Abstract
Epilepsy has been historically seen as a functional brain disorder associated with excessive synchronization of large neuronal populations leading to a hypersynchronous state. Recent evidence showed that epileptiform phenomena, particularly seizures, result from complex interactions between neuronal networks characterized by heterogeneity of neuronal firing and dynamical evolution of synchronization. Desynchronization is often observed preceding seizures or during their early stages; in contrast, high levels of synchronization observed towards the end of seizures may facilitate termination. In this review we discuss cellular and network mechanisms responsible for such complex changes in synchronization. Recent work has identified cell-type-specific inhibitory and excitatory interactions, the dichotomy between neuronal firing and the non-local measurement of local field potentials distant to that firing, and the reflection of the neuronal dark matter problem in non-firing neurons active in seizures. These recent advances have challenged long-established views and are leading to a more rigorous and realistic understanding of the pathophysiology of epilepsy.
 |
Premysl Jiruska is former Epilepsy Research UK fellow. Currently, he works at Institute of Physiology, Academy of Sciences of Czech Republic. In his research he focuses on mechanisms of ictogenesis and functional organization of epileptic foci. Marco de Curtis is Head of Pre-clinical Neuroscience Laboratories at Fondazione Istituto Neurologico Carlo Besta and John G. R. Jefferys is Professor of Neuroscience at University of Birmingham. Both share common interest in function of neuronal networks in health and disease. Catherine A. Schevon is a clinical neurophysiologist and her major research interests are the cellular and network mechanisms of seizure generation and spread, and how these relate to clinical diagnostic studies. Steven J. Schiff is Director of the Penn State Center for Neural Engineering and focuses on mechanisms of control of epilepsy, migraines, and Parkinson's disease. Kaspar Schindler is a clinical neurologist and works at Bern University Hospital and his main research interest is ictal dynamics and synchronization in human epilepsy.
Introduction
Coordinated neuronal activity and interactions between neurons and neuronal populations are the basic features of brain function. In the healthy brain, cognitive processes require precise integration of neural activity at specific spatiotemporal scales (Varela et al. 2001; Uhlhaas & Singer 2006). Such synchronization occurs at time scales ranging from milliseconds to hours, and over short and long intracerebral distances. Alteration of synchronization can be observed in several neurological and psychiatric disorders (Uhlhaas & Singer 2006).
One of the main disorders in which altered neuronal interactions play a crucial role is epilepsy. Historically, it has been seen as a disorder whose hallmark, spontaneous seizures, consists of high-amplitude, often rhythmic, activity. This has been interpreted as abnormally enhanced neuronal excitability and synchronization. Indeed, the term hypersynchrony was coined to describe this distinctive high-amplitude pathological behaviour (Penfield & Jasper 1954; Westbrook 1991; Margineanu 2010). In this review, we discuss the existing literature on seizure synchronization in light of our emerging knowledge of interactions between neuronal populations during seizures.
Synchronization and how it is measured
Synchrony implies that two systems, x(t) and y(t), will asymptotically converge so that their trajectories become identical, x(t) =y(t) (Brown & Rulkov 1997), and that such dynamics are structurally stable (Pecora & Carroll 1990). In practice, one admits the interposition of a function, f, that transforms the values from one system to the other x(t) =f(y(t)) (Rulkov et al. 1995), and typically this function f is the identity – a straight line with slope equal to 1. For non-identical systems, they can come close, but never exactly synchronize, and neurons and their populations are never identical (for review see Schiff 2012). Synchrony is traditionally inferred from linear correlation (in the time domain) or coherence (in the frequency domain; Fig. 1). Since synchrony reflects a stable functional relationship, perturbing neural systems to probe stability can be very useful when pushing the threshold of subtle synchronization (Francis et al. 2003), although this is rarely done in testing whether measures of correlation in epilepsy reflect true synchronization.
Figure 1. Application of linear correlation and coherence to measure synchronization.
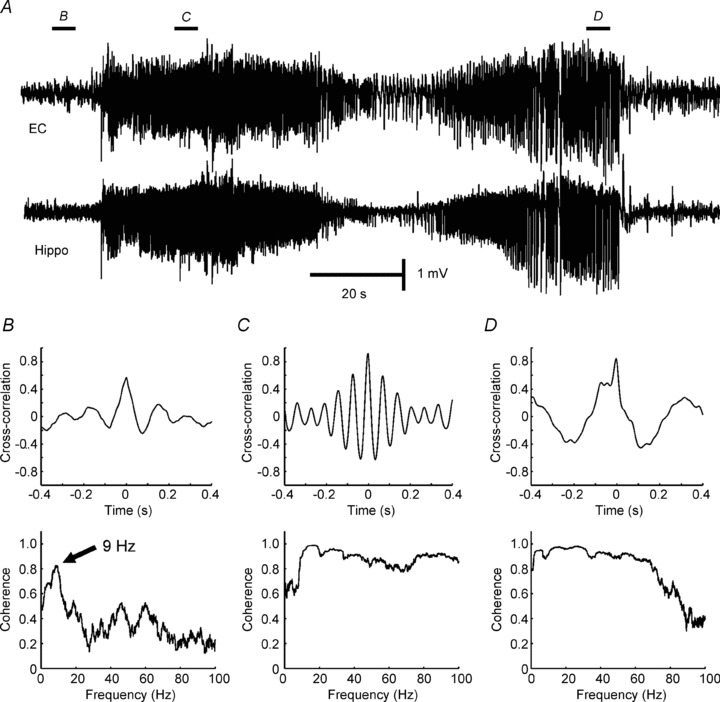
A, synchronization between the hippocampus (Hippo) and entorhinal cortex (EC) was examined before and during seizure in a tetanus toxin model of temporal lobe epilepsy (Jiruska et al. unpublished results). B, correlation of 0.5 was observed between signals from hippocampus and entorhinal cortex recorded before onset of the seizure. Coherence, often interpreted as a correlation at each frequency, shows an obvious peak in coherence at frequency 9 Hz. C, cross-correlation and coherence during early and final parts of seizure (D) (Jiruska P & Jefferys JGR; unpublished results).
