Abstract
Context:
Studies have suggested that PTH may influence mortality and progression of chronic kidney disease. However, the development of either event may influence the development of the other as a competing risk.
Objective:
The objective of the study was to examine the association of PTH with end-stage renal disease (ESRD) and pre-ESRD death using a competing risk survival model.
Design, Setting, and Patients:
A total of 10,823 participants in the Kidney Early Evaluation Program with chronic kidney disease (estimated glomerular filtration rate < 60 ml/min per 1.73m2) were examined from 2005 to 2010.
Main Outcome Measures:
The association of PTH levels with ESRD and pre-ESRD mortality was ascertained by linking Kidney Early Evaluation Program data to the Social Security Administration Death Master File and the U.S. Renal Data System.
Results:
Among the cohort, the incidence of ESRD and pre-ESRD mortality was 6.4 and 20.1 events per 1000 person-years. Higher PTH levels were associated with increasing age, black race, lack of a high school education, cardiovascular disease, hypertension, and lower glomerular filtration rate. The incidence of ESRD and pre-ESRD mortality was lowest among participants in the second PTH quintile. After multivariate adjustment, as compared with the second quintile, the risk of pre-ESRD mortality was higher in the third [subhazard ratio (SHR) 1.52 (95% confidence interval 1.04–2.22)], fourth [SHR 1.73 (95% confidence interval 1.19–2.52)], and fifth [SHR 1.86 (1.28–2.52)] quintiles, respectively. Conversely, PTH was not associated with ESRD after multivariate adjustment. The association was not modified by diabetic status, gender, race, or glomerular filtration rate status.
Conclusions:
Elevated PTH levels are associated with increased pre-ESRD mortality but not with ESRD.
Chronic kidney disease (CKD) is associated with increased mortality, largely due to cardiovascular causes (1). The increased cardiovascular risk is due to both traditional and nontraditional factors. One potential nontraditional mediator may be PTH, which becomes elevated due to stimulation of the parathyroid glands by relative hypocalcemia, phosphate retention, and vitamin D deficiency (2). Previous analyses have suggested that elevated PTH levels are associated with a high prevalence (3) and incidence of cardiovascular disease (4) among persons with moderate CKD. Furthermore, higher PTH levels have been associated with increased mortality among persons with (5, 6) and without (7, 8) CKD.
The association between PTH and CKD progression to end-stage renal disease (ESRD) has not been studied extensively (5, 6). PTH may enhance renal fibrosis (9), and medical or surgical correction of hyperparathyroidism slows down CKD progression in animals (10). Given that death before progression to ESRD is a competing outcome, the impact of PTH on CKD mortality should be considered when evaluating this association. We therefore seek to examine the impact of PTH levels on pre-ESRD mortality (mortality occurring before the development of ESRD) and ESRD.
Most survival models assume that censoring and the outcome of interest are independent (noninformative) (11). Given that death before ESRD would preclude the development of ESRD, the censoring event (death) is not independent of the outcome of interest (ESRD). Therefore, studies examining risk factors for ESRD should account for the competing event of pre-ESRD mortality (12). Conversely, a factor associated with more rapid progression to ESRD may also be associated with increased death simply through the development of ESRD. Therefore, the consideration of pre-ESRD mortality as an end point is also important (12). Studies examining the association of PTH with these outcomes among persons with CKD have examined only using traditional Cox proportional hazard models or as a combined end point (5, 6) and thus did not account for competing risks.
We explored the association of PTH with ESRD and pre-ESRD mortality in the Kidney Early Evaluation Program (KEEP), a free screening program targeting persons at high risk of CKD from a diverse nationwide sample (13, 14). KEEP measures PTH and important confounding variables among those with estimated glomerular filtration rate (GFR) less than 60 ml/min per 1.73m2 and is therefore an ideal population to study these outcomes.
Materials and Methods
Study participants
KEEP methods have been previously described (13, 14). Participants screened from November 1, 2005, through December 11, 2010, with all available measures and estimated GFR less than 60 ml/min per 1.73 m2 were included with a total sample of 10,823. The last date of follow-up was December 31, 2010. As part of KEEP screening, participants consent that information gathered from KEEP may be used for research and linked to other health databases. Determination of ESRD, defined as the requirement for dialysis or transplantation, was made by linking KEEP participants to the U.S. Renal Data System as previously described (15). Mortality was determined by linking KEEP participants to the Social Security Administration Death Master File (16). Examination of the KEEP data was approved by the Human Subjects Committee at the Minneapolis Medical Research Foundation (HSR no. 03-2262), and all participants provided informed consent.
