circled P indicates no change, and circled P with × corresponds to undetectable phosphorylation. E, FACS histograms showing means ± 1 S.D. (n = 6) of CD69 expression in Jurkat cells transfected with IDB plasmids. The ★ denotes the least statistically significant difference at p value <0.01. F, means ± 1 S.D. (n = 6) of IL-2 secretion (picograms/ml) in Jurkat cells expressing constructs encoding the same interdomain-B mutants. Compared with ITK-SYK, all mutants showed significant statistical difference at p value <0.01. The ★ denotes the least statistically significant difference at p value <0.01. n.s., statistically non-significant.
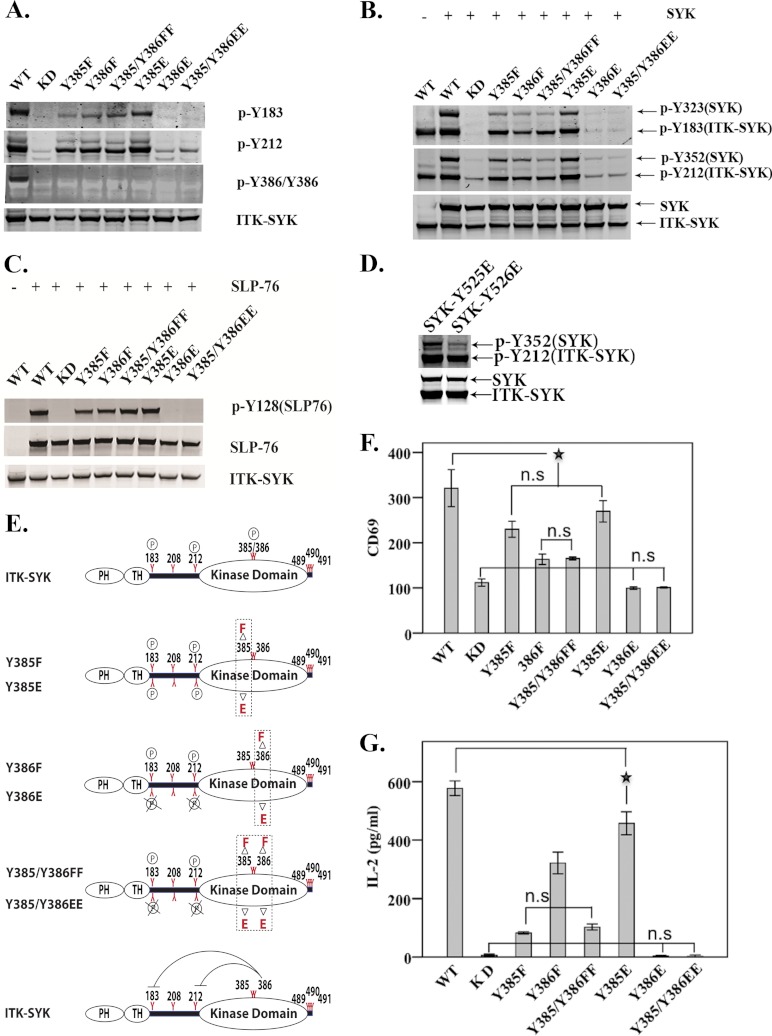
Activation Loop Tyrosines Have Differential Activity in Both ITK-SYK and SYK
ITK-SYK Tyr-385/Tyr-386 are located in the activation loop of the catalytic domain and correspond to Tyr-525/Tyr-526 of SYK (Fig. 1A). In ITK-SYK, loss of Tyr-385 or Tyr-386 subsequently led to compromised phosphorylation at Tyr-183 (Fig. 3A). Y385E, however, augmented phosphorylation at Tyr-183 (Fig. 3A). Whereas phosphorylation at Tyr-212 was comparable with that of ITK-SYK-KD, there was no detectable phosphorylation at position Tyr-183 in cells expressing mutants Y386E or Y385E/Y386E (Fig. 3A). In addition, co-expression of ITK-SYK activation loop mutants Y385F, Y386F, and Y385F/Y386F with SYK only induced weak phosphorylation of SYK at Tyr-323 and Tyr-352, in comparison with wild-type ITK-SYK. In contrast, phosphorylation of these sites was augmented in the Y385E mutant (Fig. 3B). However, phosphorylation of SYK was indistinguishable from that of ITK-SYK-KD in cells transfected with the mutants Y386E or Y385E/Y386E (Fig. 3B). Finally, with the exception of Y386E and Y385E/Y386E, all activation loop mutants maintained catalytic activity when SLP-76 was used as a substrate (Fig. 3C). Comparable results were obtained following co-expression of SYK activation loop mutants with ITK-SYK (Fig. 3D). Although SYK-Y525E was readily phosphorylated, SYK-Y526E showed compromised phosphorylation at the interdomain-B Tyr-352 (Fig. 3D).
FIGURE 3.
