Abstract
Context:
Parathyroid oxyphil cells, whose function is unknown, are thought to be derived from chief cells. Oxyphil cells increase in number in parathyroid glands of patients with chronic kidney disease (CKD) and are even more abundant in patients receiving treatment for hyperparathyroidism with calcitriol and/or the calcimimetic cinacalcet.
Objective:
We examined oxyphil and chief cells of parathyroid glands of CKD patients for differential expression of genes important to parathyroid function.
Design/Setting/Participants:
Parathyroid tissue from CKD patients with refractory hyperparathyroidism was immunostained for gene expression studies.
Main Outcome Measure:
Immunostaining for PTH, PTHrP, calcium-sensing receptor, glial cells missing 2, vitamin D receptor, 25-hydroxyvitamin D-1α-hydroxylase, and cytochrome c was quantified and expression reported for oxyphil and chief cells.
Results:
Expression of all proteins analyzed, except for the vitamin D receptor, was higher in oxyphil cells than in chief cells.
Conclusion:
Human parathyroid oxyphil cells express parathyroid-relevant genes found in the chief cells and have the potential to produce additional autocrine/paracrine factors, such as PTHrP and calcitriol. Additional studies are warranted to define the secretory properties of these cells and clarify their role in parathyroid pathophysiology.
The parathyroid glands of young adults consist primarily of chief cells. However, with age or after excessive functional stress, another cell type appears in the parathyroid gland, the oxyphil cell (1, 2). Histologically, oxyphil cells are larger than chief cells (12–20 vs. 6–8 μm), and the cytoplasm is more eosinophilic due to a high mitochondrial content. The observation of transitional oxyphil cells, which are more eosinophilic but similar in size to the chief cells, suggests that oxyphil cells are derived from chief cells. Additional evidence for a chief cell-to-oxyphil cell transdifferentiation is that transitional and oxyphil cells express PTH (3) and glial cells missing 2 (GCM2) (4), a parathyroid-specific transcription factor that is essential for parathyroid gland development.
Parathyroid oxyphil cells are markedly increased in chronic kidney disease (CKD). Interestingly, recent studies have found an association between treatment of secondary hyperparathyroidism with calcitriol and/or cinacalcet and an even higher oxyphil content of the parathyroid compared with no treatment (5–7). Although the function of the oxyphil cell is not known, it has been shown that oxyphils from CKD patients synthesize and secrete PTH (3). However, it is unclear how much PTH they secrete or whether the secretion is regulated. The expression of the calcium-sensing receptor (CaR) and vitamin D receptor (VDR) have not specifically been examined in oxyphil cells.
Parathyroid oxyphil cells have been reported to express PTHrP (8, 9), although it is unclear whether these cells secrete the hormone systemically. Given the increased mass of oxyphil cells in CKD patients, if PTHrP is secreted by these cells in high enough concentration, it could potentially contribute to renal osteodystrophy, because it acts through the same receptor as PTH. Alternatively, PTHrP could act as an autocrine/paracrine factor, regulating parathyroid growth (10) or PTH secretion (11, 12). It is noteworthy that in other cell types, PTHrP gene transcription is repressed by calcitriol (13), whereas CaR activation enhances PTHrP production in a variety of cells, including malignancies such as human prostate and breast cancer and rat Leydig cell tumor cell lines (14, 15). In contrast, in normal mammary epithelial cells, high calcium suppresses PTHrP gene expression and secretion (16–18).
Here, we compared the expression of key genes involved in parathyroid function in oxyphil and chief cells of hyperplastic parathyroid glands from patients with secondary hyperparathyroidism.
Materials and Methods
Human parathyroid gland immunostaining
As approved by the Human Research Protection Office of Washington University School of Medicine, archived parathyroid tissue obtained from 20 patients having undergone parathyroidectomy due to uremic secondary hyperparathyroidism was used for immunohistochemical analysis. A portion of tissue (formalin fixed and paraffin embedded) from a single parathyroid gland per patient was studied. Of the 20 patients analyzed, 10 were female, and 10 were male; average age was 46.1 yr old (range 29–69 yr). Race is as follows: African-American (n = 10), Caucasian (n = 8), Asian (n = 1), unknown (n = 1). Within a year before surgery, the patients had received the following treatment for secondary hyperparathyroidism: Sensipar (n = 6), Zemplar (n = 2), a combination of Sensipar and Zemplar (n = 4), no treatment (n = 7), or unknown (n = 1).
