Abstract
The T-junction of sensory neurons in the dorsal root ganglion (DRG) is a potential impediment to action potential (AP) propagation towards the CNS. Using intracellular recordings from rat DRG neuronal somata during stimulation of the dorsal root, we determined that the maximal rate at which all of 20 APs in a train could successfully transit the T-junction (following frequency) was lowest in C-type units, followed by A-type units with inflected descending limbs of the AP, and highest in A-type units without inflections. In C-type units, following frequency was slower than the rate at which AP trains could be produced in either dorsal root axonal segments or in the soma alone, indicating that the T-junction is a site that acts as a low-pass filter for AP propagation. Following frequency was slower for a train of 20 APs than for two, indicating that a cumulative process leads to propagation failure. Propagation failure was accompanied by diminished somatic membrane input resistance, and was enhanced when Ca2+-sensitive K+ currents were augmented or when Ca2+-sensitive Cl− currents were blocked. After peripheral nerve injury, following frequencies were increased in axotomized C-type neurons and decreased in axotomized non-inflected A-type neurons. These findings reveal that the T-junction in sensory neurons is a regulator of afferent impulse traffic. Diminished filtering of AP trains at the T-junction of C-type neurons with axotomized peripheral processes could enhance the transmission of activity that is ectopically triggered in a neuroma or the neuronal soma, possibly contributing to pain generation.
Key points
The peripheral terminals of sensory neurons encode physical and chemical signals into trains of action potentials (APs) and transmit these trains to the CNS.
Although modulation of this process is thought to predominantly reside at synapses, there are also indications that AP trains are incompletely propagated past points at which axons branch. One such site is the T-junction, where the single sensory neuron axon branches into peripheral and central processes.
In recordings from sensory neurons of dorsal root ganglia excised from adult rats, we identified use-dependent failure of AP propagation between the peripheral and central processes that results in filtering of rapid AP trains, especially in C-type neurons.
Propagation failure was regulated by membrane input resistance and Ca2+-sensitive K+ and Cl− currents. Following peripheral nerve injury, T-junction filtering is reduced in C-type neurons, which may possibly contribute to pain generation.
Introduction
The frequency of afferent action potential (AP) traffic is a critical feature of sensory signalling. At their peripheral termini, sensory neurons encode stimulation strength into AP frequency, such that more intense stimulation results in generation of impulse trains with higher frequencies that ultimately produce a greater percept (Burgess & Perl, 1973). Brief high-frequency trains of APs have particular importance for information transfer, as activity organized as bursts of high-frequency trains is transmitted with high synaptic reliability, while tonic discharge with the same average rate of firing may not successfully induce activity in the postsynaptic neuron (Krahe & Gabbiani, 2004). In consequence, when a fixed number of APs is generated in nociceptors of human subjects, greater pain results when the pulses are grouped with short inter-pulse intervals (Lundberg et al. 1992). High-frequency discharge is particularly effective in producing dorsal horn neuronal plasticity (Lisman, 1997), which may play a critical role supporting chronic pain states (Fang et al. 2002; Galan et al. 2004). Thus, modification of the ability of sensory neurons to conduct APs in rapid succession may fundamentally contribute to altered sensory function in pathological conditions.
Pulse trains passing to the spinal cord are shaped by limits on the ability of the axon to conduct repetitive pulses. Frequency-dependent conduction failure is in part due to the particular anatomy of the sensory neuron, in which the stem axon emerging from the soma splits into a peripheral process bound for the receptive field and a central process connected to the spinal cord. Passage of afferent APs from the periphery to the spinal cord is unreliable at this T-junction due to impedance mismatch, resulting in selective elimination of high-frequency signals. This filtering function of the T-junction has been predicted by theoretical studies (Luscher et al. 1994b; Zhou & Chiu, 2001), and has been confirmed in recordings from amphibian and embryonic mammalian dorsal root ganglia (DRGs; Stoney, 1990; Luscher et al. 1994b). Maximal propagation rates through the T-junction have been examined in healthy adult rats (Fang et al. 2005) and after peripheral inflammation in guinea pigs (Djouhri et al. 2001), but the biophysical mechanisms underlying conduction failure at this site have been only minimally explored, and the influence of nerve injury has not been examined.
Experimental depression of intracellular Ca2+ reduces propagation failure at the T-junction (Luscher et al. 1994a, 1996). We have previously noted reduced resting intracellular Ca2+ levels (Fuchs et al. 2005) and activity-induced Ca2+ influx (Hogan et al. 2000; McCallum et al. 2003) in sensory neurons following peripheral nerve injury that produces behaviour indicative of pain. We therefore hypothesized that neuronal injury may disable T-junction filtering and thereby increase the net conduction of afferent traffic. Accordingly, these experiments were designed to first confirm the existence of T-junction filtering in adult mammalian sensory neurons, and to characterize the pace at which trains of sequential APs can be conducted through the T-junction. We then tested the effect of painful nerve injury using spinal nerve ligation (SNL), a model that allows evaluation of axotomized 5th lumbar (L5) neurons separately from neighbouring intact L4 neurons. Finally, we explored possible factors that may control conduction failure, including shifts in membrane potential (Vm) during and after trains, the role of specific membrane channels, and the participation of altered membrane resistance. Our findings suggest that T-junction filtering is an important regulator of sensory traffic in adult sensory neurons, and alterations after injury may contribute to sensory dysfunction.
Methods
Ethical approval
Studies were performed on tissue from 141 male Sprague–Dawley rats (150–250 g) obtained from Charles River Laboratories Inc. (Wilmington, MA, USA), after approval from the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Animal preparation
Rats were prepared with one of two kinds of surgery. SNL (n = 79 rats) was performed during isoflurane inhalation anaesthesia (1–2% in oxygen) similarly to the previously described method of Kim & Chung (1992). Briefly, after exposure of the right paravertebral region, the sixth lumbar (L6) transverse process was removed, and the ventral rami of the right L5 and L6 spinal nerves were ligated with 6-0 silk thread and cut distal to the ligatures. In contrast to the originally described method, we did not remove paraspinous muscles or the adjacent articular processes. Other rats had only anaesthesia and lumbar skin incision (n = 62 rats). After surgery, the rats were returned to the animal colony where they were kept in individual cages under normal housing conditions.
Behavioural testing
Animals were familiarized with the testing environment for 4 h on the day prior to the first sensory evaluation. A sensory testing protocol was used in which the plantar surfaces of the hind paws were stimulated in random order with a 22-guage spinal needle applied with pressure adequate to indent but not penetrate the plantar skin (Hogan et al. 2004), using 10 touches on each foot over a 5 min test session. Each touch produced either a very brief withdrawal of the foot, or a complex, sustained behaviour that included licking, grooming or sustained elevation of the paw. Using a place-avoidance protocol, we have confirmed that this latter hyperalgesia-type behaviour selectively indicates the production of an aversive experience (Wu et al. 2010). The probability of hyperalgesia behaviour was determined on the 3rd, 8th and 15th days after surgery, and the average probability over these three test days was calculated for the right paw. The examiner did not know whether the subject had SNL or skin incision alone.
Tissue preparation
Ganglia were removed on the 17th to the 21st day after surgery. Rats were anaesthetized with isoflurane (1–2% in oxygen) and a laminectomy was performed while the surgical field was bathed with oxygenated artificial cerebrospinal fluid (aCSF), containing (in mm): NaCl, 128; KCl, 3.5; MgCl2, 1.2; CaCl2, 2.3; NaH2PO4, 1.2; NaHCO3, 24.0; glucose, 11.0; adjusted to a pH of 7.35 with CO2. The L4 and L5 ganglia and attached dorsal roots were removed, after which the animal was killed by cervical disarticulation during deep anaesthesia. The connective tissue capsule of the DRG was dissected away, and the tissue was transferred to a recording chamber and bathed with 35°C aCSF (except where otherwise specified). To evoke APs, stimulation was applied to the cut end of the dorsal root with a pair of platinum wire electrodes. Dorsal root (rather than peripheral nerve) stimulation was employed for generation of axonal APs, in order to be able to evaluate propagation in the context of peripheral nerve injury by SNL, which leaves only a very short residual peripheral nerve at the L5 level. No difference is noted in propagation failure rate when stimulating central versus peripheral axonal processes in mammalian sensory neurons (Luscher et al. 1994b).
Intracellular recording
Vm was measured in sensory neuron somata in the DRG (Fig. 1A) using microelectrodes that had resistances of 70–100 MΩ when filled with 2 m potassium acetate. To guide impalement, somata were viewed using an upright microscope equipped with differential interference contrast optics and infrared illumination. An active bridge amplifier (Axoclamp 2B; Axon Instruments, Union City, CA, USA) was used to obtain traces that were filtered at 10 kHz and digitized at 40 kHz (Digidata 1322A; Axon Instruments). Stimulation was performed with square-wave pulses 0.1–0.5 ms in duration for A-type neurons and 1.0 ms duration for C-type neurons. In each, a supramaximal stimulation intensity at twice the threshold for inducing an AP in the recorded neuron was employed. Conduction velocity (CV) was determined by dividing the distance between stimulation and recording sites by the conduction latency, which was measured as the time between the beginning of the stimulation artefact and the initiation of the AP. For certain protocols, the soma was directly depolarized by current injection through the recording electrode.
Figure 1. Depiction of the preparation and description of measured parameters.

