Abstract
Consecutive missed doses may differentially impact the efficacy of antiretroviral therapy associated with the use of a nonnucleoside reverse-transcriptase inhibitor (NNRTI) and a ritonavir-boosted protease inhibitor (PI). In a cohort of 72 subjects receiving a boosted PI, average adherence to dosage was a better predictor of human immunodeficiency virus (HIV) replication than was the duration or frequency of treatment interruption. In contrast with an NNRTI, consecutive missed doses of a boosted PI did not emerge as a major risk factor for HIV replication.
Regimens based on a ritonavir-boosted protease inhibitor (PI) and a nonnucleoside reverse-transcriptase inhibitor (NNRTI) are the most commonly prescribed highly active antiretroviral therapy (HAART) regimens in clinical practice. Although adherence remains one of the keys to realizing the benefits of either type of regimen [1–5], the impact of nonadherence has been shown to differ between PI- and NNRTI-based HAART in terms of virologic failure and antiretroviral resistance [6–9]. The percentage of missing doses and the pattern of missed doses may have different outcomes for PI- or NNRTI-based regimens. For example, consecutive missed doses in the form of a treatment interruption posed a greater risk of human immunodeficiency virus (HIV) replication on NNRTI-based antiretroviral therapy than did the same number of interspersed missed doses [10]. In particular, NNRTI-based regimens were vulnerable to treatment interruptions for >7 days [10] among patients with low-to-moderate adherence (<80%).
We examined the relationships between the level and pattern of adherence in a prospective cohort of HIV-infected individuals treated with ritonavir-boosted PI therapy. Our specific objectives were to describe the temporal association between average percent adherence, treatment interruption, and HIV replication for all subjects and for a subset of subjects with <80% adherence, to determine whether individuals with low-to-moderate adherence who were treated with a ritonavir-boosted PI are susceptible to treatment interruptions.
Methods
We conducted the analysis of data collected from 2 multicenter prospective observational cohort studies of HIV-infected patients treated with ritonavir-boosted PIs: ESPOIR (Etude et Surveillance par Pilulier électronique de l'Observance et de l'Incidence de la Réplication virale) and REACH (RE-search in Access to Care in the Homeless). The ESPOIR cohort selected consecutive patients receiving or starting twice-a-day lopinavir-ritonavir–based regimens and monitored adherence in several outpatient clinics in France. The REACH cohort selected HIV-positive homeless and marginally housed individuals in San Francisco, California. Participants from the REACH cohort who received ritonavir-boosted PI were similarly included in the analysis. The rationale for combining both cohorts was to increase statistical power and adherence-pattern diversity to make meaningful inferences. The Institutional Review Board of the University of Caen (ESPOIR) and the Committee on Human Subjects Research of the University of California, San Francisco (REACH), approved all study procedures, and patients provided written informed consent. HIV replication was defined as an HIV RNA level of ≥400 copies/mL and as an HIV RNA level of ≥50 copies/mL (investigator-defined treatment failure or 12 months of follow-up, whichever comes first). Because both HIV RNA limits provided similar results concerning predictors of viral replication, only the ≥400 copies/mL limit is reported.
Adherence was prospectively measured using 6 caps of the Medication Event Monitoring System (MEMS; Aardex). In addition, adherence was confirmed by measuring lopinavir plasma levels in the ESPOIR cohort and by having monthly unannounced pill counts in the REACH cohort. Electronic medication monitors measure patterns of missed doses with a timedate record of pill bottle opening behavior. Average percent dose adherence was defined as the number of MEMS events (pill bottle openings), divided by the number of prescribed doses, multiplied by 100. As previously reported in Parienti et al [10], we characterized patterns of missed doses by several a priori measures: (1) number of days without a dose, defined as drug discontinuation for >24 h and <48 h; (2) number of treatment interruptions lasting ≥48 h; and (3) the duration of the longest treatment interruption (in days).
Continuous variables were summarized as mean values, median values, standard deviations, and interquartile ranges (IQRs), depending of their distributions. Dichotomous data were summarized as proportions. The effect of adherence on the probability of virologic replication was estimated by calculating odds ratios (ORs) and their 95% confidence intervals (CIs) using univariate logistic regression models. To determine which adherence pattern explained the larger amount of variance in virologic outcome, we conducted forward, backward, and stepwise model selections among all 4 potential adherence patterns, with a likelihood ratio test limit of P < .05 to enter and remain in the model. In a second step, we assessed the independence of predictors. Because we were concerned by the risk of collinearity among adherence patterns, we only forced average percent adherence and longer treatment interruption in the multivariate analysis.