Immunostaining was performed using the following antibodies: CaR (1:500; developed in our laboratory) (19), VDR c-20 (sc-1008, 1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), cytochrome c (A-8, 1:50; Santa Cruz Biotechnology), 25-hydroxyvitamin D-1α-hydroxylase (1αOHase) polyclonal [raised against a synthetic peptide based on the last 14 C-terminal amino acids of the rat CYP27B1 and cross-reacts with human 1αOHase (20), accession no. AF000139, 1:500] provided by Dr. H. J. Armbrecht (St. Louis University, St. Louis, MO), PTHrP (Ab-2; Calbiochem, La Jolla, CA), PTH [1:250; (CH9) chicken anti-bovine polyclonal antibody that detects intact, middle, and COOH-terminal regions of bovine and human PTH; provided by Dr. Eduardo Slatopolsky, Washington University School of Medicine, St. Louis, MO], and GCM2 (1:250) (21). Sections designated for 1αOHase, PTHrP, GCM2, and cytochrome c were deparaffinized, rehydrated, microwaved at high intensity for 8 min in 10 mm citric acid (pH 6.0), and then allowed to cool for 10 min; tissue sections designated for PTH, CaR, and VDR immunostaining were not microwaved. For 1αOHase, sections were blocked with 0.1% Tween in PBS; for PTH, sections were blocked with 10% rabbit serum (Invitrogen); all other sections were blocked with 2.5% horse serum (Vector Laboratories, Burlingame, CA). For 1αOHase, PTHrP, GCM2, and cytochrome c, sections were incubated with the appropriate primary antibodies overnight at 4 C. For PTH, CaR, and VDR, tissue sections were incubated with the appropriate primary antibodies for 1 h at room temperature. For all except PTH, second antibodies (peroxidase-conjugated antirabbit and antimouse ImmPRESS reagents from Vector Laboratories) were applied for 30 min at room temperature, and the immune complexes were visualized with 3-amino-9-ethylcarbazole substrate-chromogen (Invitrogen, Carlsbad, CA). For PTH, tissue sections were incubated for 30 min with biotinylated rabbit antichicken second antibody (1:100; Sigma-Aldrich, St. Louis, MO) followed by streptavidin-horseradish peroxidase conjugate (Invitrogen). The immune complexes were visualized with 3-amino-9-ethylcarbazole substrate-chromogen.
Tissue sections from 20 patients with CKD were analyzed. Oxyphil cells were identified by their large size and eosinophilic cytoplasm as revealed by hematoxylin and eosin (H&E) staining and, when possible, by positive staining for cytochrome c. An encapsulated or well-defined area of chief or oxyphil cells was designated as nodular. Nonnodular areas of chief cells were designated as diffuse; nonnodular areas of oxyphil cells were designated as oxyphil clusters.
Immunostaining was quantified as previously described (19). Briefly, ×200 images of stained tissue sections were captured, converted to grayscale, and analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). The intensity of staining was quantified using the OD function of the software. The integrated OD (IOD) and area were obtained for each immunostained image, and IOD/area was calculated to express the intensity of staining per unit area. The IOD/area of the negative control immunostaining (i.e. control serum or IgG) was subtracted from IOD/area for the antibody-stained sections to obtain the corrected IOD/area. For each gene, the immunostaining of areas of diffuse chief cells in each section was considered the baseline staining. The corrected IOD/area for chief nodules, oxyphil clusters, or oxyphil nodules, if present in that section, was then calculated as percentage of the diffuse chief cell immunostaining. Generally, each patient had three to five genes analyzed on serial tissue sections. For each gene, results were reported as a percentage of baseline staining.
Serum PTHrP measurement
To determine whether parathyroid glands release detectable amounts of PTHrP, plasma was obtained from eight CKD patients before and after parathyroidectomy and from six control subjects and analyzed by Active PTHrP immunoradiometric assay from Diagnostic Systems Laboratories (DSL, Webster, TX), a Beckman Coulter Co. Blood was collected into tubes containing EDTA/aprotinin/leupeptin, as recommended by DSL. The plasma samples were also analyzed in a PTHrP enzyme immunoassay (Peninsula Laboratories, LLC, San Carlos, CA).
Statistics
One-way ANOVA with Tukey post hoc test (GraphPad Software, Inc., La Jolla, CA) was used for analysis of quantitation of the immunostaining for differential gene expression. Results (average ± sem) were reported as percent baseline staining of the chief diffuse cells.