A, the preparation, showing recording via an intracellular electrode (which in some experiments was also used for stimulation), axonal stimulation and the peripheral axonal injury at the level of the spinal nerve. Components are not to scale. B, measurements determined from action potential (AP) trace. AHP80%, duration of afterhyperpolarization until 80% recovery to baseline; AHPamp, amplitude of afterhyperpolarization; AHParea, area of the afterhyperpolarization; AHPd, afterhyperpolarization duration; APamp, amplitude of AP; APd, duration of the AP at 95% repolarization; RMP, resting membrane potential. C, AP trace (above) and differentiated wave (below) from an Ao-type neuron that lacks an inflection on the descending limb of the AP. D, AP trace (above) and differentiated wave (below) from an Ai-type neuron, showing an inflection on the descending limb of the AP, as confirmed in the differentiated trace with an interval of decreased negative slope (arrows). Note C and D have different V s−1 and time scales. E and F, somatic voltage traces during paired axonal stimulation in two different neurons. Recordings of successively shorter interstimulus intervals are superimposed. Stimuli are evident as downward deflections in the voltage traces. The neuron in E shows failure of conduction into the soma at a RP of 1.3 ms, at which interval there is a complete absence of a somatic voltage response. The neuron in F shows failure of full somatic invasion at a RP of 1.4 ms, at which interval there is a decreased somatic depolarization (single arrow), representing a passive electrotonic potential. The final complete failure of propagation of the second AP (double arrow) occurs at a shorter interstimulus interval. The electrotonic potentials (single arrow) represent AP failure in the stem axon, while complete absence of an impulse (double arrow) represents failure at the T-junction.
Neurons were excluded if they lacked an AP amplitude (APamp) equal to or greater than 40 mV. The threshold level above which neurons are excluded according to resting membrane potential (RMP) is necessarily arbitrary. We chose the level of −50 mV as a conservative boundary. Recordings with RMPs between −40 and −50 mV were a small population (8% of all recordings that had RMPs more polarized than −40 mV) for which the following frequency (418 ± 42, defined below) did not differ from the neurons used in the study with RMP more polarized than −50 mV (357 ± 13, P = 0.20). RMP was determined after stable recording was achieved, typically after 2 min. APamp was measured from RMP to the AP peak. AP duration (APd) was determined at a voltage 5% from RMP to the AP peak (Fig. 1B). Afterhyperpolarization (AHP) amplitude (AHPamp) was measured from RMP to the most hyperpolarized level of the AHP. Duration of the AHP (AHPd) was measured to the point representing 80% recovery of the AHP back to RMP. AHP area under the curve (AHParea) was determined by digital trace analysis (Axograph 4.7; Axon Instruments). The presence of a hump or inflection on the descending limb of the AP was determined by examination of the differentiated trace (Fig. 1C and D). Refractory period (RP) was determined as the longest inter-pulse interval that failed to produce two consecutive somatic depolarizations, including either an electrotonic potential or a full AP (Stoney, 1990), during paired axonal stimulation with progressively shorter interstimulus intervals (Fig. 1E and F). The following frequency was determined by evoking trains with 20 axonal stimuli at rates of 10–900 Hz, presented in a sequence of increasing frequency with 4 s intervals between trains. We arrived at this design as follows. Trains of APs numbering 10–30 impulses are typical following an incremental increase of cutaneous thermal stimulation (Bessou & Perl, 1969) or a brief noxious mechanical stimulation (Bessou et al. 1971; Koltzenburg & Handwerker, 1994; Slugg et al. 2000) in various species. Because there was a need to stimulate each neuron with repeated trains in order to define the following frequency, trains needed to be short enough that excessive Ca2+ accumulation did not occur. Finally, each impalement has a limited stable interval of recording. In order to balance these issues, trains of 20 APs at 4 s intervals were chosen as representative of natural activity while also being tolerated by the neuron. Our prior data (Gemes et al. 2010) demonstrate recovery of cytoplasmic Ca2+ in typical neurons with trains such as these within the 4 s interval used between trains. The following frequency was defined as the maximum frequency of stimulation at which each stimulus in the train produced a somatic depolarization (electrotonic potential or full AP; Fig. 2). This inclusion of electrotonic potentials for this purpose is further justified in the Results below.
Figure 2. Sample voltage traces from four different neurons showing the somatic response to axonal stimulation at the following frequency and at a higher frequency at which conduction fails (*), both at the same time and voltage scales.

The aRMP at the initiation of the 2nd and last action potentials (APs) are marked with an arrowhead. Separately, the last AP of the train is aligned with the trace of a single AP from the same cell, shown at a compressed time scale and with APs that are truncated (indicated by a double slash). Stimulus artefacts are truncated. A, a C-type neuron from the L4 DRG after SNL demonstrates a depolarizing shift in the aRMP at following frequency. B, a Control Ai neuron shows depolarization of the aRMP during the train and progressive replacement of APs by incomplete depolarizations (electrotonic potentials), indicative of conduction failure in the stem axon. At a higher frequency, complete absence of somatic depolarization is evident as well (*), indicative of propagation failure at the T-branch. C, a Control Ao neuron in which the aRMP depolarizes during the train to a potential that is depolarized relative to the original RMP, and reveals an ADP following the last AP. D, an Ao neuron from L5 after SNL develops hyperpolarization during the train.
Data for APd, APamp, and CV were tabulated for the first and last AP of trains at the following frequency for each neuron. AHP dimensions, including AHPamp, AHPd and AHParea, were measured after the 20th AP of the train and compared with dimensions after a solo AP in the absence of a train. (These parameters were not calculated for C-type neurons due to the larger artefact that produced greater uncertainty regarding voltage measurements.) During a train of APs, each AP other than the first is necessarily superimposed upon the AHP that follows the prior APs. We determined the membrane voltage at the moment of AP initiation, which we term the apparent RMP (aRMP) for that AP, for the 2nd and 20th AP in each train, and compared these to identify the pattern of shift in the aRMP during tetanic stimulation (Fig. 2).
Teased fibre recording
The maximum AP firing frequency was determined from excised uninjured L5 and L6 dorsal roots during superfusion with either aCSF as used for the intracellular DRG recordings, or a standard teased fibre recording solution (Koltzenburg et al. 1997) containing (in mm): NaCl, 123; KCl, 3.5; MgSO4, 0.7; NaH2PO4, 1.7; CaCl2, 2.0; sodium gluconate, 9.5; glucose, 5.5; sucrose, 7.5; Hepes, 10; with 290 mosmol l−1 and pH 7.45 at 32 ± 0.5°C. Results were comparable and were pooled. Single units were recorded with a silver electrode from fibres teased from the root either distally where it entered the DRG or proximally where it entered the cord. Stimulation was performed at the opposite end (average distance 12.7 ± 0.6 mm, n = 14) using either the oil-immersed bipolar system that was used for axonal stimulation during intracellular recordings noted above (pulse durations 0.5 ms, n = 7) or a monopolar contact with a remote ground, both of which were submerged in the superfusing buffer (pulse durations 1.0 ms, n = 7). Because these systems produced comparable results, the findings were pooled. Trains of stimulation pulses were generated with a current amplitude twice the threshold for initiating an AP. CV of each unit was determined by measuring AP latency and the distance between the stimulating and recording electrodes. C-fibres were identified as units with CV <1.2 m s−1 (Kwan et al. 2009). AP waveforms were analysed and saved using a Powerlab 4.0 system and Chart software (ADInstruments, Colorado Springs, CO, USA).
Each recorded unit was observed for a 1 min period to confirm the absence of spontaneous activity. To identify maximum following frequency (Hz), trains of 20 pulses were applied with progressively higher frequency (5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 Hz). The maximum frequency was determined as the rate at which evoked APs followed each pulse in the train. At high stimulation rates, the pulse artefact eventually obscured the APs such that a true maximum rate could not be identified in all units. In these cases, the maximum rate that could be directly evaluated was recorded.
Agents
Bath Ca2+ elevation was achieved by switching from a modified aCSF containing 2 mm CaCl2 and 7.2 mm MgCl2 to one containing 8 mm CaCl2 and 1.2 mm MgCl2, by which the divalent cation concentration and membrane surface charge are maintained (Hille, 2001). The Ca2+-activated K+ channel activators NS1619 and NS309, the Ca2+-activated Cl− channel blocker niflumic acid, and the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker ZD7288 were delivered by a microperfusion technique from a pipette with a 10 μm diameter tip that was positioned 200 μm from the impaled neuron, and ejected continuously by pressure applied to the back end of the pipette (Picospritzer II; General Valve Corp., Fairfield, NJ, USA). Preliminary experiments indicated an effective 5-fold dilution of pipette solution into the bath at the cell surface, so pipette solutions were prepared with agents diluted in aCSF at concentrations 5-fold greater than the desired final concentrations. Stock solutions of NS1619, NS309 and niflumic acid were prepared in dimethyl sulphoxide at concentrations such that final delivered concentrations of dimethyl sulphoxide were less than 0.1%, which itself had no measurable effects on recorded parameters (data not shown; Santos et al. 2003). ZD7288 was initially dissolved in water. All agents were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) except for ZD7288, which was purchased from Tocris Bioscience (Bristol, UK).
Data analysis and statistical testing
Neurons were classified according to dorsal root CV, such that neurons with CV <1.5 m s−1 were considered C-type, most of which are non-myelinated nociceptors (Lawson, 2002), while others were considered A-type. Further categorization of A-type neurons was based on the presence (Ai-type) or absence (Ao-type) of an inflection or hump on the descending limb of the AP, as the presence of an inflection distinguishes putative nociceptive neurons from neurons conveying non-noxious sensory information (Rose et al. 1986; Ritter & Mendell, 1992), and this categorization has been used by previous studies on the topic of branch point filtering and AP trains in sensory neurons (Stoney, 1990; Amir & Devor, 1997). Data from these different neuronal types were analysed separately. For each neuronal type, the Control group consisted of neurons from L4 and L5 DRGs after skin incision alone, which were compared with the SNL4 and SNL5 groups (neurons from L4 and L5 after SNL surgery). Additional observations on neurons from sham surgery animals in which the L4 and L5 spinal nerves were exposed but no ligation was performed (Ai: n = 25; Ao: n = 23) showed minimal differences from neurons in the Control group, and are not further reported here.