Synchronization in epilepsy and seizures is conceptually complex and both decreases and increases in synchrony are integral features. As there are several valid methods of computation, and measurements can be taken on different types of signals and across different spatial scales, the results should be always interpreted with appropriate attention to context (Varela et al. 2001; Pikovsky et al. 2003). At the cellular level, synchronization is typically inferred from a linear measure of correlated firing between neurons and can be examined between various neuronal subtypes, e.g. between principal neurons or distinct interneuronal subclasses (Salinas & Sejnowski 2001; Ziburkus et al. 2006). Interactions between neuronal populations are often examined indirectly using field potentials, which are presumed to reflect the synaptic currents arising locally from the collective activity of each of these neuronal populations. During seizures important exceptions to this assumption can occur due, for instance, to the distance between the neuronal somata and their synaptic terminals. Spatially, synchronization can be investigated on a local (micro) scale, between adjacent neurons or cortical columns, or across many centimeters of cortex. Synchronization can also be measured on different time scales. Neural firing and high-frequency oscillations are best examined on a millisecond time scale, while low-frequency synchronization requires data samples extending over tens of seconds, hours or even days (Welsh et al. 1995).
When interpreting synchronization data, it is essential to know if the synchronization is expressed as a single absolute value, or as a relative value demonstrating a rate of change. In the first case, synchronization (or desynchronization) can be determined by a measure exceeding (or falling below, respectively) a defined threshold. In the second case, synchronization is examined in terms of how it changes in time: an increasing trend in coupling can be interpreted as synchronization, while a decreasing trend can be described as desynchronization.
Rulkov et al. (1995) defined the term generalized synchronization for non-identical coupled non-linear systems. While linear correlation assesses a linear functional relationship, generalized synchronization detects a non-linear functional relationship between the dynamics of two systems. It was readily apparent that methods to detect non-linear synchronization could reveal relationships in neuronal systems to which linear methods were blind (Schiff et al. 1996). But how much functional importance lies within the hard-to-detect non-linear regime remains an open question (Netoff et al. 2006). There are a range of metrics of neuronal systems that are used to assess the degree of synchrony – time lags, phase relationships between oscillating signals, mutual information, mathematical continuity – and none has proved a clear winner for different systems and different types of coupling. The non-linear techniques tend to be more sensitive to noise than the linear ones, and noise appears to represent an important factor along with the degree of non-linearity in choosing a detection technique (Netoff et al. 2004). The role of non-linear techniques to detect synchronization in epilepsy remains an active and open area for investigation.
Finally, dynamical synchronization in noisy systems is a statistical phenomenon, and employing strict statistical null hypotheses to establish the significance of synchronization should be applied to any of the above strategies. Even determining whether systems are not coupled, given noisy electrode data with shared frequencies, can be a difficult albeit necessary question to address (Netoff et al. 2006). Recent work invoking methods from random matrix theory (Fig. 2; Li et al. 2007; Muller et al. 2008) and random networks (Zhao et al. 2011) demonstrate that our conceptual understanding of statistical synchrony continues to advance, and will further change our concept of synchrony in epileptic seizures.
Figure 2. Synchronization profile of seizure activity in the low-calcium model in hippocampal slices in vitro.
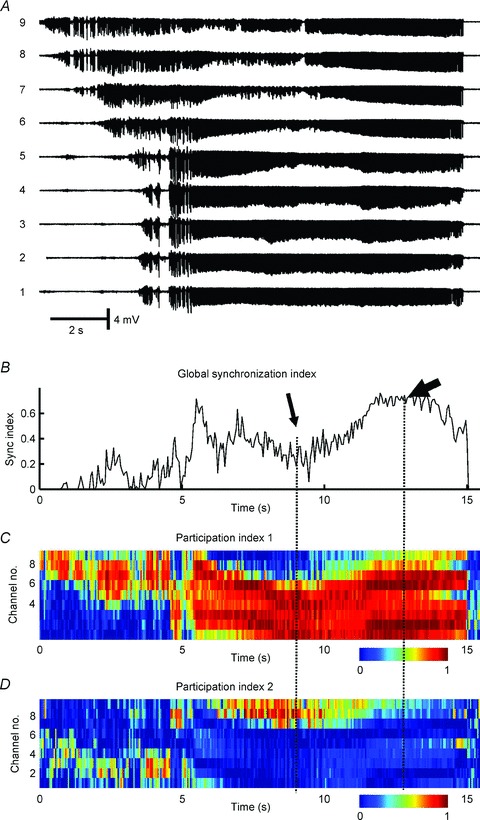
A, signals recorded from CA1 area with nine microelectrodes separated by ∼300 μm. B, wavelet phase synchronization was used to calculate the temporal profile of a global synchronization index and shows a progressive increase in synchronization which reaches maximal values towards the end of the seizure. C, random matrix analysis was applied to determine the temporal profile of the first participation index. This index identifies the largest synchronization cluster and its components. Colours indicate how much each channel contributes to the cluster at each time. Cold colours indicate a low contribution while hot colours mean a high contribution. D, the second largest cluster of synchronization. The drop in global synchronization index (thin arrow) is due to the development of two independent clusters of synchrony. During the final part of the seizure, when synchronization reaches its maximal value, ictal activity is generated by a single large cluster of synchronous activity (thick arrow) to which nearly all channels contribute (Jiruska P & Jefferys JGR; unpublished results; random matrix analysis was described in detail in Li et al. 2007).