Results
Immunostaining
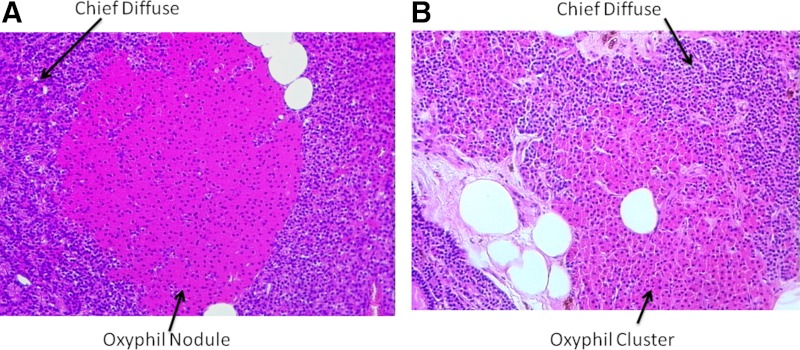
Parathyroid tissue from CKD patients was stained with H&E to identify areas of diffuse and nodular chief and oxyphil cells. Figure 1A shows a representative example of an oxyphil nodule surrounded by diffuse chief cells. Oxyphil cells are easily identified by their large size and eosinophilic staining compared with the chief cells. The eosinophilic staining is due to the large number of mitochondria in the oxyphil cells. Note the distinct borders defining the oxyphil nodule in Fig. 1A compared with the oxyphil cluster in Fig. 1B, where the delineation between oxyphil and chief cells is more diffuse/undefined.
Fig. 1.
Histology of parathyroid glands from patients with CKD. Representative H&E-stained sections are of different areas of hyperplastic human parathyroid glands. A, A well-defined oxyphil nodule surrounded by diffuse chief cells; B, a more diffuse group, or cluster, of oxyphil cells with surrounding diffuse chief cells. Magnification, ×200.
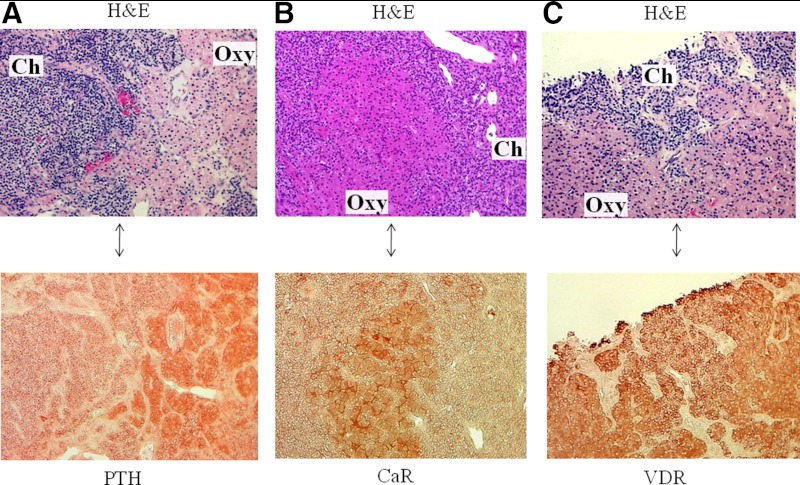
Parathyroid oxyphil cells are believed to be derived from chief cells because they continue to express PTH; however, it has been speculated that oxyphil cells may represent deactivated chief cells, secreting less PTH. We investigated the relative levels of PTH content in oxyphil and chief cells by immunostaining. As shown in Table 1, which summarizes the results of immunostaining quantitation, expression of PTH was modestly, but significantly, higher in oxyphil clusters compared with diffuse chief cells. This higher expression of PTH in an oxyphil cluster is represented in Fig. 2A; chief and oxyphil cells are delineated by H&E staining in the upper image, and PTH immunostaining in an adjacent section is shown in the lower image. As indicated in Table 1, PTH expression was the lowest in chief cell nodules (not shown in image).
Table 1.
Relative expression of gene products in chief and oxyphil cells of human parathyroid glands from patients with CKD
| Protein | Chief diffuse | Oxyphil cluster | Chief nodule | Oxyphil nodule |
|---|---|---|---|---|
| 1αOHase | 100 (n = 17) | 264.0 ± 26.7a (n = 13) | 68.8 ± 16.5a,b (n = 6) | 191.6 ± 23.7a,c (n = 7) |
| Cytochrome c | 100 (n = 10) | 921.3 ± 239a (n = 7) | 335.9 ± 195.6b (n = 5) | 1076.3 ± 306.0a,c (n = 5) |
| PTH | 100 (n = 9) | 126.4 ± 20.2a (n = 5) | 76.4 ± 10.3a,b (n = 6) | 117.8 ± 15.0c (n = 4) |
| PTHrP | 100 (n = 14) | 225.5 ± 34.3a (n = 10) | 103.8 ± 23.3b (n = 7) | 168.4 ± 23.9a,b,c (n = 6) |
| VDR | 100 (n = 11) | 124.9 ± 39.5 (n = 8) | 118.8 ± 23.6 (n = 6) | 128.8 ± 15.9 (n = 6) |
| CaR | 100 (n = 11) | 128.4 ± 17.9a (n = 9) | 94.9 ± 13.7b (n = 6) | 134.8 ± 29.3a,c (n = 5) |
| GCM2 | 100 (n = 12) | 171.5 ± 21.1a (n = 8) | 72.2 ± 13.7a,b (n = 5) | 96.0 ± 19.6b (n = 6) |
Expression of quantitated immunostaining is reported as percentage of diffuse chief. A portion of one gland per patient was analyzed; n = number of patients analyzed for that particular gene and cell type.