Differential roles of the activation loop tyrosines in ITK-SYK and SYK. Western blot analysis of COS7 cells expressing ITK-SYK activation loop tyrosine mutants alone (A) or with SYK (B). (For details regarding constructs, see Table 1.) C, SLP-76 is a direct substrate of ITK-SYK. Western blot analysis of COS7 cells co-transfected with plasmid constructs encoding SLP-76 and ITK-SYK activation loop mutants. D, SYK activation loop mutants were co-transfected with ITK-SYK, and total and phosphorylated proteins were detected as described in Fig. 1B. The effect of Y525E substitution did not differ from wild-type SYK (data not shown). E, schematic representation of phosphorylation status of ITK-SYK activation loop mutants. Phenylalanine (upper panel in each graph) is a nonphosphorylatable substitution, whereas glutamic acid (lower panel in each graph) is a phosphomimetic with regard to the altered charge. Bottom panel illustrates negative regulation of kinase activity by Y386E mutant. The circled P represents no change, and circled P with × corresponds to undetectable phosphorylation. F, FACS histogram indicates means ± 1 S.D. (n = 6) of CD69 expression of Jurkat cells with the corresponding ITK-SYK activation loop plasmids. Compared with ITK-SYK, all mutants displayed significant statistical difference at p value of <0.01. ★ denotes two of the least statistically significant differences at p value of <0.01. G, mean values ± 1 S.D. (n = 6), of IL-2 secretion (picograms/ml) in Jurkat cells. Compared with ITK-SYK, all mutants showed significant statistical differences at p value of <0.01. ★ denotes the least statistically significant difference at p value <0.01. n.s., statistically non-significant.
In Fig. 3, F and G, for clarity, only insignificant changes are indicated together with the least significant comparison giving a p value of <0.01. Thus, compared with wild-type ITK-SYK, all of the mutants had very significantly reduced activity. Expression of Y385F and Y385E in Jurkat cells modestly affected CD69 expression, and there was no statistically significant difference between these mutants (Fig. 3F). In contrast, the CD69 expression was very much reduced in cells transfected with constructs encoding Y386F or Y385F/Y386F (Fig. 3F). Moreover, the impact of phenylalanine replacements was more profound when measuring IL-2 secretion as compared with CD69 expression in Y385F- or Y385F/Y386F-transfected cells (Fig. 3G). Similar to their effects on tyrosine phosphorylation, the glutamic acid substitution mutants involving Y386E (i.e. Y386E and Y385E/Y386E) were highly deficient in CD69 expression, and IL-2 secretion was almost undetectable (Fig. 3, F and G).
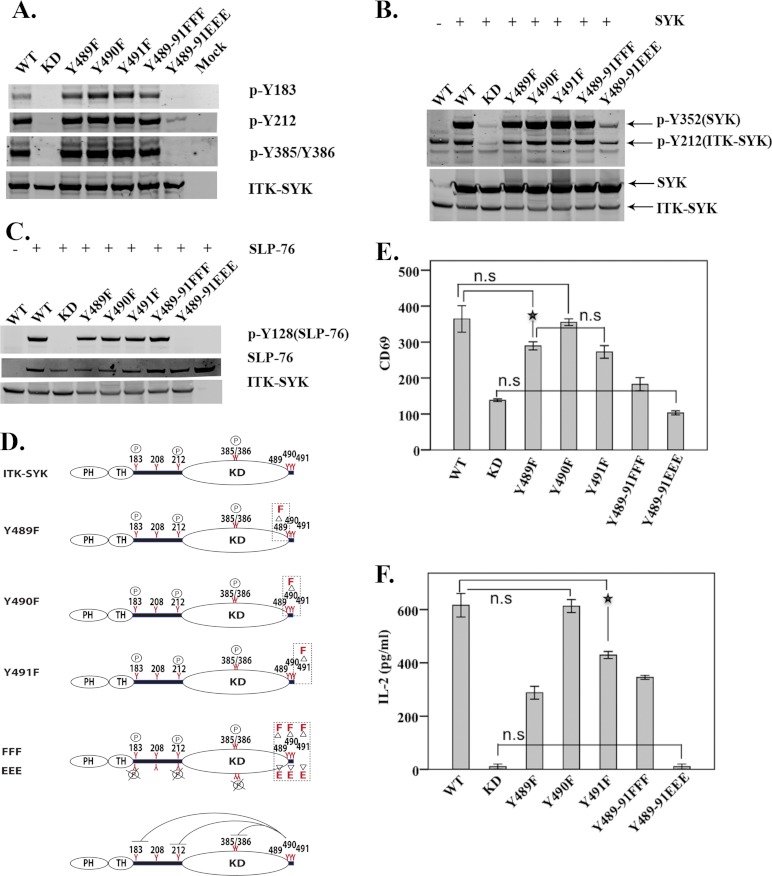
Regulatory Effect of the C-terminal Tyrosines on ITK-SYK Kinase Activity
The three C-terminal tyrosines Tyr-489, Tyr-490, and Tyr-491 of ITK-SYK correspond to the three conserved SYK C-terminal tyrosines Tyr-629, Tyr-630, Tyr-631 and to the two ZAP-70 tyrosines Tyr-596 and Tyr-597 (Fig. 1A). To investigate the role of the C-terminal tyrosines in the regulation of the ITK-SYK activation, we mutated Tyr-489, Tyr-490, and Tyr-491, singly or all three in tandem, to phenylalanine. We then expressed ITK-SYK mutants Y489F, Y490F, Y491F, and Y489F/Y490F/Y491F separately in heterologous cell lines. All these C-terminal ITK-SYK mutants were phosphorylated on the interdomain-B together with the activation loop tyrosines, similar to wild-type ITK-SYK (Fig. 4A). Similar results were also obtained when the tyrosine phosphorylation on SYK and SLP-76 was investigated (Fig. 4, B and C).
FIGURE 4.
