Abstract
During slow-wave sleep, cortical neurons display synchronous fluctuations between periods of persistent activity (‘UP states’) and periods of relative quiescence (‘DOWN states’). Such UP and DOWN states are also seen in isolated cortical slices. Recently, we reported that both spontaneous and evoked termination of UP states in slices from the rat medial entorhinal cortex (mEC) involves GABAB receptors. Here, in order to dissociate the roles of GABAB1a- and GABAB1b-containing receptors in terminating UP states, we used mEC slices from mice in which either the GABAB1a or the GABAB1b subunit had been genetically ablated. Pharmacological blockade of GABAB receptors using the antagonist CGP55845 prolonged the UP state duration in both wild-type mice and those lacking the GABAB1b subunit, but not in those lacking the GABAB1a subunit. Conversely, electrical stimulation of layer 1 could terminate an ongoing UP state in both wild-type mice and those lacking the GABAB1a subunit, but not in those lacking the GABAB1b subunit. Together with previous reports, indicating a preferential presynaptic location of GABAB1a- and postsynaptic location of GABAB1b-containing receptors, these results suggest that presynaptic GABAB receptors contribute to spontaneous DOWN state transitions, whilst postsynaptic GABAB receptors are essential for the afferent termination of the UP state. Inputs to layer 1 from other brain regions could thus provide a powerful mechanism for synchronizing DOWN state transitions across cortical areas via activation of GABAergic interneurons targeting postsynaptic GABAB receptors.
Key points
GABAB receptors containing the GABAB1a subunit contribute to spontaneous termination of UP states.
GABAB receptors containing the GABAB1b subunit are essential for afferent-evoked termination of UP states.
Introduction
During slow-wave sleep, cortical neurons participate in the slow oscillation during which these neurons synchronously fluctuate between periods of persistent activity (‘UP states’) and periods of relative quiescence (‘DOWN states’) (Steriade et al. 1993). Such UP and DOWN states can also be observed in brain slices in vitro, prepared from a variety of animal species and cortical regions such as the ferret visual cortex (Sanchez-Vives & McCormick, 2000) or, more recently, the rodent medial entorhinal cortex (mEC) (Cunningham et al. 2006; Mann et al. 2009; Tahvildari et al. 2012).
UP states are synaptically driven, with increases in both excitatory and inhibitory transmission relative to DOWN states (Sanchez-Vives & McCormick, 2000; Shu et al. 2003). During the UP state, inhibitory conductances dynamically scale to match excitatory conductances (Shu et al. 2003). Conversely, during the in vivo DOWN state, few inhibitory postsynaptic potentials are seen in intracellular recordings and fast-spiking interneurons appear to be silent (Timofeev et al. 2001). The UP state originates within the cortex but transitions between states can be triggered in vivo by sensory input (Petersen, 2003) or in vitro by electrical stimulation of synaptic inputs arising within (Shu et al. 2003) or outwith the cortex (MacLean et al. 2005).
Previous work from our group demonstrated that, in the rat mEC, electrical stimulation in layer 3 could evoke a DOWN-to-UP state transition, and subsequent stimulation in layer 1 could terminate this UP state (Mann et al. 2009). It was found that GABAA receptors balanced the UP state and modulated firing frequency, while GABAB receptors mediated the UP state termination: blockade of GABAB receptors both prolonged spontaneous UP states and prevented layer 1 stimulation from evoking an UP-to-DOWN state transition (Mann et al. 2009).
Functional GABAB receptors exist as heterodimers between GABAB1 and GABAB2 subunits, with the GABAB1 subunit existing in two isoforms, GABAB1a and GABAB1b (Bettler et al. 2004). Evidence from both the hippocampus and the neocortex suggests that GABAB receptors containing GABAB1a subunits are preferentially located presynaptically whilst those containing GABAB1b subunits are preferentially located postsynaptically (Perez-Garci et al. 2006; Vigot et al. 2006). In this study, we sought to determine whether the location of GABAB receptors affected their role in terminating the UP state. Using mice in which either the GABAB1a subunit or the GABAB1b subunit had been genetically ablated, we could dissociate the effects of GABAB receptors containing the different subunits. We found that GABAB receptors containing the GABAB1a subunit modulate the timing of the spontaneous UP state termination and those containing the GABAB1b subunit are necessary for terminating the UP state by electrical stimulation in layer 1.
