Abstract
Type A botulinum toxin blocks not only ACh release from motor nerve terminals but also central synaptic transmission, including glutamate, noradrenaline, dopamine, ATP, GABA and glycine. Neurotoxins (NTXs) are transported by both antero- and retrogradely along either motor or sensory axons for bidirectional delivery between peripheral tissues or the CNS. A newly developed type A2 NTX (A2NTX) injected into one rat foreleg muscle was transported to the contralateral muscle. This finding was consistent with the NTX traveling retrogradely via spinal neurons and then transsynaptically through motor neurons to the contralateral motor neurons within the spinal cord and on to the soleus muscle. In the present study we found that toxin injection into the rat left soleus muscle clearly induced bilateral muscle relaxation in a dose-dependent fashion, although the contralateral muscle relaxation followed the complete inhibition of toxin-injected ipsilateral muscles. The toxin-injected ipsilateral muscle relaxation was faster and stronger in A2NTX-treated rats than A1LL (BOTOX). A1LL was transported almost equally to the contralateral muscle via neural pathways and the bloodstream. In contrast, A2NTX was mainly transported to contralateral muscles via the blood. A1LL was more successfully transported to contralateral spinal neurons than A2NTX. We also demonstrated that A1LL and A2NTX were carried from peripheral to CNS and vice versa by dual antero- and retrograde axonal transport through either motor or sensory neurons.
Key points
Botulinum toxin A (BoNT/A) blocks synaptic transmission via the cleavage of SNAP-25. Axonal transport of BoNT/A (A1 type botulinum toxin (A1LL) and A2 type botulinum toxin (A2NTX)) from periphery to the CNS has been described in trigeminal nerve and foreleg muscles.
A1LL and A2NTX were injected into the ipsilateral soleus, and their effects on ipsilateral and contralateral contractions were compared as a measure of local and systemic/transport-mediated effects. Spinal transmission was also measured to determine axonal and transsynaptic transport of neurotoxin.
A2NTX induced faster and stronger muscle relaxation than A1LL. A1LL arrived at the contralateral muscle by almost equal transport via neural pathways and by the circulation.
A2NTX was mainly transported to contralateral muscles via the blood.
A1LL and A2NTX were carried from peripheral to CNS and vice versa by dual antero- and retrograde axonal transport through either motor or sensory neurons.
Our results may point to greater potential safety in A2NTX form.
Introduction
Clostridium botulinum produces neurotoxins (NTXs) that have been classified into seven serotypes, A–G, based on their immunological characteristics. These poisons are among the most potent toxic substances in nature, act at neuromuscular junctions to inhibit ACh release from motor nerve endings, and result in muscle flaccidity by inhibiting neuromuscular transmission. Currently, the pharmaceutical products of botulinum toxin have been developed as important therapeutic agents for neurological disorders, such as blepharospasm, hemifacial spasm and various dystonias (Sadick, 2003; Jankovic, 2004).
These toxins are protein complexes called progenitor toxins, and are composed of a 150 kDa NTX together with non-toxic components. Type A progenitor toxins are divided by molecular weight into three classes: LL toxin (900 kDa); L toxin (500 kDa); and M toxin (300 kDa; Sakaguchi, 1983). The M toxin consists of an NTX and a non-toxic component exhibiting no haemagglutinin (HA) activity, and the L and LL toxins are formed by conjugation of M toxin with several HA subcomponents (Montecucco et al. 1996).
Type A organisms have been serologically classified into five subtypes (A1–A5), in which the NTX components vary in their amino acid sequences (Arndt et al. 2006; Dover et al. 2009). Products based on botulinum toxin type A (BoNT/A) are used in medical treatment of neurological disorders, and are produced from LL toxin, L toxin or NTX derived from subtype A1 organisms (Dressler & Benecke, 2007). Recently, A1 type botulinum toxin (A1LL) and A2 type botulinum toxin (A2NTX) were compared in rats using tests in primary spinal cord (PSC) cells and grip strength tests. A2NTX entered PSC cells more quickly than A1LL, and the difference in endocytotic rate contributed to more potent blockade of the neuromuscular junctions (Pier et al. 2011). In addition, A2NTX was transported less effectively than A1LL in foreleg muscles (Torii et al. 2011b). A2NTX more strongly suppressed fast inhibitory synaptic transmission than excitatory transmission in CNS interneurons. The rank of A2NTX effectiveness was glycinergic IPSCs > GABAergic IPSCs >> glutamatergic EPSCs (Akaike et al. 2010; Yamaga et al. 2012). Some reports suggest that type A toxin is transported from the injected site by axonal transport. A1LL was retrogradely transported from the superior colliculus to the retina, and was anterogradely carried from the retina to the superior colliculus. Furthermore, A1LL injected into one side of the hippocampus was carried to the contralateral one (Antonucci et al. 2008; Restani et al. 2011). Our previous behavioral study also suggested that A1LL was retrogradely transported from injected muscle to spinal cord, and was then anterogradely carried from the contralateral spinal motor neurons to the nerve endings (Torii et al. 2011a). However, the mechanisms of A1LL action in spinal cord have not been identified.
In the present experiments, we examined how A2NTX is transported from ipsilateral soleus muscle to the contralateral muscle, and compared the rate of inhibition of muscle tone by measuring the isometric contraction of soleus muscle to electrical stimulation of the tibial nerve. Most of the experiments were carried out using A2NTX, but in some cases we used A1LL for comparison. To investigate whether non-axonal transport mechanism also contributed to toxin accumulation in the contralateral side, we subjected a subset of rats to colchicine- or antitoxin-treatments. Toxin transport within the spinal cord was estimated by measuring electrophysiologically the glycinergic spontaneous and evoked IPSCs (sIPSCs and eIPSCs, respectively) of the substantia gelatinosa (SG) neurons.
Methods
Purification of toxins
Botulinum type A NTXs (150 kDa; NTX) were prepared using the modification of a previously reported method (Sakaguchi et al. 1981). Clostridium botulinum type A strain Chiba-H, which belongs to subtype A2, respectively, was cultured in PYG medium (pH 7.0) containing 2% peptone, 0.5% yeast extract, 0.5% glucose and 0.025% sodium thioglycolate, by allowing them to stand at 30°C for 3 days. M toxin was purified from the culture fluid by filtration, ion-exchange chromatography and gel filtration. M toxin was adsorbed onto a DEAE Sepharose column equilibrated with 10 mm phosphate buffer (pH 7.5) and eluted with a 0–0.3 m NaCl gradient buffer for separation of NTX and non-toxic component. The purified NTXs were stored at –70°C until use. For the test control, commercial A1LL toxin (BOTOX, Allergan Inc., Irvine, CA, USA) was used.