Inhibitory roles of C-terminal tyrosines in ITK-SYK. Western blot analysis of COS7 whole cell lysates expressing C-terminal mutants of ITK-SYK alone (A) or with SYK (B). (Constructs are described in Table 1.) Native and phosphorylated proteins were detected using reagents described in Fig. 1B. C, SLP-76 was expressed in COS7 cells either alone or with C-terminal mutants of ITK-SYK. Total and phosphorylated proteins were detected using reagents described in Fig. 1B. D, graphic representation of phosphorylation status of C-terminal ITK-SYK mutants. Phenylalanine (upper panel in each graph) was used as a nonphosphorylatable mutation and glutamic acid (lower panel in each graph) as a phosphomimetic with regard to the altered charge. Bottom panel depicts the negative regulation of the molecule by triple phosphomimetic ITK-SYK-Y489E/Y490E/Y491E (EEE). The circled P represents no change, and circled P with × corresponds to undetectable phosphorylation. E, FACS histogram means ± 1 S.D. (n = 6) of CD69 expression in Jurkat cells with the corresponding C-terminal ITK-SYK mutants. Compared with ITK-SYK, all mutants, except Y490F, showed significant statistical difference at a p value of 0.01. ★ denotes the least statistically significant difference at p value of <0.01. F, means ± 1 S.D. (n = 6) of IL-2 secretion (picograms/ml) in Jurkat cells. Compared with the ITK-SYK, all mutants, except Y490F, showed significant statistical difference at p value of <0.01. ★ denotes the least statistically significant difference at p value of <0.01. n.s., statistically non-significant.
To assess the impact of the negative charge triggered during phosphorylation at the ITK-SYK C terminus, we generated an additional triple mutant Y489E/Y490E/Y491E. In stark contrast, the triple mutant displayed no detectable phosphorylation at Tyr-183, and only residual phosphorylation at the interdomain-B Tyr-212 and virtually nothing at the activation loop Tyr-385/Tyr-386 (Fig. 4A). Y489E/Y490E/Y491E slightly phosphorylated SYK but failed to phosphorylate SLP-76 (Fig. 4, B and C), suggesting that this triple mutant Y489E/Y490E/Y491E is not capable of mediating signaling to SYK and SLP-76 (Fig. 4, A–F). In Fig. 4, E and F, for clarity, only all nonsignificant changes are indicated together with the least significant comparison giving a p value of <0.01. Thus, with the exception of the Y490F mutant, which behaves similar to wild-type ITK-SYK, all other mutants show significantly reduced CD69 expression and IL-2 secretion. The Y489E/Y490E/Y491E triple mutant is similar to kinase-dead ITK-SYK (Fig. 4, E and F).
SLP-76 Is Indispensable for Elevated CD69 Expression and IL-2 Secretion
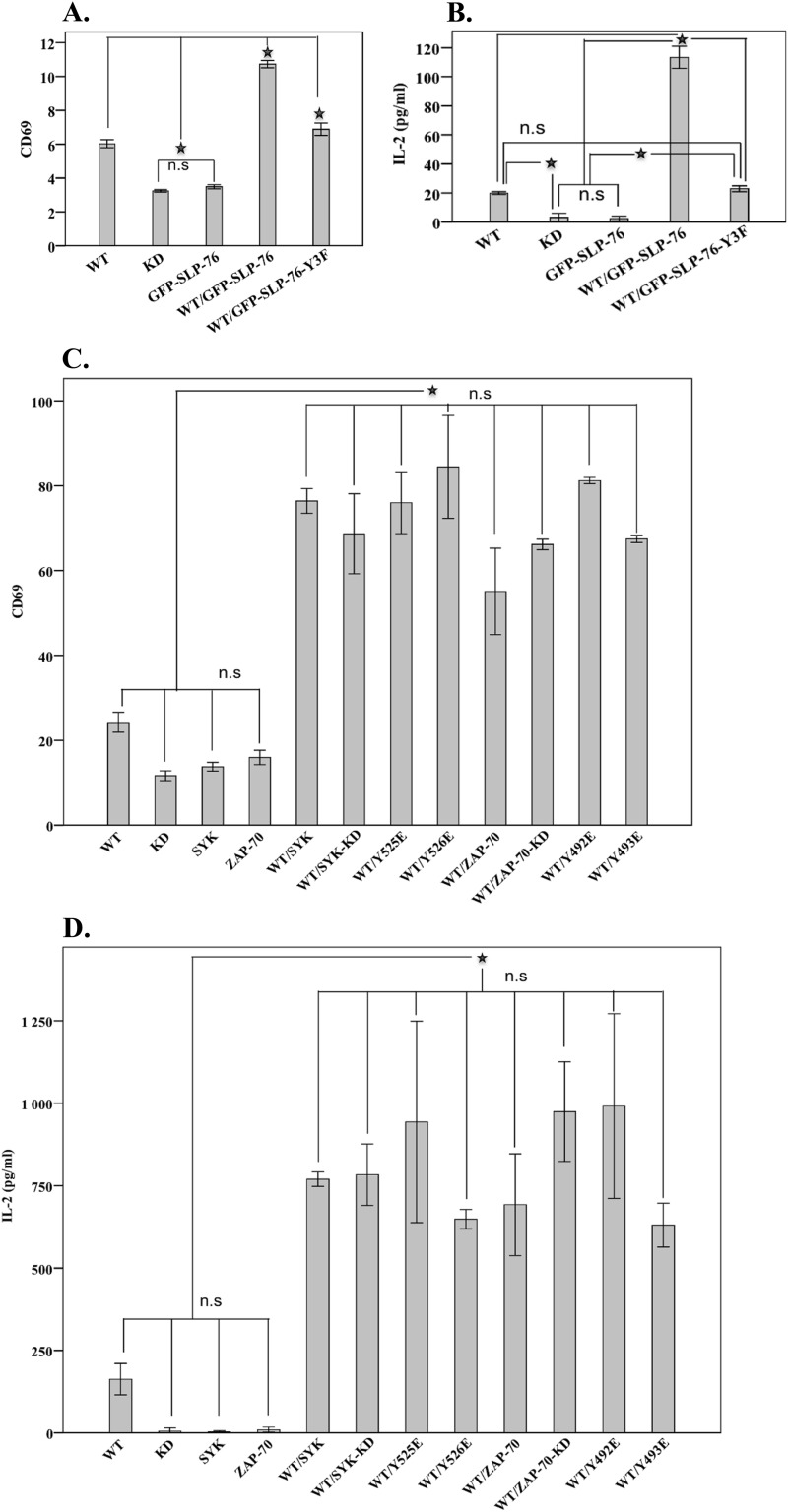
SLP-76 lacks intrinsic catalytic activity and instead regulates signal transduction by controlling the spatial and temporal availability of interacting molecules. To directly assess the requirement for SLP-76 downstream of ITK-SYK, we employed the SLP-76-deficient Jurkat T cell line (J14). ITK-SYK was co-expressed with either wild-type SLP-76 or with a SLP-76 mutant lacking all three regulatory tyrosines at the N terminus. In the absence of SLP-76, expression of ITK-SYK led to a modest expression of CD69 and IL-2 secretion (Fig. 5, A and B). In the absence of ITK-SYK, expression of SLP-76 had no effect on CD69 expression or the secretion of IL-2 (Fig. 5, A and B). However, co-expression of ITK-SYK with SLP-76 induced a dramatic elevation in CD69 expression and IL-2 secretion. We also demonstrate that the three functional tyrosines of SLP-76 are required for ITK-SYK-mediated IL-2 secretion and CD69 expression (Fig. 5, A and B). Thus, CD69 expression and IL-2 secretion are entirely dependent on the presence of an intact SLP-76 in a native T cell milieu.