Cellular activity during seizures
Penfield and Jasper hypothesized that seizures are an extreme form of synchronous brain activity, characterized by decreased inhibition and enhanced excitation, leading to a transient condition of intense, hypersynchronous neuronal activity (Penfield & Jasper 1954). Electroencephalographic (EEG) recordings during such episodes reveal high-amplitude ictal (seizure) discharges in the Berger (1–25 Hz) frequency bands (Penfield & Jasper, 1954).
It has long been assumed that sites where the EEG shows high amplitude ictal discharges are participating in the seizure; i.e. that high-amplitude waveforms and intense neural firing are spatially tightly coupled. This concept was first advanced in the 1930s (Bishop 1932), and was further supported by simultaneous intracellular and extracellular recordings from in vitro models (Jefferys & Haas 1982; Konnerth et al. 1984; Jensen & Yaari 1997). However, coupling between neuronal firing and field potentials is more complex than this. In early studies, it became obvious that not all sampled neurons participated in the tonic and clonic ictal patterns. Matsumoto & Ajmone-Marsan (1964) reported in a cat model of focal epilepsy the observed increase in neuronal firing in only one third of recorded neurons, which they termed ‘active’, describing the remaining neurons as ‘passive’. An even higher proportion of passive neurons was found in recordings from humans, using depth electrodes with microwire bundles (Wyler et al. 1982; Wyler & Ward, 1986, Babb et al. 1987), or the 96-electrode Utah array (Truccolo et al. 2011). The latter study found that neuronal firing rates across the territory sampled by the array became much more heterogeneous at the start of seizures than in the preictal period, probably contributed to by passive neurons. Using similar recording techniques but a different site selection strategy, Schevon et al. (2012) showed that core areas within the seizure onset zone demonstrate the tonic/clonic ictal patterns predicted by the animal studies. Very highly synchronous firing was detected in the clonic phase, with action potential spike timing that was tightly locked to the phase of ictal discharges in the Berger bands (Fig. 3). Yet ahead of the ictal wavefront, firing appeared heterogeneous, similar to that reported by the Truccolo et al. study (Fig. 3).
Figure 3. Early parts of a human seizure recording from two Utah array microelectrodes (3 mm apart).
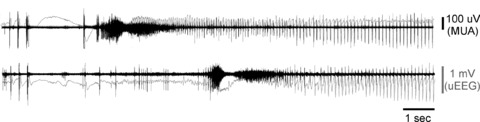
The figure shows simultaneous multi-unit activity (MUA; 300 Hz–3 kHz, 500th order FIR bandpass filter, black traces) and ‘micro’ EEG (uEEG; <50 Hz low-pass filter, grey trace). The activity in the bottom channel joins the seizure several seconds after the top channel, and shows MUA during the penumbral, tonic and clonic phases of the seizure. Note that the two MUA recordings are highly synchronized during the clonic phase, but at no other time. The penumbral phase clearly demonstrates dissociation between MUA and EEG (Schevon CA, McKhann G, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ unpublished results; for details see Schevon et al. 2012).
Calcium imaging studies permitted detailed analysis of seizure propagation in vitro. Working with a zero-Mg2+ mouse model, Trevelyan et al. found that the tonic firing phase is compressed into a narrow ictal wavefront that spreads outward from the origin and progressively recruits additional sites to the seizure (Trevelyan et al. 2006, 2007). Behind the wavefront, neuronal burst firing becomes nearly perfectly aligned across sites, due to rapid multidirectional synaptic propagation within the recruited area (Trevelyan et al. 2007). Strong postsynaptic barrages emanating from recruited sites invade the non-recruited regions ahead of the wavefront, triggering a strong, rapid feedforward inhibitory response. This response, termed ‘surround inhibition’, has previously been documented in both in vitro and in vivo seizure models (Prince & Wilder 1967, Schwartz & Bonhoeffer 2001). Because of surround inhibition, pyramidal cell firing ahead of the ictal wavefront is restrained, and a mismatch arises between firing and the postsynaptic potentials that contribute to EEG.
Because of the rapid and extensive distribution of the powerful synaptic currents generated during a seizure, the size of the penumbra (region in which local field potentials and neural firing are dissociated) can be arbitrarily large and even multilobar (Schevon et al. 2012). Spatial extension of penumbras that are large relative to the seizure focus can explain why many microelectrode studies in humans (Wyler et al. 1982; Babb et al. 1987; Truccolo et al. 2011; Bower et al. 2012) failed to record the expected neural signature of a seizure.