Patient characteristics
Age, sex, race, education, health insurance coverage, education, history of cancer, family history of kidney disease or dialysis, and tobacco use were obtained from patient-filled questionnaires. Diabetes was defined as self-reported history of hyperglycemia or diabetes mellitus, taking diabetic medications, a fasting blood glucose of 126 mg/d or greater, or a random blood glucose of 200 mg/dl or greater. Dyslipidemia was defined as a history of high cholesterol or taking cholesterol lowering medications. Cardiovascular disease was defined as having a personal history of heart angina, heart attack, heart bypass surgery, heart angioplasty, stroke, heart failure, abnormal heart rhythm, or coronary heart disease. This definition was used to be consistent with prior KEEP manuscripts (17, 18). Hypertension was defined as either a self-reported history of hypertension, taking antihypertensive medications, or measured blood pressure of 130/85 mm Hg or greater. Blood pressure, height, weight, and body mass index (BMI) were measured by trained personnel. Specific medication brands or classes were not recorded in KEEP.
Laboratory data
As part of KEEP screening, only those found to have an estimated GFR less than 60 ml/min per 1.73m2 by the Modification of Diet in Renal Disease equation (19) had additional measurements for PTH, calcium, and phosphorus (17, 18). Among those whose estimated GFR was above this range, these variables were not measured.
Main predictor
PTH was measured using the Immulite 2000 assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). This assay is a two-site, chemiluminescent, enzyme-labeled immunometric assay with intra- and interassay coefficient of variation of 4.2–5.7 and 6.3–8.8%, respectively (17, 18). The normal range for this assay is 12–65 ng/liter. This assay detects both the intact PTH (1–84) molecule and the PTH (7–84) fragment (20).
Additional laboratory data
Calcium and phosphorus were measured as previously described (17, 18). Calcium levels were not corrected for albumin because KEEP does not measure serum albumin levels. Other markers of mineral metabolism such as alkaline phosphatase, vitamin D, and fibroblast growth factor-23 (FGF-23) are not measured in KEEP. Microalbuminuria was defined as a spot urine albumin to creatinine ratio of 30 mg/g or greater. For the purpose of statistical models, GFR was estimated using the CKD Epidemiology Collaboration equation (21) over Modification of Diet in Renal Disease because prior analyses have found that this method reclassifies those at lower risk for death into higher GFR categories (16).
Statistical methods
PTH was examined as a categorical predictor and was divided into equal quintiles. Univariate associations across PTH quintiles were examined using ANOVA for continuous variables and χ2 testing for categorical variables. Cumulative incidences of ESRD and pre-ESRD mortality were estimated considering the competing risks of either event (22). Ascertainment of PTH as a predictor of ESRD, accounting for pre-ESRD death as a competing event, was performed via the method of Fine and Gray (23). Here the subdistribution, or the cumulative incidence of a particular event in the setting of competing risks, is modeled via an extension of the Cox proportional hazards model (24). In this model, the exponential of the regression coefficients are now termed the subdistribution hazard or subhazard ratios (SHRs) and represent the effects of the covariates (24). This model was also used to examine PTH as a predictor of pre-ESRD mortality with ESRD as a competing event. Models were adjusted as follows: model 1 was unadjusted; model 2 was adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), and family history of kidney disease or dialysis (yes/no); model 3 was adjusted for model 2 + smoking status (yes/no), dyslipidemia (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), hypertension (yes/no), cancer (yes/no), albuminuria (yes/no), BMI, and hemoglobin; and model 4 was adjusted for model 3 + baseline GFR, calcium, and phosphorus. Multiplicative interaction terms were generated to examine for effect modification for diabetes, gender, race (Black vs. non-Black), and GFR status (≤45 vs. > 45). A two-tailed value of P < 0.05 was considered statistically significant.
Results
A total of 10,823 KEEP participants with GFR less than 60 ml/min per 1.73 m2 were included in the analysis. For the eligible cohort, the mean age was 70.7 yr and predominantly female (68.6%) and white Caucasian (68.7%). The cohort had a predominance of diabetes, cardiovascular disease, hypertension, and cancer: 47.3, 43.5, 92.7, and 21.0%, respectively. The mean estimated GFR was 47.4 ml/min per 1.73 m2. For the entire cohort, 52.9% had PTH measurements above the normal range with 45.7% having measurements that were in the normal range. Only 1.4% had measurements below normal.
Baseline demographics across PTH quintiles are reported in Table 1. Compared with those in the first (lowest) quintile, those in the fifth (highest) quintile of PTH were older, were more frequently African-American, were less likely to have a high school education, and had a greater prevalence of cardiovascular disease, hypertension, and albuminuria. Across the spectrum of intact PTH, systolic blood pressure and BMI were higher in the fifth quintile, whereas estimated GFR, hemoglobin, calcium, and phosphorus were lower. However, the differences across the groups for calcium and phosphorus were clinically small (≤ 0.26 and ≤ 0.62 mg/dl, respectively).