Methods
Ethical approval
All experiments were conducted in accordance with the UK Animals Scientific Procedures Act (1986) and in accordance with animal protocols approved by the National Institutes of Health. Transgenic mice lacking either the GABAB1a or the GABAB1b subunit (Vigot et al. 2006), and wild-type controls (BALB/c mice; Harlan, Bicester, UK) were used.
Slice preparation and electrophysiology
Horizontal slices (400 μm) containing the mEC were prepared from postnatal day 14–21 mice of both sexes after decapitation under deep isoflurane-induced anaesthesia. Slices were cut in ice-cold (<4°C) standard artificial cerebrospinal fluid (aCSF) containing (in mm): NaCl (126), KCl (3–3.5), NaH2PO4 (1.25), MgSO4 (2), CaCl2 (2) and NaHCO3 (26), and were incubated at room temperature for 1 h in interface conditions with standard aCSF, before being transferred to modified aCSF with reduced MgSO4 (1 mm) and CaCl2 (1.2 mm). Slices were maintained in interface conditions prior to recording; they were then mounted on a coverslip (coated with 0.1% poly-l-lysine in ultrapure H2O) and transferred to a submerged-style recording chamber where they were superfused with modified aCSF at 4–5 ml min−1 at 32–34°C, conditions that promote spontaneous network activity (Hajos et al. 2009).
Whole-cell current-clamp recordings were made from principal cells in layer 3 of mEC, using glass pipettes pulled from standard borosilicate glass containing (in mm): potassium gluconate (110), Hepes (40), ATP-Mg (2), GTP (0.3), NaCl (4) and biocytin (2–4 mg ml−1) (pH 7.2–7.3, osmolarity 275–290 mosmol l−1). Membrane potential values were not corrected for the liquid junction potential. Electrical stimulation was carried out using Digitimer DS3 constant current stimulators with monopolar steel electrodes.
Data acquisition and analysis
Data were recorded using an Axon Multiclamp 700A or 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) and low-pass filtered at 2 kHz. The signal was digitized at 5 kHz using either an Axon Digidata 1322A on a PC running Axon PClamp 9 or an Instrutech ITC-18 on a PC running Igor Pro using procedures written in-house. Data acquired using PClamp were imported into Igor Pro using Neuromatic (ThinkRandom; http://www.thinkrandom.com/) for further analysis.
UP and DOWN state transitions were monitored automatically with an algorithm that detected changes in DC membrane potential and membrane potential fluctuations using a moving average window method (Craig, 2011). All detected UP states were confirmed by visual inspection. Statistical comparisons were made using analysis of variance (ANOVA) with post-hoc Bonferroni multiple-comparison correction, or Student's two-sample and paired t tests as appropriate. Unless otherwise stated, all values are given as mean ±SEM.