P < 0.05 vs. chief diffuse.
P < 0.05 vs. oxyphil cluster.
P < 0.05 vs. chief nodule.
Fig. 2.
Immunostaining of PTH, CaR, and VDR in parathyroid oxyphil and chief cells. A–C, Representative images of parathyroid tissue from three patients with CKD. Areas of chief (ch) and oxyphil (oxy) cells are identified by H&E staining (upper image in each panel); an adjacent, immunostained section is shown as the lower image of each panel. A, Expression of PTH is higher in oxyphil cells compared with chief cells; B, CaR expression is significantly higher in oxyphil cells; whereas C, VDR was not differentially expressed in the parathyroid tissue. Magnification, ×200.
PTH synthesis and secretion are regulated primarily by extracellular calcium and the activated form of vitamin D, calcitriol. Suppression of PTH by these two factors is mediated by the CaR and the VDR. As shown in Table 1, CaR expression was slightly, but significantly, higher in oxyphil clusters and nodules compared with chief cells; however, the VDR was not differentially expressed. Representative images of the expression of CaR and VDR are shown in Fig. 2, B and C, respectively. H&E staining delineates chief and oxyphil cells in the upper panels; CaR and VDR immunostaining in respective adjacent sections are illustrated in the lower panels.
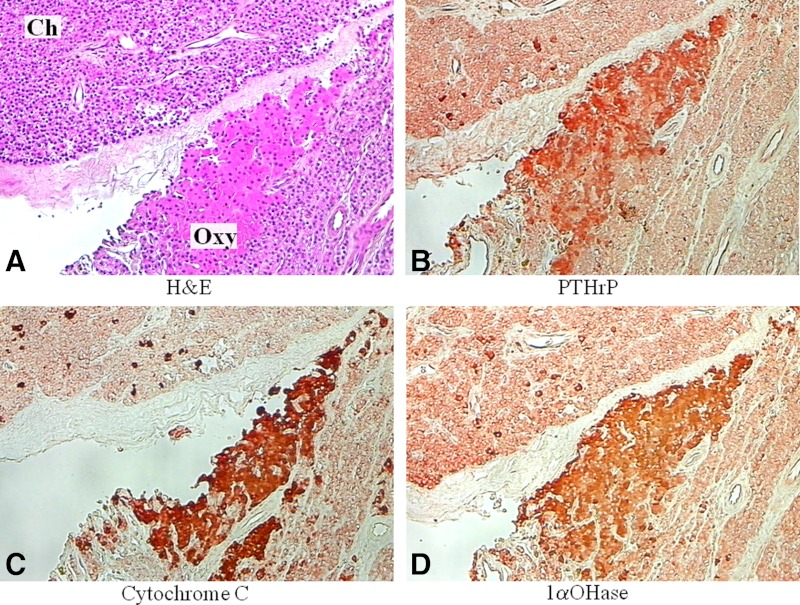
PTHrP was highly expressed in oxyphil clusters and oxyphil nodules compared with diffuse chief cells (Table 1), in agreement with earlier studies by Kitazawa and co-workers (8, 9). A representative image of the increase in PTHrP in oxyphil cell clusters compared with diffuse chief cells is shown in Fig. 3B.
Fig. 3.
Immunostaining of PTHrP, cytochrome c, and 1αOHase in parathyroid oxyphil and chief cells. A, Chief (ch) and oxyphil (oxy) cells were identified by H&E stain in a representative image of parathyroid tissue from a patient with CKD; B–D, serial sections show differential expression of PTHrP (B), cytochrome c (C), and 1αOHase (D), with higher levels in the oxyphil cells compared with chief cells. Magnification, ×200.
Oxyphil cells contain abundant mitochondria (22, 23). In confirmation of this, the mitochondrial protein cytochrome c was very highly expressed in oxyphil clusters and oxyphil nodules compared with diffuse chief cells (Table 1). Figure 3C illustrates cytochrome c staining in oxyphil clusters compared with diffuse chief cells. Similarly, in oxyphil cells, a markedly elevated expression was observed for the 1αOHase, the mitochondrial cytochrome P450 that catalyzes the formation of 1,25-dihydroxyvitamin D3 (calcitriol), the active form of vitamin D (Table 1). Figure 3D shows the staining for 1αOHase in oxyphil clusters compared with diffuse chief cells. Chief cell nodules exhibited the lowest expression of 1αOHase (not shown).