We also conducted a predefined sensitivity analysis limited to subjects with <80% adherence in order (1) to increase the variability of adherence patterns, because subjects with ≥80% adherence had homogeneous adherence patterns [10], and (2) to test whether adherence patterns still predicted viral replication at low-to-moderate adherence. In this analysis, quantitative variables were compared between groups with and without HIV replication by use of the exact Wilcoxon rank sum test, because of the uncertainty of whether the adherence data from this small sample met assumptions of the normal distribution.
Analyses were performed using PowerView, version 2.3.3 (Aardex), and SAS, version 9.1 (SAS Institute). All reported P values are 2-sided, and a P value of <.05 was considered to be statistically significant.
Results
Seventy-two patients were followed during the period from December 2006 through December 2008. The mean age (± standard deviation) was 43.9 ± 7.5 years, and 59 (82%) of the 72 patients were male. The median CD4+ cell count was 339 cells/mm3 (IQR, 210–550 cells/mm3). Of these 72 patients, 55 (76%) were treated with a combination of lopinavir and ritonavir, and 13 (18%) were treated with atazanavir boosted by ritonavir. Other baseline characteristics are shown in Table 1.
Table 1.
Baseline Characteristics of the ESPOIR and REACH Cohorts
| Characteristic | Overall (n = 72) | ESPOIR (n = 43) | REACH (n = 29) |
|---|---|---|---|
| Age, mean ± SD, years | 43.9 ± 7.5 | 43.7 ± 8.5 | 44.3 ± 5.7 |
| Male sex | 59 (82) | 35 (81) | 24 (83) |
| HIV risk factor | |||
| Heterosexual | 13 (18) | 13 (30) | 0 (0) |
| Men who have sex with men | 38 (53) | 25 (58) | 13 (45) |
| Intravenous drug use | 18 (25) | 3 (7) | 15 (52) |
| Unknown | 3 (4) | 2 (5) | 1 (3) |
| Baseline HIV RNA level | |||
| <50 copies/mL | 26 (36) | 14 (33) | 12 (41) |
| 50–1000 copies/mL | 22 (31) | 14 (33) | 8 (28) |
| >1000 copies/mL | 24 (33) | 15 (34) | 9 (31) |
| Median CD4+ cell count (IQR), cells/mm3 | 339 (210–550) | 406 (280–554) | 232 (129–507) |
| Started boosted PIs at inclusion | 21 (29) | 21 (49) | 0 |
| Treatment naive | 8 (11) | 8 (19) | 0 |
| Treatment experienced | 13 (18) | 13 (30) | 0 |
| Started boosted PIs prior to inclusion | 51 (71) | 22 (51) | 29 (100) |
| Boosted PI | |||
| Lopinavir-ritonavir | 55 (76) | 43 (100) | 12 (41) |
| Atazanavir-ritonavir | 13 (18) | 0 (0) | 13 (45) |
| Other | 4 (6) | 0 (0) | 4 (14) |
| Other antiretrovirals | |||
| Tenofovir plus emtricitabine-lamivudine | 21 (29) | 16 (37) | 5 (17) |
| Zidovudine plus lamivudine | 10 (14) | 9 (21) | 1 (3) |
| Abacavir plus lamivudine | 10 (14) | 8 (19) | 2 (7) |
| Zidovudine plus lamivudine plus abacavir | 5 (7) | 1 (2) | 4 (14) |
| Abacavir plus tenofovir | 5 (7) | 2 (5) | 3 (10) |
| Didanoside plus stavudine | 3 (4) | 1 (2) | 2 (7) |
| Other combinations | 18 (25) | 6 (14) | 12 (42) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. HIV, human immunodeficiency virus; ESPOIR, Etude et Surveillance par Pilulier électronique de l'Observance et de l'Incidence de la Réplication virale; IQR, interquartile range; PI, protease inhibitor; REACH, REsearch in Access to Care in the Homeless; SD, standard deviation.
