Abstract
Context:
The first year after transplantation is characterized by rapid bone loss.
Objective:
The aim of this study was to compare zoledronic acid (zoledronate) and alendronate for prevention of transplantation bone loss.
Design and Setting:
A randomized clinical trial was conducted at a transplantation center.
Patients:
The study included 84 adults undergoing heart or liver transplantation and a concurrently transplanted, nonrandomized reference group of 27 adults with T scores greater than −1.5.
Interventions:
Alendronate (70 mg weekly for 12 months) or one 5-mg infusion of zoledronate were both initiated 26 ± 8 d after transplantation.
Main Outcome Measures:
The primary outcome was total hip bone mineral density (BMD) 1 yr after transplantation. Secondary outcomes included femoral neck and lumbar spine BMD and serum C-telopeptide, a bone resorption marker.
Results:
In the reference group, BMD declined at the spine and hip (P < 0.001). In the randomized groups, hip BMD remained stable. Spine BMD increased in the zoledronate group and did not change in the alendronate group; at 12 months, the 2.2% difference between groups (95% confidence interval, 0.6 to 3.9%; P = 0.009) favored zoledronate. In heart transplant patients, spine BMD declined in the alendronate and increased in the zoledronate group (−3.0 vs. +1.6%, respectively; between-group difference, 4.2%; 95% confidence interval, 2.1 to 6.3%; P < 0.001). In liver transplant patients, spine BMD increased comparably in both groups. Twelve-month C-telopeptide was lower in the zoledronate group than in the alendronate group (79 vs. 49%; P = 0.04).
Conclusions:
One 5-mg infusion of zoledronate and weekly alendronate prevent bone loss at the hip and, in liver transplant patients, increase spine BMD. In heart transplant patients, spine bone BMD remained stable with zoledronate but decreased with alendronate.
Rapid bone loss is common during the first year after heart and liver transplantation (1–5). Vertebral fracture incidence ranges from 14 to 35% after heart (6–8) and 8 to 65% after liver transplantation, with recent studies showing lower rates (1–5). Several randomized trials (8–14) and our meta-analysis (15) demonstrate that bisphosphonates initiated immediately after transplantation prevent bone loss during the first year. Daily alendronate prevented bone loss after heart transplantation (8), and five iv infusions of zoledronate prevented bone loss after liver transplantation (9). Zoledronate is a long-acting bisphosphonate that increases bone mineral density (BMD) and reduces fracture incidence (16). In postmenopausal women, a single 5-mg infusion of zoledronate suppressed the bone resorption marker C-telopeptide (CTx) for 3 yr (17, 18), and in patients initiating glucocorticoids, it was associated with larger increases in BMD than daily oral risedronate (18). We hypothesized that either a single 5-mg infusion of zoledronate or weekly alendronate would prevent bone loss after heart or liver transplantation.
Patients and Methods
Design overview
In this 1-yr, double-placebo, double-masked study, patients were randomized to a single infusion of active zoledronate (5 mg), or active alendronate (70 mg weekly), or their matching placebos within 30 d of transplantation. Active alendronate or placebo was initiated the day after the infusion and continued for 12 months. The reference group included concurrently transplanted patients with T scores of −1.5 or greater; patients with T scores below −1.5 were eligible if they declined participation in the randomized study. Both randomized and reference groups received ergocalciferol (50,000 IU/d for 5 d) before randomization and calcium (945 mg) and vitamin D (1000 IU) daily after randomization.
Setting and participants
The study (www.Clinicaltrials.gov NCT00297830) was conducted in the Division of Endocrinology, the Irving Institute for Clinical and Translational Research and the Comprehensive Transplantation Center of Columbia University Medical Center (CUMC) with the approval of the Institutional Review Board. Written informed consent was obtained from all participants.
Men and women, aged 20 to 70 yr, who had undergone heart or liver transplantation at CUMC were eligible. Exclusion criteria were metabolic bone diseases, hypocalcemia, hypercalcemia, cancer (excepting atrial myxoma, hepatocellular carcinoma), thyrotoxicosis, serum creatinine above 2.0 mg/dl (176 mmol/liter) by 1 month after transplantation, active peptic ulcer disease, inflammatory diseases, hormone replacement therapy, bisphosphonates, or calcitonin.
All received iv methylprednisolone intraoperatively and d 1, followed by oral prednisone and either cyclosporine or tacrolimus. Heart transplant participants received prednisone 100 mg tapering to 10 mg by 3 months, 5 mg by 6 months, and 1–5 mg at 12 months. Liver transplant participants received prednisone 20 mg, tapering to 5 mg by 3 months, and discontinuing at 6 months. Rejection was most commonly managed with glucocorticoids. Trough blood cyclosporine levels were maintained between 250 and 350 ng/ml for the first 6 months and between 100 and 250 ng/ml for the second 6 months. Trough blood tacrolimus levels were maintained between 6 and 12 ng/dl.