FIGURE 5.
ITK-SYK-mediated CD69 elevated expression or IL2 secretion is dependent on the adapter function of SLP-76 and SYK/ZAP-70. FACS histogram means ± 1 S.D. (n = 4) for CD69 expression from J14 (deficient in SLP-76) (A) and P116 cells (C) (deficient in both SYK and ZAP-70). Means ± 1 S.D. (n = 4) of IL-2 secretion (picograms/ml) in J14 (B) and P116 cells (D). The SLP-76-deficient Jurkat cell line (A and B) was transfected with ITK-SYK alone or together with constructs encoding GFP-SLP-76 or GFP-SLP-76-Y3F (in plasmid GFP-SLP-76-Y3F, the three N-terminal tyrosines 112, 128, and 145 were replaced with phenylalanine, see Table 1). ★ denotes the statistically significant difference at p value <0.01. C and D, cells were transfected with the corresponding plasmids of ITK-SYK with or without SYK or ZAP-70 or their mutants. ★ denotes the statistically significant difference for CD69 expression or IL-2 secretion at p value of <0.01. n.s., statistically non-significant.
Adapter Function of SYK and ZAP-70 Enhances the Activation of T Cells
To determine whether SYK and ZAP-70 have functional roles in ITK-SYK-mediated T cell signaling, we employed a specific Jurkat T cell line lacking both SYK and ZAP-70 (P116). To test this hypothesis, the P116 Jurkat cell line was transfected with ITK-SYK with or without SYK or ZAP-70 mutants (Fig. 5, C and D). To this end, we found that ITK-SYK expression caused a low level CD69 expression and IL-2 secretion. ITK-SYK-KD-induced CD69 expression or IL-2 secretion was not statistically significant from the cells expressing SYK or ZAP-70 (p value <0.01) (Fig. 5, C and D). Co-expression of ITK-SYK with either of the SYK family members induced significant CD69 expression or IL-2 secretion (Fig. 5, C and D). In Fig. 5, C and D, for clarity, only all nonsignificant changes are indicated together with the least significant comparison with a p value of <0.01.
Previously, a kinase-independent adapter-function for SYK and ZAP-70 has been reported both in normal and malignant cell signaling (15, 29, 33, 34). We found that co-expression of kinase-inactive or activation loop glutamic acid mutants of SYK or ZAP-70 with ITK-SYK led to a pronounced increase in CD69 expression and IL-2 secretion (Fig. 5, C and D). The increase was comparable with that of the co-expression of wild-type SYK or ZAP-70 (Fig. 5, C and D) demonstrating the importance of the adapter function of these kinases.
Phosphorylation of ITK-SYK in T Cells
To further investigate the phosphorylation status of ITK-SYK in T cells, we transiently transfected the various constructs into Jurkat cells. To this end, we monitored the phosphorylation of four different tyrosines, Tyr-183, Tyr-212, and the Tyr-385/Tyr-386 doublet, of ITK-SYK in Jurkat cells. In steady state, phosphorylation was virtually undetectable, although traces of tyrosine phosphorylation could be seen at the interdomain-B Tyr-212 (Fig. 6A, lower panels). Similarly, a phosphorylation background was visible in cells following serum starvation. In contrast, there was no detectable phosphorylation at the other interdomain-B Tyr-183 or the activation loop Tyr-385/Tyr-386, in quiescent or in steady state conditions. We speculate that ITK-SYK is constantly dephosphorylated in Jurkat cells, which could be the underlying reason for the inability of detection of phosphorylation in these cells. Therefore, we transfected Jurkat cells with plasmids expressing ITK-SYK or ITK-SYK-KD and activated cells with serum plus pervanadate. Remarkably, in the presence of pervanadate, activation loop tyrosines of ITK-SYK, but not those of SYK, were heavily phosphorylated (Fig. 6, lower panels, lane c). This observation again supports our hypothesis that in steady state ITK-SYK maintains an open conformation.