Experimental animals
ICR/CD-1 mice (4 weeks old, female, about 20 g; Charles River Laboratories Japan, Yokohama), mature Wistar rats (8 weeks old, male, about 250 g; Kyudo, Saga, Japan) were used for the toxic activity assay and isometric muscle contraction test, respectively. Immature Wistar rats (12–18 days old, male, about 40–70 g; Kyudo) were used for measurement of glycinergic sIPSCs and eIPSCs. Animals were maintained under controlled light/dark conditions, and had free access to food and water. All experiments were performed in accordance with the Guiding Principles for Care and Use of Animals in the Field of Physiological Sciences of the Physiological Society of Japan, and were approved by the Animal Ethics Committee of Kumamoto Health Science University.
Toxic activity measurements
The toxic activities of A1LL and A2NTX were determined employing the mouse intraperitoneal (i.p.) LD50 method (Pearce et al. 1994). The mouse i.p. LD50 was determined using a seven dose assay with a dilution interval of 1.25 and 20 mice per dose. The evaluation period was the first 96 h after administration, and the LD50 was calculated using the probit method. One mouse i.p. LD50 was defined as 1 unit (U).
Measurement of isometric contraction of rat soleus muscles
Isometric muscle contractions of the A1LL- or A2NTX-injected (ipsilateral) soleus muscles and the contralateral normal muscles of mature rats were measured in vivo at 5 days after toxin injection. The toxins were serially diluted to 0.06–400 U ml−1 with physiological saline containing 0.5% human serum albumin (diluent). Rats were anaesthetized by 3% isoflurane using inhalation anaesthesia apparatus (Muromachi Kikai, Tokyo, Japan). After the disappearance of the eyelid reflex, 50 μl of toxin was injected using an insulin syringe (Becton Dickinson, Tokyo) into the ipsilateral soleus muscles separately in two parts of the proximal and distal sides of soleus muscle end-plate portion innervated by the tibial nerve branch, as shown in Fig. 1A. The isometric muscle contraction was measured as follows. Rats were anaesthetized by i.p. injection of urethane (Wako, Osaka, Japan) at 0.6 g kg−1. A surgical incision was made in the left or right lower leg, and the gastrocnemius resected to expose the soleus muscles. A distal side of tendon of soleus muscle was cut from the calcaneus. Silk suture ligated this tendon and fixed to the tension transducer (TB 611T; Nihon Kohden, Tokyo). To prevent drying of the soleus muscles, a small pool was made using surrounding skin and filled with well-oxygenated (95% O2+ 5% CO2) Krebs solution at 36°C, which was perfused at a flow rate of 5 ml min−1. The tibial nerve was cut at the proximal side of the soleus muscle. The nerve of the distal side was sucked into a glass pipette (i.d. ϕ 0.8–1 mm). Then the nerve was electrically stimulated with 80 μs duration square wave pulses using supramaximal intensity at a frequency of 1–50 Hz. The tension transducer was connected to an amplifier (AP-601G; Nihon Kohden), and the isometric muscle contraction was recorded on paper using a pen recorder (Rikadenki, Tokyo). The contraction was calculated as the amount of work (W). The object undergoes an infinitesimal displacement dt. The work done by F in this displacement is defined as the scalar product of the vectors F and dt (Zatsiorsky, 2002). W can be written as follows:
Figure 1. Methods of injections and contraction measurements from rat soleus muscle.
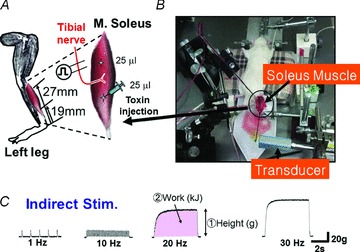
A, toxin injection site. Each toxin was injected with 25 μl at each of two sites along the left soleus muscle. B, in vivo measurement of the isometric contraction of rat soleus muscle. C, a part of tibial nerve of the control rat was stimulated with square pulses (80 μs duration) at supramaximal intensity and frequencies between 1 and 50 Hz.
where F is the muscle force, v is the muscle contraction velocity, s and e mean start and end time of muscle contraction. Therefore, W is the sum of the muscle forces. In addition, we measured time-dependent changes of the contraction after toxin injection. The toxins were diluted to 2 U ml−1 with the diluent. Anaesthetized rats were injected with 25 μl of toxin solution into each of the two parts of the ipsilateral soleus muscle. The isometric contraction of the ipsilateral muscle was measured at 0 (control), 3, 6, 12, 24 and 48 h after toxin (A1LL or A2NTX) injection.
Transport of toxin from ipsilateral injection sites to the contralateral muscle in colchicines-treated rats
To investigate whether toxins were transported solely via neural connections from ipsilateral soleus muscles to the contralateral ones, sciatic nerves of either side were pretreated with 10 μg site−1 colchicine 3 h before toxin injection. Colchicine selectively inhibits axonal transport by acting on neuronal microtubules (James et al. 1970). In our previous report (Torii et al. 2011a), the left brachial plexus was covered with agar containing 10 μg colchicine. As a result, A1LL was not transported to the contralateral foreleg, so that in colchicine treatment the grip strength was preserved in the contralateral limb. When the left brachial plexus was stimulated in colchicine-treated, non-toxin-injected rats (time control) 7 days after surgery, muscular contraction of the left foreleg appeared normal. The colchicine treatment was carried out as follows: rats underwent general anaesthesia (3% isoflurane). A surgical incision was made in the groin to expose either the left or right sciatic nerve. The nerve was covered with agar containing colchicine and the agar was covered with a small silicon tube (i.d. ϕ 3 mm) with Vaseline applied to seal both ends of the tube to prevent colchicine leakage to non-target tissues.
Colchicine-treated rats were injected with 50 μl of toxin (10 U site−1) into the ipsilateral soleus muscle. The isometric contraction of contralateral soleus muscles was measured 5 days after the toxin treatment.
Determination of toxin spread route using antitoxin
In an alternative test of whether toxins are transported to the contralateral soleus muscle via the circulating blood, type A2 antitoxin was intravenously administered 1 h after A2NTX injection. This is a time interval at which the antitoxin was effective as described previously (Torii et al. 2011a). The type A2 antitoxin is equine-derived F(ab′)2 fragment, obtained as follows; horses were immunized with A2NTX and sera were collected. The F(ab′)2 fragment was obtained by peptic digestion and ammonium sulfate precipitation of immunoglobulin from the sera. In the anaesthetized rats 50 μl of A2NTX was injected into two parts of ipsilateral soleus muscle. Then 1 U per 100 μl of type A2 antitoxin was injected to A2NTX-treated rats through the tail vein 1 h after A2NTX administration. One unit of the antitoxin neutralizes 4 log U of type A2 toxin (Jones et al. 2006). The contraction of the contralateral soleus muscle was measured at 5 days after A2NTX and type A2 antitoxin injections.
