Abstract
Mechanisms leading to the observed immune dysregulation in HIV-1 infection are not well understood. HIV-specific IL-10-positive CD8+ T cells are increased in advanced HIV disease. We have previously reported that Gag-specific IL-10-positive CD8+ T cells suppressed cytolysis. In this study we describe the suppressive effect of Nef-specific IL-10-positive CD8+ T cells. Interestingly, simultaneous removal of both Gag- and Nef-specific IL-10-positive CD8+ T cells led to higher HIV-specific cytolysis compared with the removal of Nef-specific IL-10-positive CD8+ T cells alone. We also examined the level of programmed cell death-1 (PD-1) as a measure of immune dysfunction in association with IL-10-positive suppressor CD8+ T cells. The level of PD-1 expression on CD107-positive effector CD8+ T cells was significantly increased when IL-10-positive suppressor CD8+ T cells were present (p < 0.05). Our results suggest that IL-10-positive suppressor CD8+ T cells contribute to the immune dysfunction observed in advanced HIV infection and that the concomitant presence of multiple IL-10-positive CD8+ T cell populations may have an additive suppressive effect.
T cells with regulatory functions are implicated in modulating immune responses to chronic viral infections, including HIV (1). CD4+ regulatory T cells inhibit both CD4+ and CD8+ T cell-mediated HIV-specific immune responses in vitro and may have HIV specificity (2–4). Suppressor CD8+ T cells have been shown to inhibit both CD4+ and CD8+ T cell effector functions (5, 6), although the mechanism of this inhibition is not well defined. We have previously described the presence of suppressor Gag-specific IL-10-positive CD8+ T cells that suppressed degranulation of HIV-specific CTL (7). We postulated that suppressor IL-10-positive CD8+ T cells specific to other HIV Ags also exist. In this study, we examined the suppressive function of Nef-specific IL-10-positive CD8+ T cells and investigated the concomitant suppressive effect of two IL-10-positive CD8+ T cell populations on HIV-specific effector function.
The inhibitory receptor programmed cell death-1 (PD-1)3 is highly expressed on exhausted T cells during chronic viral infection, and blockade of the PD-1–PD-1 ligand (PDL-1) pathway can restore effector functions (8). Increased PD-1 expression on CD8+ T cells is associated with immune dysfunction and HIV disease progression (9–12). PD-1 ligation has been suggested as a suppressor mechanism of regulatory T cells (13, 14). Therefore, we analyzed the relationship between PD-1 expression and the presence of suppressor HIV-specific IL-10-positive CD8+ T cells.
Materials and Methods
Study subjects and samples
HIV-positive volunteers (n = 21) were recruited from the Research in Access to Care in the Homeless (REACH) cohort in San Francisco as previously described (7, 15). Demographic information and CD4+ T cell count were obtained at the time of enrollment and blood draw. Institutional Review Board approvals were obtained from the California Department of Public Health and University of California–San Francisco Committee on Human Research, and all study participants gave written informed consent. None of the study participants had received antiretroviral therapy for at least 6 mo. HIV RNA level was determined from plasma using the Roche Amplicor 1.5 (Roche Diagnostic Systems), as per manufacturer's recommendations. PBMC were separated and cryopreserved in liquid nitrogen until assay time.
Antigens
Peptides corresponding to the sequences of clade B consensus sequences of HIV-1 for Gag and Nef (http://www.hiv.lanl.gov/content/sequence/HIV/CONSENSUS/Consensus.html) were synthesized as 15 aa overlapping by 11 aa (Mitochor Mimotopes). Synthetic peptides for Gag (total = 123) and Nef (total = 49) used for all T cell assays were pooled into one single pool of peptides with final concentration of 1 μg/ml per peptide (16). A single pool of overlapping peptides corresponding to the amino acid sequence of the PP65 protein (BD Biosciences) was used to detect human CMV-specific response (17, 18).