Randomization and interventions
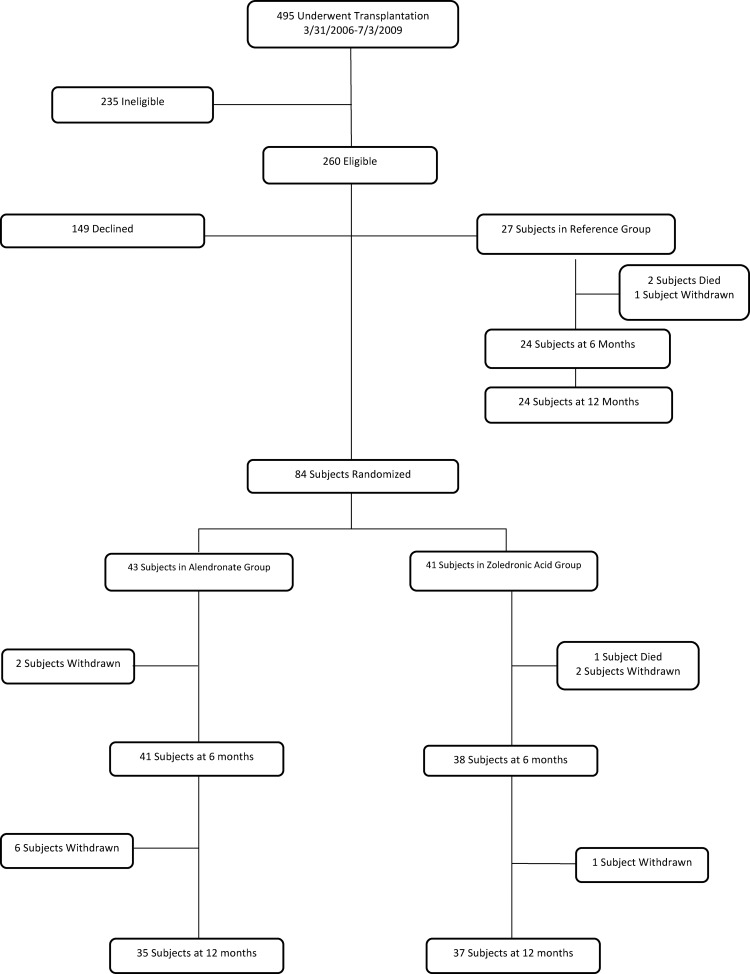
Of 495 patients transplanted between March 2006 and July 2009, 235 were ineligible (Fig. 1); the most common reasons for ineligibility were age below 20 yr, serum creatinine levels above 2.0 mg/dl, or enrollment in another clinical trial. A total of 135 patients declined or were not approached. Eighty-four were randomized, 43 to alendronate and 41 to zoledronate. Two of 27 patients in the reference group died of transplant-related causes, and one withdrew before the 6-month visit. Thirty-five patients (81%) in the alendronate group and 37 (90%) in the zoledronate group completed 12 months of treatment. Eight withdrew from the alendronate group: four for patient preference, one for excessive bone loss at 6 months, and three for newly diagnosed neoplasms. In the zoledronate group, one patient died of sepsis and two withdrew (one with Guillain-Barre, one with sepsis) before the 6-month visit; one withdrew before the 12-month visit.
Fig. 1.
Study profile.
Outcomes and follow-up
BMD, spine radiographs, and fasting morning serum were obtained before randomization. BMD was repeated at 6 and 12 months, radiographs at 12 months, and serum at 2, 6, and 12 months. The primary efficacy variable was percentage change in total hip BMD. Primary safety endpoints included serum calcium and creatinine levels at 2, 6, and 12 months. Secondary outcomes included percentage change in BMD of the lumbar spine and femoral neck and serum CTx.
BMD was measured by dual-energy x-ray absorptiometry (QDR-4500 densitometer; Hologic, Inc., Bedford, MA); short-term in vivo coefficient of variation is 0.68% (spine) and 1.36% (femoral neck). T scores were generated using gender-specific databases provided by the manufacturer. Spine radiography was performed as described (19). Incident fractures were defined as a 20% or 4-mm decrease in any vertebral height (20). Serum was collected on the day of randomization, after the participants had received 5 d of ergocalciferol. Serum calcium, creatinine, and albumin were measured by autoanalyzer (Technicon Instruments, Tarrytown, NY). Serum was archived at −80 C for analysis of 25-hydroxyvitamin D and PTH, as previously described (8), and of CTx and N-terminal propeptides of procollagen type 1 (P1NP) by ELISA (IDS Ltd., Scottsdale, AZ).
Adverse events
Medications, side effects of study drugs, adverse events, clinical fractures, and compliance with alendronate/placebo were documented at each visit by interview and chart review. Nonvertebral fractures were ascertained by radiograph review. Gastrointestinal symptoms, common after transplantation, are also associated with alendronate (18). Gastrointestinal symptoms that resolved after discontinuation and recurred after resumption of alendronate were considered likely related to alendronate. If symptoms were intolerable, alendronate or matching placebo was discontinued, but the patient remained in the study.
Statistical analysis
The study was designed to test the two-sided superiority of zoledronate relative to alendronate and, secondarily, the one-sided superiority of zoledronate relative to the reference group. The primary outcome was the percentage change in total hip BMD at 12 months. Based on preliminary data that total hip BMD would decrease by 1.5 ± 3.7% on alendronate and increase by 1.0 ± 3.7% on zoledronate, we estimated that 35 subjects per group would provide 80% power and 5% α to detect a 2.5 ± 3.7% difference between randomized groups. Anticipating a 20% dropout, 84 subjects were randomized. The first 27 subjects who consented were enrolled as the reference group. Visits were coordinated with transplant appointments; minor timing variations were addressed by annualizing BMD changes. Data collected outside visit windows were treated as missing. No missing values were imputed.
Group differences at baseline were assessed with Student's t tests for continuous variables and χ2 or Fisher's exact test for categorical variables. BMD percentage change from baseline was tested with linear mixed models for repeated measures; covariates were fixed effect of treatment (to test overall differences between treatments), interaction between treatment and time (to test for differences between the groups in percentage changes at 6 and 12 months), random effects of patient and error, and baseline BMD. The primary analysis compared BMD outcomes between the randomized groups. Separate models that controlled for age were constructed for secondary analyses comparing between-group differences between each randomized group and the reference group. Models were repeated separately for heart and liver recipients. Post hoc comparison of between-group differences at specific follow-up times and within-group differences at different follow-up times were calculated from model estimated mean differences and se of the differences using the method of simultaneous confidence limits. Group differences in biochemistries and immunosuppression were evaluated with linear mixed models as described above, except for serum calcium levels, which used a compound symmetry covariance structure. Differences in safety events between randomized groups were estimated with Fisher's exact test. All results are presented as means ± se values and 95% confidence intervals (CIs). All hypotheses were tested by intention-to-treat. All analyses used SAS version 9.2 MIXED, FREQ, or TTEST procedures (SAS Institute, Cary, NC).