Mechanical dissociation of the spinal SG neurons
Wistar rats (12–18 days old) were decapitated under pentobarbital anaesthesia (50 mg kg−1, i.p.). The spinal cord (L4–L6) was quickly removed and immersed in an ice-cold incubation medium (see below), saturated with 95% O2 and 5% CO2. Slices of spinal cord (400 μm thick) containing SG region were prepared with a vibrating microtome (VT 1200S; Leica, Nussloch, Germany). The slices were then incubated in a medium oxygenated with 95% O2 and 5% CO2 at room temperature (21–24°C) for at least 1 h before mechanical dissociation. For mechanical dissociation, slices were transferred into a 35 mm culture dish (Primaria 3801; Becton Dickinson, Rutherford, NJ, USA) containing the standard external solution (see below), and the regions of SG were identified under a binocular microscope (SMZ645; Nikon, Tokyo). Details of this selective, mechanical dissociation procedure have been described previously (Rhee et al. 1999; Akaike & Moorhouse, 2003). Briefly, SG neurons were mechanically dissociated using a fire-polished glass pipette oscillating at 50–60 Hz, and whose tip was lightly placed on the SG region on the slice surface and vibrated horizontally (0.1–0.2 mm displacement) for about 2 min. Following dissociation, the slices were removed from the dish and the isolated SG neurons settled and adhered to the bottom of the dish within 15 min, and recordings commenced.
Electrical measurements
All electrical measurements were made on the soma membrane of isolated SG neurons in conventional whole-cell patch recording configuration under voltage-clamp (Multiclamp 700B; Molecular Devices, Sunnyvale, CA, USA). The holding potential (VH) was 0 mV for spontaneous and evoked glycinergic sIPSCs and eIPSCs at room temperature (21–24°C).
Patch pipettes were made from borosilicate capillary glass (1.5 mm o.d., 0.9 mm i.d., G-1.5; Narishige, Tokyo, Japan) in two stages on a vertical pipette puller (PP-830; Narishige). The resistance of the recording pipettes filled with internal (patch pipette) solution was 3–6 MΩ. Isolated SG neurons were observed under phase contrast on an inverted microscope (Diapot; Nikon). Neurons were continuously monitored on a computer monitor and an oscilloscope (DCS-7040; Kenwood, Melrose, MA, USA). All membrane currents were filtered at 3 kHz (E-3201A Decade Filter; NF Electronic Instruments, Tokyo), and stored on a computer using pCLAMP 10.2 (Axon Instruments, Union City, CA, USA). Hyperpolarizing step pulses (5 mV, 30 ms duration) were used to monitor access resistance, and any neurons that changed the recording more than 20% were rejected.
Focal electrical stimulation of single glycinergic boutons
Focal electrical stimulation of a single bouton adherent to mechanically dissociated SG neurons has been described previously (Akaike et al. 2002; Akaike & Moorhouse, 2003). The stimulating pipette was made from θ glass tubes filled with normal external test solution. The stimulating electrode was placed close to the postsynaptic soma membrane while recording from the isolated single SG neuron. The stimulating pipette was then carefully moved along the surface membrane of soma or dendrites. In individual neurons, test current pulses (duration, 100 μs; intensity, 0.1–0.2 mA; interval, 30–60 ms) induced glycinergic eIPSCs with about 10–40% of failures at a frequency of 1 stim per 5 s from an isolator (SS-202J; Nihon Koden). As the stimulation pipette was moved, glycine-gated outward currents appeared in all-or-none fashion at a VH of 0 mV (Akaike et al. 2002), indicating that the stimulating pipette was positioned just above a single glycinergic bouton.
Solutions
Several different solutions were used in the present study. The ionic composition of the incubation medium consisted of (in mm): NaCl, 124; KCl, 5; KH2PO4, 1.2; NaHCO3, 24; CaCl2, 2.4; MgSO4, 1.3; glucose, 10; saturated with 95% O2 and 5% CO2. The pH was adjusted to 7.45. The standard external solution used for recordings contained (in mm): NaCl, 150; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 10; Hepes, 10. All external solutions were adjusted to pH 7.4 with Tris-base. The composition of internal (patch pipette) solution for voltage-clamp experiments was (in mm): caesium methanesulfonate, 80; CsCl, 5; CsF, 65; tetraethylammonium (TEA)-Cl, 5; EGTA, 2; HEPES, 10. The pH of the pipette solution was adjusted to 7.2 with Tris-base. Both sIPSCs and eIPSCs were isolated from GABAergic ones by using ATP-free internal solution (Shirasaki et al. 1992). In addition, glutamatergic EPSCs were blocked with 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline acid (CNQX) and 2-amino-5-phosphonovaleric acid (d-AP5).
Drugs
The drugs and chemicals used in the present study included: TEA, d-AP5, EGTA and colchicine purchased from Sigma (St Louis, MO, USA); Vaseline and urethane from Wako Pure Chemicals (Osaka); CNQX from Tocris (Eilisville, MO, USA); isoflurane from Abbott (Tokyo); and pentobarbital from Kyoritsu Seiyaku (Tokyo). All test solutions containing drugs were applied by ‘Y-tube system’ for rapid solution exchange within 20 ms (Akaike & Harata, 1994).
Data analysis
Glycinergic sIPSCs were counted and analysed in pre-set epochs before, during and after each test condition using the MiniAnalysis Program (Synaptosoft, NJ, USA). Briefly, the events were initially screened automatically using an amplitude threshold of 5 pA, and then visually accepted or rejected based upon their 10–90% rise and 90–37% decay times. The average values of both frequency and amplitude of synaptic events during the control period (5 min) were calculated, and the frequency and amplitude of all events during toxin application were normalized to these values. The effects of toxins were quantified as percentage changes in frequency and amplitude as synaptic events and compared with the individual control.
Glycinergic eIPSCs elicited by focal electrical stimulation were counted and analysed in pre-set epochs before, during and after each test condition using MiniAnalysis and pCLAMP 10.2. The amplitude and failure rate (Rf) of eIPSCs were analysed with pCLAMP 10.2 (Katsurabayashi et al. 2004; Yamaga et al. 2012). The effects of A2NTX on the current amplitude, Rf and paired-pulse ratio (PPR) for eIPSCs were normalized as percentage changes from their respective controls. Numerical values are reported as means ± SEM of these normalized values.