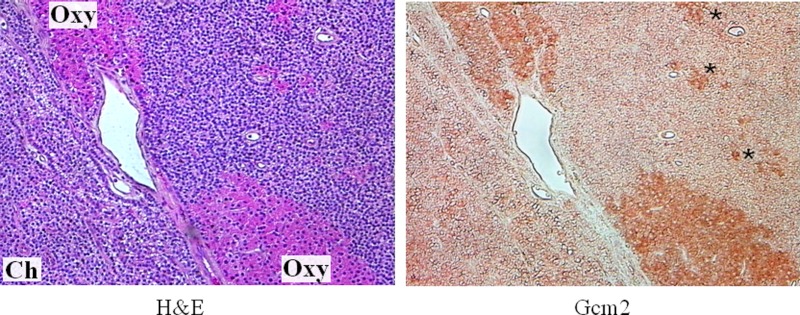
The parathyroid-specific transcription factor GCM2 has been shown to be essential for completion of parathyroid gland development (24) and continues to be expressed in mature parathyroids (25, 26). We found GCM2 to be significantly increased in oxyphil clusters (Table 1 and Fig. 4), suggesting it may be responsible, in part, for the higher CaR and PTH in these cells. As indicated in Table 1, chief cell nodules expressed the least amount of GCM2.
Fig. 4.
Immunostaining of GCM2 in parathyroid oxyphil and diffuse chief cells. Chief (ch) and oxyphil (oxy) cells were identified by H&E stain in a representative image of parathyroid tissue from a CKD patient (left). An adjacent section (right) shows differential expression of GCM2, with higher levels in the oxyphil cells. Even smaller groups of oxyphil cells (asterisks) interspersed among the chief cells express higher levels of GCM2. Magnification, ×200.
Serum PTHrP measurement
Plasma PTHrP was measured by immunoradiometric assay and enzyme immunoassay. Levels in all normal subjects, and before and after parathyroidectomy in the CKD patients, were undetectable in both assays (data not shown).
Discussion
The chief cells of the parathyroid glands play a central role in calcium homeostasis by sensing changes in extracellular calcium and releasing the appropriate amount of PTH to correct or maintain normal blood calcium levels. Although chief cells are the major cell type of the parathyroid glands of young, healthy subjects, another cell type, the oxyphil cell, appears with age and increases dramatically in number in patients with CKD (1). More recently, evidence indicates that treatment of hyperparathyroidism in CKD patients increases the content of oxyphil cells in the glands (5–7). Importantly, oxyphil content was highest in patients receiving the calcimimetic cinacalcet, suggesting a role for CaR activation in the genesis of these cells. Also of note is the report by Allen and Thorburn (27) that oxyphil cell content is positively associated with mean serum calcium in cases of primary multiple-gland hyperparathyroidism, consistent with a role for CaR activation in their formation. Although these data support the hypothesis of the involvement of the CaR in oxyphil development, additional studies are needed to fully define the factors involved in the transdifferentiation of chief to oxyphil cell.
The function of oxyphil cells is not known, but it has been speculated that they may be deactivated chief cells. The parathyroid glands become hyperplastic when the existing chief cells cannot produce sufficient PTH to correct external stimuli (i.e. low blood calcium, high blood phosphate, or low calcitriol levels). However, when these stimuli are corrected, or masked in the case of cinacalcet treatment for secondary hyperparathyroidism, the (apparent) need for the additional chief cells declines. Parathyroid chief cells do not readily undergo apoptosis, and therefore, conversion to a non-PTH-secreting cell type could prevent excessive secretion of the hormone. However, our current findings indicate that parathyroid oxyphil cells contain high levels of immunoreactive PTH, indicating that these cells continue to produce the hormone. Furthermore, CaR and VDR are expressed by oxyphil cells, suggesting continued regulation of PTH by calcium, calcimimetics, and vitamin D compounds. Tanaka et al. (3) previously showed that heterotransplantation of human parathyroid nodules that consisted exclusively of oxyphil or chief cells into nude mice were able to secrete intact human PTH. Both cell types were determined to have similar PTH secretory activity. In the present study, we show that immunoreactive PTH is significantly higher in oxyphil cells compared with chief cells, with chief cell nodules expressing the least amount of PTH. It is not known whether this increased expression of PTH in oxyphil cells is reflective of intact PTH, inactive PTH fragments, or antagonistic fragments [e.g. PTH (7–84)]. Future studies with isolated oxyphil cells will better assess their secretory properties.