Drugs and chemicals
CGP55845 was purchased from Tocris Bioscience (Bristol, UK). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Results
Electrical stimulation in mouse mEC can evoke UP and DOWN state transitions
Whole-cell recording from layer 3 pyramidal cells was used to monitor UP and DOWN states, which occurred spontaneously at a frequency of 3.1 ± 0.5 min−1 in wild-type BALB/c mice (n= 5). As previously reported in the rat (Mann et al. 2009), electrical stimulation (100–250 μA for 100–150 μs) in layer 3 of the mEC in BALB/c mice could evoke an UP state (Fig. 1A and B). UP states evoked by layer 3 stimulation had a similar duration and firing frequency to those occurring spontaneously (UP state duration, spontaneous vs L3 stimulation: 1.9 ± 0.15 s vs 1.5 ± 0.14 s; P > 0.05; UP state firing frequency, 4.6 ± 1.24 s−1 vs 3.7 ± 0.95 s−1; P > 0.05; n= 11; Fig. 1C). Stimulation in layer 1 (150–250 μA for 100–150 μs) 500 ms after layer 3 stimulation could then terminate the evoked UP state (Fig. 1A and B). Layer 1 stimulation significantly shortened the duration of the evoked UP state (UP state duration, L3 stimulation vs L3 + L1 stimulation: 1.5 ± 0.14 s vs 0.8 ± 0.07 s; P < 0.01; n= 11; one-way ANOVA; Fig. 1C). The duration and firing frequency of UP states displayed a large degree of variation within an individual slice. Figure 1D and E display the UP state duration (Fig. 1D) and firing frequency (Fig. 1E) for 20 consecutive, spontaneously occurring UP states observed in three different slices. The range of the coefficient of variation (CV) in these examples was 0.36–0.66 for UP state duration, and 0.35–0.86 for firing frequency. These results confirm that the mouse mEC shows UP and DOWN states with properties similar to those of the rat mEC.
Figure 1. Electrical stimulation in the mouse mEC can turn on and off persistent activity.
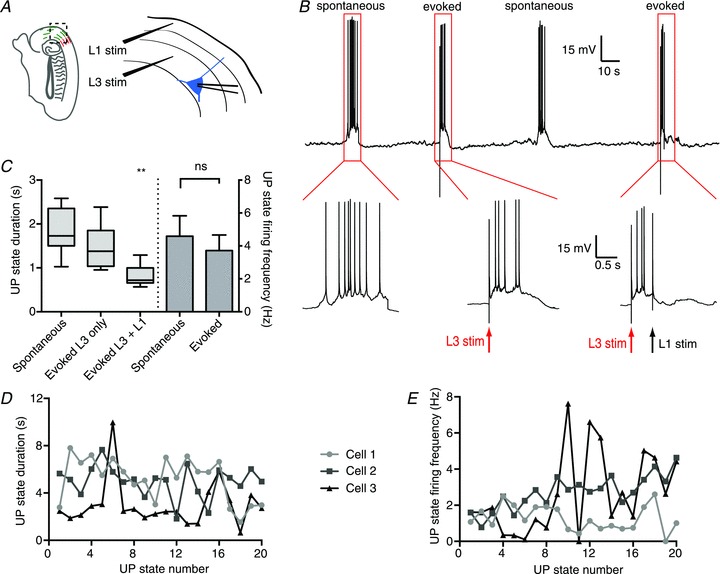
A, recording schematic. Whole-cell current-clamp recordings were made from principal cells in layer 3 of the mEC. UP states were evoked by stimulating in layer 3 within 200 μm of the principal cell soma, and subsequent stimulation in layer 1 was used to terminate the UP state. B, representative trace with expansions showing a spontaneous UP state (lower left), an UP state evoked by layer 3 stimulation (lower middle) and an UP state evoked with layer 3 stimulation and terminated with layer 1 stimulation (lower right). C, duration and firing frequency of UP states evoked by layer 3 stimulation were not statistically significant from spontaneous UP states, but layer 1 stimulation significantly shortened the UP state. The whiskers in the boxplots represent the minimum and maximum values D, UP state duration plotted for 20 consecutive spontaneous UP states for three different neurons. E, UP state firing frequency plotted for the same neurons and UP states as D. **P < 0.01.
GABAB receptor-mediated inhibition contributes to the spontaneous termination of UP states, as well as afferent stimulation-evoked DOWN state transitions (Mann et al. 2009). As GABAB receptors exist in at least two forms, those containing the GABAB1a subunit and those containing the GABAB1b subunit, respectively (Vigot et al. 2006), we sought to determine whether these receptors were differentially involved in terminating the UP state. This was done by comparing the effects of a GABAB receptor antagonist and layer 1 stimulation in wild-type mice with those in mice genetically engineered to lack either the GABAB1a or the GABAB1b subunit (Vigot et al. 2006).