FIGURE 6.
Phosphorylation of ITK-SYK in T lymphocytes and in SYF cells. A, Western blot analysis of Jurkat cells transfected with ITK-SYK or ITK-SYK-KD. Following transfection, cells were processed for immunoblotting and decorated with phosphospecific antibodies as described in Fig. 1B. Cells were either serum-starved (2 h) or treated with pervanadate (100 μm, 20 min on ice). Minus serum (lane a), steady state (lane b), and pervanadate (lane c). The upper panel shows global tyrosine phosphorylation, and the lower panel shows phosphorylation of critical tyrosines in endogenous SYK, ITK-SYK, and kinase-inactive ITK-SYK. B, Western blot analysis of SYF cells, lacking SFKs, transfected with ITK-SYK and ITK-SYK mutants. (For details regarding constructs, see Table 1.) Lysates from COS7 cells transfected with ITK-SYK or ITK-SYK-KD were run in parallel as control for detection of ITK-SYK phosphorylation at Tyr-212.
Constitutive Activation of ITK-SYK Is Independent of SFKs
To investigate the role of SFKs in ITK-SYK activation and signaling, a fibroblast cell line (SYF) lacking all members of the SFKs was used. SYF cells are deficient for the three major fibroblast-specific members of the SFKs, SRC, YES, and FYN (35). Because, according to our earlier observations, the kinase activity of ITK-SYK was not affected following loss of putative SFK-phosphorylated tyrosines, we envisaged that activation and phosphorylation of ITK-SYK was entirely independent of SFKs. To this end, constructs encoding ITK-SYK were transfected together with a mammalian expression vector encoding SLP-76 in SYF cells. Interestingly, ITK-SYK, but not kinase-defective ITK-SYK (KD), readily phosphorylated SLP-76 (Fig. 6B). Surprisingly, there was no detectable phosphorylation of ITK-SYK at Tyr-183, Tyr-212, or Tyr-385/Tyr-386 (even in the presence of pervanadate) following overexpression of ITK-SYK in SYF cells (Fig. 6B). Lysates of COS7 cells expressing ITK-SYK or ITK-SYK-KD were run in a parallel experiment as a control of phosphorylation. These data lend credit to our previous observations, consistent with the notion that ITK-SYK is in an open conformation and is capable of phosphorylating its substrates independent of SFKs.
DISCUSSION
Phosphorylation is a key regulatory mechanism controlling various aspects of proteins, including function, stability, subcellular localization, and protein-protein interaction. Tyrosine kinases are regulated by addition or removal of phosphates at certain tyrosine residues. Phosphorylation switches the kinase from low enzymatic activity to elevated catalysis, notwithstanding that in certain cases the same post-translational modification at other residues instead leads to inhibition (16). In general, kinases are kept at a low basal state of activity through various auto-inhibitory mechanisms as well as through the action of protein-tyrosine phosphatases. Depending on their modular structure, different families of tyrosine kinases employ different activation strategies. For example, in SFKs the underlying mechanism is referred to as a “graded” switch because they are activated in steps, and each step results in catalytic elevation, although TFKs, like ITK, need two different steps for their activation, and this mechanism is denoted as an “AND” switch (16). SYK and ZAP-70 belong to a family of kinases with “OR” switches, because they can be activated in two different modes, SH2 domain-binding to immunoreceptor tyrosine-based activation motifs or phosphorylation of their interdomains. Because of the nature of its structure, ITK-SYK seems to defy the classic inhibitory and activation mechanisms. This fusion kinase has acquired plasma membrane-targeting ability due to the presence of a PH-TH domain as well as an open conformation created by lack of the two SH2 domains of SYK. In wild-type SYK, the tandem SH2 domains are responsible for maintaining the conformation of the kinase in an inactive state (15).
In our study, we used phenylalanine as a nonphosphorylatable replacement of tyrosine and glutamic acid to provide negative charge to mimic the effects of a phosphorylated tyrosine. The negative charge associated with glutamic acid might indeed mimic Tyr(P) in contexts where phosphorylation destabilizes one conformation to favor another conformation. In contrast, when Tyr(P) is part of a ligand-binding site, and this may not be well mimicked by the tyrosine to glutamic acid substitution. Thus, IL-2 secretion and elevation in CD69 expression in glutamic acid substitutions may not completely reproduce the effects of phosphorylations due to suboptimal ligand binding.
Mutation analysis of ITK-SYK revealed interesting features of ITK-SYK, including an essentially maximal constitutive kinase activity in the native state and a hierarchical phosphorylation pattern. Whereas the ITK-SYK-Y183F mutant is demonstrated to be associated with modestly reduced IL-2 secretion and CD69 expression, mutation of the corresponding tyrosine 323 in SYK results in a gain-of-function phenotype (23). However, Tyr-208 and Tyr-212 displayed a hierarchical phosphorylation pattern (Fig. 2A). Sequential phosphorylations have also been observed in many other proteins, where phosphorylation at one site primes them for subsequent phosphorylation at another site (36). Because ITK-SYK might presumably exist in an open, active conformation, loss of phosphorylation at interdomain-B most likely influences its interactions, yet it does not interrupt downstream signaling events. The corresponding interdomain-B glutamic acid mutations lead to enhanced phosphorylation of the tyrosines in both this linker and in the activation loop. However, downstream signaling was essentially unaffected, suggesting that because, in steady state, the conformation of the fusion protein is already open, the interdomain-B region remains basically inert. Thus, amino acid replacements of potentially regulatory tyrosines in this region will not have any major impact on ITK-SYK kinase function.