Although the overall levels of expression of the CaR and VDR are decreased in hyperplastic parathyroid tissue compared with normal tissue (19, 28–33), they are still highly expressed, although heterogeneously, throughout the tissue. Grzela et al. (34) found that protein expression of the CaR and VDR in tertiary hyperparathyroidism was expressed in a markedly diverse manner throughout the parathyroid, with areas of very intense staining for CaR or VDR being surrounded by areas of minimal staining. Likewise, Välimäki et al. (35) found that expression of CaR, VDR, and PTH mRNA were heterogeneously expressed in parathyroid glands of patients with secondary hyperparathyroidism, varying widely not only within the same gland but also between glands. However, neither study reported expression with respect to cell type. In the present study, we found that the CaR was differentially expressed in hyperplastic parathyroid tissue and was significantly higher in oxyphil cells. Expression of the VDR was also heterogeneously expressed in the tissue, but the difference between chief and oxyphil cells was found not to be significant. It is tempting to speculate that treatments for hyperparathyroidism (i.e. calcitriol, calcimimetics, or vitamin D analogs) may alter expression of the CaR and/or VDR, resulting in less variation of expression in the tissue. In fact, Mizobuchi et al. (36) found that the calcimimetic compound NPS R-568 up-regulates CaR expression levels in parathyroid glands of rats with chronic renal insufficiency. Additional studies correlating differential gene expression with treatment for hyperparathyroidism in human glands are in progress.
Consistent with the finding of CaR and PTH in oxyphil cells is our observation that they also express GCM2. This parathyroid-specific transcription factor is critical for parathyroid development (24) and functions to maintain high levels of expression of PTH and CaR (21). Silencing GCM2 in human parathyroid cells reduces the expression of the CaR (21) and PTH (Brown, A. J., and C. S. Ritter, manuscript in preparation), indicating a continued role for GCM2 in parathyroid function. Nonaka (4) recently investigated GCM2 as a diagnostic marker of parathyroid lesions, and found positive expression in chief, oxyphil, and clear cells. However, they reported that expression was uniform throughout the parathyroid tissue. In the present study, we found a heterogeneous expression of GCM2, with expression highest in oxyphil clusters and lowest in chief cell nodules. To see whether this difference in observed expression (uniform vs. heterogeneous) was due to different GCM2 antibodies, we tested our parathyroid tissue with two additional GCM2 antibodies (Santa Cruz; GCM2 S-19 and C-20) and still found the expression to be heterogeneous (unpublished observation).
Parathyroid oxyphil cells were previously reported to express PTHrP (8, 9), and our findings confirm this observation. However, it is unclear whether the PTHrP is secreted by the cells. Given that the mass of oxyphil cells in these enlarged parathyroid glands can be substantial, we investigated whether PTHrP was released systemically in concentrations that had the potential to influence bone turnover. We found that plasma PTHrP levels in eight CKD patients studied were undetectable both before and after parathyroidectomy, the same as for normal individuals. This was true even in two of the CKD patients who each had an estimated 1.2 g oxyphilic parathyroid tissue (one patient had a total of 6 g parathyroid tissue removed, 20% of which was estimated to be oxyphilic according to H&E evaluation; the second patient had a total of 3 g parathyroid tissue removed, 40% of which was estimated to be oxyphilic). Thus, if PTHrP is secreted by oxyphil cells, it does not appear to leave the parathyroid glands and may act only in an autocrine/paracrine fashion. In fact, exogenous PTHrP has been shown to enhance PTH release in response to low calcium in rats and in rat parathyroid gland cultures (11, 12), indicating the potential of PTHrP actions in human glands as well.
Oxyphil cells have abundant mitochondria, suggesting a need for energy production. Mitochondria are also the site of vitamin D metabolism, and we recently reported that the vitamin D 1αOHase is very highly expressed in oxyphil cells (20). Calcitriol produced by extrarenal tissues does not typically enter the circulation to regulate mineral metabolism. Instead, it is usually made in response to cell-specific stimuli and carries out autocrine/paracrine functions. We have recently demonstrated that high calcium increases the production of 1αOHase in human parathyroid monolayers and that this enzyme is active (20). Therefore, we speculate that parathyroid oxyphil cells, formed by transdifferentiation in response to CaR activation, may produce calcitriol to reduce PTH synthesis, providing an additional potential mechanism for control of PTH by calcimimetics.
In conclusion, the parathyroid oxyphil cells that accumulate in substantial numbers in CKD patients express parathyroid-relevant genes found in the chief cells (PTH, VDR, CaR, and GCM2), strongly suggesting that oxyphil cells are not simply deactivated chief cells. The presence of VDR and CaR further suggests that oxyphil cells release PTH in a regulated fashion, but this must be determined empirically using enriched cell populations. Parathyroid oxyphil cells also have the potential to produce autocrine/paracrine factors, such as PTHrP and calcitriol. Comparative analysis of the proteins released by oxyphil and chief cells (secretomes) may identify additional novel secreted factors of pathophysiological importance. The fact that oxyphil cells are increased in the parathyroid glands of CKD patients, being highest in patients receiving conventional treatment for hyperparathyroidism, suggests that they have a function distinct from that of chief cells. Additional studies are required to clearly define the role of these cells in parathyroid pathophysiology.
Acknowledgments
We thank Nicole Hagan, R.M.A., for patient coordination, in addition to the surgical fellows and nursing staff of the Department of Otolaryngology/Surgery at Barnes-Jewish Hospital for help in obtaining parathyroid tissue and Dr. H. J. Armbrecht of St. Louis University and St. Louis Veterans Affairs Center for the antibody to the 1α-hydroxylase.