Possible differences in the work, Rf and PPR distribution were tested by Student's paired two-tailed t test and individual absolute values. Values of P < 0.05 were considered significant.
Results
Time-dependent relaxation of isometric contraction in toxin-injected ipsilateral soleus muscles
Contractions in ipsilateral soleus muscles of A1LL- or A2NTX-treated rats (0.1 U site−1) were measured at 0, 3, 6, 12, 24 and 48 h after these toxin injection The contractions calculated as relative work (%) were 71.89 ± 17.7, 15.9 ± 7.85, 4.28 ± 4.24 and 0.22 ± 1.22% of control for A2NTX-treated soleus muscles at 6, 12, 24 and 48 h after the toxin injection, while the ones in A1LL-treated rats were 67.95 ± 18.05, 33.36 ± 5.6, 18.11 ± 8.7 and 8.93 ± 4.3% of control (Fig. 2). Effects of A2NTX reached a steady-state inhibition level about 25 h after the toxin injection (n= 8). However, A1LL required over 50 h to reach a steady-state inhibition (n= 8).
Figure 2. Time after toxin injection to inhibition of isometric contraction of the ipsilateral soleus muscle.
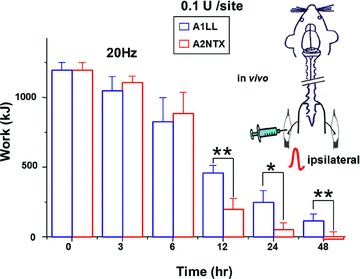
Either A1LL (blue) or A2NTX (red) was injected (0.1 U site−1) in the rat left soleus muscles (ipsilateral side, see inset figure). The contractions induced by 20 Hz electrical stimuli were measured in toxin-administered ipsilateral muscles at 0, 3, 6, 12, 24 and 48 h after the toxin injection. Each column is the mean ± SEM from 8 soleus muscles. *P < 0.05, **P < 0.01
Dose-dependent inhibition of ipsi- and contralateral soleus muscle contraction of A2NTX-treated rats
A2NTX at 0.003–20 U site−1 was injected to the ipsilateral soleus muscle, and isometric contraction was measured in both ipsi- and contralateral soleus muscles after 5 days (Fig. 3C). With A2NTX, the ipsilateral muscle inhibition occurred at low concentrations (0.003–0.1 U site−1) (Fig. 3A). However, muscle inhibition of the contralateral muscles required higher toxin concentrations (1–20 U site−1) (Fig. 3B). Complete inhibition of ipsilateral and contralateral muscle contraction was observed at 0.1 and 20 U site−1 A2NTX, respectively. Such results might be consistent with A2NTX transported by either axon or blood circulation from ipsilateral to contralateral soleus muscles.
Figure 3. A2NTX inhibition of contraction of both the ipsilateral and contralateral soleus muscles.
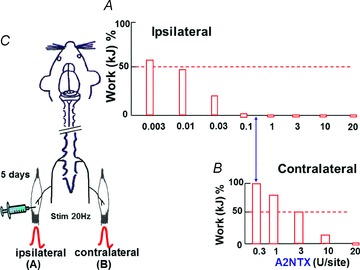
A2NTX (0.003–20 U site−1) was injected in the left soleus (ipsilateral) muscle (C). The contraction elicited by electrical stimuli of 20 Hz was measured in both toxin-injected ipsilateral and contralateral soleus muscles of rats at 5 days after injection of toxin at various concentrations (A and B). The measured ipsilateral muscle contraction decreased at 0.003–0.1 U site−1 range in a concentration-dependent fashion. In the contralateral muscles, the inhibition of A2NTX on contraction appeared at much higher doses between 1 and 20 U site−1. Data were obtained from 6 muscles.
Toxin transport in colchicines-treated rats
In control rats, stimulation at 1–50 Hz produced characteristic tetanic contractures of soleus muscles (Fig. 1C), which describe a sigmoidal contraction curve relating to stimulation frequency (Fig. 4). The toxins A1LL or A2NTX at 10 U site−1 nearly eliminated this contractile response at all stimulus frequencies in the contralateral muscle after 5 days (Fig. 4). However, colchicine pretreatment (10 μg site−1) on sciatic nerve of the toxin injection side (Fig. 4A) or on the contralateral sciatic nerve (Fig. 4B) attenuated the contractile inhibition. The effect was much greater using A1LL than A2NTX (Fig. 4). The inhibition ratio of contraction appeared equally in the treatment of ipsilateral and contralateral sciatic nerve by colchicine for each toxin. The results were summarized in Fig. 5. The results suggest that A1LL is nearly equally transported via axonal transport and blood circulation to the contralateral soleus muscles, while A2NTX travels mainly via blood circulation.
Figure 4. Colchicine attenuates A1LL but not A2NTX inhibition of contraction of contralateral soleus muscles.
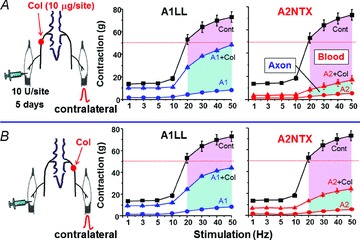
A, the rat left side (ipsilateral) sciatic nerve was treated with colchicine (10 μg site−1), and then the rats received 10 U site−1 of A1LL or A2NTX in the ipsilateral soleus muscles. In the contralateral soleus muscles of colchicine model rats, A1LL inhibition on the muscles was greater than that of A2NTX. In colchicine, contralateral inhibition was considered to be due to toxin delivered via the blood. B, the rat right side (contralateral) sciatic nerve was treated with colchicine, and then the rats received 10 U site−1 of A1LL or A2NTX in the left (ipsilateral) soleus muscles. The results gave similar findings as seen in A. Each symbol shows the average value from 6–8 muscles.
Figure 5. A1LL and A2NTX delivered in one muscle had effects from toxin in the contralateral muscle.
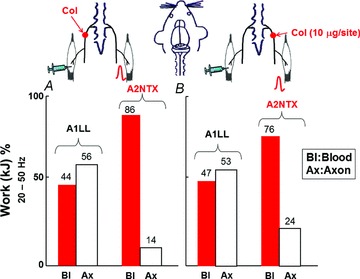
Histograms shows mean work at 20–50 Hz from Fig. 4. Both toxins were transported via axonal transport and blood from ipsilateral soleus muscles to the contralateral ones. The transportation ratio was higher in nerve for A1LL. A2NTX was also transported by not only nerve but also by blood circulation, though the main pathway was blood circulation. Each column is the average from 6–8 muscles neurons, and the data were quoted from Fig. 4. Ax, axon; Bl, blood.