Table 1.
Patient population/demographics by PTH quintile
| First quintile (≤41) (n = 2218) | Second quintile (>41 to 59) (n = 2180) | Third quintile (>59 to 80) (n = 2176) | Fourth quintile (>80 to 114) (n = 2100) | Fifth quintile (>114) (n = 2149) | P value (for trend) | |
|---|---|---|---|---|---|---|
| Age (mean ± sd) | 69.8 ± 10.5 | 70.1 ± 10.7 | 71.3 ± 10.5 | 71.6 ± 10.6 | 71.1 ± 11.9 | <0.0001 |
| Male (%) | 32.1 | 32.3 | 32.2 | 33.6 | 32.2 | 0.65 |
| Race (%) | <0.0001 | |||||
| White | 76.5 | 73.4 | 69.0 | 66.9 | 57.6 | |
| Black | 14.2 | 17.2 | 20.7 | 24.7 | 33.0 | |
| Other | 9.4 | 9.4 | 10.3 | 8.4 | 9.4 | |
| Smoker (%) | 0.98 | |||||
| Never | 59.4 | 60.2 | 59.5 | 60.3 | 59.5 | |
| Quit | 35.6 | 34.6 | 35.5 | 34.8 | 35.1 | |
| Current | 5.0 | 5.2 | 5.0 | 4.9 | 5.4 | |
| High school education (%) | 86.3 | 85.3 | 82.5 | 82.6 | 78.8 | <0.0001 |
| Health insurance (%) | 92.3 | 90.9 | 91.6 | 91.2 | 90.7 | 0.16 |
| Family history of kidney disease or dialysis (%) | 15.1 | 15.7 | 15.7 | 17.5 | 17.0 | 0.04 |
| Comorbidities | ||||||
| Diabetes (%) | 52.0 | 43.7 | 45.4 | 45.6 | 49.7 | 0.42 |
| Cardiovascular dz (%) | 40.1 | 39.4 | 43.2 | 44.6 | 50.6 | <0.0001 |
| Cancer (%) | 19.8 | 21.1 | 21.2 | 21.5 | 21.3 | 0.22 |
| Hypertension (%) | 91.4 | 91.7 | 91.7 | 93.5 | 95.7 | <0.0001 |
| Dyslipidemia (%) | 63.9 | 63.4 | 60.9 | 62.2 | 61.6 | 0.07 |
| Albuminuria (%) | 16.1 | 17.0 | 22.2 | 26.0 | 38.1 | <0.0001 |
| Physical measurements | ||||||
| Systolic BP (mm Hg) (mean ± sd) | 135.8 ± 19.4 | 137.2 ± 19.5 | 137.5 ± 20.7 | 137.9 ± 20.0 | 139.1 ± 21.7 | <0.0001 |
| BMI (kg/m2) (mean ± sd) | 29.7 ± 6.1 | 29.6 ± 5.9 | 29.6 ± 6.1 | 30.3 ± 6.5 | 31.3 ± 7.1 | <0.0001 |
| Laboratory measurements | ||||||
| eGFR | 49.6 ± 8.4 | 49.6 ± 8.3 | 48.7 ± 8.8 | 47.3 ± 9.4 | 41.9 ± 12.3 | <0.0001 |
| Hgb | 13.4 ± 1.5 | 13.4 ± 1.4 | 13.3 ± 1.5 | 13.2 ± 1.5 | 12.8 ± 1.6 | <0.0001 |
| Calcium | 9.8 ± 0.5 | 9.7 ± 0.4 | 9.6 ± 0.4 | 9.6 ± 0.5 | 9.5 ± 0.6 | <0.0001 |
| Phosphorus | 3.74 ± 0.58 | 3.70 ± 0.56 | 3.69 ± 0.58 | 3.65 ± 0.58 | 3.67 ± 0.70 | <0.0001 |
dz, Disease; BP, blood pressure; eGFR, estimated GFR; Hgb, hemoglobin.