This work was supported by grants from Abbott Laboratories and the Center for D-Receptor Activation Research (CeDAR). Histology services were supported in part by the Washington University School of Medicine Diabetes Research Training Center (DK-20579).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CaR
- Calcium-sensing receptor
- CKD
- chronic kidney disease
- GCM2
- glial cells missing 2
- H&E
- hematoxylin and eosin
- IOD
- integrated OD
- 1αOHase
- 25-hydroxyvitamin D-1α-hydroxylase
- VDR
- vitamin D receptor.
References
- 1. Christie AC. 1967. The parathyroid oxyphil cells. J Clin Pathol 20:591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamperl H. 1962. [Onkocytes and onkocytoma]. Virchows Arch Pathol Anat Physiol Klin Med 335:452–483 (German) [PubMed] [Google Scholar]
- 3. Tanaka Y, Funahashi H, Imai T, Seo H, Tominaga Y, Takagi H. 1996. Oxyphil cell function in secondary parathyroid hyperplasia. Nephron 73:580–586 [DOI] [PubMed] [Google Scholar]
- 4. Nonaka D. 2011. Study of parathyroid transcription factor Gcm2 expression in parathyroid lesions. Am J Surg Pathol 35:145–151 [DOI] [PubMed] [Google Scholar]
- 5. Lomonte C, Martino R, Selvaggiolo M, Bona RM, Cazzato F, Milano R, Chiarulli G, Basile C. 2003. Calcitriol pulse therapy and histology of parathyroid glands in hemodialysis patients. J Nephrol 16:716–720 [PubMed] [Google Scholar]
- 6. Lomonte C, Vernaglione L, Chimienti D, Bruno A, Cocola S, Teutonico A, Cazzato F, Basile C. 2008. Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol 3:794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sumida K, Nakamura M, Ubara Y, Marui Y, Tanaka K, Takaichi K, Tomikawa S, Inoshita N, Ohashi K. 2011. Histopathological alterations of the parathyroid glands in haemodialysis patients with secondary hyperparathyroidism refractory to cinacalcet hydrochloride. J Clin Pathol 64:756–760 [DOI] [PubMed] [Google Scholar]
- 8. Kitazawa R, Kitazawa S, Fukase M, Fujita T, Kobayashi A, Chihara K, Maeda S. 1992. The expression of parathyroid hormone-related protein (PTHrP) in normal parathyroid: histochemistry and in situ hybridization. Histochemistry 98:211–215 [DOI] [PubMed] [Google Scholar]
- 9. Kitazawa R, Kitazawa S, Maeda S, Kobayashi A. 2002. Expression of parathyroid hormone-related protein (PTHrP) in parathyroid tissue under normal and pathological conditions. Histol Histopathol 17:179–184 [DOI] [PubMed] [Google Scholar]
- 10. Matsushita H, Hara M, Endo Y, Shishiba Y, Hara S, Ubara Y, Nakazawa H, Suzuki N, Kawaminami K, Kido T, Li Q, Grimelius L. 1999. Proliferation of parathyroid cells negatively correlates with expression of parathyroid hormone-related protein in secondary parathyroid hyperplasia. Kidney Int 55:130–138 [DOI] [PubMed] [Google Scholar]
- 11. Lewin E, Almaden Y, Rodriguez M, Olgaard K. 2000. PTHrP enhances the secretory response of PTH to a hypocalcemic stimulus in rat parathyroid glands. Kidney Int 58:71–81 [DOI] [PubMed] [Google Scholar]
- 12. Lewin E, Garfia B, Almaden Y, Rodriguez M, Olgaard K. 2003. Autoregulation in the parathyroid glands by PTH/PTHrP receptor ligands in normal and uremic rats. Kidney Int 64:63–70 [DOI] [PubMed] [Google Scholar]
- 13. Kremer R, Sebag M, Champigny C, Meerovitch K, Hendy GN, White J, Goltzman D. 1996. Identification and characterization of 1,25-dihydroxyvitamin D3-responsive repressor sequences in the rat parathyroid hormone-related peptide gene. J Biol Chem 271:16310–16316 [DOI] [PubMed] [Google Scholar]
- 14. MacLeod RJ, Chattopadhyay N, Brown EM. 2003. PTHrP stimulated by the calcium-sensing receptor requires MAP kinase activation. Am J Physiol Endocrinol Metab 284:E435–E442 [DOI] [PubMed] [Google Scholar]
- 15. Chattopadhyay N. 2006. Effects of calcium-sensing receptor on the secretion of parathyroid hormone-related peptide and its impact on humoral hypercalcemia of malignancy. Am J Physiol Endocrinol Metab 290:E761–E770 [DOI] [PubMed] [Google Scholar]
- 16. VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. 2004. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. Journal of Clin Invest 113:598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ardeshirpour L, Dann P, Pollak M, Wysolmerski J, VanHouten J. 2006. The calcium-sensing receptor regulates PTHrP production and calcium transport in the lactating mammary gland. Bone 38:787–793 [DOI] [PubMed] [Google Scholar]