Continuous data that were normally distributed are reported as mean ± SEM. Two groups were compared either by Student's t test for two groups, by one-way ANOVA for three groups, or by two-way ANOVA (between main effect for injury groups, within main effect for repeated measures comparing the 1st and 20th AP, and a main effect for interaction). Post hoc comparisons between groups were performed with Tukey's honest significant difference test (Statistica 6.0; StatSoft, Tulsa, OK, USA) when a significant main effect was identified. Continuous data that were not normally distributed are expressed as median (25th percentile/75th percentile), and were analysed by univariate non-parametric Mann–Whitney test for two groups or Kruskal–Wallis ANOVA by Ranks for three or more groups (Prism 4; GraphPad Software, Inc., San Diego, CA, USA). When a significant main effect was confirmed, planned post hoc comparisons were performed using Dunn's multiple comparisons test. A repeated measures model was used to compare between the 1st and 20th AP (Wilcoxon signed-rank test). Nominal data, such as rates of occurrence of electrophysiological features, were evaluated by cross-tabulation with significance tested by maximum-likelihood χ2 (Statistica 6.0; StatSoft). When a main effect was significant, post hoc comparisons were tested by Fisher's exact test corrected for the number of comparisons. Significance levels were set at P < 0.05. For cases in which a significant main effect for injury is identified but post hoc analysis shows no significantly different pairs, we interpreted this to mean there is an overall influence of injury without definitive evidence of an influence on any specific group.
Results
Behavioural testing
The probability of a hyperalgesia response that included licking, grooming and sustained withdrawal from noxious stimulation was increased ipsilateral to SNL injury (32.2 ± 4.1%, n = 79) compared with the response after skin incision alone (1.9 ± 0.9%, n = 62; P < 0.0001).
Effect of injury on AP parameters
Axotomy by ligation/section of the L5 spinal nerve (SNL) produced expected electrophysiological changes (Tables 1 and 2) similar to our previous findings (Sapunar et al. 2005). Specifically, SNL5 neurons had smaller diameters for Ai-type neurons, slower CV (Ao), depolarized RMP (Ai), decreased APamp (Ao), increased APd (Ai and Ao) and increased AHParea (Ai) compared with Control. Apart from an increased APamp in Ao-type neurons, the neurons from the L4 DRG after SNL resembled Control neurons.
Table 1.
AP parameters following the 1st and 20th AP of a train
| ANOVA main effects P | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell type | Control | SNL4 | SNL5 | Injury | AP repeat | Injury × AP repeat | ||
| N | Ai | 55 | 25 | 41 | ||||
| Ao | 91 | 44 | 28 | |||||
| C | 31 | 6 | 12 | |||||
| Diameter (μm) | Ao | 38.9 ± 0.8 | 39.3 ± 1.0 | 37.7 ± 1.6 | 0.73 | |||
| Ai | 41.7 ± 0.8 | 39.4 ± 1.8 | 37.6 ± 0.9* | 0.007 | ||||
| C | 32.5 ± 1.7 | 33.7 ± 2.0 | 31.2 ± 1.7 | 0.61 | ||||
| Initial RMP | Ao | −60.8 ± 0.9 | −62.7 ± 1.0 | −61.3 ± 1.0 | 0.4 | |||
| Ai | −65.7 ± 0.9 | −63.2 ± 1.3 | −58.6 ± 0.8 | <0.001 | ||||
| C | −66.7 ± 1.5 | −65.8 ± 3.0 | −66.1 ± 1.4 | 0.93 | ||||
| CV | Ao | 1st (m s−1) | 22.1 ± 0.9 | 20.5 ± 1.2 | 16.5 ± 1.1 | 0.006 | <0.001 | 0.07 |
| 20th (m s−1) | 19.5 ± 0.8§§ | 18.7 ± 1.2§§ | 14.8 ± 1.0§§ | |||||
| Δ (%) | −11.4 ± 0.9 | −9.3 ± 1.5 | −10.0 ± 1.8 | 0.4 | ||||
| Ai | 1st (m s−1) | 17.9 ± 1.1 | 14.0 ± 1.3 | 15.2 ± 1.0 | 0.1 | <0.001 | 0.26 | |
| 20th (m s−1) | 15.7 ± 0.9§§ | 12.4 ± 1.0§§ | 14.08 ± 1.0§§ | |||||
| Δ (%) | −9.9 ± 1.6 | −10.5 ± 2.0 | −7.5 ± 1.2 | 0.37 | ||||
| C | 1st (m s−1) | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.46 | 0.001 | 0.24 | |
| 20th (m s−1) | 0.7 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.0§ | |||||
| Δ (%) | −8.5 ± 3.8 | −11.5 ± 2.7 | −15.9 ± 6.2 | 0.56 | ||||
| APamp | Ao | 1st (mV) | 69.2 ± 1.5 | 75.7 ± 1.8* | 71.4 ± 2.3 | 0.07 | 0.55 | 0.24 |
| 20th (mV) | 70.9 ± 1.5 | 75.5 ± 2.1* | 71.0 ± 2.4 | |||||
| Δ (%) | 2.8 ± 1.21 | −0.0 ± 1.90 | −0.3 ± 1.67 | 0.26 | ||||
| Ai | 1st (mV) | 80.9 ± 1.7 | 85.7 ± 2.1 | 71.7 ± 1.8† | <0.001 | <0.001 | 0.001 | |
| 20th (mV) | 79.6 ± 1.7 | 80.8 ± 3.1 | 62.2 ± 2.6§§**†† | |||||
| Δ (%) | −1.2 ± 1.5 | −5.8 ± 2.5* | −13.6 ± 2.3** | <0.001 | ||||
| C | 1st (m s−1) | 87.2 ± 2.2 | 85.0 ± 4.4 | 79.7 ± 3.2 | 0.34 | 0.95 | 0.21 | |
| 20th (m s−1) | 82.2 ± 2.4 | 78.9 ± 6.0 | 77.8 ± 4.0 | |||||
| Δ (%) | −4.2 ± 2.5 | −0.8 ± 3.4 | −2.9 ± 4.3 | 0.84 | ||||
| APd | Ao | 1st (ms) | 0.8 ± 0.0 | 0.72 ± 0.0 | 1.0 ± 0.1 | 0.005 | <0.001 | 0.15 |
| 20th (ms) | 1.1 ± 0.1§§ | 1.0 ± 0.1§§ | 1.3 ± 0.1§§ | |||||
| Δ (%) | 48.7 ± 2.9 | 41.6 ± 4.1 | 30.8 ± 4.4** | 0.007 | ||||
| Ai | 1st (ms) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.6 ± 0.1* | <0.001 | <0.001 | 0.8 | |
| 20th (ms) | 1.5 ± 0.1§§ | 1.5 ± 0.1§§ | 2.0 ± 0.1§§* | |||||
| Δ (%) | 47.3 ± 5.4 | 39.9 ± 7.1 | 31.1 ± 3.3 | 0.06 | ||||
| C | 1st (m s−1) | 3.3 ± 0.2 | 3.1 ± 0.2 | 3.6 ± 0.3 | 0.83 | 0.03 | 0.25 | |
| 20th (m s−1) | 2.8 ± 0.1§§ | 3.2 ± 0.3 | 3.2 ± 0.4 | |||||
| Δ (%) | −13.9 ± 4.0 | −6.8 ± 3.9 | −7.6 ± 6.6 | 0.59 | ||||
Values are expressed as mean ± SEM. Injury effect P indicates the ANOVA main effect of injury for the parameter in that row. For post hoc comparisons: different from Control *P < 0.05, **P < 0.01; different from SNL4 †P < 0.05, ††P < 0.01; 20th different from 1st §P < 0.05, §§P < 0.01. AP, action potential; APamp, action potential amplitude; APd, action potential duration; CV, conduction velocity; n, number of cells; RMP, resting membrane potential; SNL, spinal nerve ligation; SNL4, 4th lumbar ganglion after SNL; SNL5, 5th lumbar ganglion after SNL.
Table 2.
AHP parameters following single APs or a train of APs
| Cell type | Control | SNL4 | SNL5 | Injury effect P | ||
|---|---|---|---|---|---|---|
| n | Ai | 55 | 26 | 41 | ||
| Ao | 91 | 44 | 28 | |||
| AHPamp | Ai | Single (mV) | 12.8[8.8/15.3] | 12.7[7.9/15.9] | 8.4[6.7/10.7]* | 0.002 |
| 20th (mV) | 10.5[6.3/13.4]§§ | 9.0[5.3/12.5]§§ | 6.1[4.0/8.3]§§* | 0.002 | ||
| Δ (%) | −17.7[−5.1/−29.7] | −24.5[−14.7/−28.7] | −28.7[−12.6/−48.7] | 0.55 | ||
| Ao | Single (mV) | 12.5 [8.9/15.0] | 13.0 [9.6/14.7] | 8.8 [6.9/11.6] | 0.038 | |
| 20th (mV) | 9.1 [4.5/10.9]§§ | 9.6[6.6/11.4]§§ | 7.4 [4.8/11.0] | 0.34 | ||
| Δ (%) | −26.6[−12.8/−49.8] | −24.1[−7.1/−39.7] | −8.7[−2.6/−24.3]* | 0.014 | ||
| AHPd | Ai | Single (ms) | 29.8[12.2/45.5] | 27.7 [17.1/38.4] | 7.6 [4.8/13.3]**†† | <0.001 |
| 20th (ms) | 129.0[45.5/215.5] | 168.0[72.3/309.5]§ | 46.5[11.0/138.5]**† | <0.01 | ||
| Δ (%) | 300[83/690] | 358[197/856] | 309[44/1015]† | 0.02 | ||
| Ao | Single (ms) | 7.2[4.2/12.3] | 5.9 [4.0/12.4] | 8.4[5.5/19.6] | 0.75 | |
| 20th (ms) | 61.0[17.3/117.4]§§ | 36.1[14.0/93.5]§§ | 50.4[21.7/99.0]§§ | 0.38 | ||
| Δ (%) | 476[115/1342] | 299[120/1114] | 369[144/1158] | 0.44 | ||
| AHParea | Ai | Single (ms mV) | 363 [176/552] | 294 [172/519] | 62 [33/131]**†† | <0.001 |
| 20th (ms mV) | 1211[580/2112]§§ | 993[369/1936] | 87 [−25/418]**† | <0.001 | ||
| Δ (%) | −241[−81/475] | −194[−24/−485] | −50[−580/147]** | <0.001 | ||
| Ao | Single (ms mV) | 75 [39/154] | 88 [52/151] | 51 [29/141] | 0.23 | |
| 20th (ms mV) | 77[119/1159]§§ | 453[196/920]§§ | 624[145/1523]§§ | 0.54 | ||
| Δ (%) | −364[−98/−759] | −351[−87/−680] | −609[−113/−1209] | 0.38 |
Values are expressed as median [25th/75th percentile]. Amplitude and area hyperpolarized compared with the RMP are considered positive. Injury effect P indicates the ANOVA main effect of injury for the parameter in that row. For post hoc comparisons: different from Control *P < 0.05, **P < 0.01; different from SNL4 †P < 0.05, ††P < 0.01; 20th different from single §P < 0.05, §§P < 0.01. AHPamp, afterhyperpolarization amplitude; AHParea, area of the afterhyperpolarization; AHPd, afterhyperpolarizarion duration; n, number of cells; SNL, spinal nerve ligation; SNL4, 4th lumbar ganglion after SNL; SNL5, 5th lumbar ganglion after SNL.