Although the activation loop Y386E mutation was inhibitory, the adjacent Y385E displayed no obvious effect on kinase activity. Similar results were observed in the double mutant Y385E/Y386E, which failed to phosphorylate SYK and ZAP-70 and was unable to induce CD69 expression or enhanced IL-2 secretion (Fig. 3, A–G). To this end, the paired activation loop tyrosines of ITK-SYK 385/386 correspond to 525/526 tyrosines of SYK. The crystal structure of SYK shows that tyrosine 525 faces outside the “turn motif,” whereas tyrosine 526 is located in the inner side of the motif being close to the positively charged amino acids Lys-517 and Arg-493 (27). It is tempting to speculate that it is the close physical association of Tyr(P)-386 with Lys-377 and/or Arg-353, which might be responsible for impairing the catalytic activity. In ITK-SYK, phosphorylation of Tyr-386 seems to be tightly coupled to that of Tyr-385. It is possible that the situation is reminiscent to that of the insulin receptor tyrosine kinase, where activation loop tyrosines are sequentially phosphorylated. Thus, if a negative charge or phosphorylation appears first at Tyr-386 or simultaneously at both Tyr-385 and Tyr-386, it could lead to inhibition, because this does not follow a sequential order of phosphorylation. Nevertheless, undesired effects of glutamic acid replacement of Tyr-386 cannot be ignored. Further studies are warranted to better understand the differential regulation of activation loop tyrosines in ITK-SYK. According to a recent report, loss of the C-terminal tyrosines in SYK led to enhanced phosphorylation in the activation loop tyrosines (30). In contrast, ITK-SYK was equally phosphorylated at the corresponding activation loop tyrosines 385/386 in the absence or presence of functional C-terminal tyrosines (Fig. 4A). Moreover, unlike SYK, loss of C-terminal tyrosines did not result in further enhancement of phosphorylation at the activation loop tyrosines in ITK-SYK (Fig. 4A). These data suggest that, because ITK-SYK likely adopts an open conformation, the inhibitory role of the C-terminal tyrosines is normally not operational. However, in the triple mutant Y489E/Y490E/Y491E, inhibition is complete suggesting that negatively charged residues will significantly change the three-dimensional configuration of the fusion protein.
The degree of phosphorylation is often used as a general measure of the catalytic status of a kinase. However, from the analysis of the ITK-SYK mutants, it is clear that phosphorylation may differ from the catalytic activity on downstream substrates and subsequent biological readouts. Thus, although phosphorylations correlate with these phenomena, it is not identical. As an example, the Y208E mutant showed increased phosphorylations on its other residues as compared with wild-type ITK-SYK, yet SLP-76 phosphorylation did not differ, and the downstream activation markers, CD69 and IL-2, were in fact reduced, even if very modestly. A remarkable observation was that ITK-SYK could phosphorylate SLP-76 in SYF cells, which lack SFKs. This shows that ITK-SYK does not need to be switched on by SFKs, which is frequently seen for other kinases, such as for TFKs themselves.
Whether the conformation of the PH-TH domain has some influence in the context of ITK-SYK is presently unknown. However, functional expression of an ITK-SYK PH domain mutant (R29C) in mice was shown to be associated with perhaps an even increased transformation potential and tumorigenicity in the animals (6). Thus, rather than membrane tethering, we favor the idea that it is the open conformation of ITK-SYK per se, which is the key for its oncogenic activity. This is also supported by its capacity to phosphorylate activation loop tyrosine residues in the absence of SLP-76. Also, it is interesting that SLP-76 is required for the phosphorylation of SYK at the activation loop tyrosines 525/526 but not for the interdomain-B tyrosines. The open conformation is further supported by the fact that enhanced activity of ITK-SYK-induced downstream signaling was not readily obtained in any of the mutants.
With respect to the finding that the adapter function of SYK/ZAP-70 is critical in the downstream signaling of ITK-SYK, there is precedence. For example, in chronic lymphocytic leukemia B cells, ZAP-70-mediated enhancement of B cell receptor signaling is independent of its kinase function (34). Likewise, in normal cells, the importance of the adapter function of ZAP-70 has been shown for the development of immature CD4+CD8+ thymocytes having limited expression of LCK (37). In SYK, the importance of an adapter function for calcium mobilization was demonstrated in the chicken cell line DT-40 cells as well as in mouse B lymphocytes (29, 33). In a recent report, suppression of Treg cells was also demonstrated to be independent of ZAP-70 kinase activity (38). Altogether, the essential adapter activity of SYK/ZAP-70 in ITK-SYK-mediated signaling is not a unique phenomenon per se, but ITK-SYK may be the only example where the adapter effect was equally demonstrated for SYK and ZAP-70.
The primary objective of this study was to investigate activation and signaling of ITK-SYK. However, in the event that certain observations that have been documented in the various ITK-SYK mutants were replicated in earlier experiments involving SYK and ZAP-70, this would of course be of general interest. We have so far only analyzed potential glutamic acid substitutions in the SYK Tyr-525/Tyr-526 pair. However, interestingly, a related effect to what was observed in ITK-SYK was also noted when Tyr-526 in SYK (corresponding to Tyr-386 in ITK-SYK) was replaced with glutamic acid, namely that the interdomain-B phosphorylation was negatively affected (Fig. 3D). We speculate that this may also be the case for ZAP-70. Our data also suggest that the same may be true for glutamic acid substitutions in the C-terminal tail of native SYK and ZAP-70. Further studies are currently in progress to investigate the corresponding C-terminal glutamic acid substitutions in SYK and ZAP-70.