- 18. Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. 2008. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem 283:24435–24447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown AJ, Ritter CS, Finch JL, Slatopolsky EA. 1999. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int 55:1284–1292 [DOI] [PubMed] [Google Scholar]
- 20. Ritter CS, Haughey BH, Armbrecht HJ, Brown AJ. 2012. Distribution and regulation of the 25-hydroxyvitamin D3 1α-hydroxylase in human parathyroid glands. J Steroid Biochem Mol Biol 130:73–80 [DOI] [PubMed] [Google Scholar]
- 21. Mizobuchi M, Ritter CS, Krits I, Slatopolsky E, Sicard G, Brown AJ. 2009. Calcium-sensing receptor expression is regulated by glial cells missing-2 in human parathyroid cells. J Bone Miner Res 24:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munger BL, Roth SI. 1963. The cytology of the normal parathyroid glands of man and Virginia deer; a light and electron microscopic study with morphologic evidence of secretory activity. J Cell Biol 16:379–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Müller-Höcker J, Schäfer S, Copeland WC, Wiesner R, Seibel P. 1998. Immunohistochemical detection of human mtDNA polymerase γ and of human mitochondrial transcription factor A in cytochrome-c-oxidase-deficient oxyphil cells of hyperfunctional parathyroids. Virchows Archiv 433:529–536 [DOI] [PubMed] [Google Scholar]
- 24. Günther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G. 2000. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 406:199–203 [DOI] [PubMed] [Google Scholar]
- 25. Correa P, Akerstrom G, Westin G. 2002. Underexpression of Gcm2, a master regulatory gene of parathyroid gland development, in adenomas of primary hyperparathyroidism. Clin Endocrinol (Oxf) 57:501–505 [DOI] [PubMed] [Google Scholar]
- 26. Kebebew E, Peng M, Wong MG, Ginzinger D, Duh QY, Clark OH. 2004. GCMB gene, a master regulator of parathyroid gland development, expression, and regulation in hyperparathyroidism. Surgery 136:1261–1266 [DOI] [PubMed] [Google Scholar]
- 27. Allen TB, Thorburn KM. 1981. The oxyphil cell in abnormal parathyroid glands. A study of 114 cases. Arch Pathol Lab Med 105:421–427 [PubMed] [Google Scholar]
- 28. Fletcher S, Brownjohn AM, Dunwell C, Harnden P. 1997. Immunocytochemical detection of a reduction in 1,25 dihyroxyvitamin D3 receptor expression in uraemic parathyroid tissue. Nephrol Dial Transplant 12:93–96 [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez M, Nemeth E, Martin D. 2005. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol 288:F253–F264 [DOI] [PubMed] [Google Scholar]
- 30. Kifor O, Moore FD, Jr, Wang P, Goldstein M, Vassilev P, Kifor I, Hebert SC, Brown EM. 1996. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab 81:1598–1606 [DOI] [PubMed] [Google Scholar]
- 31. Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, Drüeke TB. 1997. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 51:328–336 [DOI] [PubMed] [Google Scholar]
- 32. Korkor AB. 1987. Reduced binding of [3H]1,25-dihydroxyvitamin D3 in the parathyroid glands of patients with renal failure. N Engl J Med 316:1573–1577 [DOI] [PubMed] [Google Scholar]
- 33. Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y. 1993. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 92:1436–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grzela T, Chudzinski W, Lasiecka Z, Niderla J, Wilczynski G, Gornicka B, Wasiutynski A, Durlik M, Boszczyk A, Brawura-Biskupski-Samaha R, Dziunycz P, Milewski L, Lazarczyk M, Lazarczyk M, Nawrot I. 2006. The calcium-sensing receptor and vitamin D receptor expression in tertiary hyperparathyroidism. Int J Mol Med 17:779–783 [PubMed] [Google Scholar]
- 35. Välimäki S, Farnebo F, Forsberg L, Larsson C, Farnebo LO. 2001. Heterogeneous expression of receptor mRNAs in parathyroid glands of secondary hyperparathyroidism. Kidney Int 60:1666–1675 [DOI] [PubMed] [Google Scholar]
- 36. Mizobuchi M, Hatamura I, Ogata H, Saji F, Uda S, Shiizaki K, Sakaguchi T, Negi S, Kinugasa E, Koshikawa S, Akizawa T. 2004. Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol 15:2579–2587 [DOI] [PubMed] [Google Scholar]