Failure of the somatic depolarization reflects propagation failure at the T-junction
Prior observations in neurons from amphibians, embryonic rat DRGs and adult rabbit nodose ganglia (Stoney, 1990; Ducreux et al. 1993; Luscher et al. 1994b) have established the T-junction as the site with the lowest safety factor for propagation of APs between the opposing processes of the sensory neuron. We extended these findings to adult mammalian DRG neurons by employing collision experiments in which we monitored somatic membrane depolarizations induced by converging APs that were triggered in the peripheral and central processes (Fig. 3). We observed that whenever the second of a pair of peripheral pulses produced either an incomplete somatic depolarization (the electrotonic residue of a distant AP) or a full somatic AP, this is accompanied by blockade of the AP coming from the central process. Thus, both types of somatic depolarization are evidence for successful transit of the impulse between the peripheral and central processes (Fig. 3), which confirms findings in other preparations (Stoney, 1990). We further established that when the time between a pair of peripheral pulses was reduced to an interval at which the second AP fails to produce any type of somatic depolarization, this simultaneously allowed the arrival of an impulse generated in the central process. This indicates that whenever the AP approaching the T-junction from peripheral process fails to enter the stem axon, it also fails to enter the central process. Therefore, failure of longitudinal conduction from one process to the other can be inferred from complete loss of the somatic depolarization. On the basis of these observations, only complete failure of somatic depolarization was regarded as evidence of longitudinal propagation failure for the purpose of determining RP and following frequency.
Figure 3. Confirmation by collision experiments that somatic potential recordings indicate T-junction events.

L5 DRGs were removed with the sciatic nerve attached, which was used for peripheral process stimuli (P1 and P2), while central process stimulation (C) was performed at the dorsal root (A). The interval between P2 and C stimuli was held constant, while the timing of the preceding peripheral pulse (P1) was variable. Somatic events resulting from these stimuli are labelled beneath the depolarization. In this recording of an Ao neuron (central CV = 12 m s−1, peripheral CV = 14 m s−1), stimulus artefacts are shown in B and C, but were subtracted in other panels. B, both P1 and P2 stimuli (arrows) result in full somatic APs. (No C stimulus was provided.) C. a shorter P1–P2 interstimulus interval (isi) results in a reduced P2 depolarization, representing the electrotonic potential from an AP that failed in the stem axon before invading the soma. (Again, no C stimulus was provided.) D. a single central stimulus produces a somatic AP. (No P stimuli were provided.) E. the C depolarization is absent due to collision of the C AP in the central process with the P2 AP, which has successfully transited the T-junction. F, a shorter P1–P2 interval produces only an electrotonic P2 potential in the soma, but the C depolarization is still absent, indicating passage of the P2 AP into the central process. G, a still shorter P1–P2 interval results in complete failure of P2 somatic depolarization, accompanied by the arrival of an unblocked C AP in the soma. This demonstrates that a somatic electrotonic potential represents an AP transiting the T-junction, while complete failure of somatic depolarization represents AP propagation failure at the T-branch. Findings in three other neurons were the same, including one in which polarities were reversed (paired central stimuli colliding with a late peripheral stimulus).
Pulse repetition rates at which propagation fails in neuronal subgroups and the influence of injury
The RP defines the minimum interval at which a neuron can successfully conduct a second AP. For Control neurons, RPs differed between neuronal categories, in the rank order of C-type ≫ Ai > Ao (ANOVA P < 0.0001; P < 0.001 for all paired comparisons; Fig. 4A). In general, the effects of injury on RP were small (Fig. 4A), although there was significant prolongation of RP in L5 after SNL in both Ai and Ao neurons. The RP of C-type neurons was not affected by injury.
Figure 4. Influence of neuronal type and injury on impulse propagation.
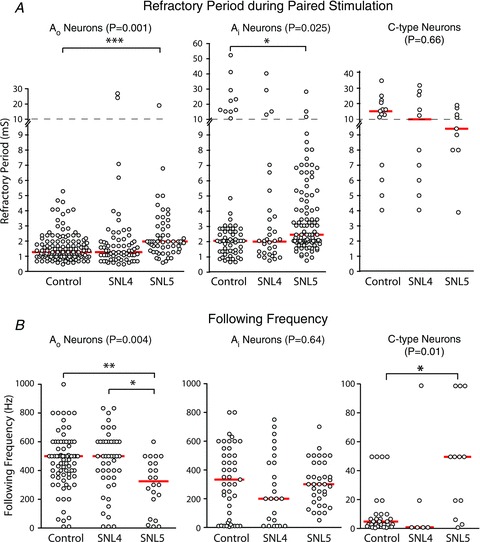
A, RP of sensory neurons during paired stimulation. Panels show data according to neuron type and the injury group. Spinal nerve ligation (SNL)4 and SNL5 are neurons from the L4 and L5 ganglions from animals after SNL surgery. The central indicator bars represent the median value. The P-value indicates the probability of a main effect for injury, and significant post hoc comparisons are shown by connecting brackets. *P < 0.05, ***P < 0.001. Note the broken y-axis with two different scales. B, the maximal stimulation rate (the following frequency) that leads to conduction into the stem axon of all APs in a train of 20 APs. **P < 0.01.
Following frequency indicates the ability of a neuron to successfully conduct all APs in a train, and thereby imposes a greater demand on neuronal AP propagation than the two-pulse sequence of an RP test. For Control neurons, we found following frequencies that were distinct for different neuronal categories, in the rank order of Ao > Ai ≫ C-type (ANOVA P < 0.0001; Ao vs. Ai, P < 0.05; Aovs. C, P < 0.001; Aivs. C, P < 0.001; Fig. 4B). These findings are similar to those of Fang et al. (2005), except that they identified following frequencies for C-type neurons that are relatively faster than those we report here. This is attributable to their measurement of the rate at which 80% of APs successfully invaded the stem axon, whereas we used a 100% endpoint. Additionally, for rates less than 100 Hz, their constant duration (200 ms) trains encompassed fewer pulses than the 20 in the trains that we used.
Although SNL injury did not affect following frequency in Ai neurons, following frequency was decreased in Ao SNL5 neurons (Fig. 4B). In contrast, C-type neurons developed an ability to conduct AP trains at a 10-fold higher rate following axotomy (SNL5 group). These findings indicate a neuron type-specific effect of injury on T-junction filtering, and suggest amplified filtering of non-nociceptive afferent signals but facilitated passage of nociceptive AP trains following injury.
Following frequency in dorsal root fibres
To confirm that AP propagation fails at the T-junction rather than as it approaches in the axon between the site of stimulation and the T-junction, we measured following frequencies in dorsal root axons using an in vitro teased fibre technique (Fig. 5A). Rates were comparable when determined by recording at the point where the root enters the DRG and stimulating at the end transected close to the spinal cord (54 ± 7 Hz, n = 13) or when stimulating and recording sites were reversed (44 ± 9 Hz, n = 5; P = 0.48). Following frequency recorded in fibres was also independent of the use of bipolar versus monopolar stimulation (see Methods). These rates (Fig. 5A) significantly exceeded those recorded in the soma (Fig. 4B; P < 0.0001). Together with the collision data, these findings strongly suggest that the T-junction is the point of failure for both entry into the stem axon and transit to the opposing process.
Figure 5. Site and mechanism of impulse propagation failure.

A, testing maximum axonal firing rate in C-type units in excised dorsal roots. A sample trace (CV = 1.03 m s−1) during stimulation (90 Hz) of the dorsal root segment at the end transected from the spinal cord and recorded from fibres teased from the end transected from the DRG. The first 3 and last 7 APs (arrows) of the train are shown. Stimulation rate is 90 Hz, and the conduction latency is 27.1 ms for the first AP and 29.7 ms for the 20th AP. Summary data from this and other C-type units (n = 18) are shown in the right panel, in which open circles represent recordings in which the rate is assigned as the highest observed following frequency prior to the AP being occluded by the stimulus artefact at higher frequencies, thus making these data possible underestimates. Filled circles represent data for which failure was directly observed at the next higher frequency. The central indicator bars represent the median value for the entire group. B, comparison of the maximum rate at which APs in C-type neurons can propagate through the axonal T-junction (‘Axon’), versus the maximum rate at which the same neuron's soma can fire all of a train of 20 APs during direct somatic current injection (‘Soma’). Conduction through the T-junction was confirmed by the generation of a depolarization, including either an AP or an incomplete electrotonic depolarization, during axonal stimulation. The central indicator bars represent the median value. Main effect of Injury Group P < 0.01; main effect of Stimulation Site P < 0.001; significant post hoc comparisons are shown by connecting brackets, *P < 0.05. C, sensitivity of following frequency to modulation of various Ca2+-sensitive channels. Repeat determinations show stable responses in time controls. Increasing Ca2+ influx during neuronal activation by elevating bath Ca2+ concentration from baseline (2 mm) to 8.0 mm decreased maximal following frequency. Activation of SK and IK subtypes of Ca2+-activated K+ channels with NS309 (5 μm) also decreased following frequency. Activation of the BK subtype of Ca2+-activated K+ channels with NS1619 (10 μm) had no effect on following frequency. Blockade of Ca2+-activated Cl− channels with niflumic acid (Nif A; 100 μm) decreased following frequency. Blockade of the HCN channels with ZD7288 (10 μm) had no effect. Bars show mean ± SEM. Significance testing was done by Student's t test before converting to percentage change, *P < 0.05, **P < 0.01; numbers in the bar are n.