Acknowledgments
We thank Dr. Arthur Weiss for the kind gifts of Jurkat knock-out cell lines and Dr. Stephen C. Bunnell for the kind gifts of GFP-SLP-76 plasmids. We are very grateful to Iram Bilal for assistance with in silico analyses.
This work was supported in part by the Swedish Cancer Society, the Swedish Research Council, European Council FP7 Grant EURO-PAD net, and the regional agreement on medical training and clinical research (Avtalet mellan Landstinget och staten om samarbete om Läkarutbildning och Forskning) between Stockholm County Council and Karolinska Institutet.
- ITK
- IL-2 inducible T cell kinase
- SYK
- spleen tyrosine kinase
- SH2
- domain containing leukocyte protein of 76 kDa
- PH
- pleckstrin homology
- TH
- Tec homology
- SH
- Src homology
- ZAP-70
- ζ-chain-associated protein kinase 70 kDa
- IL-2
- interleukin-2
- TCR
- T cell receptor
- TFK
- TEC family kinase
- SFK
- SRC family kinase
- SLP-76
- SH2 domain-containing leukocyte protein of 76 kDa.
REFERENCES
- 1. Streubel B., Vinatzer U., Willheim M., Raderer M., Chott A. (2006) Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20, 313–318 [DOI] [PubMed] [Google Scholar]
- 2. Huang Y., Moreau A., Dupuis J., Streubel B., Petit B., Le Gouill S., Martin-Garcia N., Copie-Bergman C., Gaillard F., Qubaja M., Fabiani B., Roncador G., Haioun C., Delfau-Larue M. H., Marafioti T., Chott A., Gaulard P. (2009) Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 33, 682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussain A., Faryal R., Nore B. F., Mohamed A. J., Smith C. I. (2009) Phosphatidylinositol-3-kinase-dependent phosphorylation of SLP-76 by the lymphoma-associated ITK-SYK fusion protein. Biochem. Biophys. Res. Commun. 390, 892–896 [DOI] [PubMed] [Google Scholar]
- 4. Rigby S., Huang Y., Streubel B., Chott A., Du M. Q., Turner S. D., Bacon C. M. (2009) The lymphoma-associated fusion tyrosine kinase ITK-SYK requires pleckstrin homology domain-mediated membrane localization for activation and cellular transformation. J. Biol. Chem. 284, 26871–26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pechloff K., Holch J., Ferch U., Schweneker M., Brunner K., Kremer M., Sparwasser T., Quintanilla-Martinez L., Zimber-Strobl U., Streubel B., Gewies A., Peschel C., Ruland J. (2010) The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J. Exp. Med. 207, 1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dierks C., Adrian F., Fisch P., Ma H., Maurer H., Herchenbach D., Forster C. U., Sprissler C., Liu G., Rottmann S., Guo G. R., Katja Z., Veelken H., Warmuth M. (2010) The ITK-SYK fusion oncogene induces a T-cell lymphoproliferative disease in mice mimicking human disease. Cancer Res. 70, 6193–6204 [DOI] [PubMed] [Google Scholar]
- 7. Yu L., Smith C. I. (2011) Tec family kinases. FEBS J. 278, 1969. [DOI] [PubMed] [Google Scholar]
- 8. Hussain A., Yu L., Faryal R., Mohammad D. K., Mohamed A. J., Smith C. I. (2011) TEC family kinases in health and disease–loss-of-function of BTK and ITK and the gain-of-function fusions ITK-SYK and BTK-SYK. FEBS J. 278, 2001–2010 [DOI] [PubMed] [Google Scholar]
- 9. Salim K., Bottomley M. J., Querfurth E., Zvelebil M. J., Gout I., Scaife R., Margolis R. L., Gigg R., Smith C. I., Driscoll P. C., Waterfield M. D., Panayotou G. (1996) Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 15, 6241–6250 [PMC free article] [PubMed] [Google Scholar]
- 10. Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G., et al. , (1993) Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261, 358–361 [DOI] [PubMed] [Google Scholar]
- 11. Mohamed A. J., Yu L., Bäckesjö C. M., Vargas L., Faryal R., Aints A., Christensson B., Berglöf A., Vihinen M., Nore B. F., Smith C. I. (2009) Bruton's tyrosine kinase (Btk). Function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228, 58–73 [DOI] [PubMed] [Google Scholar]
- 12. Thomas J. D., Sideras P., Smith C. I., Vorechovský I., Chapman V., Paul W. E. (1993) Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261, 355–358 [DOI] [PubMed] [Google Scholar]
- 13. August A., Sadra A., Dupont B., Hanafusa H. (1997) Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the pleckstrin homology domain of inducible T cell kinase. Proc. Natl. Acad. Sci. U.S.A. 94, 11227–11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geahlen R. L. (2009) Syk and pTyr'd. Signaling through the B cell antigen receptor. Biochim. Biophys. Acta 1793, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulathu Y., Grothe G., Reth M. (2009) Autoinhibition and adapter function of Syk. Immunol. Rev. 232, 286–299 [DOI] [PubMed] [Google Scholar]
- 16. Bradshaw J. M. (2010) The Src, Syk, and Tec family kinases. Distinct types of molecular switches. Cell. Signal. 22, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 17. Groesch T. D., Zhou F., Mattila S., Geahlen R. L., Post C. B. (2006) Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J. Mol. Biol. 356, 1222–1236 [DOI] [PubMed] [Google Scholar]