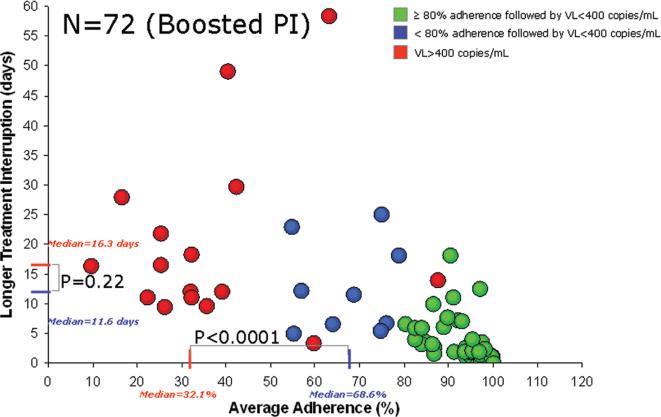
The percentages of individuals with HIV replication are depicted in Figure 1, by different adherence strata and different levels of HIV RNA. All adherence measurements, including the frequency of short-term interruptions (24–48 h), significantly predicted HIV replication >400 copies/mL, as shown in Table 2. The average percent adherence was the only adherence pattern retained in the logistic model predictive of viral replication (likelihood ratio, 55.4; P < .001). In multivariate analysis, average percent adherence (OR, 0.30; 95% CI, 0.15–0.60; P < .001), not longer treatment interruption (OR, 1.07; 95% CI, 0.96–1.18; P = .22), was associated with viral replication. Figure 2 shows the relationship between average percent adherence and the longer durations of treatment interruption in days, in a dual-scale adherence plot. Subjects with >80% average adherence also had treatment interruptions, and all except one achieved virologic suppression.
Figure 1.
Virologic suppression rates, based on the average percent adherence to boosted protease inhibitor therapy.
Table 2.
Risk of Replication of Human Immunodeficiency Virus (HIV) of >400 Copies/mL by Average Percent and Patterns of Adherence
| Variable | HIV controla (n = 56) | HIV replication (n = 16) | ORb (95% CI) | P |
|---|---|---|---|---|
| Adherence rate,c mean ± SD, % | 89.2 (11.6) | 36.8 (19.4) | 0.27 (0.14–0.53) | <.001 |
| No. of days without dose,d mean ± SD | 8 (10) | 38 (27) | 1.08 (1.04–1.12) | <.001 |
| No. of TIs,e mean ± SD | 3.2 (5.1) | 11.7 (5.8) | 1.24 (1.09–1.39) | .001 |
| Duration of longer TIs, mean ± SD, days | 4.9 (5.6) | 20.0 (14.9) | 1.22 (1.10–1.36) | .002 |
NOTE. CI, confidence interval; OR, odds ratio; SD, standard deviation; TIs, treatment interruptions.
HIV RNA level of <400 copies/mL at the end of adherence monitoring; results were similar with an HIV RNA level of <50 copies/mL.
Computed by use of logistic regression. An OR of >1 means an increased probability of HIV replication.
OR and 95% CI are provided for a 10% increase in adherence rate.
Defined as discontinuation of drugs for >24 h and <48 h.
Defined as discontinuation of drugs for >48 h.
Figure 2.
Relationship between average percent adherence to boosted protease inhibitor (PI) therapy and longer treatment interruption among subjects with (red) and without (green and blue) HIV replication. The red lines on the X-axis and the Y-axis correspond to the median average adherence rate and the duration of treatment interruption, respectively, among subjects with a subsequent HIV RNA level (ie, viral load [VL]) of ≥400 copies/mL. The blue lines on the X-axis and the Y-axis correspond to the median average adherence rate and the duration of treatment interruption, respectively, among subjects with a subsequent VL of <400 copies/mL and low-to-moderate adherence (<80%). The difference in average percent adherence, but not treatment interruption duration, is statistically significant between those with a VL of ≥400 copies/mL and those with a VL of <400 copies/mL.
Among subjects with low-to-moderate (<80%) adherence, the median difference in longest treatment interruption between those with and without HIV replication was 4.7 days (16.3 days vs 11.6 days, respectively), which was statistically nonsignificant (P = .22), whereas the median difference in average percent adherence between those with and without HIV replication was 36.5% (32.1% vs 68.6%, respectively), which was statistically significant (P < .001) (Figure 2).