Conduction fails at longer interstimulus intervals in a train than in a pair of pulses
To identify if repetitive firing in trains can maintain successful conduction at the same inter-pulse intervals as for the initial pair, we compared the maximum following frequency for 20 AP trains to the same neuron's maximum instantaneous firing rate (1 per inter-pulse interval) for a pair of APs during the RP protocol. These two measures are correlated (P < 0.001, R2 = 0.52 for all neurons, n = 303), but the maximum frequency is substantially slower for a train of 20 APs compared with a pair of APs (train rate per pair rate of 0.66 ± 0.03 for Ao-type fibres, n = 154; 0.62 ± 0.03 for Ai-type, n = 128; and 0.18 ± 0.04 for C-type, n = 21; P < 0.001 for C-type vs. Ao and Ai; no effect of injury groups). This reveals that sustained firing progressively generates a condition that impedes successful AP propagation through the T-junction.
Maximal somatic firing rate during conducted trains is regulated at a site other than the soma
We hypothesized that if failed propagation through the T-junction is the cause of limited somatic firing rates in response to axonal pulse trains, rather than features of the somatic membrane, this would predict that direct somatic excitation could produce firing rates that exceed the maximum following frequency of conducted APs. We tested this hypothesis in C-type neurons in which the characteristic of slow following frequencies provides the best conditions to identify differences in somatic versus T-junction rates. Specifically, we compared the maximal rate at which 20 somatic APs could be evoked by current injection through the recording electrode to the maximal rate of impulse conduction through the T-junction (i.e. the following frequency), determined in random sequence for each neuron. Thirty-seven of 42 (88%) C-type neurons had a maximal firing frequency during somatic stimulation that exceeded the following frequency (Fig. 5B). Although injury increased following frequency for C-type neurons, maximal somatic firing rates during direct stimulation were not different between injured SNL5 and Control neurons. This indicates that the increased ability of C-type neurons to conduct trains of APs after injury is due to altered properties at the T-junction rather than effects on excitability of the somatic membrane.
Additional evidence that the T-junction regulates following frequency independent of somatic excitability is the lack of relationship between following frequency and the rheobase current necessary to initiate an AP by direct somatic depolarization via current injection through the recording electrode (R2 = 0.01, P = 0.13, n = 188). Further support for the view that events in the axon control following frequency comes from a significant inverse relationship between CV slowing during the train and following frequency (P = 0.0015, n = 294). The weakness of this influence (R2 = 0.03) indicates that the T-junction is regulated by additional factors other than those that control axonal CV.
Ca2+-activated channels regulate propagation through the T-junction
We have previously shown that trains of APs similar to those used in the present recordings substantially elevate cytoplasmic Ca2+ in adult sensory neurons in intact DRGs (Gemes et al. 2010), while others have identified the critical role of cytoplasmic Ca2+ in regulating conduction through the T-junction in embryonic rat DRGs (Luscher et al. 1994a, 1996). We tested whether cytoplasmic Ca2+ regulates AP propagation in our model of adult DRGs by elevating the Ca2+ concentration in the bath solution, which increases cytoplasmic Ca2+ accumulation during sensory neuron activation (Fuchs et al. 2007; Lirk et al. 2008). Whereas time control recordings showed no effect (Fig. 5C), elevating bath Ca2+ by switching from aCSF containing 2 mm Ca2+ to one containing 8 mm Ca2+ (in a separate experiment) decreased the following frequency (Fig. 5C), which is consistent with a sensitivity of T-junction propagation to cytoplasmic Ca2+ concentration.
Possible molecular sites of action of cytoplasmic Ca2+ include Ca2+-dependent membrane channels. Sensory neurons express Ca2+-activated K+ (KCa) channels with pharmacological features of large, intermediate and small conductance (BK, IK and SK) subtypes (Sarantopoulos et al. 2003), which respectively contribute to the early, intermediate and late phases of the AHP. We tested the role of KCa channels on T-junction conduction by determining the following frequency in sensory neurons before and after pharmacological activation of these channels. NS309 (5 μm), which increases current through the IK and SK channels (Pedarzani et al. 2005; Strobaek et al. 2006), decreased following frequency (Fig. 5C) and concurrently expanded the somatic AHParea following the train (baseline: 1760 ± 4733 ms mV; NS309: 5799 ± 1464 ms mV; P < 0.01). However, NS1619 (10 μm), which selectively increases current through BK channels (Olesen et al. 1994; Zhang et al. 2003), had no effect on following frequency (Fig. 5C). It did, however, increase AHPamp at the end of the train (baseline: 6.1 ± 1.4 mV; NS1619: 7.5 ± 1.2 mV; P < 0.05), confirming that this feature is regulated by BK channels.
Sensory neurons also possess Ca2+-sensitive Cl− channels (Mayer, 1985; Currie et al. 1995), which influence sensory neuron excitability (Liu et al. 2010). Blockade of these channels with the standard blocker niflumic acid (100 μm) decreased following frequency (Fig. 5C). Finally, we evaluated a possible contribution of HCN channels that produce the depolarizing H-type current. These channels may augment the AHP (Maccaferri & McBain, 1996), are activated by cytoplasmic Ca2+ (Schwindt et al. 1992; Pan, 2003), and regulate sensory neuron excitability (Hogan & Poroli, 2008). However, blockade with ZD7288 (10 μm) resulted in no change in following frequency (Fig. 5C). Together, these pharmacological observations indicate that propagation of APs through the T-junction is sensitive to regulation by Ca2+-activated IK/SK and Cl− channels.
Role of Vm
AP trains in our model produced the expected changes in AP dimensions and CV. Specifically, we observed a slower CV for the last AP of the train compared with the first for all neuronal types during axonal stimulation at the following frequency (Table 1), similar to prior studies (Luscher et al. 1994a; Waikar et al. 1996). Additional injury-induced CV slowing appeared to be additive with activity-dependent slowing in Ao neurons. We also confirmed previous observations (Bielefeldt & Jackson, 1993) of APd prolongation during trains in both Ai and Ao neurons, with further prolongation attributable to injury (Table 1). In C-type neurons, however, the APd was shortened during the train, although this effect was eliminated by injury. APamp was decreased by repetitive firing in injured Ai neurons.
Because the AHP drives the Vm further from firing threshold and regulates neuronal excitability (Gold et al. 1996; Sapunar et al. 2005), we measured changes in AHP during trains as a possible contribution to regulation of following frequency. AHParea was greatly increased after the last AP of the train compared with a single AP in both Ai and Ao neurons of the Control group (Table 2). This was the result of a large increase in AHPd despite a decrease in AHPamp observed in all groups. To reveal the incremental pattern of AHP change during repetitive firing, we recorded the AHP generated after a variable number of preceding APs (Fig. 6). This showed a progressive loss of amplitude in the early AHP component, while the late AHP increased in amplitude and duration (Fig. 6A). The early AHP component was completely ablated by the train in a subset of neurons (51/259, 20%, no effect of A-fibre type or injury; Fig. 6B). The large conductance (BK) Ca2+-activated K+ current, which generates the early AHP (Scholz et al. 1998; Swensen & Bean, 2003), inactivates with time during depolarization (Raman & Bean, 1999; Khaliq et al. 2003), consistent with the incrementally diminishing AHP amplitude observed here.
Figure 6. Superimposed AHPs following trains composed of from 1 to 20 action potentials (APs) in two different neurons.

In one (A), the amplitude of the fast AHP shows progressive decrement, concurrent with the development of a slower AHP component with greater number of preceding APs. Other neurons, such as that shown in B, show a progressive decrease of the fast component amplitude and its eventual disappearance, along with the development of a slower component. The AHP will influence the membrane voltage at which the next AP in a train is initiated (aRMP) according to the interstimulus interval. Rates that place the following APs initiation to the left of the dotted arrows will result in progressive depolarization of the aRMP as the fast AHP diminishes. Slow rates will have AP initiations to the right of the dotted arrow, and result in progressive hyperpolarization of the aRMP. The scale bars apply to both neurons.
Although the AHParea increases during the train, our data do not support this as a factor regulating propagation failure. First, although we found that AHParea is inversely related to the following frequency (P = 0.04 for the regression), this relationship accounts for very little of the variance in following frequency (R2 = 0.01). Second, although axotomy (SNL5 group) nearly eliminated the train-induced AHP expansion in Ai neurons (Table 2), the following frequency was unaffected (Fig. 4). Third, the following frequency was not different in neurons in which the early component of the AHP was ablated during the train (data not shown). Fourth, although niflumic acid decreased following frequency (Fig. 5C), this was accompanied by decreased AHParea (baseline: 1141 ± 303 ms mV; niflumic acid: 480 ± 131 ms mV; n = 15, P < 0.05). Finally, in a subset of neurons, the impulse train was followed by an afterdepolarization (ADP; Fig. 2C), which in other neural tissues contributes to generation of burst firing (Brumberg et al. 2000; Su et al. 2001; Lancaster et al. 2002). In our sensory neuron recordings, an ADP after a train was observed in Ai neurons after injury (Control 0/55; SNL4 3/26 (12%), P = 0.04 vs. Control; SNL5 12/40 (30%), P < 0.001 vs. Control), and in Ao neurons independent of injury at an overall rate of 8% (13/163). However, the presence of an ADP had no effect on following frequency (without ADP, 348 ± 12 Hz; with ADP, 353 ± 45 Hz). Thus, the AHP and ADP have minimal direct influence upon conduction through the T-junction.