These studies highlight that EEG and local field potential synchronization (reflecting non-local subthreshold inputs) may be dissociated from spike synchronization (reflecting local suprathreshold neuronal outputs). This context is important to keep in mind when investigating the spatial heterogeneity of seizure activity, and for interpreting observed synchronization of EEG signals between sites at differing spatial scales. This phenomenon is analogous to the neuronal dark matter problem, as eloquently discussed by Shoham et al. (2006). In seizures, however, we now have evidence that such dark matter regions, where neurons are receiving synaptic inputs but not necessarily producing output spikes, is a spatiotemporal process. Seizing neurons spend much of their time listening and the level of neuronal participation during seizures is regulated by the level of inhibitory tone and surround inhibition. Metrics of synchrony, depending upon whether they measure spikes or local field potentials, will bear complex spatiotemporal relationships to such seizure evolution, as we now discuss.
Synchronization and seizure onset
The large-scale spatial structure of seizure-generating sites is complex. Some evidence suggests that the seizure onset zone is functionally organized into small neuronal clusters. Microelectrode studies of in vivo and in vitro models of epilepsy and seizures have revealed synchronous firing clustered in tiny (<1 mm diameter) microdomains. Activity in these microdomains was present during epileptogenesis and ictogenesis, and manifested in extracellular recordings as high-frequency signals (Bragin et al. 2000; Jiruska et al. 2010a). In human surface and depth microelectrode recordings, highly localized EEG events termed microseizures have been detected, most prominently in the seizure onset zone (Schevon et al. 2008, 2010; Stead et al. 2010). While microseizures are good candidates for being characteristic markers of the seizure onset zone, their precise role in seizure genesis and propagation has yet to be established. For example, it is possible that instead of expanding outward from a single constrained cortical site, seizures develop from coalescing of spatially separate epileptic microdomains (Bragin et al. 2000; Bikson et al. 2003; Jiruska et al. 2010a). The coalescing of distributed cortical microdomains has been postulated to underlie the transition to higher-amplitude ictal discharges and the emergence of a macroseizure from microseizures (Jiruska et al. 2010a; Stead et al. 2010). Several mechanisms for this transition have been proposed, including: synchronous excitatory synaptic input, decreased volume of the extracellular space, increased extracellular potassium and loss of inhibitory restraint (Fox et al. 2007; Jiruska et al. 2010a; Trevelyan et al. 2007). Inhibition can promote synchronization through paradoxical mechanisms: by GABAergic depolarization resulting from imbalance of chloride (reviewed by Pavlov et al. 2013, in this issue) or by synchronous recovery from inhibition of a larger population of principal neurons (Klaassen et al. 2006).
Desynchronization during seizures
The notion that seizures may arise from multiple, distributed cortical microdomains, and affect cortical regions outside the seizure focus, can explain a finding that at first glance appears to be counterintuitive: desynchronization at the initiation of seizures, followed by increasing large-scale synchronization as the seizure progresses. This desynchronization has been observed in both neural activity and local field potentials in vitro and in vivo (Netoff & Schiff 2002; Cymerblit-Sabba & Schiller 2012) and has also been well documented in human intracranial and extracranial recordings (Fig. 4; Le van Quyen et al. 2001; Chavez et al. 2003; Mormann et al. 2003; Wendling et al. 2003; Schiff et al. 2005).
Figure 4. Desynchronization at the onset of a seizure recorded with intracranial electrodes in a patient with temporal lobe epilepsy.
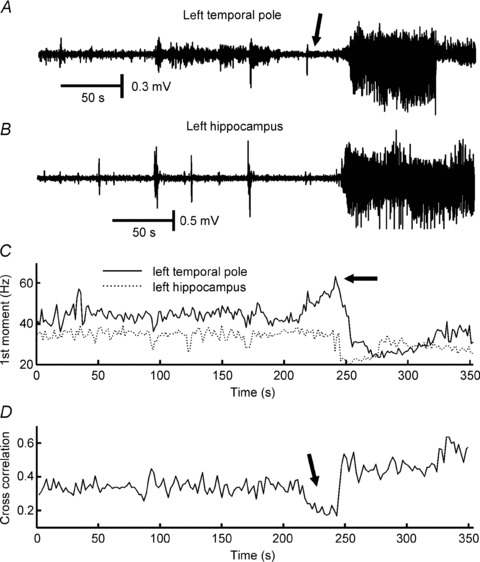
A, recording from the left temporal pole contact located at the seizure onset zone. Ictal onset is characterized by the presence of gamma and fast gamma activity (60–120 Hz; arrow). B, simultaneous recording from the left hippocampus. C, first spectral moment within the 2–200 Hz frequency band demonstrates a temporal dependence of the spectral content. The presence of a high-frequency onset is characterized by an increase in the first spectral moment (arrow). D, temporal evolution of cross-correlation between signals from temporal pole and hippocampus: the initial part of the seizure is characterized by decorrelation (desynchronization; arrow). Unpublished data (Jiruska P, Jefferys JGR, Marusic P) from case reported by Jiruska et al. 2008.
The spatial scale of synchronization between neuronal populations must always be considered. For example, in the low-calcium in vitro model, neuronal clusters are associated with high-frequency local field potential signals at the onset of seizures. As the seizure progresses, adjacent clusters (∼150 μm diameter separation) demonstrate increased synchronization (Jiruska et al. 2010a). In contrast, synchronization between more widely separated clusters (>300 μm) was low interictally and strengthened and expanded with the progression of each seizure (Jiruska et al. 2010a). The interpretation of early ictal desynchronization in human recordings depends strongly on electrode location relative to the recruited seizure territory. If synchrony is measured between two regions at a time when one is generating seizure activity while the second remains unaffected, then the measure will indicate desynchronization (Le van Quyen et al. 2001).