Antitoxin interrupts intravenous toxin transfer
To test more directly whether A2NTX was transported via the blood circulation, A2-antitoxin (1 U per 100 μl) was injected intravenously via the lateral tail vein 1 h after A2NTX administration. Intravenous injection of antitoxin successfully prevented A2NTX inhibition of contralateral soleus muscle contraction over all stimulus frequencies (Fig. 6).
Figure 6. Effect of A2 antitoxin on the contralateral muscles contraction.
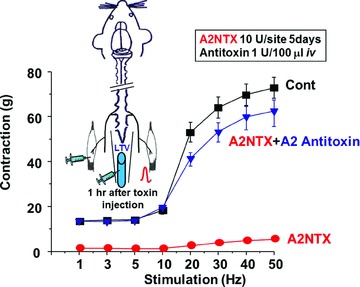
Rats were injected 10 U site−1 of A2NTX in the left (ipsilateral) soleus muscles. Then A2 antitoxin (1 U per 100 μl) was injected into the rat lateral tail vein (LTV) 1 h after A2NTX injection in the ipsilateral soleus muscles. The contralateral muscle contraction was measured at 5 days after the toxin treatment. A2 antitoxin markedly removed A2NTX-induced inhibition on contralateral muscle. Each symbol is the mean ± SEM from 8 muscles.
Modulation of glycinergic sIPSCs by NTXs
A1LL and A2NTX were transferred from toxin-injected ipsilateral soleus muscles to the contralateral muscle via both axonal transport and blood circulation. The colchicine results suggest that A1LL is carried by transsynaptic transport from ipsilateral spinal neurons to the contralateral spinal neurons as well as blood circulation. In fact, Matak et al. (2011) showed A1LL crossing contralaterally in the CNS using immunofluorescence. Retrograde axonal transport of A1LL from the periphery to the spinal motoneurons was also reported by Antonucci et al. (2008). Moreover, we previously showed that A2NTX markedly suppressed glycinergic sIPSCs and eIPSCs from nerve terminals projecting to spinal sacral dorsal commissural nucleus (SDCN) neurons that are second-order afferent sensory neurons (Akaike et al. 2010). These findings suggest that toxins are transported antero- and retrogradely through sciatic nerve fibres – likely both efferent motor nerves as well as afferent sensory nerves. We, therefore, examined whether these toxins affected glycinergic sIPSCs and eIPSCs in second-order sensory SG neurons in layer II of spinal dorsal horn via sciatic nerve axons from ipsilateral soleus muscles of toxin-injected rats.
Figure 7A shows a schematic illustration of second-order sensory SG neuron projected directly by glutamatergic sensory neurons and indirectly by the other nerve endings such as glutamatergic, glycinergic and GABAergic interneurons. Our mechanical isolation yields SG neurons with preserved excitatory and inhibitory nerve inputs. Recordings (n= 60–80) from isolated neurons from bilateral SG regions of spinal cord of control normal rats showed widely varying basal frequencies of sIPSCs. Most neurons showed high basal frequencies of sIPSCs (1.0–1.5 Hz), but some had quite low frequency (<0.1 Hz; Fig. 7Ba). The distribution of instantaneous frequencies was plotted for each experiment and the mean frequency obtained. Some experiments had low (Fig. 7Ba, upper panel) or high (Fig. 7Ba, lower panel) frequencies. The mean frequency from these experiments was binned into four arbitrary bands from 0.1 to 1.5. Most experiments had a higher frequency of sIPSCs, and there was a strong positive correlation between frequency and the relative number of experiments. This correlation coefficient (CC = r) was 0.94 (Fig. 7Bb), and the mean frequency was 0.74 ± 0.13.
Figure 7. Glycinergic spontaneous IPSCs (sIPSCs) in the normal rat spinal substantia gelatinosa (SG) neurons.
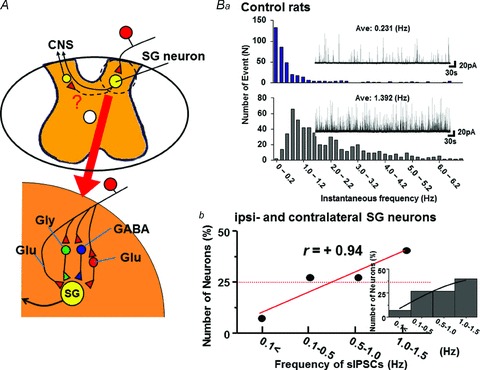
A, schematic illustration of rat spinal slice preparation and glutamatergic afferent input to SG neuron. The SG neuron is also projected by inhibitory glycinergic and GABAergic interneurons, and by excitatory glutamatergic ones. Ba, histogram shows the number of instantaneous frequency (bin size of 0.2 Hz) in neurons with low-frequency sIPSCs (Ave. 0.231 Hz, upper panel) and high-frequency sIPSCs (Ave. 1.392 Hz, lower panel). The sIPSCs were recorded from ‘synaptic bouton’ preparation of two ipsilateral SG neurons at spinal L3 level of normal rat without toxin treatment. Insets show typical currents. Bb, the sIPSCs were obtained from 60–80 ipsi- and contralateral SG neurons at spinal L3–5 level of 7 normal rats. The y-axis shows event number of glycinergic sIPSCs, while the x-axis shows sIPSC frequency. Inset shows a histogram fitted with Gaussians. There was a high positive CC (+0.94) between event number and frequency of sIPSCs.