Spontaneous UP states do not differ significantly between wild-type, GABAB1a−/− and GABAB1b−/− mice
Before examining the role of receptor type in terminating the UP state, we compared the properties of spontaneous UP states between the three genotypes. Representative recordings from wild-type, GABAB1a−/− and GABAB1b−/− mice are presented in Fig. 2A–C. We observed no significant differences in the incidence, duration or firing frequency of spontaneous UP states between the wild-type and knockout mice (wildtype (n= 5) vs GABAB1a−/− (n= 10) vs GABAB1b−/− (n= 5); UP state incidence: 3.1 ± 0.5 min−1 vs 2.6 ± 0.3 min−1 vs 4.4 ± 1.2 min−1; P > 0.05; one-way ANOVA; Fig. 2D; UP state duration: 3.7 ± 0.6 s vs 2.1 ± 0.2 s vs 3.2 ± 0.7 s; P > 0.05; one-way ANOVA; Fig. 2E; UP state firing frequency: 3.1 ± 0.5 Hz vs 3.0 ± 0.5 Hz vs 4.4 ± 1.2 Hz; Fig. 2F).
Figure 2. Recordings from layer 3 principal cells.
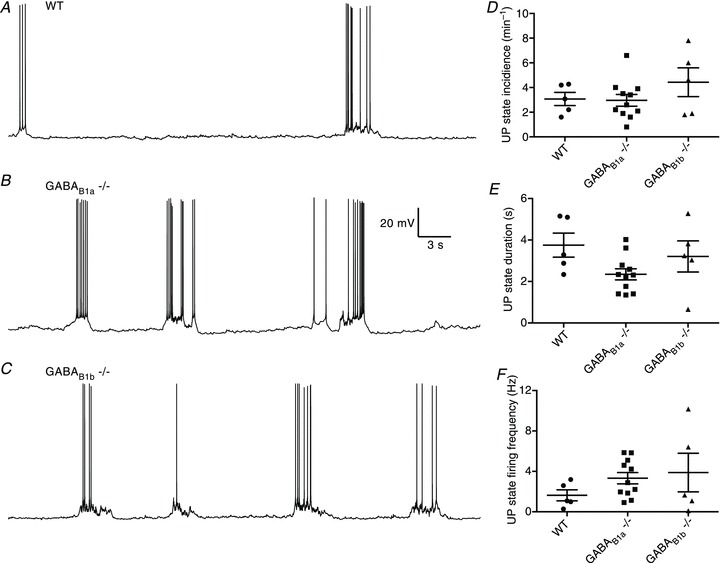
A–C, representative recordings made from layer 3 principal cells for 60 s for wild-type (WT) mice (A), GABAB1a−/− mice (B) and GABAB1b−/− mice (C). D–F, overall, no significant differences in UP state incidence (D), duration (E) or firing frequency (F) were observed between the three groups.
GABAB1a receptors modulate the duration of the UP state
Pharmacological blockade of GABAB receptors increases the duration of spontaneous as well as evoked UP states (Mann et al. 2009). We therefore investigated the effects on UP state duration of a GABAB receptor blocker in wild-type as well as GABAB1a−/− and GABAB1b−/− mice (Fig. 3A). As expected, blockade of GABAB receptors using 1 μm CGP55845, a selective GABAB receptor antagonist, significantly prolonged the UP state duration in wild-type mice (UP state duration relative to baseline, DMSO vs 1 μm CGP55845: 96 ± 6.7%vs 142 ± 14.9%; P= 0.0217; Student's t test). Similar to wild-type controls, 1 μm CGP55845 significantly prolonged the UP state duration in GABAB1b−/− mice (UP state duration relative to baseline, DMSO vs 1 μm CGP55845: 102 ± 10.0%vs 139 ± 7.7%; P= 0.012; Student's t test) but not in GABAB1a−/− mice (UP state duration relative to baseline, DMSO vs 1 μm CGP55845: 104 ± 8.7%vs 116 ± 7.4%; P= 0.311; Student's t test). These data are summarized in Fig. 3B and indicate that GABAB receptors containing the GABAB1a but not the GABAB1b subunit are responsible for the effect of CGP55845 on the duration of the UP state, suggesting that presynaptic GABAB receptors contribute to the spontaneous termination of UP states.