Desynchronization has been described during seizure onsets characterized by the presence of signals in the beta and gamma ranges (15–40 Hz) classified as low-amplitude fast activity (Wendling et al. 2003; de Curtis & Gnatkovsky 2009) recorded in seizures originating from both neocortex and entorhinal cortex in humans and in an in vitro guinea pig model of seizures (Wendling et al. 2003; Gnatkovsky et al. 2008; de Curtis & Gnatkovsky 2009). They found suppressed action potential firing at seizure initiation, while local (mainly somatic-projecting) interneurons demonstrate sustained and intensive discharges resulting in prominent and fast inhibitory postsynaptic potentials in principal cells (Wendling et al. 2002; de Curtis & Gnatkovsky 2009). The most plausible explanation for these findings is the ‘inhibitory veto’ in the area ahead of the ictal wavefront, described above (Trevelyan et al. 2006, 2007), i.e. the recordings were penumbral and the seizure focus was located elsewhere.
The concept of spike synchronization needs to be understood within a framework where synaptic conduction pathways have finite conduction velocity and each path is of a different length. Synchrony, the concept of a stable functional relationship where elements do similar things at the same time, needs to be broadened to that of polychronicity in neural networks, when time lags must be incorporated into the firing time of each neuron as population activity is considered (Izhikevich 2006). The epileptic brain possesses myriad of mechanisms that may synchronize the action potential firing on a millisecond time scale, involving both synaptic and non-synaptic mechanisms. There is a large amount of literature reviewing these mechanisms, which are beyond the scope of the present review (Dudek et al. 1986; Jefferys, 1995; Carlen et al. 2000; Jefferys et al. 2012a,b; Zorec et al. 2012).
Caution must, however, be observed in extrapolating these results to all types of seizures. Differences exist between experimental models and between epilepsy syndromes. Several distinct and region-specific seizure onset patterns are known and may have different cellular, network and synchronization mechanisms (Bragin et al. 2005, 2009; Bertram 2009; de Curtis & Gnatkovsky 2009). Human seizures, as reflected in the complex and varied clinical semiological findings, coupled with the distinctive electrographic patterns of various seizure types, all bespeak to the likelihood of different dynamical mechanisms in these ictal events.
The impact of spatial scale on synchronization has been well demonstrated in the genesis of interictal pathological high-frequency oscillations (Jefferys et al. 2012). In normal brain exposed to pro-epileptic conditions, populations of bursting cells can synchronize their action potential firing (Foffani et al. 2007). This collective firing manifests in local field potentials as high-frequency oscillations, with spectral peaks corresponding to the inter-spike frequency of individual neurons firing at 200–300 Hz (Dzhala & Staley 2004). In contrast, in the chronic epileptic brain, interictal high-frequency oscillations with frequencies up to 800 Hz (fast ripples) have been recorded (Staba et al. 2002; Bragin et al. 2004; Jiruska et al. 2010b). It has been suggested that oscillations at frequencies far higher than the maximum firing rate of a pyramidal cell can be explained by an emergent network phenomenon generated by independent (out-of-phase) neuronal populations (Bikson et al. 2003; Foffani et al. 2007; Ibarz et al. 2010). This epileptic phenomenon demonstrates the importance of spatial scale and the nuances of synchronization measures. Although temporally uncorrelated, the out-of-phase firing may still be regarded as synchronized, or at least have a polychronous relationship, in terms of having a stable phase (Rosenblum et al. 1996) or temporal lags (Rosenblum et al. 1997). Demont-Guignard et al. (2012) examined in detail the role of pyramidal cell population sizes, the spatial distribution of hyperexcitable pyramidal cells and the level of synchronization between action potentials in forming interictal high-frequency oscillations. In their combined computational and in vitro study they showed that a weak level of synchronization between afferent action potentials can manifest as extracellular fast ripples, while the progressive increase in the synchronization between action potentials resulted in a shift from fast ripple oscillations to an interictal EEG spike discharge.
Synchronization and seizure termination
Synchronization reaches its peak during the final stages of the seizure (Fig. 2). A consistent observation across different in vitro and in vivo models and human seizures is that they terminate simultaneously over large areas of the brain (Topolnik et al. 2003; Timofeev & Steriade 2004; Schindler et al. 2007b; Truccolo et al. 2011). Therefore, it has been suggested that either enhancing or disrupting synchronization may promote seizure termination and may potentially be used as abortive therapy.
Fires stop when there is nothing left to ignite; epileptic seizures might stop when there is nothing left to excite. Thus, one possible way in which seizures terminate is the creation of an extended area of hypoexcitable tissue, or a ‘zone of relative refractoriness’. An extensive series of in vivo experiments used multi-site intracellular and extracellular neuronal recordings and local cortical field potential recordings to investigate initiation, development and cessation of spontaneous spike-wave seizures in cats under ketamine–xylazine anaesthesia (Timofeev & Steriade 2004). Early after seizure onset, the propagation time between field potentials recorded at multiple sites of neocortex progressively decreased while long-range synchrony increased and reached a maximum towards the end of the seizure (Topolnik et al. 2003). When all the affected neuronal pools are drawn into highly synchronous paroxysmal activity the seizure terminates. Increasing synchronization towards the end of seizures has not only been noted in animal models, but also in several studies of intracranially recorded seizures in humans (Schiff et al. 2005; Schindler et al. 2007a,b,c; Kramer et al. 2010). In the cat model of spontaneous spike-wave seizures, the synchronous activity towards the end of the seizures was in the form of ∼1 Hz oscillations synchronized by EPSPs, but paced by intrinsic neuronal oscillations. Each cycle of the oscillation is initiated by hyperpolarization-activated depolarizing current (Ih) and then enhanced by Na+ and Ca2+ currents. These depolarizing currents then activate potassium currents (IK), which have a hyperpolarizing effect. The idea is that Ca2+-dependent activation of the hyperpolarizing currents eventually overcomes the depolarizing effect of the Ih component of the oscillation. As this effect occurs simultaneously it results in seizure termination across the entire synchronized territory (Timofeev & Steriade, 2004).