Flow-based intracellular IL-10 and TGF-β staining
Detection of HIV-specific IL-10 and TGF-β production was performed using PBMC (1 × 106) incubated with Gag or Nef peptide pools for 2 h at 37°C in 5% CO2 in the presence of costimulatory anti-CD49d and anti-CD28 (1 μg/ml, BD Biosciences), followed by Golgi-stop (BD Pharmingen) for 12–14 h as previously described (19, 20). LPS (1 ng/ml; Sigma-Aldrich) and PMA (50 ng/ml) and ionomycin (1 μg/ml) (Sigma-Aldrich) were used as positive controls for IL-10 and TGF-β production, respectively. Media alone without Ag stimulation was used as negative control. PBMC were stained with an amine-reactive viability dye as a dead cell exclusion marker (Molecular Probes) (21). Cells were stained with the following Abs: CD3 PerCP Cy5.5, CD4 PE Cy7, and anti-CD8 Pacific blue (BD Pharmingen). PBMC were then permeabilized and stained with anti-TGF-β PE (Biotest Diagnostics) and anti-IL-10 APC (BD Pharmingen) and analyzed by flow cytometry as we have previously described (22). A minimum of 30,000 CD3+ cells per sample were acquired using a six-color flow cytometer (BD LSR II, BD Biosciences), and analysis was performed by FlowJo software (Tree Star). Results were expressed as percentage IL-10- or TGF-β-positive CD8+ T cells (% positive = % Agspecific – % negative control). Responses ≥0.1% and 2× the background were considered positive. All volunteers demonstrated significant IL-10 and TGF-β production following LPS and PMA/ionomycin stimulation. Background expression was <0.05%.
MACS cytokine secretion/separation assay
IL-10-positive cells were isolated using the MACS cytokine secretion/separation kit following the manufacturer's protocol (Miltenyi Biotec) (7). Briefly, PBMC were stimulated with Nef B or both Gag B and Nef B peptides for 6 h and then labeled with anti-IL-10 PE detection surface Ab followed by magnetic labeling with anti-PE microbeads. IL-10-positive cells were isolated by magnetic separation using the MACS columns. Purity of the IL-10-negative PBMC was consistently <99%. Unfractionated and eluted IL-10-negative PBMC were stimulated for an additional 6 h with Nef B peptides in a CD107a degranulation assay as described below.
Flow-based CD107a degranulation assay
Degranulation assay was performed as previously described (23). Cytotoxic CD8+ T cell function has been shown to correlate directly with T cell degranulation, which is a prerequisite process of perforin-granzyme-mediated lytic function (24), and can be measured by increased expression of surface CD107 (25). Briefly, unfractionated PBMC or IL-10-negative PBMC (1 × 106) were incubated with 1 μg/ml of anti-CD49d and anti-CD28 (BD Biosciences), anti-CD107a (BD Biosciences), and HIV or human CMV peptide pools in a 0.2 ml final volume for 6 h in the presence of Golgi-stop. Staphylococcus enterotoxin B (1 μg/ml, Sigma-Aldrich) and media alone were used as positive and negative controls, respectively. PBMC were stained with an amine-reactive viability dye as a dead cell exclusion marker (Molecular Probes) (21). Cells were then stained with the following Abs: CD4 PE Cy7, CD3 PerCP Cy5.5, PD-1 allophycocyanin, and CD8 Pacific blue (BD Pharmingen). A minimum of 30,000 CD3+ cells per sample were acquired using a six-color flow cytometer (BD LSR II) and analysis was performed by FlowJo software. Results were expressed as percentage CD107a-positive CD8+ T cells (% positive = % Ag-specific – % negative control). Responses ≥0.1% and 2× the background were considered positive. All volunteers demonstrated significant CD107a expression following Staphylococcus enterotoxin B stimulation. Background expression was ≤0.1%.
Statistical analysis
Groups were compared using the Mann-Whitney U test and analysis was performed with PRISM software version 4.02 (GraphPad).
Results
Removal of Nef-specific IL-10-positive CD8+ T cells is associated with increased HIV-specific cytolysis
We have previously observed the presence of Gag-specific IL-10-positive CD8+ T cells that directly suppress CD8 cytolysis (7). In this study, we assessed whether IL-10-positive CD8+ T cells specific to other HIV Ags may also exist. We measured the frequency of Nef-specific, as well as Gag-specific, IL-10-positive CD8+ T cells in 21 HIV-1-infected volunteers (20, 22). Volunteers had a median age of 45 years (range, 31–61), CD4 T cell count of 141.5 cells/mm3 (range, 11–233), and median HIV plasma RNA of 130,000 copies/ml (range, 19,000–780,000). Ten volunteers demonstrated significant Gag- and Nef-specific IL-10-positive CD8+ T cell responses (median = 0.2% (range, 0.1–0.4%) and 0.17% (range, 0.1–0.4%) for Gag and Nef, respectively). Differences in the frequencies of Gag- and Nef-specific IL-10-positive CD8+ T cell responses were not significant (p = 0.14). No Gag- or Nef-specific IL-10-positive CD4+ T cell responses were detected (data not shown). No significant differences in age, CD4 count, or plasma HIV RNA were observed between volunteers with or without HIV-specific IL-10-positive CD8+ T cell responses (p = 0.48, p = 0.97, and p = 0.54 for age, CD4 count, and plasma HIV RNA, respectively; data not shown).