Glycinergic sIPSCs in toxin-treated rats
To investigate the contralateral transport of NTXs across the spinal cord interior, A1LL (1, 4 U site−1) or A2NTX (4 U site−1) was injected into one soleus muscle. The ipsilateral and the contralateral SG neurons of 5 days toxin-treated rats were mechanically dissociated, and glycinergic sIPSCs were recorded from each side of SG neurons. Interestingly, CC values between number of events (y-axis) and frequency (x-axis) of sIPSCs reversed from positive in SG neurons of normal rats to negative in bilateral SG ones after 5 days treatment of 1 or 4 U site−1 A1LL (Fig. 8). The negative CC value was greater at 4 U site−1 than 1 U site−1. The average frequency of sIPSCs was lower at 4 U site−1 (0.24 ± 0.05 Hz) than 1 U site−1 (0.37 ± 0.04 Hz), and the peak of Gaussian distribution is shifted leftward, and the values of ipsilateral SG neurons of the toxin-injected side (−0.71 and −0.98 for 1 U site−1 and 4 U site−1, respectively) were slightly more negative than those of contralateral SG neurons (−0.67 for 1 U site−1 and −0.93 for 4 U site−1). In the case of 1 U site−1 A2NTX, ipsilateral SG neurons gave −0.72 CC (average frequency of sIPSCs, 0.46 ± 0.07 Hz) value, but the contralateral neurons still showed a slight positive CC value of +0.49 (0.68 ± 0.09 Hz) and had the peak of Gaussian distribution in the right side. These results indicate that A2NTX crossing from the ipsilateral to contralateral SG neurons in the spinal cord is either slower or less effectively taken up into central terminals compared with A1LL. As a next step, we investigated the effect of A2NTX on action potential dependent glycinergic eIPSCs.
Figure 8. Effects of A1LL and A2NTX on spontaneous IPSCs (sIPSCs) of ipsi- and contralateral SG neurons.
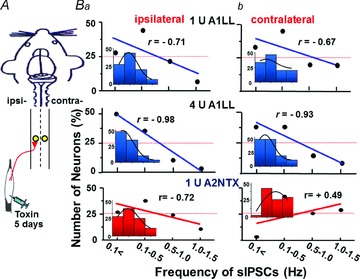
A, A1LL and A2NTX were injected in ipsilateral soleus muscles. Five days after the toxin treatment, ‘synaptic bouton’ preparations of bilateral SG neurons were obtained with mechanical dissociation. B, CC value between number of events (y-axis) and sIPSC frequency (x-axis) reversed from positive seen in SG neurons of normal rats to negative in both ipsi- (a) and contralateral (b) SG neurons after 5 days treatment of 1 and 4 U site−1 A1LL. In the case of A2NTX, the contralateral SG neurons still showed a little positive CC value. Each CC value was obtained from 70–115 SG neurons. All insets show histograms fitted with Gaussians. Each column in the histograms is the same as the x-axis of sIPSCs frequency.
Glycinergic eIPSCs in A2NTX-treated rats
To test for functional changes in synaptic transmission with A2NTX injections, we isolated single ipsilateral SG neurons (Fig. 9A and B) from normal and A2NTX-treated rats. Typical glycinergic eIPSCs from SG neuron of normal rats showed little change in the P1 and P2 current amplitude evoked by paired-pulse focal electrical stimulation (Fig. 9Ca). In control SG neurons, the PPR (P2/P1) of glycinergic eIPSCs was 1.08 ± 0.04 (n= 11 boutons). However, in SG neurons of A2NTX-treated rats (Fig. 9Ca (2) and (3)), the PPR increased significantly to 1.35 ± 0.09 (P < 0.05, n= 28 boutons). Likewise, Rf of P1 increased significantly from 0.21 ± 0.02 in control SG neurons (n= 11) to 0.28 ± 0.02 of toxin-treated SG neurons (n= 28). The results indicate that A2NTX inhibits synaptic glycine release machinery from glycinergic presynaptic terminals to second-order sensory afferent SG neurons.
Figure 9. A2NTX inhibits glycinergic evoked IPSCs (eIPSCs) recorded from ipsilateral SG neurons of A2NTX injected into the ipsilateral muscle.
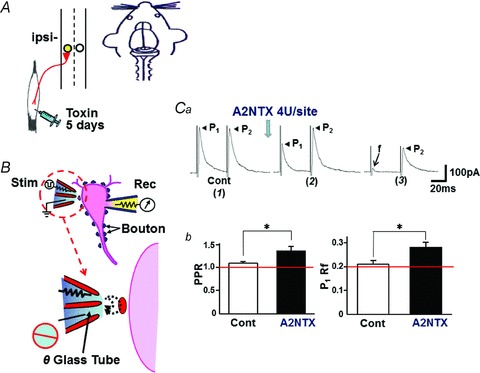
A and B, as shown in the schematic illustration, a single glycinergic nerve terminal (bouton) was activated by paired-pulse focal electrical stimulation to know how the toxin acts with synaptic transmitter release machinery. Ca, typical first (P1) and second (P2) evoked eIPSCs of ipsilateral SG neurons evoked by focal paired-pulse stimulation. Data were obtained from normal rat (1) and 5 days-A2NTX (1 U site−1)-treated rat ((2) and (3)). Recordings of (2) and (3) were obtained from SG neurons of different animals. f means failure of P1 eIPSC response. Cb, A2NTX (1 U site−1) significantly increased the P2/P1 ratio (PPR) and Rf. The results indicate clearly that A2NTX inhibits significantly the synaptic activity in spinal SG neurons in in vivo condition. Data were obtained from 11 to 28 boutons.
Discussion
In the present study, we compared subtype of BoNT/A NTXs and their inhibition of rat soleus muscle contraction. A2NTX caused stronger inhibition than A1LL. Our previous behavioral study using grip strength and twitch tension assays also showed that A2NTX was more effective than A1LL in rats (Torii et al. 2011b). The common dosage recommendation for A1LL injected to soleus muscle is 75 U site−1 (body weight 60 kg) as treatment for human soleus muscle spasms. If the dose is extrapolated simply from human to rat, A1LL is injected 0.3 U site−1 to rat soleus muscle (body weight 250 g). In the present study, complete inhibition of the isometric contraction of toxin-injected rat soleus muscles appeared at 0.1–1 U site−1 for A2NTX and A1LL, respectively (Figs 2 and 3). Because the therapeutic dose of human soleus muscle spasms provide for adequate inhibition of contraction for toxin-injected rat muscles, the differences in muscle relaxation of A2NTX and A1LL for rat seem directly applicable to humans. Consequently, A2NTX may require lower doses than A1LL as a treatment for human muscle spasm (Fig. 2).