Results
Study population
Participants were predominantly male and white (Table 1). Randomized groups did not differ significantly by organ transplanted, days between transplantation and randomization, age, gender, menopausal status, race or ethnic group, type of heart or liver disease, or baseline BMD. Lumbar spine, femoral neck, or total hip T score was below −2.5 in 26% of the women and 20% of the men. The reference group was significantly younger than the zoledronate group, had proportionately fewer postmenopausal women than both randomized groups, although the differences were not significant, and had higher BMD than both randomized groups.
Table 1.
Baseline characteristics of study participants
| Characteristic | Alendronate | Zoledronate | Reference |
P values |
||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| Sample size (n) | 43 | 41 | 27 | |||
| Heart transplant | 26 (60) | 27 (66) | 17 (63) | |||
| Liver transplant | 17 (40) | 14 (34) | 10 (37) | |||
| Days from transplantation to randomization | 26 ± 7 | 27 ± 9 | NA | 0.65 | ||
| Age (yr) | 54 ± 10 | 55 ± 8 | 48 ± 15 | 0.41 | 0.03 | 0.08 |
| Gender | ||||||
| Male | 35 (81) | 30 (73) | 20 (74) | 0.37 | 0.94 | 0.47 |
| Female | 8 (19) | 11 (27) | 7 (26) | |||
| Postmenopausal | 6 (75) | 10 (91) | 3 (43) | 0.86 | 0.09 | 0.51 |
| Race/ethnic group | ||||||
| Non-Hispanic White | 27 (63) | 31 (76) | 19 (70) | 0.10 | 0.76 | 0.79 |
| Non-Hispanic Black | 4 (9) | 6 (15) | 3 (11) | |||
| Hispanic | 12 (28) | 4 (9) | 5 (19) | |||
| Cardiac diagnosis | ||||||
| Dilated | 13 (50.0) | 14 (51.9) | 11 (64.7) | 1.00 | 0.73 | 0.38 |
| Ischemic | 9 (34.6) | 9 (33.3) | 3 (17.6) | |||
| Congenital | 0 (0) | 1 (3.7) | 1 (5.9) | |||
| Other | 4 (15.4) | 3 (11.1) | 2 (11.8) | |||
| Liver diagnosis | ||||||
| Cholestatic | 1 (5.9) | 1 (7.2) | 1 (10.0) | 0.42 | 0.41 | 0.78 |
| Viral | 11 (64.7) | 6 (42.8) | 8 (80.0) | |||
| Alcohol | 3 (17.6) | 1 (7.2) | 0 (0) | |||
| Other liver disease | 1 (5.9) | 3 (21.4) | 1 (10.0) | |||
| Viral and alcohol | 1 (5.9) | 3 (21.4) | 0 (0) | |||
| BMD (g/cm2) | ||||||
| Lumbar spine | 0.976 ± 0.167 | 0.947 ± 0.113 | 1.111 ± 0.143 | 0.37 | <0.001 | <0.001 |
| T score | −1.1 ± 1.5 | −1.3 ± 1.0 | 0.1 ± 1.3 | 0.37 | <0.001 | <0.001 |
| Z score | −0.5 ± 1.6 | −0.6 ± 1.0 | 0.6 ± 1.4 | 0.69 | <0.001 | 0.01 |
| Femoral neck | 0.790 ± 0.120 | 0.742 ± 0.113 | 0.912 ± 0.113 | 0.07 | <0.001 | <0.001 |
| T score | −1.1 ± 0.8 | −1.4 ± 0.8 | −0.1 ± 0.9 | 0.08 | <0.001 | <0.001 |
| Z score | −0.3 ± 0.9 | −0.5 ± 0.7 | 0.5 ± 0.8 | 0.18 | <0.001 | 0.01 |
| Total hip | 0.941 ± 0.136 | 0.904 ± 0.133 | 1.064 ± 0.122 | 0.22 | <0.001 | <0.001 |
| T score | −0.6 ± 0.8 | −0.9 ± 0.8 | 0.3 ± 0.8 | 0.23 | <0.001 | <0.001 |
| Z score | −0.2 ± 0.9 | −0.4 ± 0.8 | 0.6 ± 0.8 | 0.43 | <0.001 | 0.01 |
Data are expressed as mean ± sd or number (percentage). NA, Not applicable. P values: A, Alendronate vs. zoledronate; B, zoledronate vs. reference; C, Alendronate vs. reference.
Immunosuppression
Prednisone dose was lower at randomization in the zoledronate group than the alendronate group, whether heart and liver transplant patients were considered together or separately (Table 2). By 2 months, prednisone doses were similar between groups, but they were higher in heart than liver transplant patients thereafter. At 12 months, most heart transplant patients were on prednisone (43 of 44; 99%), whereas most liver transplant patients were not (25 of 28; 89%). Cyclosporine doses declined comparably over 12 months in all three groups: by 42% in the alendronate group, 51% in the zoledronate group, and 41% in the reference group (P = 0.04; data not shown). Tacrolimus doses remained stable and did not differ among groups (data not shown). Neither cyclosporine nor tacrolimus doses differed by transplant.
Table 2.