Figure 3. GABAB1a-containing receptors contribute to the spontaneous termination of UP states.
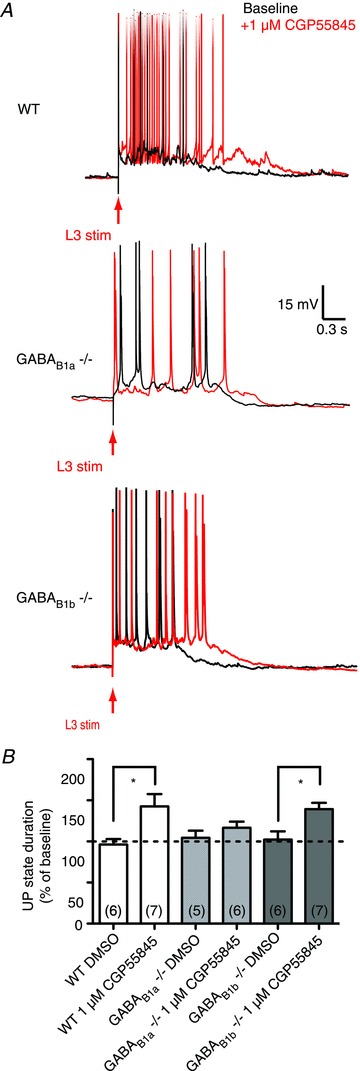
A, representative traces taken from wild-type (WT), GABAB1a−/− and GABAB1b−/− mice. B, the selective GABAB receptor antagonist CGP55845 (1 μm) significantly prolonged the UP state in wild-type and GABAB1b−/− mice, but not in GABAB1a−/− mice. Error bars are SEM; number of slices in parentheses; *P < 0.05; Student's t test.
GABAB1b receptors are necessary for afferent termination of the UP state
Next, we investigated the effect of layer 1 stimulation in GABAB1a−/− and GABAB1b−/− mice, compared to the effect in wild-type animals. As in the rat, stimulation in layer 1 significantly shortened an evoked UP state in wild-type mice and this effect could be blocked by 1 μm CGP55845 (reduction in UP state duration, baseline (n= 11) vs DMSO (n= 6) vs 1 μm CGP55845 (n= 7): 43 ± 4.5%vs 56 ± 4.6%vs 12 ± 8.2%; P= 0.0002; one-way ANOVA, Fig. 4A–C). Under baseline conditions, layer 1 stimulation also significantly shortened the UP state in GABAB1a−/− mice, an effect that was also blocked by 1 μm CGP55845 (reduction in UP state duration, baseline (n= 8) vs DMSO (n= 5) vs 1 μm CGP55845 (n= 6): 59 ± 4.3%vs 61 ± 4.7%vs 4.7 ± 5.7%; P < 0.0001; one way ANOVA, Fig. 4A–C). In contrast, layer 1 stimulation did not terminate an evoked UP state in GABAB1b−/− mice in any condition (reduction in UP state duration, baseline (n= 8) vs DMSO (n= 5) vs 1 μm CGP55845 (n= 11): −1.8 ± 3.6%vs−2.2 ± 7.8%vs 5.3 ± 4.3%; P > 0.05; one-way ANOVA, Fig. 4A–C). From these results, we conclude that GABAB1a subunit-containing receptors are not required for the afferent-evoked termination of the UP state and that this effect is mediated via GABAB1b subunit-containing GABAB receptors.
Figure 4. GABAB1b-containing receptors are necessary for afferent-evoked termination of the UP state.