Several adverse effects of botulinum toxin products have been reported. For example, the relaxation of off-target muscles is of concern. In the present study, excessive doses of A2NTX well beyond the recommended dose triggered off-target, contralateral inhibition of muscle contraction (1–20 U site−1). Such a toxin spread to distant contralateral muscle might be due to toxin transfer either via body fluid or nerves (Wiegand et al. 1976; Tang-Liu et al. 2003; Dressler & Adib Saberi, 2005). Wiegand et al. (1976) reported that the retrograde axonal transport of toxin to the 6th lumbar spinal cord vertebra (L6) takes 48 h after the NTX injection into the cat gastrocnemius muscle, but that the toxin might inactivate during the long transport time. Inactivation appears not to be likely as the toxin retains its activity for prolonged periods (Antonucci et al. 2008; Matak et al. 2011; Torii et al. 2011a). When we injected 10 U site−1 of A1LL to ipsilateral soleus muscle after colchicine treatment of the ipsilateral sciatic nerve, the toxin transportation of A1LL to the contralateral muscle was reduced considerably (Fig. 4A). This suggests that A1LL was transported to the contralateral muscle almost equally via both nerve and blood. In contrast, A2NTX in the same tests was mainly transported via blood. This result is supported by the finding that A2NTX-induced relaxation of the contralateral muscle was ineffective when A2-antitoxin was injected intravenously soon (1 h) after ipsilateral toxin injection (Fig. 6). When A1LL or A2NTX was injected after treatment of colchicine at the ipsilateral or contralateral sciatic nerves, the decrease of the relaxation of contralateral muscle contraction was quite similar (Fig. 4A and B). This suggests that the toxins are carried by both the retrograde and anterograde transportation via efferent motor nerves and afferent sensory nerves from injected ipsilateral soleus muscle to spinal cord, and then the toxins are also transported anterogradely via efferent sciatic nerve from contralateral motor neurons to the contralateral soleus muscles.
Five days after A1LL or A2NTX injection to the left side (ipsilateral) soleus muscle, there was a remarkable decrease in the frequency of glycinergic sIPSCs recorded in the SG neurons bilaterally. The sIPSC frequency was highly correlated to the event number (r=+0.94) in control SG neurons, but negatively correlated for A1LL (r=−0.71) and A2NTX (r=−0.72) in 1 U site−1 injected ipsilateral SG neurons. The IPSC results suggest that suppression of glycinergic transmission to SG neuron results from the toxin transportation to glycinergic interneuron terminals that project to SG neurons. Moreover, the CC value (r=−0.71) between sIPSC frequency and event number of ipsilateral SG neurons in the rats treated with 1 U site−1 A1LL was almost the same in contralateral SG neurons (r=−0.67). However, in the case of 1 U site−1 A2NTX, the CC value of sIPSC frequency was r=−0.72 in SG neurons of toxin-injected ipsilateral side and r=+0.49 in the contralateral neurons. These results suggest that both A1LL and A2NTX were transferred similarly to toxin-injected ipsilateral glycinergic interneurons projecting to SG neurons, but differently to contralateral ones: i.e. A1LL was more easily transferred to contralateral SG interneurons than A2NTX. In in vitro conditions, the frequency of glycinergic sIPSCs markedly decreased and the sIPSCs finally ceased in the presence of A1LL or A2NTX (Akaike et al. 2010). Decreases of amplitude and frequency of sIPSCs, and amplitude decreases and Rf increases of eIPSC in the presence of A1LL or A2NTX might have resulted from inhibition of the transmitter release machinery, an idea reinforced by the increased P2/P1 ratio (PPR; Yamaga et al. 2012) and present experiments. BoNT/A was bidirectionally transported in CNS and motor nerves (Antonucci et al. 2008; Restani et al. 2011; Torii et al. 2011a). However, the pathway of toxin in spinal cord was unclear. Our current electrophysiological findings suggested that the toxin was transported from ipsilateral to contralateral sides within the spinal cord. Transsynaptic transport of A1LL is greater than that of A2NTX.
It is evident that A1LL and A2NTX were transferred from SG neurons of toxin injected ipsilateral side to the contralateral SG neurons. There is a report that when A1 type toxin was injected into one side of the hippocampus, the toxin was transported from the toxin-injected side to the contralateral hippocampus (Antonucci et al. 2008). However, active transport of toxins in spinal neurons might occur more broadly through various neuronal circuits within the spinal interior. Future experiments may help to better define the circumstances and transport mechanisms of botulinum toxin in the CNS.
In the present experiments, colchicines interrupted axonal transport of toxins from the toxin-injected ipsilateral soleus muscles to the contralateral muscles through sciatic nerve. Previously, it was assumed that botulinum toxin traveled retrogradely via motor nerve axons into the CNS. However, Matak et al. (2011) reported that the toxin is also transported anterogradely through peripheral sensory nerves to the CNS. Thus, our present findings indicate that the toxin traveled bidirectionally from toxin-injected ipsilateral soleus muscles using both motor and sensory nerves.
A2NTX shares 89% amino acid sequence homology with A1NTX, 95% in light chains and 87% in heavy chains. The difference in the transport pathway between A1LL and A2NTX injected to the soleus muscle may be caused by variations in the amino acid sequence (or by differences in molecular weight). Botulinum toxins have been developed for use as important therapeutic agents. However, the adverse effects of the toxins may reduce their utility. A2NTX caused less muscle flaccidly to non-toxin-treated muscle than A1LL in our previously report (Torii et al. 2011a, 2011b). On the other hand, A2NTX was transported to contralateral via blood. If overdoses of A2NTX were injected, the occurrence of a side-effect is able to be blocked using antitoxin. Therefore, the safety profile of A2NTX may be superior to A1LL as the latter has about 55% of the toxin transported to contralateral via nerve.
Conclusion
In this study, we evaluated the routes of A2NTX or A1LL transport from ipsilateral soleus muscle to the contralateral muscle, and found that the muscle relaxation resulting from decreasing neuromuscular transmission was more effective in A2NTX than A1LL. This result suggests that A2NTX may be a new tool for clinical treatment for requirement of a large amount or a high concentration of toxin treatment for human muscle spasm. We also found that A1LL and A2NTX were carried from the toxin-injected peripheral side to CNS resulting in one of the side-effects. A1LL was almost equally transported via both neural pathway and blood circulation to the contralateral muscle. In contrast, A2NTX was mainly transported to contralateral muscles via blood. The results suggest that toxins given to one side muscle at a high dose in clinical might cause reduction in contraction of the contralateral muscle. Such adverse effects of A2NTX on the contralateral muscle side might be easily removed by the injection of A2-antitoxin, suggesting that A2NTX usage is much safer than A1LL, and that A2NTX may offer new promise as a therapeutic agent.
Acknowledgments
The authors sincerely thank Dr Michael C. Andresen for his advice and comments. The work was supported by The Science Research Promotion Fund for N. Akaike, and Grants-in Aid from Kumamoto Health Science University for M.C. Shin and N. Akaike.