Discussion
Consistent with several previous studies, average percent adherence to boosted PIs was closely associated with viral suppression [11, 12]. Greater than 95% adherence conferred 100% sensitivity to predict HIV RNA suppression <50 copies/mL. However, viral suppression was also common among patients with >70% average adherence: all except 1 (98%) had an HIV RNA level <400 copies/mL, and all except 4 (92%) had an HIV RNA level <50 copies/mL. Consequently, this implies that <95% adherence to boosted PIs, as opposed to unboosted PIs, can lead to viral suppression [13]. When the analysis was restricted to patients with low-to-moderate adherence (ie, <80%), average percent adherence remained statistically associated with HIV RNA replication <400 copies/mL. In contrast to Shuter et al [14], our data suggest that the level of adherence is closely associated with HIV replication, even at an adherence rate of <80%. Treatment interruptions also predicted virologic replication in a univariate logistic regression model; however, treatment interruptions were no longer statistically significantly associated with virologic replication in the multivariate logistic regression analysis, adjusting for average adherence, or among patients with low-to-moderate adherence.
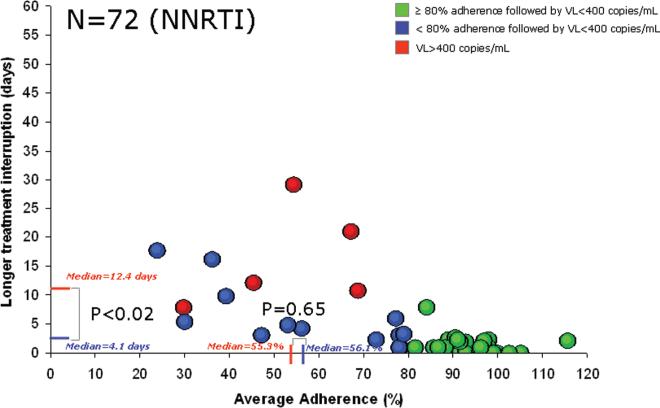
As adherence rates decrease, different patterns of missed doses are possible, as shown in Figure 2. Missed doses can either occur as sustained interruptions or as more regularly interspersed missed doses. Unlike NNRTI-based regimens (Figure 3, adapted from Parienti et al [10]), the characteristics of treatment interruption did not emerge as major risk factors explaining virologic outcome with ritonavir-boosted PI-based regimens.
Figure 3.
Relationship between average percent adherence to nonnucleoside reverse-transcriptase inhibitor (NNRTI) and longer treatment interruption among subjects with (red) and without (green and blue) HIV replication. The red lines on the X-axis and the Y-axis correspond to the median average percent adherence rate and duration of treatment interruption, respectively, among subjects with a subsequent HIV RNA level (ie, viral load [VL]) of ≥400 copies/mL. The blue lines on the X-axis and the Y-axis correspond to the median average adherence percent rate and the duration of treatment interruption, respectively, among subjects with a subsequent VL of <400 copies/mL and low-to-moderate adherence (<80%). The difference in duration of treatment interruption, but not average percent adherence rate, is statistically significant between those with a VL of ≥400 copies/mL and those with a VL of <400 copies/mL. This figure was adapted from Figure 2 in Parienti et al [10].
Although the assessment of MEMS-defined adherence patterns was precise, both the sample size and the number of events were small. In addition, we did not measure drug resistance, and the risk of resistance after the initial virologic failure of ritonavir-boosted PI-based therapy is predictably low [11, 15]. Finally, differences in study design may limit the comparison between current and previous work [10]. We believe that the biological plausibility of our observational findings compensates for these limitations. Boosted PIs have a short half-life. For this reason, regularly interspersed missed doses may pose more problems for ritonavir-boosted PIs than for NNRTI-based regimens [10, 16]. On the other hand, the emergence of drug resistance in the presence of suboptimal plasma drug levels is unlikely given the short-half life of the drug [9], the poor viral fitness [17] of the mutants, and the high genetic barrier of boosted PIs. These results may not extrapolate to darunavir-ritonavir–based regimens, because of the unique pharmacodynamics conferred by a very long fixation to the protease enzyme [18]. Lopinavir-ritonavir was more sensitive than darunavir-ritonavir to a suboptimal average adherence of <95% in term of HIV RNA replication [12]. Finally, patterns of adherence for all the components of combination antiretroviral therapy, including the boost, need to be considered [19]. Because only 1 medication was monitored, we were unable to assess the impact of differential adherence, which is impossible with regimens composed of a single fixed-dose combination dosage form.