End-stage renal disease
Among the cohort, 166 of 10,823 participants (1.5%) developed ESRD over 25,794 person-years of follow-up with 15, 9, 18, 19, and 105 events in the first, second, third, fourth, and fifth PTH quintiles, respectively. Incidence rates of ESRD were 3.0, 1.8, 3.4, 3.6, and 20.6 per 1000 patient-years for the first, second, third, fourth, and fifth PTH quintiles (P < 0.0001), respectively (Fig. 1A). On unadjusted analyses (model 1), those in the fifth PTH quintile had a nearly 12-fold higher risk of developing ESRD [SHR 11.79 (5.97–11.88), P < 0.0001)] compared with those in the second quintile (Table 2). The first, third, and fourth quintiles were not associated with a higher risk of ESRD compared with the second quintile. After adjustment for demographic variables (model 2), the risk of ESRD in the fifth quintile was similar [SHR 11.03 (5.55–21.93), P < 0.0001] but was attenuated after adjustment for medical comorbidities (model 3) [SHR 5.22 (2.37–11.41, P < 0.0001]. Upon adjustment for renal function, calcium, and phosphorus (model 4), the higher risk of ESRD in the fifth PTH quintile was further attenuated and no longer statistically significant[SHR 2.06 (0.91–4.69, P = 0.08].
Fig. 1.
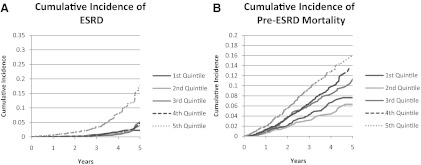
A and B, Cumulative probability of ESRD and pre-ESRD across PTH quintiles.
Table 2.
Competing-risk model for PTH and incident ESRD (SHR + 95% confidence interval)
| SHR | 95% CI | P value | |
|---|---|---|---|
| Model 1 | |||
| First quintile | 1.71 | 0.75–3.90 | 0.21 |
| Second quintile | 1.00 | ||
| Third quintile | 1.94 | 0.87–4.32 | 0.10 |
| Fourth quintile | 2.06 | 0.93–4.56 | 0.07 |
| Fifth quintile | 11.79 | 5.97–23.30 | <0.0001 |
| Model 2 | |||
| First quintile | 1.45 | 0.61–3.44 | 0.40 |
| Second quintile | 1.00 | ||
| Third quintile | 1.84 | 0.80–4.21 | 0.15 |
| Fourth quintile | 1.77 | 0.77–4.04 | 0.18 |
| Fifth quintile | 11.03 | 5.55–21.93 | <0.0001 |
| Model 3 | |||
| First quintile | 1.49 | 0.56–3.92 | 0.43 |
| Second quintile | 1.00 | ||
| Third quintile | 1.77 | 0.71–4.41 | 0.23 |
| Fourth quintile | 1.45 | 0.58–3.62 | 0.42 |
| Fifth Quintile | 5.20 | 2.37–11.41 | <0.0001 |
| Model 4 | |||
| First quintile | 1.42 | 0.54–3.77 | 0.48 |
| Second quintile | 1.00 | ||
| Third quintile | 1.62 | 0.65–4.05 | 0.30 |
| Fourth quintile | 0.98 | 0.39–2.48 | 0.97 |
| Fifth quintile | 2.06 | 0.91–4.69 | 0.08 |
Model 1 is unadjusted. Model 2 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), and family history of kidney disease or dialysis (yes/no). Model 3 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), family history of kidney disease or dialysis (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), hypertension (yes/no), cancer (yes/no), dyslipidemia, albuminuria (yes/no), BMI, baseline, Hgb level, and smoking status. Model 4 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), family history of kidney disease or dialysis (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), hypertension (yes/no), cancer (yes/no), dyslipidemia, albuminuria (yes/no), BMI, Hgb level, smoking status, baseline eGFR, calcium, and phosphorus. CI, Confidence interval; Hgb, hemoglobin; eGFR, estimated GFR.
Mortality
Among the sample, 524 of 10,823 (4.8%) died before the development of ESRD over 26,012 person-years of follow-up with 71, 56, 106, 121, and 170 events for first, second, third, fourth, and fifth PTH quintiles, respectively. Incidence rates for pre-ESRD mortality were 14.0, 10.9, 20.0, 23.0, and 32.5 per 1000 patient-years for the first, second, third, fourth, and fifth PTH quintiles (P < 0.0001), respectively (Fig. 1B). On unadjusted analyses (model 1), compared with the second quintile, there was a greater risk of death among the participants in the third [SHR 1.82 (1.32–2.52), P = 0.0003], fourth [SHR 2.09 (1.52–2.87), P < 0.0001], and fifth [SHR 2.97 (2.19–4.01), P < 0.0001] quintiles (Table 3). Furthermore, these associations were only mildly attenuated and remained significant after adjustment for demographic variables (model 2); medical comorbidities (model 3); and renal function, calcium, and phosphorus (model 4) (Table 3). In the fully adjusted model, compared with the second quintile, the risk of pre-ESRD mortality was higher in the third [SHR 1.52 (95% confidence interval 1.04–2.22)], fourth [SHR 1.73 (95% confidence interval 1.19–2.52)], and fifth [SHR 1.86 (1.28–2.52)] quintiles, respectively.