Alternatively, Ca2+-activated channel opening might influence propagation failure by causing a progressive shift in the baseline Vm during the train (Debanne et al. 2011). Each AP is initiated during the AHP from the preceding pulse (Fig. 2), so we characterized the cumulative effect of this phenomenon by comparing the somatic Vm at the moment of AP initiation (which we termed the aRMP) for the second and the last APs in the train. Most Control neurons (Ai: 26/40, 65%; Ao: 56/72, 78%) showed a pattern in which the aRMP became progressively more depolarized (Figs 2A–C and 7). We considered whether this pattern could result from summation of ADPs (Sanchez-Vives & Gallego, 1994). However, only very few neurons (4 Ai, 8 Ao, of 292 neurons, 4%) showed an ADP following a single AP, so the depolarizing patterns observed in most neurons during trains evolved in the absence of a single AP ADP. Nerve injury had no effect on the average change of aRMP during trains (Fig. 7), although a depolarizing pattern was less common in Ao neurons after injury (10/21, 48%; P = 0.01 vs. Control).
Figure 7. Shift of the apparent resting membrane potential (aRMP) during a stimulus train at each neuron's following frequency.

The ordinate indicates the aRMP of the 20th AP minus the aRMP of the 2nd AP in a train of 20 APs. The P-value indicates the probability of a main effect for injury. Ai neurons in the Control group and neurons from the L5 ganglion after spinal nerve ligation (SNL5 group) show a depolarizing shift of the aRMP during repetitive firing that is significantly greater than zero (one-sample Student's t test P < 0.01), as do Ao neurons in the Control and SNL4 group. The central indicator bars represent the median value.
Before considering whether changing aRMP during the train contributes to propagation failure, we note that contrasting data were reported by Amir & Devor (1997), who found that the progressive RMP shift during trains of APs produced by direct somatic stimulation was in the hyperpolarizing direction for 90% of DRG neurons, rather than depolarizing as we found. Because that study employed prolonged stimulation (10 s), we tested additional Control neurons to determine whether hyperpolarization might eventually predominate if stimulation (axonal) is extended for 10 s. Of these 16 neurons, 15 (94%) showed a depolarizing shift during prolonged stimulation at following frequency. This indicates that our findings are not specific for a particular duration of stimulation. A more relevant factor may be the firing rate during the train, which was generally slower in the work by Amir and Devor than in the present report, in which we focused on the maximal following frequency. The aRMP is set by the amplitude of the preceding AHP at the moment of the initiation of the next AP. Because the early AHP typically diminishes with repetition but the late AHP expands (Fig. 6), we predicted that AP initiation in trains with short interstimulus intervals will occur at a progressively more depolarized Vm, while repetition at slower rates will result in APs being initiated during the slow AHP that is expanding during the train, producing hyperpolarization of the aRMP. We confirmed this by comparing patterns of aRMP in individual neurons at different stimulation rates (Fig. 8A and B), which showed a shift from hyperpolarization to depolarization with increasing rates. Finally, Amir and Devor identified depolarization of inactive sensory neuron somata during stimulation of adjacent fibres, termed ‘cross-depolarization’. We sought evidence of this phenomenon as a possible contribution to depolarization in our recordings. We observed, however, that in the absence of conducted APs, Vm recovered to the pre-train RMP along the trajectory of the AHP (Fig. 8C), suggesting that only active events for the recorded neuron contribute to shifts in aRMP.
Figure 8. The effect of firing rate on membrane voltage (Vm) during a train of APs.

A, recording of somatic Vm during axonal stimulation. Conducted APs are initiated at a Vm that is influenced by the firing rate. Traces are shown at the same vertical scale, and APs are truncated except for traces at 10 Hz, 450 Hz and 500 Hz. The thin horizontal line indicates the Vm at the initiation of the second AP, thereby providing a reference to identify progressive hyper- or depolarization during the train. Original RMP is evident by a short segment at the beginning of each trace. In this Control Ao neuron, hyperpolarization is apparent up to a rate of 100 Hz, while depolarization develops thereafter. At 450 Hz and 500 Hz, some stimuli are followed by complete failure of conduction through the T-junction (marked by *). Other APs invade the stem axon, but either fail to generate an AP in the soma (producing only an electrotonic potential, ‘e’) or initiate an incomplete somatic component (‘i’). AP failure and electrotonic potentials lack a full AHP, which therefore interrupts the pattern of depolarization. Dotted straight-line segments fill gaps in the traces where stimulus artefacts were removed for clarity. B, summary data for a sample of 9 neurons (7 Ao, 2 Ai), showing a dependence of direction of shift of the apparent resting membrane potential (aRMP) upon firing rate. Data are mean ± SEM. C, in another neuron (Ao), periods of failed conduction (marked by *) interrupt hyperpolarization (at 50 Hz) and depolarization (150 Hz and 200 Hz), during which Vm recovers towards resting Vm along the trajectory of the previous AHP. This indicates that shifts in aRMP require membrane activation and are not the result of ongoing stimulation of adjacent neurons. Note also the recovery of the fast AHP (dotted arrow) after a brief period of membrane quiescence. Downward lines are stimulation artefacts.
It is unlikely that the shift in aRMP accounts for the eventual failure of AP propagation through the T-junction as failure evolves in the context of both depolarizing and hyperpolarizing patterns. We nonetheless asked whether the pattern of change of the aRMP during a train regulates the frequency at which the conduction fails. Neurons with a depolarizing pattern of aRMP during a train had higher following frequencies compared with those that had unchanging or hyperpolarizing patterns, for both Ai neurons (208 ± 15 vs. 85 ± 14 s−1, P < 0.001) and Ao neurons (374 ± 15 vs. 203 ± 14 s−1, P < 0.001). However, the relationship between firing rate and the pattern of aRMP shift described above dictates that neurons with fast following frequencies will show a depolarizing pattern as a result of those firing rates, so cause and effect cannot be distinguished on this basis. Further evidence that the pattern of aRMP shift does not regulate conduction failure is provided by the findings that NS309 and niflumic acid altered the following frequency but had no effect on the pattern of aRMP during trains (data not shown).
Role of membrane resistance
An established mechanism by which Ca2+-activated channels may modulate membrane excitability is by decreasing membrane resistance, which shunts current and causes failure of AP propagation at critical sites such as the T-junction. We examined this in the somata of 17 A-type neurons by comparing input resistance (Rin) after single APs and after trains of 20 APs at following frequency. Rin was calculated from the membrane voltage change induced by a brief hyperpolarizing current injection (0.5 nA, for 7 ms). A larger and more sustained decrease in Rin developed during a train of APs than after a single AP (Fig. 9), which was consistent regardless of whether the neuron showed a depolarizing pattern during the train (n = 11), a hyperpolarizing pattern (n = 4) or no change in aRMP (n = 2). These observations support Rin loss as a possible cause of eventual AP propagation failure during a train.
Figure 9. The effect of an action potential (AP) train on subsequent input resistance in an uninjured Ao neuron.

Input resistance, determined as the voltage change divided by current injected during a brief hyperpolarizing current injection, is depressed after a single AP (A), but recovers promptly to baseline. Following a train of 20 APs at 200 Hz (B), the same neuron shows a prolonged recovery of input resistance. The scale bars apply to both A and B. The current protocol for determining input resistance (C) consists of 0.5 nA hyperpolarizing current pulses lasting 7 ms, every 50 ms, starting 10 ms after the last AP. Summary data (mean ± SEM) of 15 A-type neurons (D) show a significantly longer recovery after a 20 pulse train than a single AP with significant differences (P < 0.05) up to 360 ms after the initiation of the AHP. Recovery from a train fits a biexponential with time constants 46 ms and 1578 ms. The time scale in D applies also to the other panels.
Discussion
Although the pseudounipolar design of adult mammalian peripheral sensory neurons removes the electrical load of the soma from the direct conduction path between the central and peripheral processes of the neuron, the resulting T-junction still introduces an electrical obstacle to impulse propagation. The purpose of our study was to determine whether this site of impedance mismatch has a functional consequence in adult mammalian sensory neurons. The main finding from this investigation is that the T-junction is a preferential site of failure for afferent traffic. Furthermore, this low-pass filtering function is Ca2+-dependent, and filtering is lost selectively in C-type neurons after peripheral nerve injury.
Regulation of T-junction conduction
In our model, AP propagation can potentially fail at several locations between the axonal stimulation site and the somatic recording site. During activation with short interstimulus intervals, incomplete somatic depolarizations may appear that display a distinct amplitude, lack an AHP, and fail in an all-or-none fashion (Figs 1E and F and 2B), suggesting that these depolarizations represent the passive electrotonic residue of a distant AP that fails to activate somatic AP generation (Stoney, 1990; Luscher et al. 1994b). Our collision experiments agree with others (Stoney, 1990; Luscher et al. 1994b; Zhou & Chiu, 2001) in showing that these incomplete depolarizations originate from APs that successfully propagate longitudinally into the opposing process and into the stem axon, but fail to activate the somatic membrane. Complete loss of the somatic depolarization represents failure of the AP to propagate into either the stem axon or to propagate longitudinally. Using this endpoint, we observed failure of propagation through the T-junction at AP repetition rates that are slower than the rates at which the soma and axon can be excited. These combined findings indicate that the T-junction acts as a low-pass filter such that fewer sensory signals may reach the spinal cord than are generated in the periphery.
Rate-dependent propagation failure at neuronal branch points has been identified in other systems, including vertebrate central and peripheral neurons, for which several mechanisms have been proposed (see Debanne et al. 2011). There is evidence to support membrane depolarization resulting from extracellular K+ accumulation, which in turn leads to inactivation of Na+ channels. In other systems, propagation failure has been attributed to hyperpolarization due to activation of Ca2+-dependent channels and intracellular Na+ accumulation, which shift Vm away from the threshold for AP initiation. However, our present findings in mammalian sensory neurons show no consistent relationship between shifts in Vm and propagation failure at the T-junction (detailed in Results).