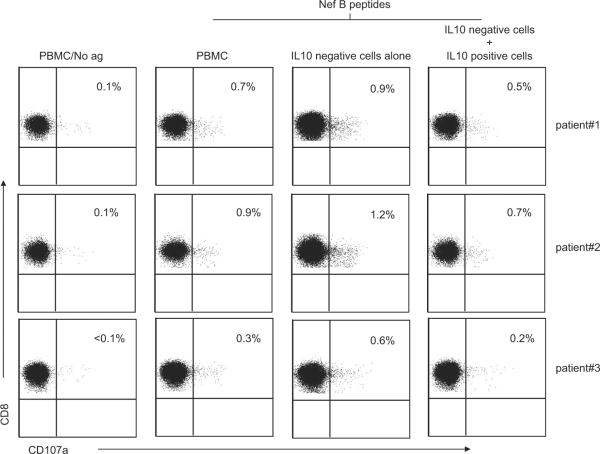
Next, we examined the effect of in vitro removal of the Nef-specific IL-10-positive CD8+ T cells from three HIV-positive volunteers with detectable Nef-specific IL-10-positive CD8+ T cell responses (data not shown). Eluted IL-10-negative PBMC were evaluated for Nefspecific cytolysis using the CD107a degranulation assay (7). Removal of the IL-10-positive CD8+ T cells resulted in a significantly increased frequency of Nef-specific CD107a-positive CD8+ T cells compared with unfractionated PBMC (Fig. 1).
FIGURE 1.
The removal of Nefspecific IL-10-positive CD8+ T cells is associated with increased HIV-specific cytolysis. PBMC were stimulated with Nef B peptides and then IL-10-positive cells were isolated. Eluted IL-10-negative PBMC were stimulated for an additional 6 h with Nef B peptides in the presence of anti-CD107a FITC and then stained with anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, and anti-CD8 Pacific blue and analyzed by flow cytometry. Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of CD107a were determined. The results were expressed as the percentage of Nef-specific CD8 T cells expressing CD107a in the presence or absence of IL-10-positive cells after subtraction of the background values. Plots are from three independent experiments yielding similar results.
The addition of these autologous Nef-specific IL-10-positive CD8+ T cells to the eluted IL-10-negative PBMC significantly lowered the frequency of CD107a-positive CD8+ T cells compared with IL-10-negative PBMC alone, and reduced the CD107a-positive CD8+ T cell frequency to the level observed in unfractionated PBMC. These results suggest that distinct populations of HIV-specific IL-10-positive CD8+ T cells display similar suppressive function.
HIV-specific IL-10-positive CD8+ T cells do not produce TGF-β
CD8+ T cells have been shown to express high IL-10 and TGF-β levels in HIV infection (22, 26, 27). We determined whether the HIV-specific IL-10-positive CD8+ T cells produce TGF-β. PBMC were stimulated with Gag or Nef peptides and the extent of IL-10 and TGF-β production by the HIV-specific CD8+ T cells was assessed from 21 HIV-positive volunteers. Representative plots of the frequency of the Ag-specific IL-10- and TGF-β-positive CD8+ T cells are shown in Fig. 2. Seven and nine volunteers demonstrated significant Gag- and Nef-specific TGF-β-positive CD8+ T cell responses, respectively (median = 0.3% (range, 0.21–0.67%) and 0.16% (range = 0.1–0.4%) for Gag and Nef, respectively). No TGF-β production was detected in HIV-specific IL-10-positive CD8+ T cells. These results suggest that the HIV-specific IL-10-positive CD8+ T cells have a distinct cytokine production profile compared with other non-HIV suppressor CD8+ T cells.
FIGURE 2.
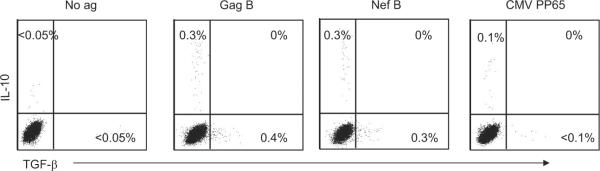
HIV-specific IL-10-positive CD8+ T cells do not produce TGF-β. PBMC were stimulated with Ag and then stained with anti-TGF-β PE, anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, anti-IL-10 allophycocyanin, and anti-CD8 Pacific blue and analyzed by flow cytometry. Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of IL-10- and TGF-β-positive CD8+ T cells were determined. Representative plots of Ag-specific CD8+ T cells expressing IL-10 and TGF-β after subtraction of the background values.