Table 3.
Competing-risk model for PTH and pre-ESRD mortality (SHR + 95% confidence interval)
| SHR | 95% CI | P value | |
|---|---|---|---|
| Model 1 | |||
| First quintile | 1.31 | 0.92–1.85 | 0.14 |
| Second quintile | 1.00 | ||
| Third quintile | 1.82 | 1.32–2.52 | 0.0003 |
| Fourth quintile | 2.09 | 1.52–2.87 | <0.0001 |
| Fifth quintile | 2.97 | 2.19–4.01 | <0.0001 |
| Model 2 | |||
| First quintile | 1.34 | 0.91–1.96 | 0.14 |
| Second quintile | 1.00 | ||
| Third quintile | 1.70 | 1.20–2.43 | 0.003 |
| Fourth quintile | 2.01 | 1.42–2.83 | <0.0001 |
| Fifth quintile | 2.66 | 1.90–3.71 | <0.0001 |
| Model 3 | |||
| First quintile | 1.19 | 0.79–1.81 | 0.41 |
| Second quintile | 1.00 | ||
| Third quintile | 1.55 | 1.06–2.28 | 0.02 |
| Fourth quintile | 1.80 | 1.24–2.60 | 0.002 |
| Fifth quintile | 1.99 | 1.38–2.86 | 0.0002 |
| Model 4 | |||
| First quintile | 1.20 | 0.79–1.82 | 0.40 |
| Second quintile | 1.00 | ||
| Third quintile | 1.52 | 1.04–2.24 | 0.03 |
| Fourth quintile | 1.73 | 1.19–2.52 | 0.004 |
| Fifth quintile | 1.86 | 1.28–2.52 | 0.001 |
Model 1 is unadjusted. Model 2 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), and family history of kidney disease or dialysis (yes/no). Model 3 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), family history of kidney disease or dialysis (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), hypertension (yes/no), cancer (yes/no), dyslipidemia, albuminuria (yes/no), BMI, baseline, Hgb level, and smoking status. Model 4 is adjusted for age, race, gender, high school education (yes/no), health insurance (yes/no), family history of kidney disease or dialysis (yes/no), diabetes (yes/no), cardiovascular disease (yes/no), hypertension (yes/no), cancer (yes/no), dyslipidemia, albuminuria (yes/no), BMI, Hgb level, smoking status, baseline eGFR, calcium, and phosphorus. CI, Confidence interval; Hgb, hemoglobin; eGFR, estimated GFR.
Effect modification
For ESRD, there was no effect modification for diabetes (P = 0.53), gender (P = 0.34), race (P = 0.88), and GFR status (P = 0.86). For pre-ESRD mortality, there was no effect modification for diabetes (P = 0.06), gender (P = 0.46), race (P = 0.28), and GFR status (P = 0.52).
Discussion
In this study, we sought to examine the association between serum PTH levels obtained during KEEP screening and longitudinal progression to ESRD and pre-ESRD mortality. Our primary finding is that higher PTH levels are associated with increased risk of pre-ESRD death but not with progression to ESRD. Furthermore, the association with pre-ESRD mortality was only mildly attenuated by demographic variables and comorbidities and subsequent adjustment for renal function, calcium, and phosphorus. Our findings that calcium and phosphorus had a clinically small range in this cohort further suggest that PTH in moderate CKD may predict pre-ESRD mortality.
The results of our study are similar those of Schumock et al. (6), who examined the association of PTH with ESRD among a population of predialysis diabetic CKD population. In that study, they found nearly a 7-fold higher risk of ESRD among those with secondary hyperparathyroidism. These results are similar to ours after adjustment for demographics and medical comorbidity. However, no further adjustment for renal function, calcium, or phosphorus was made in that study, which resulted in a significant attenuation of the association in our study. Furthermore, direct measurement of PTH was not made in that study, which limits the comparison with ours.
The lack of association may be due to relatively few ESRD episodes. We speculate that the low number developing ESRD is likely due to the relatively short duration of follow-up at an average of 2.4 yr. Additionally, our findings may be explained by divergent actions of PTH on the kidney. As stated previously, PTH may accelerate renal fibrosis (9). Furthermore, in uremic rats, medical or surgical parathyroidectomy results in a slowing in the progression of CKD with an associated reduction in interstitial fibrosis and glomerulosclerosis (10). However, it is possible that these findings might be partly explained by better blood pressure control in animals after parathyroidectomy. Conversely, PTH may maintain renal blood flow and GFR due to its vasodilatory effect on the afferent arteriole (25). Unlike uremic animals, renal function has been reported to remain stable after parathyroidectomy in humans with CKD, with some experiencing a decline coinciding with the initiation of calcium and vitamin D analogs (26). Given these discordant findings, it is likely that the effects of PTH on renal function are complex and multifaceted and may explain the lack of association of PTH with CKD progression in our study. It is additionally possible that the higher risk of death among those with higher PTH levels may have simply precluded this outcome.