Prednisone dose (mg/d)
| Drug | Baseline | 2 months | 6 months | 12 months | Comparisons |
||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| Heart and liver | |||||||
| Alendronate | 24.2 ± 1.2a | 10.9 ± 1.2 | 5.9 ± 1.2 | 3.6 ± 1.3 | 0.07 | 0.48 | 0.83 |
| Zoledronate | 17.8 ± 1.2a | 9.2 ± 1.3 | 5.1 ± 1.3 | 2.5 ± 1.3 | |||
| Reference | 21.5 ± 2.0 | 10.1 ± 1.4 | 5.5 ± 1.3 | 3.8 ± 1.0 | |||
| Heart | |||||||
| Alendronate | 29.0 ± 1.7a | 14.1 ± 1.7 | 7.6 ± 1.7 | 5.8 ± 1.9 | 0.16 | 0.43 | 0.93 |
| Zoledronate | 20.9 ± 1.6a,b | 11.5 ± 1.7 | 6.3 ± 1.7 | 3.6 ± 1.8 | |||
| Reference | 26.3 ± 2.6b | 12.8 ± 1.8 | 7.4 ± 1.7 | 5.5 ± 1.3 | |||
| Liver | |||||||
| Alendronate | 16.8 ± 1.0a | 6.0 ± 1.0 | 3.3 ± 1.0 | 0.3 ± 1.1 | 0.19 | 0.67 | 0.56 |
| Zoledronate | 13.6 ± 1.1a | 5.4 ± 1.1 | 3.3 ± 1.1 | 0.7 ± 1.1 | |||
| Reference | 14.0 ± 1.0 | 4.7 ± 0.7 | 1.9 ± 0.6 | 0.3 ± 0.3 | |||
Data are expressed as mean ± se. Boldface data are statistically different from baseline within group. A shared superscript indicates a significant difference between the groups at that time-point. Comparisons: A, Alendronate vs. zoledronate group by time interaction; B, zoledronate vs. reference group by time interaction; C, alendronate vs. reference group by time interaction.
Bone mineral density
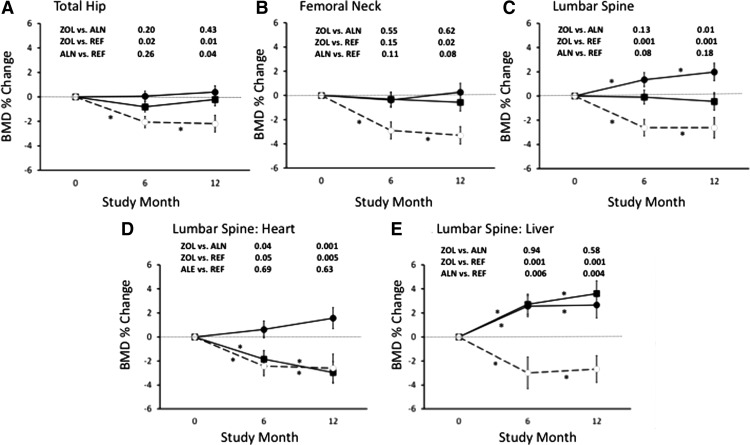
In the reference group (Fig. 2), BMD declined by −2.2% at the total hip (95% CI, −3.6 to −0.7; P < 0.001), −3.3% at the femoral neck (95% CI, −4.8 to −1.8; P < 0.001), and −2.6% at the spine (95% CI, −4.4 to −0.9; P < 0.001). The amount and pattern of bone loss were similar in heart and liver patients (data not shown).
Fig. 2.
Intention-to-treat analysis of mean (±se) percentage change in BMD. Asterisks, Significant differences from baseline; closed circles, zoledronic acid (ZOL); closed squares, alendronate (ALN); open circles, reference group (REF). A, Total hip (heart + liver); B, femoral neck (heart + liver); C, lumbar spine (heart + liver); D, lumbar spine: heart; E, lumbar spine: liver.
In the groups randomized to zoledronate or alendronate, total hip and femoral neck BMD did not change significantly from baseline (Fig. 2, A and B). In contrast, lumbar spine BMD increased significantly from baseline by 2.0% (95% CI, 0.6 to 3.4%) in the zoledronate group and did not change significantly (0.5%; 95% CI, −1.9 to 1.0%) in the alendronate group (Fig. 2C). At 12 months, the between-group differences were not significant at the total hip (0.5%; 95% CI, −0.7 to 1.7%; P = 0.43) or femoral neck (0.4%; 95% CI, −1.2 to 2.0%; P = 0.62). In contrast, the 2.2% between-group difference at the spine (95% CI, 0.6 to 3.9%; P = 0.009) was significant, favoring zoledronate.
Secondary analyses revealed greater total hip bone loss from baseline at 12 months in the reference than the zoledronate (estimated difference, 2.1%; 95% CI, 0.8 to 3.4%; P = 0.002) and alendronate groups (estimated difference, 1.6%; 95% CI, 0.1 to 3.2%; P = 0.04). Femoral neck bone loss was greater in the reference than the zoledronate (estimated difference, 2.4%; 95% CI, 0.6 to 4.3%; P = 0.02), but not the alendronate group (estimated difference, 1.7%; 95% CI, −0.2 to 3.6%; P = 0.08). Spine bone loss was greater in the reference group than the zoledronate group (estimated difference, 4.2%; 95% CI, 2.5 to 6.0%; P < 0.001), but not the alendronate group (estimated difference, 1.4%; 95% CI, −0.7 to 3.5%; P = 0.18).
Change in spine BMD differed by transplant type. In heart transplant patients (Fig. 2D), spine BMD increased by 1.6% in the zoledronate group (95% CI, −0.2 to 3.3%; P = 0.08) and decreased by 3.0% in the alendronate group (95% CI, −4.7 to −1.2%; P = 0.001). The between-group difference was 4.2% (95% CI, 2.1 to 6.3%; P < 0.001), favoring zoledronate. In liver transplant patients (Fig. 2E), spine BMD increased by 3.6% in the alendronate group (95% CI, 1.5 to 5.7%; P = 0.017) and 2.7% in the zoledronate group (95% CI, 0.5 to 4.8%; P = 0.02). The estimated between-group difference was −0.7% (95% CI, −3.2 to 1.8%; P = 0.58). Heart and liver patients did not differ at the hip (data not shown).
Inclusion of prednisone dose at randomization and at later time points as a covariate in the analysis of BMD change did not affect the results either in the combined heart and liver transplant group analyses or in the separate heart and liver transplant group analyses.