We cannot conclusively establish a mechanism for sensory neuron propagation failure, but we note that somatic input resistance is consistently decreased during trains of repetitive APs regardless of the trends in Vm during repetitive firing. Other findings from our study support membrane resistance as a controlling factor, including increased conduction failure with pharmacological opening of IK/SK subtypes of KCa channels, and substantial train-induced expansion of the AHP that is generated by opening KCa channels in these neurons. Furthermore, we have previously documented that AP trains lead to the accumulation of intracellular Ca2+ (Gemes et al. 2010), which resolves with a time constant of approximately 1 s, in accordance with the time for the input resistance to recover (Fig. 9D). Nonetheless, the participation of multiple mechanisms contributing to propagation failure is likely. For instance, we show that niflumic acid slows the following frequency even though it increases membrane resistance (Currie et al. 1995). This finding, however, is consistent with prior observations that Ca2+-activated Cl− currents excite sensory neurons (Liu et al. 2010). It is also likely that the mechanisms contributing to propagation failure differ between sensory neuron subgroups.
A limitation of our data is that recordings of Vm in the soma may not fully reflect membrane events at the T-junction. Some assurance is offered by the recognition that axons are likewise equipped with voltage-gated Ca2+ channels and Ca2+-activated conductances (Scholz et al. 1993; Luscher et al. 1996; Bender & Trussell, 2009; Yu et al. 2010). Furthermore, recordings by others from axonal segments lacking T-junctions are consistent with our key findings, including activity-induced depression of both membrane excitability (Bostock & Grafe, 1985; Waikar et al. 1996) and input resistance (David et al. 1995) during trains, and contribution of KCa channel opening to AP propagation failure (Bielefeldt & Jackson, 1993).
Potential influence of T-junction filtering on sensory function
Successful transmission of APs through the T-junction is a critical step in the chain of events leading to sensory perception following natural sensory stimulation. The range of maximal impulse conduction rates we have found for Control Ao neurons overlaps with peak rates of impulse generation recorded in peripheral processes during natural stimulation of low-threshold mechanoreceptors. Specifically, maximum instantaneous firing frequencies between 300 Hz and 600 Hz are reported for cutaneous receptors in various mammalian species, including human (Burgess & Perl, 1973; Knibestol, 1973; Iggo & Ogawa, 1977). Instantaneous rates may exceed rates within a sustained train, which is less often reported. However, Leem et al. (1993) have noted complete entrainment of AP trains in low-threshold mechanoreceptors of rats at stimulation rates up to 500 Hz for periods of 10 s. In human subjects, sustained trains have been recorded in peripheral nerve from muscle afferents at rates up to 400 Hz (Vallbo, 1970) and from cutaneous mechanoreceptors at rates up to 550 Hz (Knibestol & Vallbo, 1970; Johansson et al. 1988). These are levels at which we observed T-junction filtering, which may therefore contribute to shaping the frequency profile of afferent traffic in non-nociceptive A-type neurons.
In C-type fibres, maximal instantaneous firing recorded in peripheral processes in response to natural noxious stimulation have been reported at rates from 20 Hz to above 80 Hz for mechanical stimuli (Koltzenburg et al. 1997; Slugg et al. 2000; Chen & Levine, 2003), and from 50 Hz to above 100 Hz for thermal stimuli (Bessou & Perl, 1969; Long, 1977; Kress et al. 1992). Sustained firing for 20 s at 20 Hz can be induced by cold (Leem et al. 1993). As we observed typical following frequencies at 5 Hz for C-type fibres, T-junction filtering may represent a critical mechanism regulating the afferent transmission of pain signals, possibly with distinct effects in subgroups of C-units. Conduction failure in a burst follows initial conduction success and occurs progressively with higher frequencies, so the expected perceptual effect would be akin to adaptation, such that sensations generated by activity at the high end of a neuron's dynamic range will be lessened in intensity and duration. This may serve to avoid an overwhelming or distracting percept, and to protect neuronal somata from extreme Ca2+ loads, particularly in the setting of pathological conditions such as exposure to irritants that elevate maximal firing rates (Kress et al. 1992).
T-junction filtering after injury
Our data show that Ao neurons are able to transmit trains of APs only at reduced rates following axotomy (SNL5 group), whereas the following frequency was not affected in Ai neurons. In contrast, APs in the typically nociceptive C-type population are able to transit the T-junction at considerably higher frequencies after axotomy. This effect of nerve injury resembles a similar acceleration of following frequencies in C-type neurons during peripheral tissue inflammation (Djouhri et al. 2001). Reduced T-junction filtering after axotomy may result from decreased activation of K(Ca) currents due to diminished Ca2+ influx through voltage-gated Ca2+-channels, as we (McCallum et al. 2006) and others (Abdulla & Smith, 2001) have observed in small sensory neurons after peripheral nerve injury. Additionally, Ca2+-activated K+ channels are themselves reduced after nerve injury (Sarantopoulos et al. 2007), including the IK and SK subtypes that support T-junction filtering. Teleologically, the presence of filtering offers a means by which C-fibre afferent traffic to the CNS can be rapidly escalated at the onset of inflammation and nerve injury, promptly triggering protective behaviour.
Diminished T-junction filtering in C-type nociceptors after injury may enhance CNS delivery of nociceptive traffic originating in traumatized peripheral nerves, thereby potentiating neuropathic pain. A question arises regarding the source of afferent activity in axotomized sensory neurons (SNL5 group in our model) as they are detached from their receptive fields. Furthermore, we and others (Ma et al. 2003; Djouhri et al. 2006; although not all, e.g. Meyer et al. 1985; Serra et al. 2012) fail to see spontaneous activity in axotomized C-type units. However, various observations make it likely that in the behaving animal, ectopic activity is generated in axotomized neurons at the site of neuroma formation and in their somata. First, naturally generated activity in the receptive fields of the dorsal primary ramus of the L5 spinal nerve, which remains intact after SNL, may excite axotomized ventral ramus neurons in the same DRG by the process of cross-excitation (Devor & Wall, 1990). There may be particularly high activity in these surviving afferents as they innervate skin, muscle bone and joints damaged by SNL surgery. Second, axotomized neurons, including C-type units, acquire mechanical sensitivity both in the DRG and at the neuroma (Wall & Devor, 1983; Blumberg & Janig, 1984; Rivera et al. 2000), which reside in the highly mobile environment of the intervertebral foramen and the lumbar paravertebral space in the case of SNL. Finally, sympatho-somatic coupling may drive ectopic activity in the DRG and neuroma after axotomy (Blumberg & Janig, 1984; Burchiel, 1984), generating firing rates that may exceed 20 Hz (Habler et al. 1987). Suppression of autonomic activity by deep barbiturate anaesthesia as used during recording (Ma et al. 2003; Djouhri et al. 2006) would prevent the occurrence of this sympathetically driven firing. In awake animals, we have noted substantial global sympathetic activation induced by noxious mechanical stimulation (Rigaud et al. 2011). Although sensory neuron recording in the active animal is not currently possible, generation of ectopic C-fibre activity in axotomized neurons is supported by observations in human subjects in which pain is induced when axotomized neurons are exposed to mechanical stimulation or catecholamines (Chabal et al. 1992; Lin et al. 2006).
In contrast to our findings that AP propagation is diminished by injury in Ao units but enhanced in C-type units, others (Amir et al. 1999) have found increased spontaneous activity and depolarization-induced AP genesis after injury in Ao sensory neurons, while Ai and C-type neurons are unaffected. These distinct patterns of effects highlight the likelihood that the mechanisms regulating AP propagation versus AP initiation are distinct. A favourable consequence of our present findings is that novel therapies may be envisioned that target the Ca2+-dependent events governing AP propagation at the point of conduction failure within the DRG. For example, amplified T-junction filtering might be induced by augmenting voltage-gated Ca2+ channel conductance, elevating extracellular Ca2+ levels or injecting a Ca2+ ionophore into the DRG, thereby limiting the access of high-frequency trains of APs to the spinal cord dorsal horn. This approach could compensate for excessive nociceptive traffic regardless of the underlying natural or pathogenic events that produce high-frequency trains.
Acknowledgments
The reported research was funded by NIH grants NS042150 to Q.H.H. and NS070711 to C.L.S. None of the authors have a conflict of interest in accordance with the policy of this journal. This work was supported in part by grants no. NS-42150 (to Q.H.H.) and NS-40538 (to C.S.) from the National Institutes of Health, Bethesda, MD, USA.
Glossary
- aCSF
artificial cerebrospinal fluid
- ADP
afterdepolarization
- AHP
afterhyperpolarization
- AHPamp
afterhyperpolarization amplitude
- AHParea
area of the afterhyperpolarization
- AHPd
afterhyperpolarization duration
- AP
action potential
- APamp
action potential amplitude
- APd
action potential duration
- aRMP
apparent resting membrane potential
- CV
conduction velocity
- DRG
dorsal root ganglion
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- L4
L5, L6, lumbar 4th, 5th and 6th segmental level
- RMP
resting membrane potential
- RP
refractory period
- SNL
spinal nerve ligation
Author contributions
The experiments were performed in the laboratories of C.S. (teased fibre recording) and Q.H.H., MD (for everything else). The contributions of the authors are as follows. Conception and design of the experiments: G.G., D.V., S.R.G., C.S. and Q.H.H. Collection, analysis and interpretation of data: G.G., A.K., M.R., P.L., D.S., M.L.B., D.V., S.R.G., M.L., S.J.M., C.L.S. and Q.H.H. Drafting the article or revising it critically for important intellectual content: G.G., D.V., S.G., C.L.S. and Q.H.H. All authors approved the final version of the manuscript.