Although no association with PTH and ESRD was observed when controlling for renal function, calcium, and phosphorus, elevated PTH levels were independently associated with increased mortality. In prior studies in KEEP, elevated PTH levels were associated with prevalent cardiovascular disease (3). Furthermore, other studies in CKD have demonstrated an increased risk of cardiovascular events (4) and mortality (5, 6). Studies examining mortality have examined this association among predialysis diabetics (6) and men (5) with CKD. Our findings did not find effect modification for gender or diabetes, thus extending this association to nondiabetics and women with CKD. Our study is also unique in that it is the first to examine the association of PTH with pre-ESRD mortality accounting for the competing risk of ESRD.
It has been speculated that one potential mechanism of toxicity with PTH may be due to increasing intracellular calcium concentrations in the cardiac myocytes via L-type calcium channels (27, 28). The intracellular calcium overload may then lead to increased oxidative stress and cardiac myocyte necrosis leading to scar (29). The presence of scar subsequently may lead to an increased propensity toward dysrhythmia and sudden cardiac death (30). Indeed, high PTH levels have been associated with sudden cardiac death but not death from other cardiovascular causes (7). Other potential mechanisms of PTH toxicity include the promotion of vascular calcification (31), aortic valve calcification (32), and arterial stiffness (33).
These findings suggest that PTH may be a modifiable risk factor for cardiovascular disease and that therapies designed to lower PTH levels could be of benefit. A recent randomized trial among predialysis CKD patients examined whether paricalcitol, an active vitamin D analog potent at suppressing PTH, would prevent progression of left ventricular hypertrophy (34). Although paricalcitol was not effective in preventing progression compared with placebo, cardiovascular events were reduced with this therapy. Whether this occurred due to PTH suppression or other pathways of vitamin D receptor activation is not known.
It is important to consider that the measured level of PTH in the blood is a reflection of multiple factors influencing the synthesis and release of this hormone. Elevated levels of PTH are causative in metabolic bone disease but may not be in the causal progression of kidney or heart disease that results in death. In this sense, PTH may be viewed as a response variable, indicating that it is a reflection of multiple pathogenic processes but itself is not an etiological factor in either outcome in this study. Although adjustments were made for important confounders such as calcium, phosphorus, and GFR, it is possible that other unmeasured factors contributing to PTH elevations may have contributed. Low 25-hydroxyvitamin D levels are associated with increased mortality among those with CKD (35). Thus, it is possible that the association of higher PTH and mortality may be a reflection of vitamin D deficiency. However, recent studies in populations largely without CKD have shown an independent association of PTH with cardiovascular mortality in a population without vitamin D deficiency (8). Furthermore, among men with CKD, higher PTH has been shown to be associated with increased mortality independent of vitamin D analog treatment (5). Unfortunately, KEEP screening did not measure 25-hydroxyvitamin D levels, which limits our conclusions regarding an independent effect. Other potential confounders or intermediaries may be FGF-23 and alkaline phosphatase. PTH acts on bone cells to increase FGF-23 (36) and alkaline phosphatase (37). FGF-23 is thought to contribute to the development of left ventricular hypertrophy in CKD (38). Alkaline phosphatase may promote vascular calcification (39) and has been associated with increased mortality in persons with CKD (40). Unfortunately, neither variable was measured in KEEP; thus, we were unable to determine their effects.
Strengths of this study include a large, diverse population with CKD and available measures, including ascertainment of mortality and ESRD. The use of a competing risk model is an additional strength. The study has important limitations, however. First, KEEP screening does not measure known confounders and intermediaries such as 25-hydroxyvitamin D, FGF-23, or alkaline phosphatase. Furthermore, due to the retrospective nature of this study, additional unknown unmeasured confounding may also be playing a role. Second, linkage to the Social Security Administration Death Master File does not provide the cause of death, and thus, we could not additionally examine the association of PTH with cardiovascular mortality. The lack of determination of the cause of death is therefore a major limitation to our study. Third, single measurements for covariates such as serum creatinine and PTH as well as patient recall may have led to misclassification bias. In such situations, when there are multiple levels of exposures (e.g. PTH quintiles), the risk estimate for the highest level of exposure (e.g. fifth quintile) would be biased to the null (41). If misclassification did occur, this may additionally potentially explain the lack of association of PTH with ESRD. Fourth, the PTH assay used in KEEP screening also detects PTH fragments. Whether this influenced our results and whether these fragments have a physiological role leading to death or ESRD requires further study. Fifth, because the regulation of PTH secretion is multifaceted (2), inclusion of all of the covariates may have led to overadjustment, particularly in the final model. However, given that the association of PTH and these covariates may be bidirectional, it is impossible to determine which factors are confounders and which are part of the causal pathway toward the events of interest, particularly in the context of competing risks, and thus, all variables were included in the final model. Furthermore, the association of PTH and pre-ESRD mortality was not attenuated and remained highly significant in the final model. Finally, KEEP does not collect detailed medication lists from its participants. We could not adjust for use of medications such as vitamin D or vitamin D analogs, phosphate binders, calcimimetics, and calcium supplements, all of which may influence PTH levels. Additional information regarding angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or statin therapy would have strengthened our study but were not recorded in KEEP. However, despite these limitations, we believe that the findings of the study are valuable and further add to the fund of knowledge regarding PTH and mortality in CKD.