Fractures
Paired spine radiographs were available for 33 patients in the zoledronate group, 36 patients in the alendronate group, and 21 patients in the reference group. Two patients in the alendronate group (5.6% of those with radiographs) and no patients in the zoledronate or reference groups sustained incident vertebral fractures. Nonvertebral fractures occurred in one patient in the zoledronate group, three in the alendronate group, and none in the reference group.
Biochemical indexes of mineral metabolism and bone remodeling
Mean serum calcium remained normal in all groups (Table 3). Serum creatinine was slightly higher at baseline in the reference group and remained stable, whereas there were slight but significant increases in the randomized groups. Serum 25-hydroxyvitamin D did not change. Serum PTH increased significantly from baseline in the zoledronate and alendronate groups and remained stable in the reference group. There were no differences between heart and liver transplant patients (data not shown).
Table 3.
Biochemistries
| Analyte | Randomization | 2 Months | 6 Months | 12 Months | Comparisons |
||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| Calcium | |||||||
| Alendronate | 9.1 ± 0.3a | 8.8 ± 0.2 | 9.2 ± 0.1 | 9.6 ± 0.4 | 0.03 | 0.29 | 0.12 |
| Zoledronate | 8.9 ± 0.3 | 9.1 ± 0.2 | 9.4 ± 0.1a | 9.6 ± 0.4 | |||
| Reference | 9.0 ± 0.1a | 9.0 ± 0.1 | 8.8 ± 0.1a | 8.9 ± 0.2 | |||
| Creatinine | |||||||
| Alendronate | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.7 ± 0.2 | 0.68 | 0.60 | 0.58 |
| Zoledronate | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.2 | |||
| Reference | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | |||
| 25-hydroxyvitamin D | |||||||
| Alendronate | 37 ± 5a | 32 ± 3 | 39 ± 2 | 50 ± 7 | 0.80 | 0.87 | 0.78 |
| Zoledronate | 31 ± 5a | 27 ± 3a | 37 ± 2 | 46 ± 7a | |||
| Reference | 43 ± 3 | 38 ± 3a | 41 ± 3 | 46 ± 4a | |||
| PTH | |||||||
| Alendronate | 12 ± 12 | 35 ± 7a | 42 ± 6 | 65 ± 17 | 0.46 | 0.40 | 0.21 |
| Zoledronate | 19 ± 12 | 38 ± 7b | 43 ± 6 | 57 ± 16 | |||
| Reference | 28 ± 3 | 31 ± 3a,b | 31 ± 3 | 32 ± 3 | |||
| Serum CTx | |||||||
| Alendronate | 0.68 ± 0.12 | 0.40 ± 0.07a | 0.30 ± 0.06a | 0.21 ± 0.18 | 0.001 | 0.001 | 0.65 |
| Zoledronate | 0.78 ± 0.12 | 0.22 ± 0.08a,b | 0.22 ± 0.06b | 0.07 ± 0.17a | |||
| Reference | 0.64 ± 0.06 | 0.51 ± 0.07b | 0.50 ± 0.06a,b | 0.48 ± 0.06a | |||
| Serum P1NP | |||||||
| Alendronate | 55.1 ± 5.4 | 43.2 ± 5.4 | 54.7 ± 8.2 | 42.3 ± 4.6a | 0.51 | 0.001 | 0.007 |
| Zoledronate | 55.3 ± 4.2 | 37.8 ± 3.6 | 41.6 ± 6.4a | 42.6 ± 6.6b | |||
| Reference | 45.4 ± 4.9 | 48.2 ± 5.5 | 61.5 ± 7.1a | 74.4 ± 7.5a,b | |||
Data are expressed as mean ± se. Boldface data are statistically different from baseline within group. A shared superscript indicates a significant difference between the groups at that time-point. Comparisons: A, Alendronate vs. zoledronate group by time interaction; B, zoledronate vs. reference group by time interaction; C, alendronate vs. reference group by time interaction.
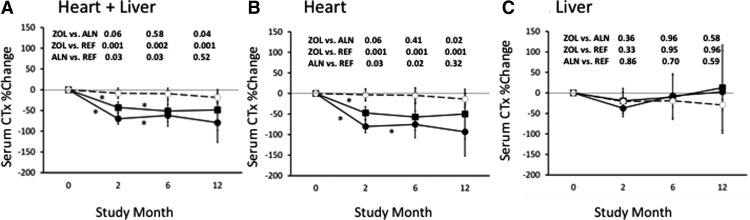
By 2 months, serum CTx decreased by 43% in the alendronate and 70% in the zoledronate group (Fig. 3A). At 12 months, serum CTx, expressed as percentage change from baseline, was significantly lower in the zoledronate than the alendronate group. In heart transplant patients, serum CTx tended to decline more in those randomized to zoledronate than alendronate (80 vs. 47%; P = 0.06). Serum P1NP followed a similar pattern. At 12 months, CTx was significantly lower in heart transplant patients who received zoledronate than the reference group (0.15 ± 0.22 vs. 0.51 ± 0.07 pg/ml, respectively; P < 0.001), whereas those who received alendronate did not differ from the reference group (0.35 ± 0.23 vs. 0.51 ± 0.07 pg/ml, respectively; P = 0.26; Fig. 3B). In liver transplant patients, serum CTx and P1NP did not change in any group.
Fig. 3.
Mean (±se) percentage change in serum CTx. Asterisks, Significant differences from baseline; closed circles, zoledronic acid (ZOL); closed squares, alendronate (ALN); open circles, reference group (REF). A, Heart + liver; B, heart; C, liver.