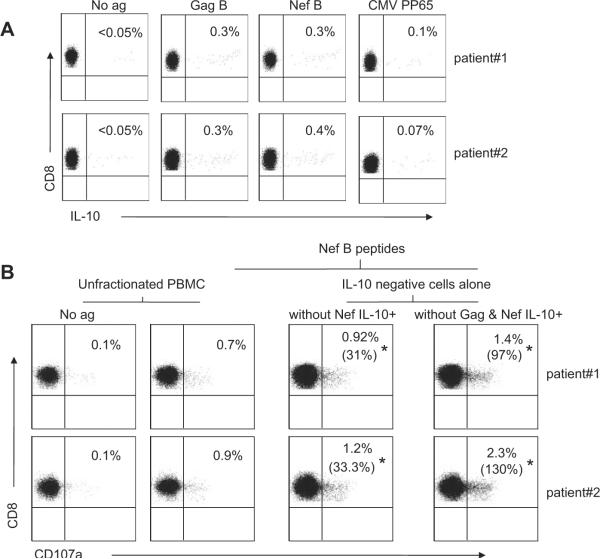
Simultaneous removal of two HIV-specific IL-10-positive CD8+ T cell populations is associated with an additive increase in HIV-specific cytolysis
Next, we examined the effect of simultaneous removal of both Gag- and Nef-specific IL-10-positive CD8+ T cells on HIV-specific cytolysis compared with the removal of Nef-specific IL-10-positive CD8+ T cells alone. Nef-specific cytolysis using the CD107a degranulation assay was measured in the eluted IL-10-negative PBMC from two HIV-positive volunteers (Fig. 3A). Simultaneous removal of the Gag- and Nef-specific IL-10-positive CD8+ T cells resulted in significantly higher frequency of Nef-specific CD107a-positive CD8+ T cells compared with the removal of Nef-specific IL-10-positive CD8+ T cells alone (Fig. 3B). The addition of autologous IL-10-positive CD8+ T cells, either Gag- and Nef-specific or Nef-specific alone, to the eluted IL-10-negative PBMC significantly reduced the frequency of CD107a-positive CD8+ T cells to a comparable level of those observed in unfractionated PBMC stimulated with Nef or with both Gag and Nef peptides (data not shown). These results suggest that the Gag-specific IL-10-positive CD8+ T cells augmented the suppressive effect of the Nef-specific IL-10-positive CD8+ T cells.
FIGURE 3.
Simultaneous removal of multiple HIV-specific IL-10-positive CD8+ T cell populations is associated with an additive increase in HIV-specific cytolysis. A, PBMC were stimulated with Ag and then stained with anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, anti-IL-10 allophycocyanin, and anti-CD8 Pacific blue and analyzed by flow cytometry. Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of CD8+ T cells were determined. The results were expressed as Ag-specific CD8+ T cells expressing IL-10 after subtraction of the background values. B, PBMC were stimulated with Nef B peptides alone or with Gag B and Nef B peptides and then IL-10-positive cells were isolated. Eluted IL-10-negative PBMC were stimulated for an additional 6 h with Nef B peptides in the presence of anti-CD107a FITC and then stained with anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, and anti-CD8 Pacific blue and analyzed by flow cytometry. Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of CD107a were determined. The results were expressed as the percentage of Nef-specific CD8 T cells expressing CD107a after subtraction of the background values. * Percentage values in parentheses represent the percentage increase in CD107a expression compared with the unfractionated PBMC stimulated with Nef peptides alone or with both Gag and Nef peptides. Plots are from two independent experiments yielding similar results.
Presence of suppressor HIV-specific IL-10-positive CD8+ T cells is associated with increased number of HIV-specific PD-1-positive CD8+ T cells
Increased PD-1 expression on CD8+ T cells is associated with T cell dysfunction and HIV disease progression (9–12). We have previously observed the association of HIV-specific IL-10-positive CD8+ T cells with advanced disease (22). We postulated that the presence of suppressor HIV-specific IL-10-positive CD8+ T cells is associated with increased PD-1 expression on HIV-specific effector CD8+ T cells. We assessed degranulation and PD-1 expression on Gag- and Nef-specific CD8+ T cells. All volunteers demonstrated significant Gag-specific CD107a responses, while Nef-specific CD107a responses were detected in 20 of 21 volunteers. The frequency of CD107a-positive or PD-1-positive CD8+ T cells after Gag- or Nef-specific stimulation between volunteers with detectable IL-10-positive CD8+ T cells compared with volunteers with undetectable IL-10-positive CD8+ T cells was not significantly different (p = 0.21 and p = 0.34 for CD107a and PD-1 after Gag-specific stimulation, respectively; p = 0.06 and p = 0.85 for CD107a and PD-1 after Nef-specific stimulation, respectively; data not shown).