In conclusion, we found that higher PTH levels are associated with increased pre-ESRD mortality but not ESRD. These findings emphasize the importance of recognizing PTH levels as a nontraditional risk factor for mortality among the CKD population, including in the early stages of renal dysfunction. Additional randomized trials aimed at reducing PTH levels are required to further address this issue.
Acknowledgments
This work was supported by Grant AG040638 (to A.W.C.), the Veterans Affairs Merit System Grant CDA-2 (to A.W.C.), and the American Society of Nephrology–Association of Specialty Professors Development Grant in Geriatric Nephrology (to A.W.C.) supported by a T. Franklin Williams Scholarship Award. Additional funding was provided by Atlantic Philanthropies, Inc.; the John A. Hartford Foundation; the Association of Specialty Professors; and the American Society of Nephrology.
Disclosure Summary: The authors have no conflicts to disclose.
Footnotes
- BMI
- Body mass index
- CKD
- chronic kidney disease
- ESRD
- end-stage renal disease
- FGF-23
- fibroblast growth factor-23
- GFR
- glomerular filtration rate
- KEEP
- Kidney Early Evaluation Program
- SHR
- subhazard ratio.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 2. Kumar R, Thompson JR. 2011. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol 22:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhuriya R, Li S, Chen SC, McCullough PA, Bakris GL. 2009. Plasma parathyroid hormone level and prevalent cardiovascular disease in CKD stages 3 and 4: an analysis from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 53:S3–S10 [DOI] [PubMed] [Google Scholar]
- 4. Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. 2012. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int Urol Nephrol 44:541–547 [DOI] [PubMed] [Google Scholar]
- 5. Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. 2008. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73:1296–1302 [DOI] [PubMed] [Google Scholar]
- 6. Schumock GT, Andress DL, Marx SE, Sterz R, Joyce AT, Kalantar-Zadeh K. 2009. Association of secondary hyperparathyroidism with CKD progression, health care costs and survival in diabetic predialysis CKD patients. Nephron Clin Pract 113:c54–c61 [DOI] [PubMed] [Google Scholar]
- 7. Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. 2011. Vitamin D, parathyroid hormone, and sudden cardiac death. Hypertension 58:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J. 2009. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771 [DOI] [PubMed] [Google Scholar]
- 9. Guo Y, Yuan W, Wang L, Shang M, Peng Y. 2011. Parathyroid hormone-potentiated connective tissue growth factor expression in human renal proximal tubular cells through activating the MAPK and NF-κB signalling pathways. Nephrol Dial Transplant 26:839–847 [DOI] [PubMed] [Google Scholar]
- 10. Ogata H, Ritz E, Odoni G, Amann K, Orth SR. 2003. Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors. J Am Soc Nephrol 14:959–967 [DOI] [PubMed] [Google Scholar]
- 11. Siannis F, Copas J, Lu G. 2005. Sensitivity analysis for informative censoring in parametric survival models. Biostatistics 6:77–91 [DOI] [PubMed] [Google Scholar]
- 12. Forsblom C, Harjutsalo V, Thorn LM, Wadén J, Tolonen N, Saraheimo M, Gordin D, Moran JL, Thomas MC, Groop PH. 2011. Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 22:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown WW, Collins A, Chen SC, et al. 2003. Identification of persons at high risk for kidney disease via targeted screening: the NKF Kidney Early Evaluation Program. Kidney Int Suppl S50–S55 [DOI] [PubMed] [Google Scholar]
- 14. Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM. 2003. Early detection of kidney disease in community settings: the kidney early evaluation program (KEEP). Am J Kidney Dis 42:22–35 [DOI] [PubMed] [Google Scholar]