Compliance and adverse events
In terms of compliance, 91% of the randomized subjects took more than 80% of dispensed doses of alendronate or its matching placebo. Transplantation-associated adverse events did not differ among the groups (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). There was no difference between the two intervention groups in gastrointestinal symptoms, serum creatinine, serum creatinine levels over 3.0 mg/dl, hypocalcemia, postinfusion flu-like symptoms, or arrhythmias. More patients in the alendronate group (n = 7) than the zoledronic acid group (n = 1) experienced increases in serum creatinine above 2.0 mg/dl, but the difference was not statistically significant (P = 0.09). In three patients, the increase was transient, most commonly associated with sepsis. Of the five patients in whom the serum creatinine remained above 2.0 mg/dl, one had biopsy-proven nephropathy related to hepatitis C infection and diabetes; one developed primary hyperparathyroidism, hypercalciuria, and dehydration; one with a baseline serum creatinine of 2.0 mg/dl later developed sepsis and dehydration; one developed cellulitis and urosepsis; and one had no apparent etiology other than cyclosporine A toxicity.
Discussion
We compared a single 5-mg infusion of zoledronate with 12 months of weekly oral alendronate for prevention of bone loss during the first year after heart or liver transplantation. The primary analysis revealed no bone loss at the total hip and femoral neck with either intervention. At the spine, however, there was a significant 2.2% between-group difference at 12 months that favored zoledronate. Secondary analyses revealed that this was limited to heart transplant patients, in whom spine BMD declined in the alendronate group and remained stable in the zoledronate group. In contrast, spine BMD increased comparably and significantly in liver transplant patients treated with both drugs. The reference group sustained significant bone loss at all sites. The zoledronate group sustained less bone loss at all sites than the reference group, whereas the alendronate group sustained less bone loss than the reference group only at the total hip. These results suggest that a single infusion of zoledronate prevents bone loss at the femoral neck and total hip and improves spine BMD in both heart and liver transplant recipients. In contrast, weekly alendronate prevents bone loss at the total hip and femoral neck in heart and liver transplant recipients, but is associated with significant spine bone loss in heart transplant recipients, and increases spine BMD only in liver transplant recipients.
Previous studies of posttransplantation bone loss suggest that it is related to concomitant therapy with high-dose glucocorticoids and calcineurin inhibitors (15, 21) and associated with biochemical evidence of uncoupled bone remodeling, with decreased formation and increased resorption markers (9, 22, 23). As prednisone doses decrease, bone formation recovers, and rates of bone loss diminish (23). This transient uncoupling of bone remodeling is associated with relatively rapid bone loss during the first 6 months, after which BMD declines less rapidly, stabilizes, or improves (8, 23–25).
Oral bisphosphonates reduce rates of bone loss and fracture in glucocorticoid-induced osteoporosis (18, 26–28), likely by suppressing bone resorption. In heart transplant patients randomized to daily alendronate, spine BMD remained stable despite small but significant decreases at the total hip (1.5%) and femoral neck (1.7%) (8). In liver transplant patients randomized to weekly alendronate (29), BMD increased by 5.1% at the spine and 3.6% at the femoral neck, larger increases than observed in our study (3.5% at the spine, 0.1% at the femoral neck). Although gender, baseline BMD, and prednisone doses were similar to our study, their patients were younger and received concurrent calcitriol 0.5 μg daily. Alendronate also prevents bone loss immediately after kidney transplantation (30) and improves BMD in long-term kidney transplant recipients (31, 32).
Intravenous bisphosphonates have also been studied after organ transplantation. In male heart transplant recipients randomized to quarterly ibandronate or placebo, BMD remained stable in the ibandronate group and fell by 23–25% in the control group (12). Incident vertebral fractures were significantly lower in the ibandronate group than the control group (13 vs. 53%, respectively) (12). However, glucocorticoid doses were much higher than in our study. Quarterly ibandronate infusions also prevented bone loss and reduced vertebral fractures after kidney transplantation (13). Crawford et al. (9) randomized liver transplant patients to placebo or a much higher dose of zoledronate than we used: 4 mg at 1 wk and at 1, 3, 6, and 9 months after transplantation. Rates of bone loss were significantly lower in the zoledronate group. In another randomized study, patients who received eight infusions of zoledronate (4 mg) during the first year after liver transplantation had significantly less bone loss and fewer vertebral fractures (8.5 vs. 22.5%) (33).
When liver and heart transplants were analyzed together, zoledronate lowered bone turnover markers to a greater extent and for a longer period than alendronate. However, this was limited to heart transplant recipients. CTx did not decrease significantly in our liver transplant recipients treated with zoledronate and alendronate for reasons that are unclear. However, although the decline in CTx in our liver transplant patients was not significant, it was comparable to the decline in deoxypyridinoline observed by Crawford et al. (9). Bodingbauer et al. (33) also found no decline in resorption markers after liver transplantation, despite high doses of zoledronate. Despite the lack of decline in CTx in our liver transplant recipients, however, they experienced comparable increases in spine and maintenance of hip BMD, whereas BMD declined at all sites in untreated patients. In heart transplant patients, less potent suppression of bone resorption by alendronate than zoledronate provided less benefit at the spine. The differing response to alendronate at the lumbar spine in heart and liver transplant patients may have been related to less prednisone exposure at randomization and thereafter in the latter.
Alendronate and zoledronate were well tolerated and appeared safe. Mild postinfusion hypocalcemia and flu-like symptoms occurred in more patients in the zoledronate group than the alendronate group, but the differences were not significant. Mild deterioration in renal function affected both randomized groups comparably, and to an extent similar to that commonly seen after organ transplantation (34, 35). Serum creatinine exceeded 2.0 mg/dl at some point during the first posttransplant year in seven patients in the alendronate group and one in the zoledronic acid group; in three, the increase was transient, and in four it stabilized between 2.0 and 3.0 mg/dl; and one patient developed worsening diabetic and hepatitis C-related nephropathy that necessitated kidney transplantation. In all, there was a known etiology of declining renal function. Gastrointestinal side effects and incidence of atrial fibrillation did not differ.