Glossary
- A1LL
A1 type botulinum toxin (BOTOX)
- A2NTX
A2 type botulinum toxin
- BoNT/A
botulinum toxin A
- CC
correlation coefficient
- CNQX
6-cyano-2,3-dihydroxy-7-nitro-quinoxaline acid
- d-AP5
2-amino-5-phosphonovaleric acid
- eIPSCs
evoked IPSCs
- HA
haemagglutinin
- NTX
neurotoxin
- PPR
paired-pulse ratio
- PSC
primary spinal cord
- Rf
failure rate
- SG
substantia gelatinosa
- sIPSCs
spontaneous IPSCs
- TEA
tetraethylammonium
- SDCN
sacral dorsal commissural nucleus
Author contributions
Participated in research design: N.A., and M.C.S. Conducted experiments: M.C.S., and M.W. Performed data analysis: M.C.S., Y.T., and M.W. Wrote or contributed to the writing of the manuscript: N.A., M.C.S., M.W., T.H., Y.T., A.G., K.K., R.K., and S.K. All authors approved the final version of the manuscript.
References
- Akaike N, Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- Akaike N, Ito Y, Shin MC, Nonaka K, Torii Y, Harakawa T, Ginnaga A, Kozaki S, Kaji R. Effects of A2 type botulinum toxin on spontaneous miniature and evoked transmitter release from the rat spinal excitatory and inhibitory synapses. Toxicon. 2010;56:1315–1326. doi: 10.1016/j.toxicon.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. doi: 10.1016/s0165-6147(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Akaike N, Murakami N, Katsurabayashi S, Jin YH, Imazawa T. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci Res. 2002;42:187–195. doi: 10.1016/s0168-0102(01)00320-0. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt JW, Jacobson MJ, Abola EE, Forsyth CM, Tepp WH, Marks JD, Johnson EA, Stevens RC. A structural perspective of the sequence variability within botulinum neurotoxin subtypes A1-A4. J Mol Biol. 2006;29:733–742. doi: 10.1016/j.jmb.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Dover N, Barash JR, Arnon SS. Novel Clostridium botulinum toxin gene arrangement with subtype A5 and partial subtype B3 botulinum neurotoxin genes. J Clin Microbiol. 2009;47:2349–2350. doi: 10.1128/JCM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3–9. doi: 10.1159/000083259. [DOI] [PubMed] [Google Scholar]
- Dressler D, Benecke R. Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil. 2007;29:1761–1768. doi: 10.1080/09638280701568296. [DOI] [PubMed] [Google Scholar]
- James KAC, Bray JJ, Morgan IG, Austin L. The effect of colchicine on the transport of axonal protein in the chicken. Biochem J. 1970;177:767–771. doi: 10.1042/bj1170767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RGA, Corbel MJ, Sesardic D. A review of WHO international standards for botulinum antitoxins. Biologicals. 2006;34:223–226. doi: 10.1016/j.biologicals.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Katsurabayashi S, Kubota H, Moorhouse AJ, Akaike N. Differential modulation of evoked and spontaneous glycine release from rat spinal cord glycinergic terminals by the cyclic AMP/protein kinase A transduction cascade. J Neurochem. 2004;91:657–666. doi: 10.1111/j.1471-4159.2004.02741.x. [DOI] [PubMed] [Google Scholar]
- Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G, Rossetto O. The mechanism of action of tetanus and botulinum neurotoxins. Arch Toxicol Suppl. 1996;18:342–354. doi: 10.1007/978-3-642-61105-6_32. [DOI] [PubMed] [Google Scholar]
- Pearce LB, Borodic GE, First ER, MacCallum RD. Measurement of botulinum toxin activity: evaluation of the lethality assay. Toxicol Appl Pharmacol. 1994;128:69–77. doi: 10.1006/taap.1994.1181. [DOI] [PubMed] [Google Scholar]
- Pier CL, Chen C, Tepp WH, Lin G, Janda KD, Barbieri JT, Pellett S, Johnson EA. Botulinum neurotoxin subtype A2 enters neuronal cells faster than subtype A1. FEBS Lett. 2011;585:199–206. doi: 10.1016/j.febslet.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31(44):15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic nerve terminals projecting to meynert neurons of the rat. J Neurochem. 1999;72:800–807. doi: 10.1046/j.1471-4159.1999.0720800.x. [DOI] [PubMed] [Google Scholar]
- Sadick NS. Botulinum toxin type B. Dermatol Surg. 2003;29:348–351. doi: 10.1046/j.1524-4725.2002.29083.x. [DOI] [PubMed] [Google Scholar]
- Sakagichi G. Clostridium botulinum toxins. Pharmacol Ther. 1983;19:165–194. doi: 10.1016/0163-7258(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G, Ohishi I, Kozaki S. Biomedical aspects of botulism. In: Lewis GE, editor. Purification and Oral Toxicities of Clostridium Botulinum Progenitor Toxins. New York: Academic Press; 1981. pp. 21–34. [Google Scholar]
- Shirasaki T, Aibara K, Akaike N. Direct modulation of GABAA receptor by intracellular ATP in dissociated nucleus tractus solitarii neurons of rat. J Physiol. 1992;449:551–572. doi: 10.1113/jphysiol.1992.sp019101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Liu DD, Aoki KR, Dolly JO, de Paiva A, Houchen TL, Chasseaud LF, Webber C. Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon. 2003;42:461–469. doi: 10.1016/s0041-0101(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Torii Y, Akaike N, Harakawa T, Kato K, Sugimoto N, Goto Y, Nakahira S, Kohda T, Kozaki S, Kaji R, Ginnaga A. Type A1 but not type A2 botulinum toxin decreases the grip strength of the contralateral foreleg through axonal transport from the toxin-treated foreleg of rats. J Pharmacol Sci. 2011a;117:275–285. doi: 10.1254/jphs.11121fp. [DOI] [PubMed] [Google Scholar]
- Torii Y, Kiyota N, Sugimoto N, Mori Y, Goto Y, Harakawa T, Nakahira S, Kaji R, Kozaki S, Ginnaga A. Comparison of effects of botulinum toxin subtype A1 and A2 using twitch tension assay and rat grip strength test. Toxicon. 2011b;57:93–99. doi: 10.1016/j.toxicon.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Wiegand H, Erdmann G, Wellhöner HH. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:161–165. doi: 10.1007/BF00498587. [DOI] [PubMed] [Google Scholar]
- Yamaga T, Aou S, Shin MC, Wakita M, Akaike N. Neurotoxin A2NTX blocks fast inhibitory and excitatory transmitter release from presynaptic terminals. J Pharmacol Sci. 2012;118:75–81. doi: 10.1254/jphs.11124FP. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky MV. Kinetics of Human Motion. Champaign, IL: Human Kinetics; 2002. [Google Scholar]