- 18. Carsetti L., Laurenti L., Gobessi S., Longo P. G., Leone G., Efremov D. G. (2009) Phosphorylation of the activation loop tyrosines is required for sustained Syk signaling and growth factor-independent B-cell proliferation. Cell. Signal. 21, 1187–1194 [DOI] [PubMed] [Google Scholar]
- 19. Zeitlmann L., Knorr T., Knoll M., Romeo C., Sirim P., Kolanus W. (1998) T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J. Biol. Chem. 273, 15445–15452 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y., Oh H., Burton R. A., Burgner J. W., Geahlen R. L., Post C. B. (2008) Tyr130 phosphorylation triggers Syk release from antigen receptor by long-distance conformational uncoupling. Proc. Natl. Acad. Sci. U.S.A. 105, 11760–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potter M. D., Barbero S., Cheresh D. A. (2005) Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and β-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 280, 31906–31912 [DOI] [PubMed] [Google Scholar]
- 22. Ota Y., Beitz L. O., Scharenberg A. M., Donovan J. A., Kinet J. P., Samelson L. E. (1996) Characterization of Cbl tyrosine phosphorylation and a Cbl-Syk complex in RBL-2H3 cells. J. Exp. Med. 184, 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hong J. J., Yankee T. M., Harrison M. L., Geahlen R. L. (2002) Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase. J. Biol. Chem. 277, 31703–31714 [DOI] [PubMed] [Google Scholar]
- 24. Zhao Q., Weiss A. (1996) Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol. Cell. Biol. 16, 6765–6774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deindl S., Kadlecek T. A., Brdicka T., Cao X., Weiss A., Kuriyan J. (2007) Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 129, 735–746 [DOI] [PubMed] [Google Scholar]
- 26. Brdicka T., Kadlecek T. A., Roose J. P., Pastuszak A. W., Weiss A. (2005) Intramolecular regulatory switch in ZAP-70. Analogy with receptor tyrosine kinases. Mol. Cell. Biol. 25, 4924–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atwell S., Adams J. M., Badger J., Buchanan M. D., Feil I. K., Froning K. J., Gao X., Hendle J., Keegan K., Leon B. C., Müller-Dieckmann H. J., Nienaber V. L., Noland B. W., Post K., Rajashankar K. R., Ramos A., Russell M., Burley S. K., Buchanan S. G. (2004) A novel mode of Gleevec binding is revealed by the structure of spleen tyrosine kinase. J. Biol. Chem. 279, 55827–55832 [DOI] [PubMed] [Google Scholar]
- 28. Jin L., Pluskey S., Petrella E. C., Cantin S. M., Gorga J. C., Rynkiewicz M. J., Pandey P., Strickler J. E., Babine R. E., Weaver D. T., Seidl K. J. (2004) The three-dimensional structure of the ZAP-70 kinase domain in complex with staurosporine. Implications for the design of selective inhibitors. J. Biol. Chem. 279, 42818–42825 [DOI] [PubMed] [Google Scholar]
- 29. Kulathu Y., Hobeika E., Turchinovich G., Reth M. (2008) The kinase Syk as an adaptor controlling sustained calcium signaling and B-cell development. EMBO J. 27, 1333–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Castro R. O., Zhang J., Jamur M. C., Oliver C., Siraganian R. P. (2010) Tyrosines in the carboxyl terminus regulate Syk kinase activity and function. J. Biol. Chem. 285, 26674–26684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furlong M. T., Mahrenholz A. M., Kim K. H., Ashendel C. L., Harrison M. L., Geahlen R. L. (1997) Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim. Biophys. Acta 1355, 177–190 [DOI] [PubMed] [Google Scholar]
- 32. Keshvara L. M., Isaacson C. C., Yankee T. M., Sarac R., Harrison M. L., Geahlen R. L. (1998) Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J. Immunol. 161, 5276–5283 [PubMed] [Google Scholar]
- 33. Abudula A., Grabbe A., Brechmann M., Polaschegg C., Herrmann N., Goldbeck I., Dittmann K., Wienands J. (2007) SLP-65 signal transduction requires Src homology 2 domain-mediated membrane anchoring and a kinase-independent adaptor function of Syk. J. Biol. Chem. 282, 29059–29066 [DOI] [PubMed] [Google Scholar]
- 34. Chen L., Huynh L., Apgar J., Tang L., Rassenti L., Weiss A., Kipps T. J. (2008) ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood 111, 2685–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klinghoffer R. A., Sachsenmaier C., Cooper J. A., Soriano P. (1999) Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edelman A. M., Blumenthal D. K., Krebs E. G. (1987) Protein serine/threonine kinases. Annu. Rev. Biochem. 56, 567–613 [DOI] [PubMed] [Google Scholar]
- 37. Ashe J. M., Wiest D. L., Abe R., Singer A. (1999) ZAP-70 protein promotes tyrosine phosphorylation of T cell receptor signaling motifs (ITAMs) in immature CD4(+)8(+) thymocytes with limiting p56(lck). J. Exp. Med. 189, 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Au-Yeung B. B., Levin S. E., Zhang C., Hsu L. Y., Cheng D. A., Killeen N., Shokat K. M., Weiss A. (2010) A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat. Immunol. 11, 1085–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]