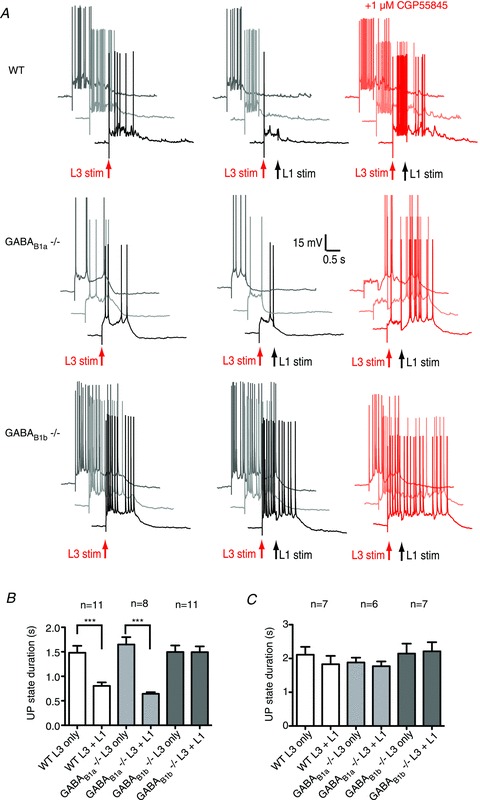
A, representative traces taken from wild-type (WT), GABAB1a−/− and GABAB1b−/− mice. Three trials taken from the same neuron are presented for each condition. B, layer 1 stimulation shortened the UP state in wild-type and GABAB1a−/− mice but not in GABAB1b−/− mice. C, the selective GABAB receptor antagonist CGP55845 (1 μm) prevented layer 1 stimulation from shortening the UP state. Error bars are SEM. ***P < 0.001; paired t test.
Discussion
Here we have dissociated the contributions of GABAB1a- and GABAB1b-containing GABAB receptors to the termination of UP states in the mEC in vitro. For all excitatory synapses that have been analysed for the location of GABAB1a and GABAB1b subunits (hippocampal CA3–CA1, hippocampal mossy fibre – CA3, thalamic and cortical inputs to the lateral amygdala, thalamus and neocortex), the GABAB1a subunit has predominantly been found to be presynaptic and the GABAB1b subunit predominantly postsynaptic (Gassman & Bettler, 2012). While the synaptic location of these subunits in the entorhinal cortex has not been studied in detail, we may assume that the distribution will be similar, although we cannot rule out that either receptor may exist in both locations. Hence we conclude that receptors containing the GABAB1a subunit, presumably presynaptic, help control the UP state duration by modulating spontaneous UP-to-DOWN state transitions, whereas receptors containing the GABAB1b subunit, most likely located postsynaptically, are necessary for afferent-evoked DOWN state transitions.
Presynaptic GABAB receptor activation can inhibit the release of both excitatory and inhibitory neurotransmitters (e.g. Pérez-Garci et al. 2006; Olah et al. 2009). As UP states are characterized by a balanced increase in both synaptic excitation and inhibition (Shu et al. 2003), it is possible that a gradual build up of extracellular GABA during the UP state progressively inhibits transmitter release via GABAB receptors at both excitatory and inhibitory synapses, and that the blockade of presynaptic GABAB receptors prolongs the UP state by preventing this presynaptic inhibition. As the blockade of GABAB receptors can prolong UP states not only in mEC but also in other cortical areas (Wang et al. 2010), this might imply that GABAB receptor modulation of spontaneous termination of the UP state is a shared mechanism across cortical areas. Given our current findings, one might have expected to see a prolongation in spontaneous UP state duration in the GABAB1a−/− mice compared to GABAB1b−/− and wild-type mice. However, the large degree of variation of UP state properties observed within individual slices (Fig. 1) and between slices from the same genotype (Fig. 2) could have occluded these differences, necessita-ting the use of GABAB receptor antagonists to unmask the contribution of receptor location to spontaneous termination, or compensatory mechanisms might have developed in GABAB1a−/− mice.