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Spike-evoked suppression and burst patterning in dorsal root ganglion neurons of the rat. J Physiol. 1997;501:183–196. doi: 10.1111/j.1469-7793.1997.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. J Neurophysiol. 1993;70:284–298. doi: 10.1152/jn.1993.70.1.284. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Janig W. Discharge pattern of afferent fibers from a neuroma. Pain. 1984;20:335–353. doi: 10.1016/0304-3959(84)90111-8. [DOI] [PubMed] [Google Scholar]
- Bostock H, Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci. 2000;20:4829–4843. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to alpha-adrenergic stimulation and hypoxia. Exp Neurol. 1984;85:257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- Burgess P, Perl E. Cutaneous mechanoreceptors and nociceptors. In: Iggo A, editor. Handbook of Sensory Physiology. New York: Springer; 1973. pp. 29–78. [Google Scholar]
- Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain response to perineuromal injection of normal saline, epinephrine, and lidocaine in humans. Pain. 1992;49:9–12. doi: 10.1016/0304-3959(92)90181-A. [DOI] [PubMed] [Google Scholar]
- Chen X, Levine JD. Altered temporal pattern of mechanically evoked C-fibre activity in a model of diabetic neuropathy in the rat. Neuroscience. 2003;121:1007–1015. doi: 10.1016/s0306-4522(03)00486-x. [DOI] [PubMed] [Google Scholar]
- Currie KP, Wootton JF, Scott RH. Activation of Ca2+-dependent Cl− currents in cultured rat sensory neurones by flash photolysis of DM-nitrophen. J Physiol. 1995;482:291–307. doi: 10.1113/jphysiol.1995.sp020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol. 1995;489:141–157. doi: 10.1113/jphysiol.1995.sp021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Dawbarn D, Robertson A, Newton R, Lawson SN. Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci. 2001;21:8722–8733. doi: 10.1523/JNEUROSCI.21-22-08722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fibre nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducreux C, Reynaud JC, Puizillout JJ. Spike conduction properties of T-shaped C neurons in the rabbit nodose ganglion. Pflugers Arch. 1993;424:238–244. doi: 10.1007/BF00384348. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Lirk P, Stucky C, Abram SE, Hogan QH. Painful nerve injury decreases resting cytosolic calcium concentrations in sensory neurons of rats. Anesthesiology. 2005;102:1217–1225. doi: 10.1097/00000542-200506000-00023. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Rigaud M, Hogan QH. Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats. Anesthesiology. 2007;107:106–116. doi: 10.1097/01.anes.0000267538.72900.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Gemes G, Rigaud M, Koopmeiners AS, Poroli MJ, Zoga V, Hogan QH. Calcium signalling in intact dorsal root ganglia: new observations and the effect of injury. Anesthesiology. 2010;113:134–146. doi: 10.1097/ALN.0b013e3181e0ef3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca2+-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett. 1996;205:161–164. doi: 10.1016/0304-3940(96)12401-0. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci Lett. 1987;82:35–40. doi: 10.1016/0304-3940(87)90167-4. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 2001. [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, Bosnjak ZJ. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86:43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Hogan QH, Poroli M. Hyperpolarization-activated current (Ih) contributes to excitability of primary sensory neurons in rats. Brain Res. 2008;1207:102–110. doi: 10.1016/j.brainres.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977;266:275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Trulsson M, Olsson KA, Westberg KG. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res. 1988;72:204–208. doi: 10.1007/BF00248518. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modelling study. J Neurosci. 2003;23:4899–4912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Knibestol M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. J Physiol. 1973;232:427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestol M, Vallbo AB. Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand. 1970;80:178–195. doi: 10.1111/j.1748-1716.1970.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Handwerker HO. Differential ability of human cutaneous nociceptors to signal mechanical pain and to produce vasodilatation. J Neurosci. 1994;14:1756–1765. doi: 10.1523/JNEUROSCI.14-03-01756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Oh EJ, Gover T, Weinreich D. Calcium and calcium-activated currents in vagotomized rat primary vagal afferent neurons. J Physiol. 2002;540:543–556. doi: 10.1113/jphysiol.2001.013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or Aα/β-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Lin EE, Horasek S, Agarwal S, Wu CL, Raja SN. Local administration of norepinephrine in the stump evokes dose-dependent pain in amputees. Clin J Pain. 2006;22:482–486. doi: 10.1097/01.ajp.0000202980.51786.ae. [DOI] [PubMed] [Google Scholar]
- Lirk P, Poroli M, Rigaud M, Fuchs A, Fillip P, Huang CY, Ljubkovic M, Sapunar D, Hogan Q. Modulators of calcium influx regulate membrane excitability in rat dorsal root ganglion neurons. Anesth Analg. 2008;107:673–685. doi: 10.1213/ane.0b013e31817b7a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RR. Sensitivity of cutaneous cold fibers to noxious heat: paradoxical cold discharge. J Neurophysiol. 1977;40:489–502. doi: 10.1152/jn.1977.40.3.489. [DOI] [PubMed] [Google Scholar]
- Lundberg LE, Jorum E, Holm E, Torebjork HE. Intra-neural electrical stimulation of cutaneous nociceptive fibres in humans: effects of different pulse patterns on magnitude of pain. Acta Physiol Scand. 1992;146:41–48. doi: 10.1111/j.1748-1716.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Lipp P, Luscher HR, Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996;490:319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Streit J, Lipp P, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. J Neurophysiol. 1994a;72:634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- Luscher C, Streit J, Quadroni R, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994b;72:622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol. 1996;497:119–130. doi: 10.1113/jphysiol.1996.sp021754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum JB, Kwok WM, Mynlieff M, Bosnjak ZJ, Hogan QH. Loss of T-type calcium current in sensory neurons of rats with neuropathic pain. Anesthesiology. 2003;98:209–216. doi: 10.1097/00000542-200301000-00032. [DOI] [PubMed] [Google Scholar]
- McCallum JB, Kwok WM, Sapunar D, Fuchs A, Hogan QH. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons. Anesthesiology. 2006;105:160–168. doi: 10.1097/00000542-200607000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Raja SN, Campbell JN, Mackinnon SE, Dellon AL. Neural activity originating from a neuroma in the baboon. Brain Res. 1985;325:255–260. doi: 10.1016/0006-8993(85)90321-x. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. Kappa-opioid receptor-mediated enhancement of the hyperpolarization-activated current (Ih) through mobilization of intracellular calcium in rat nucleus raphe magnus. J Physiol. 2003;548:765–775. doi: 10.1113/jphysiol.2002.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Abram SE, Dean C, Hopp FA, Stucky CL, Eastwood D, Tarima S, Seagard J, Hogan QH. Pain tests provoke modality-specific cardiovascular responses in awake, unrestrained rats. Pain. 2011;152:274–284. doi: 10.1016/j.pain.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Rivera L, Gallar J, Pozo MA, Belmonte C. Responses of nerve fibres of the rat saphenous nerve neuroma to mechanical and chemical stimulation: an in vitro study. J Physiol. 2000;527:305–313. doi: 10.1111/j.1469-7793.2000.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RD, Koerber HR, Sedivec MJ, Mendell LM. Somal action potential duration differs in identified primary afferents. Neurosci Lett. 1986;63:259–264. doi: 10.1016/0304-3940(86)90366-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Gallego R. Calcium-dependent chloride current induced by axotomy in rat sympathetic neurons. J Physiol. 1994;475:391–400. doi: 10.1113/jphysiol.1994.sp020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103:360–376. doi: 10.1097/00000542-200508000-00020. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos C, McCallum B, Sapunar D, Kwok WM, Hogan Q. ATP-sensitive potassium channels in rat primary afferent neurons: the effect of neuropathic injury and gabapentin. Neurosci Lett. 2003;343:185–189. doi: 10.1016/s0304-3940(03)00383-5. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos CD, McCallum JB, Rigaud M, Fuchs A, Kwok WM, Hogan QH. Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons. Brain Res. 2007;1132:84–99. doi: 10.1016/j.brainres.2006.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Reid G, Vogel W, Bostock H. Ion channels in human axons. J Neurophysiol. 1993;70:1274–1279. doi: 10.1152/jn.1993.70.3.1274. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Effects of intracellular calcium chelation on voltage-dependent and calcium-dependent currents in cat neocortical neurons. Neuroscience. 1992;47:571–578. doi: 10.1016/0306-4522(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fibre nociceptors in the monkey to controlled-force stimuli. J Neurophysiol. 2000;83:2179–2191. doi: 10.1152/jn.2000.83.4.2179. [DOI] [PubMed] [Google Scholar]
- Stoney SD., Jr Limitations on impulse conduction at the branch point of afferent axons in frog dorsal root ganglion. Exp Brain Res. 1990;80:512–524. doi: 10.1007/BF00227992. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Hougaard C, Johansen TH, Sorensen US, Nielsen EO, Nielsen KS, Taylor RD, Pedarzani P, Christophersen P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol. 2006;70:1771–1782. doi: 10.1124/mol.106.027110. [DOI] [PubMed] [Google Scholar]
- Su H, Alroy G, Kirson ED, Yaari Y. Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J Neurosci. 2001;21:4173–4182. doi: 10.1523/JNEUROSCI.21-12-04173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci. 2003;23:9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB. Slowly adapting muscle receptors in man. Acta Physiol Scand. 1970;78:315–333. doi: 10.1111/j.1748-1716.1970.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Thalhammer JG, Raymond SA, Huang JH, Chang DS, Strichartz GR. Mechanoreceptive afferents exhibit functionally-specific activity dependent changes in conduction velocity. Brain Res. 1996;721:91–100. doi: 10.1016/0006-8993(96)00165-5. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11:280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Maureira C, Liu X, McCormick D. P/Q and N channels control baseline and spike-triggered calcium levels in neocortical axons and synaptic boutons. J Neurosci. 2010;30:11858–11869. doi: 10.1523/JNEUROSCI.2651-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chiu SY. Computer model for action potential propagation through branch point in myelinated nerves. J Neurophysiol. 2001;85:197–210. doi: 10.1152/jn.2001.85.1.197. [DOI] [PubMed] [Google Scholar]