Our results, combined with previous studies [10, 20], may have implications for the choice of antiretroviral therapy among patients at risk of treatment interruptions [21, 22]. Of note, drug supply shortage or difficulties in transportation are the most frequent reasons for treatment failure in developing countries [20]. NNRTI-based regimens are the most commonly used regimen class in resource-limited settings. We know that treatment failure, as a consequence of NNRTI interruptions, is associated with HIV RNA rebound and drug resistance [4, 23]. Because, HIV RNA monitoring and genotyping are not routinely available, pharmacy refill monitoring has been proposed as a substitute for viral load monitoring [24]. Such monitoring may be more effective for PI regimens than NNRTI regimens because it does not capture interruption patterns. The use of boosted PIs may confer several advantages in this setting, including improved performance of pharmacy refill monitoring for virologic suppression and a reduction in the impact of interrupted therapy, which is a common form of missing doses in resource-limited settings.
In summary, maximal average percent adherence confers the highest probability of sustained viral suppression in HIV-infected patients treated with boosted PI-based regimens. In contrast to NNRTI-regimens [10], ritonavir-boosted PI regimens do not appear to be specifically vulnerable to treatment interruptions.
Acknowledgments
We thank Véronique Ronat, Isabelle Suaud, Anne-Catherine Cordier, and Pascale Goubin for their help in data collection and Fabien Chaillot for his expert administrative and regulatory support. We are grateful for the scientific collaboration of Isabelle Cohen-Codar and Richard A. Rhode. The ESPOIR cohort was supported by an Abbott Laboratories unrestricted grant (to Caen Côte de Nacre University hospital). The REACH cohort was supported by the National Institute of Mental Health (grant RO-54907) and the National Institute on Alcohol Abuse and Alcoholism (grant K-24 015287). We also acknowledge helpful suggestions regarding wording of the summary findings by 2 anonymous reviewers.
Footnotes
The sponsors of this study had no role in study design, data collection, data analysis, data interpretation, or decision to publish. Comments were received from Abbott Laboratories, but the authors were not obliged to incorporate these.
Presented in part: 4th International Conference on HIV Treatment Adherence, Miami, Florida, 5–7 April 2009 (abstract 151).
Potential conflicts of interest. J.-J.P. reports that he has received travel grants, honoraria for presentation at workshops, and consultancy honoraria from Abbott, Boehringer Ingelheim, and Bristol-Myers Squibb; Y.Y. reports that he has received travel grants, honoraria for presentation at workshops, and consultancy honoraria from Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Merck, Pfizer, Roche, and Tibotec. All other authors: no conflicts.
References
- 1.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 4.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 5.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor–based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53:696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 7.Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8:282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 8.Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24:1263–1268. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 9.Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23:1035–1046. doi: 10.1097/QAD.0b013e32832ba8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King MS, Brun SC, Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naive, HIV-1–infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191:2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 12.Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 13.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 15.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 16.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 18.King NM, Prabu-Jeyabalan M, Nalivaika EA, Wigerinck P, de Bethune MP, Schiffer CA. Structural and thermodynamic basis for the binding of TMC114, a next-generation human immunodeficiency virus type 1 protease inhibitor. J Virol. 2004;78:12012–12021. doi: 10.1128/JVI.78.21.12012-12021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner EM, Sharma S, Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22:75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 21.Das-Douglas M, Riley ED, Ragland K, et al. Implementation of the Medicare Part D prescription drug benefit is associated with antiretroviral therapy interruptions. AIDS Behav. 2009;13:1–9. doi: 10.1007/s10461-008-9401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murri R, Guaraldi G, Lupoli P, et al. Rate and predictors of self-chosen drug discontinuations in highly active antiretroviral therapy-treated HIV-positive individuals. AIDS Patient Care STDS. 2009;23:35–39. doi: 10.1089/apc.2007.0248. [DOI] [PubMed] [Google Scholar]
- 23.Fox Z, Phillips A, Cohen C, et al. Viral resuppression and detection of drug resistance following interruption of a suppressive non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2008;22:2279–2289. doi: 10.1097/QAD.0b013e328311d16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]