Computer modelling of neuronal networks supports the counterintuitive theory that ongoing network activity can be both initiated and terminated by synchronous excitatory synaptic input. Membrane impedance shunting, due to massively increased membrane conductance, disrupts synaptic integration and results in decreased efficacy of excitatory transmission that can promote termination of synchronized neural bursting (Gutkin et al. 2001). Another candidate mechanism for seizure termination is inhibitory transmission. Inhibition mediated by interneurons, their mutual interconnectivity and their connectivity with principal cells may provide a mechanism by which synchronized excitation may cause potent synchronization of inhibition (Pavlov et al. 2013, in this issue). This phenomenon has been reported during interictal spikes (Lebovitz 1979; de Curtis et al. 2001) and ictal discharges (Lado & Moshe 2008), possibly contributing to their synchronous post-excitatory depression. Most recently, a framework to unify the dynamics of seizure termination has been offered characterizing termination as a discontinuous critical transition or bifurcation (Kramer et al. 2012).
Post-excitatory depression is also mediated by non-synaptic mechanisms, particularly by changes in the extracellular environment that may outlast the recurrent synaptic inhibition. In the piriform cortex of the isolated guinea pig brain preparation, rhythmic interictal spikes are followed by a prolonged extracellular alkaline shift that closely correlates with inter-spike periodicity (de Curtis et al. 1998). Such pH changes induced by synchronous discharging of neurons during an interictal spike may promote synchronous post-spike depression of principal cell activity via decoupling of gap junctions. A similar phenomenon may, in principle, occur during the periodic bursting typical of the clonic (late) seizure phase, and could eventually contribute to seizure termination via synchronization of inhibition.
These diverse mechanisms all share the involvement of progressively greater large-scale synchronization of neuronal activity as seizures progress, which may be considered as an emergent self-regulatory mechanism for seizure termination.
Epilepsy and hypersynchrony
The term ‘hypersynchrony’ appeared to originate from early studies on human electroencephalogram and epilepsy, and was initially used to describe the diffuse spatial distribution of a normal monomorphic 3–5 Hz rhythm occurring (hypnagogic hypersynchrony) in children during drowsiness (Kellaway & Fox 1952). The term hypersynchrony seemed to be first applied to seizures by Penfield & Jasper (1954), and referred to an underlying mechanism of excessive synchronization of activity in a large population of neurons manifesting as high-amplitude rhythmic epileptic discharges. It has since been extrapolated to indicate synchronization of both field potentials and neuronal activity at larger spatial scales. This review demonstrates that synchronization in epilepsy is very complex, and may appear different depending on the spatial scale, the definition of synchrony and the signals being measured (i.e. neuronal spikes vs. low-frequency field potentials). As this review demonstrates, seizures are far more complex, dynamical phenomena than they were postulated to be when hypersynchrony was first suggested to be an adequate organizing principle to account for seizures. Although the term hypersynchrony has embedded itself within the accepted professional knowledge base of epilepsy, the accumulating evidence now suggests that a simple metric of increased synchronization is inadequate to account for seizures.
Conclusions
Synchronization in epilepsy is a much more complex phenomenon than often presented in the literature. The study of ictal synchronization is a rapidly evolving field, which benefited from introduction of more sophisticated statistical methods of synchronization characterization. Recent advances and observations on ictal synchronizations/desynchronizations have substantially contributed to our understanding of its pathophysiology, creating novel insights into the organization of epileptic networks and ictogenesis. Further, these observations may open new therapeutic methods for promoting seizure termination.
Acknowledgments
This work was supported by a Czech Ministry of Health Grant IGA NT/11460-4, Epilepsy Research UK grant (P1102), MRC Grant G0802162, Swiss National Science Foundation (project no. SNF 320030_122010), NIH grant 1R01EB014641-01 US-German Collaborative Research in Computational Neuroscience (CRCNS), and Medical Research Council grant (G0802162). The authors declare no conflict of interest.