- 15. Peralta CA, Norris KC, Li S, Chang TI, Tamura MK, Jolly SE, Bakris G, McCullough PA, Shlipak M. 2012. Blood pressure components and end-stage renal disease in persons with chronic kidney disease: the Kidney Early Evaluation Program (KEEP). Arch Intern Med 172:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens LA, Li S, Kurella Tamura M, Chen SC, Vassalotti JA, Norris KC, Whaley-Connell AT, Bakris GL, McCullough PA. 2011. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 57:S9–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saab G, Whaley-Connell A, Bombeck A, Kurella Tamura M, Li S, Chen SC, McFarlane SI, Sowers JR, Norris K, Bakris GL, McCullough PA. 2011. The association between parathyroid hormone levels and the cardiorenal metabolic syndrome in non-diabetic chronic kidney disease. Cardiorenal Med 1:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saab G, Whaley-Connell A, McFarlane SI, Li S, Chen SC, Sowers JR, McCullough PA, Bakris GL. 2010. Obesity is associated with increased parathyroid hormone levels independent of glomerular filtration rate in chronic kidney disease. Metabolism 59:385–389 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 20. Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M, Langman C, Malluche HH, McCarthy JT, Massry SG, Mehls O, Salusky IB, Silver JM, Smogorzewski MT, Slatopolsky EM, McCann L. 2004. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis 43:558–565 [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. 2011. United States Renal Data Systems annual data report. http://www.usrds.org/2011/view/v2_00_appx.asp
- 23. Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509 [Google Scholar]
- 24. Tai BC, Wee J, Machin D. 2011. Analysis and design of randomised clinical trials involving competing risks endpoints. Trials 12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Endlich K, Massfelder T, Helwig JJ, Steinhausen M. 1995. Vascular effects of parathyroid hormone and parathyroid hormone-related protein in the split hydronephrotic rat kidney. J Physiol 483(Pt 2):481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collier VU, Mitch WE. 1980. Accelerated progression of chronic renal insufficiency after parathyroidectomy. JAMA 244:1215–1218 [PubMed] [Google Scholar]
- 27. Rutledge M, Farah V, Adeboye A, Seawell M, Bhattacharya S, Weber K. 29 February 2012. Parathyroid hormone, a crucial mediator of pathologic cardiac remodeling in aldosteronism. Cardiovasc Drugs Ther 10.1007/s10557-012-6378-0 [DOI] [PubMed] [Google Scholar]
- 28. Smogorzewski M, Zayed M, Zhang YB, Roe J, Massry SG. 1993. Parathyroid hormone increases cytosolic calcium concentration in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 264:H1998–H2006 [DOI] [PubMed] [Google Scholar]
- 29. Borkowski BJ, Cheema Y, Shahbaz AU, Bhattacharya SK, Weber KT. 2011. Cation dyshomeostasis and cardiomyocyte necrosis: the Fleckenstein hypothesis revisited. Eur Heart J 32:1846–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. 2011. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators/clinical perspective. Circ Arrhythm Electrophysiol 4:324–330 [DOI] [PubMed] [Google Scholar]
- 31. Graciolli FG, Neves KR, dos Reis LM, Graciolli RG, Noronha IL, Moysés RM, Jorgetti V. 2009. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol Dial Transplant 24:1416–1421 [DOI] [PubMed] [Google Scholar]
- 32. Iwata S, Walker MD, Di Tullio MR, Hyodo E, Jin Z, Liu R, Sacco RL, Homma S, Silverberg SJ. 2012. Aortic valve calcification in mild primary hyperparathyroidism. J Clin Endocrinol Metab 97:132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker MD, Fleischer J, Rundek T, McMahon DJ, Homma S, Sacco R, Silverberg SJ. 2009. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab 94:3849–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. 2012. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307:674–684 [DOI] [PubMed] [Google Scholar]
- 35. Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ, Jr, Simon JF, Srinivas TR, Nally JV., Jr 2011. Low 25-hydroxyvitamin D levels and mortality in non dialysis-dependent CKD. Am J Kidney Dis 58:536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E. 2011. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 80:475–482 [DOI] [PubMed] [Google Scholar]
- 37. Yee JA. 1985. Stimulation of alkaline phosphatase activity in cultured neonatal mouse calvarial bone cells by parathyroid hormone. Calcif Tissue Int 37:530–538 [DOI] [PubMed] [Google Scholar]
- 38. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. 2011. FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. 2008. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int 73:1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kovesdy CP, Ureche V, Lu JL, Kalantar-Zadeh K. 2010. Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant 25:3003–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Birkett NJ. 1992. Effect of nondifferential misclassification on estimates of odds ratios with multiple levels of exposure. Am J Epidemiol 136:356–362 [DOI] [PubMed] [Google Scholar]