While GABAB receptors containing the GABAB1a subunit contribute to the spontaneous termination of UP states, those containing the GABAB1b subunit are necessary for afferent-evoked DOWN state transitions. It might seem surprising that, whilst essential for afferent-evoked DOWN state transition, GABAB1b subunit-containing receptors do not appear to contribute to spontaneous DOWN state transition. A parsimonious explanation would be that those interneurons that target these GABAB receptors are not activated to a large degree by the local circuitry during an UP state, but are rather activated by external afferents. Indeed, it was recently reported that neuropeptide-Y-positive interneurons in layer 2/3 are silent during mEC UP states in vitro (Tahvildari et al. 2012). Neurogliaform cells are immunoreactive for neuropeptide-Y (Price et al. 2005), making them an attractive candidate for mediating afferent termination of the UP state. Neurogliaform cells can elicit combined GABAA and GABAB receptor-mediated responses from single action potentials (Tamas et al. 2003), and, even at a low density, they can exert a large inhibitory influence by acting via volume transmission on extrasynaptic GABAB receptors (Olah et al. 2009). Neurogliaform cells are present in layer 1 of the neocortex (Hestrin & Armstrong, 1996), where they receive little or no input from superficial pyramidal cells but can exert an inhibitory influence over both excitatory (Wozny & Williams, 2011) and inhibitory cells (Christophe et al. 2002). While further work is needed to determine the source of the GABAB receptor-mediated inhibition responsible for afferent-evoked termination of the UP state, it is likely that the GABAB receptors terminate the UP state through activation of inwardly rectifying K+ (GIRK or Kir3) channels (Bettler et al. 2004) and/or by inhibiting dendritic Ca2+ channels of pyramidal cells (Perez-Garci et al. 2006).
Several mechanisms have been suggested to contribute to the spontaneous DOWN state transitions, including disfacilitation of the network (Contreras et al. 1996) or a build up of intrinsic activity-dependent K+ conductances (Sanchez-Vives & McCormick, 2000; Cunningham et al. 2006). However, more recent in vivo studies suggest that the UP state can be actively terminated: it has been reported that UP-to-DOWN state transitions occur more synchronously than DOWN-to-UP state transitions (Volgushev et al. 2006), and another study examining the electroencephalogram in human patients suggested that a DOWN state transition could occur independently of a preceding UP state (Cash et al. 2009).
If the UP state is actively terminated, then our results suggest that one mechanism could be through inputs arriving in layer 1 activating GABAergic interneurons acting on postsynaptic GABAB receptors. The question of where these inputs arrive from has yet to be addressed. In vivo, UP state propagation is fast, in the order of 1.5–7 m s−1 (Massimini et al. 2004), which is faster than the reported local spread of the oscillation through cortical tissue, which approaches 100 mm s−1 in vivo (Amzica & Steriade, 1995) and 11 mm s−1 in vitro (Sanchez-Vives & McCormick, 2000). This suggests that local propagation is inconsistent with the synchrony of UP and DOWN state transitions observed in vivo. The thalamus could play a role in synchronizing the slow oscillation in vivo (Crunelli & Hughes, 2010): the slow oscillation can be spontaneously generated in thalamocortical neurons and also neurons of the nucleus reticularis thalami (Crunelli & Hughes, 2010), and stimulation of the thalamus in vitro has been shown to trigger UP states that are indistinguishable from those generated spontaneously (MacLean et al. 2005). Applying muscimol to the thalamus of the rat greatly reduced the incidence of UP states (Doi et al. 2007), and an early in vivo study demonstrated that electrical stimulation of the thalamus could evoke a DOWN state transition in cortical neurons (Contreras & Steriade, 1995). Together, these results suggest that the thalamus may be able to synchronize cortical state transitions. As the thalamus projects extensively to layer 1 of most neocortical regions (Rubio-Garrido et al. 2009) as well as the mEC (Herkenham, 1978), thalamic activation of layer 1 interneurons could provide a plausible mechanism for the active termination of the UP state. Other studies have shown that cortico-cortical inputs also converge on layer 1 cells (e.g. Anderson & Martin, 2006), and interhemispheric projections to layer 1 are capable of mediating a long-lasting inhibition of cortical neuron firing, in a mechanism dependent on GABAB receptors on apical dendrites activated via layer 1 interneurons (Palmer et al. 2012).