References
- Babb TL, Wilson CL, Isokawa-Akesson M. Firing patterns of human limbic neurons during stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr Clin Neurophysiol. 1987;66:467–482. doi: 10.1016/0013-4694(87)90093-9. [DOI] [PubMed] [Google Scholar]
- Bertram EH. Temporal lobe epilepsy: where do the seizures really begin. Epilepsy Behav. 2009;14:32–37. doi: 10.1016/j.yebeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Fox JE, Jefferys JGR. Neuronal aggregate formation underlies spatiotemporal dynamics of nonsynaptic seizure initiation. J Neurophysiol. 2003;89:2330–2333. doi: 10.1152/jn.00764.2002. [DOI] [PubMed] [Google Scholar]
- Bishop G. Cyclic changes in excitability of the optic pathway of the rabbit. American Journal of Physiology-Legacy. 1932;103:213–224. [Google Scholar]
- Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia. 2012;53:807–816. doi: 10.1111/j.1528-1167.2012.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Engel J., Jr The cause of the imbalance in the neuronal network leading to seizure activity can be predicted by the electrographic pattern of the seizure onset. J Neurosci. 2009;29:3660–3671. doi: 10.1523/JNEUROSCI.5309-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Fields T, Fried I, Engel J., Jr Analysis of seizure onset on the basis of wideband EEG recordings. Epilepsia. 2005;46:59–63. doi: 10.1111/j.1528-1167.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- Brown R, Rulkov NF. Designing a coupling that guarantees synchronization between identical chaotic systems. Phys Rev Lett. 1997;78:4189–4192. [Google Scholar]
- Carlen PL, Skinner F, Zhang L, Naus C, Kushnir M, Perez Velazquez JL. The role of gap junctions in seizures. Brain Res Brain Res Rev. 2000;32:235–241. doi: 10.1016/s0165-0173(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Chavez M, Le Van Quyen M, Navarro V, Baulac M, Martinerie J. Spatio-temporal dynamics prior to neocortical seizures: amplitude versus phase couplings. IEEE Trans Biomed Eng. 2003;50:571–583. doi: 10.1109/TBME.2003.810696. [DOI] [PubMed] [Google Scholar]
- Cymerblit-Sabba A, Schiller Y. Development of hypersynchrony in the cortical network during chemoconvulsant-induced epileptic seizures in vivo. J Neurophysiol. 2012;107:1718–1730. doi: 10.1152/jn.00327.2011. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia. 2009;50:2514–2525. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Librizzi L, Biella G. Discharge threshold is enhanced for several seconds after a single interictal spike in a model of focal epileptogenesis. Eur J Neurosci. 2001;14:174–178. doi: 10.1046/j.0953-816x.2001.01637.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci. 1998;18:7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demont-Guignard S, Benquet P, Gerber U, Biraben A, Martin B, Wendling F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform bursts. Adv Neurol. 1986;44:593–617. [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la PL. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Fox JE, Bikson M, Jefferys JGR. The effect of neuronal population size on the development of epileptiform discharges in the low calcium model of epilepsy. Neurosci Lett. 2007;411:158–161. doi: 10.1016/j.neulet.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Francis JT, Gluckman BJ, Schiff SJ. Sensitivity of Neurons to Weak Electric Fields. J Neurosci. 2003;23:7255–7261. doi: 10.1523/JNEUROSCI.23-19-07255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Gutkin BS, Laing CR, Colby CL, Chow CC, Ermentrout GB. Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J Comput Neurosci. 2001;11:121–134. doi: 10.1023/a:1012837415096. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la PL. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Polychronization: computation with spikes. Neural Comput. 2006;18:245–282. doi: 10.1162/089976606775093882. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, de la Prida LM, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300:448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. Oxford University Press; 2012. pp. 176–189. [Google Scholar]
- Jensen MS, Yaari Y. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J Neurophysiol. 1997;77:1224–1233. doi: 10.1152/jn.1997.77.3.1224. [DOI] [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JGR. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010a;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JG. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010b;133:1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Tomasek M, Netuka D, Otahal J, Jefferys JG, Li X, Marusic P. Clinical impact of a high-frequency seizure onset zone in a case of bitemporal epilepsy. Epileptic Disord. 2008;10:231–238. doi: 10.1684/epd.2008.0207. [DOI] [PubMed] [Google Scholar]
- Kellaway P, Fox BJ. Electroencephalographic diagnosis of cerebral pathology in infants during sleep. I. Rationale, technique, and the characteristics of normal sleep in infants. J Pediatr. 1952;41:262–287. doi: 10.1016/s0022-3476(52)80003-4. [DOI] [PubMed] [Google Scholar]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci U S A. 2006;103:19152–19157. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. Slow transmission of neural activity in hippocampal area CA1 in absence of active chemical synapses. Nature. 1984;307:69–71. doi: 10.1038/307069a0. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Kolaczyk ED, Zepeda R, Eskandar EN, Cash SS. Coalescence and fragmentation of cortical networks during focal seizures. J Neurosci. 2010;30:10076–10085. doi: 10.1523/JNEUROSCI.6309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Truccolo W, Eden UT, Lepage KQ, Hochberg LR, Eskandar EN, Madsen JR, Lee JW, Maheshwari A, Halgren E, Chu CJ, Cash SS. Human seizures self-terminate across spatial scales via a critical transition. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1210047110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado FA, Moshe SL. How do seizures stop. Epilepsia. 2008;49:1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Martinerie J, Navarro V, Baulac And M, Varela FJ. Characterizing neurodynamic changes before seizures. J Clin Neurophysiol. 2001;18:191–208. doi: 10.1097/00004691-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM. Autorhythmicity of spontaneous interictal spike discharge at hippocampal penicillin foci. Brain Res. 1979;172:35–55. doi: 10.1016/0006-8993(79)90894-1. [DOI] [PubMed] [Google Scholar]