This study has several limitations. We did not include a randomized untreated group because we believed that high fracture rates reported in untreated patients required an active comparator design. The reference group was younger and had higher BMD than the randomized groups. However, we controlled analyses for baseline BMD and age, and we believe the data provide a benchmark for interpreting the effects of the interventions. The study was conducted in a single institution, and results may not apply to other centers. Heart and liver transplant recipients differ in terms of pretransplant bone disease (usually high bone turnover in heart failure and low bone turnover in liver failure) and exposure to prednisone. However, as rates and patterns of bone loss were comparable in untreated heart and liver transplant patients, this heterogeneity may broaden generalizability of the results. Because vitamin D deficiency is common in transplant recipients (36) and has been associated with profound hypocalcemia after zoledronate (37), we administered ergocalciferol to all randomized and reference participants without a history of nephrolithiasis; this may have resulted in less bone loss than prior studies. Fracture rates were also lower than previously reported (8, 9), and there were none in the reference group, likely related to their higher BMD and lower age. Moreover, the study was not powered to detect differences in fracture rates, an important clinical outcome but one that would require a large comparative effectiveness trial. Thus, it is unclear whether the two interventions differ enough to make global recommendations based upon this outcome, particularly because alendronate is available as a generic and less expensive treatment than zoledronate.
In summary, a single 5-mg infusion of zoledronate, a much smaller dose than used in prior studies, and 12 months of weekly alendronate prevented bone loss at the hip during the first year after heart and liver transplantation. At the spine, there was a significant 2.2% between-group difference, favoring zoledronate. When the type of organ transplant was considered, zoledronate and alendronate were associated with comparable improvements in liver transplant patients, but zoledronate was superior to alendronate in heart transplant patients. The biological relevance of the overall 2.2% difference in lumbar spine bone loss between the zoledronate and alendronate groups may depend upon the condition of the patient at the time of transplantation. For the 26% of women and 20% of men who already had osteoporosis at baseline in our study, for those with T scores below −1.5 who are of necessity embarking upon a course of glucocorticoid therapy, and others who are at high risk of fracture (postmenopausal women, those with a prior osteoporotic fracture), a further loss of 2.2%, particularly when it occurs over a relatively short period of time (6 months), might have considerable impact. Such patients may benefit from a more aggressive approach (zoledronate in this case). In contrast, for patients with relatively normal BMD or those at low risk of fracture, a 2.2% loss might not have serious consequences. In addition, the medication burden is high in most transplant recipients, and a single dose of zoledronate may be easier to comply with than weekly alendronate. Thus, the demonstration that a single 5-mg infusion of zoledronate lowers serum markers of bone resorption and prevents bone loss at all sites during the first posttransplant year should be of interest to organ transplantation programs.
Supplementary Material
Acknowledgments
The study was investigator-initiated. The principal investigators designed and conducted the study. Novartis Pharmaceuticals, USA, supplied zoledronate and alendronate and their matching placebos and provided financial support. Statisticians at Columbia University Medical Center performed all analyses. Novartis Pharmaceuticals reviewed the manuscript, without modifying it. This study was also supported by the Thomas L. Kempner Jr. and Katheryn C. Patterson Foundation [K24 AR052665 (to E.S.), K23 AR054127 (to A.C.), and K23 DK084337 (E.M.S.)], and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 RR024156. The content is solely the responsibility of the authors.
Clinical Trial registration no.: www.Clinicaltrials.gov NCT00297830.
Disclosure Summary: A.C., E.M.S., D.J.M., C.Z., P.Y., K.P., R.B.S., E.C.V., S.R., and D.M. have nothing to declare. E.S. is the Principal Investigator of a grant from Novartis Pharmaceuticals to Columbia University that provided funding for this study. R.B. has consulted for Novartis Pharmaceuticals.
Footnotes
- BMD
- Bone mineral density
- CI
- confidence interval
- CTx
- C-telopeptide
- P1NP
- N-terminal propeptides of procollagen type 1.
References
- 1. Cohen A, Ebeling P, Sprague S, Shane E. 2006. Transplantation osteoporosis. In: Favus M, ed. Primer on the metabolic bone diseases and disorders of bone and mineral metabolism. Washington, DC: American Society for Bone and Mineral Research; 302–309 [Google Scholar]
- 2. Cohen A, Shane E. 2003. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int 14:617–630 [DOI] [PubMed] [Google Scholar]
- 3. Compston JE. 2003. Osteoporosis after liver transplantation. Liver Transpl 9:321–330 [DOI] [PubMed] [Google Scholar]
- 4. Stein E, Compston J, Shane E. 2009. Transplantation osteoporosis. In: Bilezikian JP, ed. Osteoporosis in men. London: Elsevier; 443–452 [Google Scholar]
- 5. Stein E, Ebeling P, Shane E. 2007. Post-transplantation osteoporosis. Endocrinol Metab Clin North Am 36:937–963; viii [DOI] [PubMed] [Google Scholar]
- 6. Guichelaar MM, Schmoll J, Malinchoc M, Hay JE. 2007. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatol 46:1198–1207 [DOI] [PubMed] [Google Scholar]
- 7. Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, Otto G, Lange R, Theilmann L, Zimmerman R, Pritsch M, Ziegler R. 2001. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet 357:342–347 [DOI] [PubMed] [Google Scholar]
- 8. Shane E, Addesso V, Namerow PB, McMahon DJ, Lo SH, Staron RB, Zucker M, Pardi S, Maybaum S, Mancini D. 2004. Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med 350:767–776 [DOI] [PubMed] [Google Scholar]