While further work is needed to determine both the origin of the input to layer 1 and the cell type(s) mediating the effect, the present results provide further evidence that GABAB receptors may play a powerful role in regulating persistent network activity, and show that receptors containing GABAB1a and GABAB1b subunits have different roles in this regulation.
Acknowledgments
This work was supported by the Wellcome Trust OXION initiative (M.T.C., O.P.) and a National Institute of Child Health and Human Development (NICHD) intramural award (C.J.M.). M.T.C. held a Wellcome Trust Prize Studentship, received travel funding from the British Embassy Science and Innovation Division, and is an NIH Visiting Fellow. E.W.M. is supported by the NIH MD/PhD Partnership Training programme and by the Rhodes Trust. We are grateful to Olivia Shipton for useful discussions and help with animal breeding.
Glossary
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- mEC
medial entorhinal cortex
Author contributions
The experiments were carried out in the Laboratory of Cellular and Synaptic Neurophysiology at the National Institutes of Health, Bethesda, MD, and in the Department of Physiology, Development and Neuroscience at the University of Cambridge. M.T.C.: conception and design of experments, collection, analysis and interpretation of data, drafting and revising the manuscript. E.W.M.: conception and design of experiments, collection of data. B.B.: critically revising manuscript for important intellectual content. O.P.: conception and design of experiments, interpretation of data, drafting and revising manuscript. C.J.M.: conception and design of experiments, intepretation of data, drafting and revising manuscript. All authors approved the final version of the manuscript.
References
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol. 1995;73:20–38. doi: 10.1152/jn.1995.73.1.20. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Martin KA. Synaptic connection from cortical area V4 to V2 in macaque monkey. J Comp Neurol. 2006;495:709–721. doi: 10.1002/cne.20914. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I. The human K-complex represents an isolated cortical down-state. Science. 2009;324:1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E. Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol. 2002;88:1318–1327. doi: 10.1152/jn.2002.88.3.1318. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MT. The cortical slow oscillation: the role of slow GABAergic inhibition in mediating the UP-to-DOWN state transition. Oxford: University of Oxford; 2011. DPhil thesis. [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Mizuno M, Katafuchi T, Furue H, Koga K, Yoshimura M. Slow oscillation of membrane currents mediated by glutamatergic inputs of rat somatosensory cortical neurons: in vivo patch-clamp analysis. Eur J Neurosci. 2007;26:2565–2575. doi: 10.1111/j.1460-9568.2007.05885.x. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP–DOWN states of mouse neocortex. J Neurophysiol. 2010;104:596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M, Bettler B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–94. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE. Morphology and physiology of cortical neurons in layer I. J Neurosci. 1996;16:5290–5300. doi: 10.1523/JNEUROSCI.16-17-05290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABAB-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The barrel cortex – integrating molecular, cellular and systems physiology. Pflugers Arch. 2003;447:126–134. doi: 10.1007/s00424-003-1167-z. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Garrido P, Perez-de-Manzo F, Porrero C, Galazo MJ, Clasca F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 2009;19:2380–2395. doi: 10.1093/cercor/bhn259. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildari B, Wölfel M, Duque A, McCormick DA. Selective functional interactions between excitatory and inhibitory cortical neurons and differential contribution to persistent activity of the slow oscillation. J Neurosci. 2012;32:12165–12179. doi: 10.1523/JNEUROSCI.1181-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Lörincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep–wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations. J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neubauer FB, Lüscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur J Neurosci. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]