- Li X, Cui D, Jiruska P, Fox JE, Yao X, Jefferys JG. Synchronization measurement of multiple neuronal populations. J Neurophysiol. 2007;98:3341–3348. doi: 10.1152/jn.00977.2007. [DOI] [PubMed] [Google Scholar]
- Margineanu DG. Epileptic hypersynchrony revisited. Neuroreport. 2010;21:963–967. doi: 10.1097/WNR.0b013e32833ed111. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol. 1964;9:305–326. doi: 10.1016/0014-4886(64)90026-3. [DOI] [PubMed] [Google Scholar]
- Mormann F, Kreuz T, Andrzejak RG, David P, Lehnertz K, Elger CE. Epileptic seizures are preceded by a decrease in synchronization. Epilepsy Res. 2003;53:173–185. doi: 10.1016/s0920-1211(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Müller M, Baier G, Rummel C, Schindler K. Estimating the strength of genuine and random correlations in non-stationary multivariate time series. Europhys Lett. 2008;84:10009. [Google Scholar]
- Netoff TI, Carroll TL, Pecora LM, Schiff SJ. Detecting coupling in the presence of noise and nonlinearity. In: Schelter BI, Winterhalder M, Timmer J, editors. Handbook of Time Series Analysis. Wiley-VCH Verlag GmbH & Co. KGaA; 2006. pp. 265–282. [Google Scholar]
- Netoff TI, Pecora LM, Schiff SJ. Analytical coupling detection in the presence of noise and nonlinearity. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:017201. doi: 10.1103/PhysRevE.69.017201. [DOI] [PubMed] [Google Scholar]
- Netoff TI, Schiff SJ. Decreased neuronal synchronization during experimental seizures. J Neurosci. 2002;22:7297–7307. doi: 10.1523/JNEUROSCI.22-16-07297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Kaila K, Kullmann DM, Miles R. Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions. J Physiol. 2013;591:765–774. doi: 10.1113/jphysiol.2012.237958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecora L, Carroll T. Synchronization in chaotic systems. Phys Rev Lett. 1990;64:821–824. doi: 10.1103/PhysRevLett.64.821. [DOI] [PubMed] [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little Brown; 1954. [Google Scholar]
- Pikovsky A, Rosenblum M, Kurths J. Synchronization: A Universal Concept in Nonlinear Sciences. Cambridge University Press; 2003. [Google Scholar]
- Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996;76:1804–1807. doi: 10.1103/PhysRevLett.76.1804. [DOI] [PubMed] [Google Scholar]
- Rosenblum MG, Pikovsky AS, Kurths J. From phase to lag synchronization in coupled chaotic oscillators. Phys Rev Lett. 1997;78:4193–4196. [Google Scholar]
- Rulkov NF, Sushchik MM, Tsimring LS, Abarbanel HDI. Generalized synchronization of chaos in directionally coupled chaotic systems. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1995;51:980–994. doi: 10.1103/physreve.51.980. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Goodman RR, McKhann G, Jr, Emerson RG. Propagation of epileptiform activity on a submillimeter scale. J Clin Neurophysiol. 2010;27:406–411. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Jr, Waziri A, Branner A, Sosunov A, Schroeder CE, Emerson RG. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Jr, Goodman RR, Yuste R, Emerson RG, Trevelyan AJ. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ. Neural Control Engineering: The Emerging Intersection between Control Theory and Neuroscience. Cambridge: MIT Press; 2012. [Google Scholar]
- Schiff SJ, Sauer T, Kumar R, Weinstein SL. Neuronal spatiotemporal pattern discrimination: the dynamical evolution of seizures. NeuroImage. 2005;28:1043–1055. doi: 10.1016/j.neuroimage.2005.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, So P, Chang T, Burke RE, Sauer T. Detecting dynamical interdependence and generalized synchrony through mutual prediction in a neural ensemble. Phys Rev E. 1996;54:6708–6724. doi: 10.1103/physreve.54.6708. [DOI] [PubMed] [Google Scholar]
- Schindler K, Elger CE, Lehnertz K. Changes of EEG synchronization during low-frequency electric stimulation of the seizure onset zone. Epilepsy Res. 2007a;77:108–119. doi: 10.1016/j.eplepsyres.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: evidence from status epilepticus. Clin Neurophysiol. 2007b;118:1955–1968. doi: 10.1016/j.clinph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Schindler K, Leung H, Elger CE, Lehnertz K. Assessing seizure dynamics by analysing the correlation structure of multichannel intracranial EEG. Brain. 2007c;130:65–77. doi: 10.1093/brain/awl304. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med 7. 2001:1063–1067. doi: 10.1038/nm0901-1063. [DOI] [PubMed] [Google Scholar]
- Shoham S, O’Connor DH, Segev R. How silent is the brain: is there a “darkmatter” problem in neuroscience. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:777–784. doi: 10.1007/s00359-006-0117-6. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van GJ, Worrell GA. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003;13:883–893. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nat Neurosci. 2011;14:635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, Bourien J, Chauvel P. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain. 2003;126:1449–1459. doi: 10.1093/brain/awg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, Chauvel P. Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur J Neurosci. 2002;15:1499–1508. doi: 10.1046/j.1460-9568.2002.01985.x. [DOI] [PubMed] [Google Scholar]
- Westbrook GL. Seizures and epilepsy. In: Kandel ER, Jessel TM, Schwartz JH, editors. Principles of Neural Science. New York: McGraw-Hill; 1991. [Google Scholar]
- Wyler AR, Ojemann GA, Ward AA., Jr Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann Neurol. 1982;11:301–308. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Ward AA., Jr Neuronal firing patterns from epileptogenic foci of monkey and human. Adv Neurol. 1986;44:967–989. [PubMed] [Google Scholar]
- Zhao L, Beverlin B, Netoff T, Nykamp DQ. Synchronization from second order network connectivity statistics. Front Comput Neurosci. 2011;5:28. doi: 10.3389/fncom.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4:e00080. doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]