- 9. Crawford BA, Kam C, Pavlovic J, Byth K, Handelsman DJ, Angus PW, McCaughan GW. 2006. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:239–248 [DOI] [PubMed] [Google Scholar]
- 10. Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H. 2003. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14:2669–2676 [DOI] [PubMed] [Google Scholar]
- 11. De Sévaux RG, Hoitsma AJ, Corstens FH, Wetzels JF. 2002. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. J Am Soc Nephrol 13:1608–1614 [DOI] [PubMed] [Google Scholar]
- 12. Fahrleitner-Pammer A, Piswanger-Soelkner JC, Pieber TR, Obermayer-Pietsch BM, Pilz S, Dimai HP, Prenner G, Tscheliessnigg KH, Hauge E, Portugaller RH, Dobnig H. 2009. Ibandronate prevents bone loss and reduces vertebral fracture risk in male cardiac transplant patients: a randomized double-blind, placebo-controlled trial. J Bone Miner Res 24:1335–1344 [DOI] [PubMed] [Google Scholar]
- 13. Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, Strey C, Kirste G, Olschewski M, Reichelt A, Rump LC. 2001. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol 12:1530–1537 [DOI] [PubMed] [Google Scholar]
- 14. Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, Cockwell P, Sweny P, Banks LM, Hall-Craggs M, Noonan K, Andrews C, Cunningham J. 2009. Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. Am J Kidney Dis 53:856–865 [DOI] [PubMed] [Google Scholar]
- 15. Stein EM, Ortiz D, Jin Z, McMahon DJ, Shane E. 2011. Prevention of fractures after solid organ transplantation: a meta-analysis. J Clin Endocrinol Metab 96:3457–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. 2007. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822 [DOI] [PubMed] [Google Scholar]
- 17. Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR. 2010. Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res 25:2251–2255 [DOI] [PubMed] [Google Scholar]
- 18. Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN. 2009. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373:1253–1263 [DOI] [PubMed] [Google Scholar]
- 19. Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San Valentin R, Cummings SR. 1995. Comparison of methods for defining prevalent vertebral deformities: the Study of Osteoporotic Fractures. J Bone Miner Res 10:890–902 [DOI] [PubMed] [Google Scholar]
- 20. Black DM, Palermo L, Nevitt MC, Genant HK, Christensen L, Cummings SR. 1999. Defining incident vertebral deformity: a prospective comparison of several approaches. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 14:90–101 [DOI] [PubMed] [Google Scholar]
- 21. Ebeling PR. 2009. Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab 94:1483–1490 [DOI] [PubMed] [Google Scholar]
- 22. Sambrook PN, Kelly PJ, Fontana D, Nguyen T, Keogh A, Macdonald P, Spratt P, Freund J, Eisman JA. 1994. Mechanisms of rapid bone loss following cardiac transplantation. Osteoporos Int 4:273–276 [DOI] [PubMed] [Google Scholar]
- 23. Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, Mancini D, Michler RE, Aaronson K, Addesso V, Lo SH. 1997. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 82:1497–1506 [DOI] [PubMed] [Google Scholar]
- 24. Monegal A, Navasa M, Guañabens N, Peris P, Pons F, Martinez de Osaba MJ, Ordi J, Rimola A, Rodés J, Muñoz-Gómez J. 2001. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int 12:484–492 [DOI] [PubMed] [Google Scholar]
- 25. Monegal A, Navasa M, Guañabens N, Peris P, Pons F, Martínez de Osaba MJ, Rimola A, Rodés J, Muñoz-Gómez J. 2001. Bone mass and mineral metabolism in liver transplant patients treated with FK506 or cyclosporine A. Calcif Tissue Int 68:83–86 [DOI] [PubMed] [Google Scholar]
- 26. Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, Kendler DL, Lentle B, Olszynski W, Ste-Marie LG, Tenenhouse A, Chines AA. 1997. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 337:382–387 [DOI] [PubMed] [Google Scholar]
- 27. Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA. 1999. Risedronate therapy prevents corticosteroid-induced bone loss. Arthritis Rheum 42:2309–2318 [DOI] [PubMed] [Google Scholar]
- 28. Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. 1998. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339:292–299 [DOI] [PubMed] [Google Scholar]
- 29. Atamaz F, Hepguler S, Karasu Z, Kilic M, Tokat Y. 2006. The prevention of bone fractures after liver transplantation: experience with alendronate treatment. Transplant Proc 38:1448–1452 [DOI] [PubMed] [Google Scholar]
- 30. Abediazar S, Nakhjavani MR. 2011. Effect of alendronate on early bone loss of renal transplant recipients. Transplant Proc 43:565–567 [DOI] [PubMed] [Google Scholar]
- 31. Trabulus S, Altiparmak MR, Apaydin S, Serdengecti K, Sariyar M. 2008. Treatment of renal transplant recipients with low bone mineral density: a randomized prospective trial of alendronate, alfacalcidol, and alendronate combined with alfacalcidol. Transplant Proc 40:160–166 [DOI] [PubMed] [Google Scholar]
- 32. Lan G, Peng L, Xie X, Peng F, Wang Y, Yu S. 2008. Alendronate is effective to treat bone loss in renal transplantation recipients. Transplant Proc 40:3496–3498 [DOI] [PubMed] [Google Scholar]
- 33. Bodingbauer M, Wekerle T, Pakrah B, Roschger P, Peck-Radosavljevic M, Silberhumer G, Grampp S, Rockenschaub S, Berlakovich G, Steininger R, Klaushofer K, Oberbauer R, Mühlbacher F. 2007. Prophylactic bisphosphonate treatment prevents bone fractures after liver transplantation. Am J Transplant 7:1763–1769 [DOI] [PubMed] [Google Scholar]
- 34. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. 2003. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349:931–940 [DOI] [PubMed] [Google Scholar]
- 35. Verna EC, Farrand ED, Elnaggar AS, Pichardo EM, Balducci A, Emond JC, Guarrera JV, Brown RS., Jr 2011. Basiliximab induction and delayed calcineurin inhibitor initiation in liver transplant recipients with renal insufficiency. Transplantation 91:1254–1260 [DOI] [PubMed] [Google Scholar]
- 36. Stein EM, Cohen A, Freeby M, Rogers H, Kokolus S, Scott V, Mancini D, Restaino S, Brown R, McMahon DJ, Shane E. 2009. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 23:861–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breen TL, Shane E. 2004. Prolonged hypocalcemia after treatment with zoledronic acid in a patient with prostate cancer and vitamin D deficiency. J Clin Oncol 22:1531–1532 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.