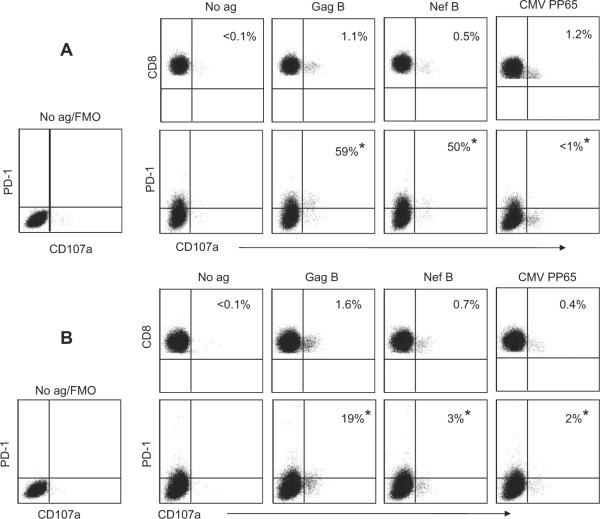
Next, we examined the effect of the presence of IL-10-positive CD8+ T cells on the number of HIV-specific CD107a-positive CD8+ T cells that expressed PD-1. Representative plots of the frequencies of the Ag-specific CD8+ T cells expressing CD107a and PD-1 from two volunteers with detectable and undetectable HIV-specific IL-10-positive CD8+ T cells are shown in Fig. 4, A and B, respectively. The presence of HIV-specific IL-10-positive CD8+ T cells was associated with significantly higher numbers of PD-1+CD107a-positive Gag- (Fig. 5A), and Nef- (Fig. 5B) specific CD8+ T cells ( p = 0.01 and p = 0.02 for Gag and Nef, respectively).
FIGURE 4.
PD-1 expression on HIV-specific CD107a-positive CD8+ T cells. PBMC were stimulated with HIV or CMV peptides in the presence of anti-CD107a FITC and then stained with anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, anti-PD-1 allophycocyanin, and anti-CD8 Pacific blue and analyzed by flow cytometry. Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of CD107a were determined and the extent of PD-1 expression was examined. Gating on PD-1-positive cells was performed using the fluorescence-minus-one (FMO) control for PD-1. Representative plots of the frequencies of the Ag-specific CD8 T cells expressing CD107a and PD-1 from two volunteers with (A) detectable or (B) undetectable HIV-specific IL-10-positive CD8+ T cells. The values marked with an asterisk (*) represent the fraction of CD107a-positive cells that express PD-1 over the total number of CD107a-positive cells (equivalent to 100%).
FIGURE 5.
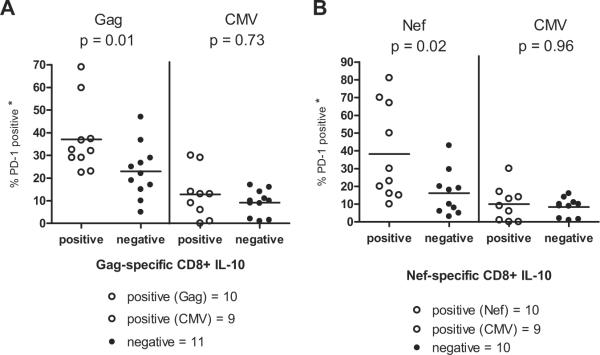
The presence of suppressor HIV-specific IL-10-positive CD8+ T cells is associated with an increased number of HIV-specific PD-1-positive CD8+ T cells. PBMC were stimulated with HIV or CMV peptides in a CD107a degranulation assay and the extent of PD-1 expression on the CD107a-positive CD3+CD8+ T cells was determined. The values marked with an asterisk (*) represent the fraction of CD107a-positive cells that express PD-1 over the total number of CD107a-positive cells (equivalent to 100%). Data are from individuals with significant (A) Gag B or (B) Nef B CD107a responses only. Bars represent median values. Differences in the frequency of Gag- and Nef-, but not of CMV-, specific PD-1+CD107a+ CD8+ T cells between IL-10-positive and IL-10-negative volunteers were statistically significant (p = 0.01, p = 0.03, and p < 0.05 for Gag, Nef, and CMV, respectively).
To determine whether the presence of IL-10-positive HIV-specific CD8+ T cells was associated with altered PD-1 expression on CD8+ T cells stimulated with non-HIV Ags, we analyzed human CMV-specific responses in the same study population. PP65-specific CD107a-positive responses were detected in 9 of 10 volunteers with HIV-specific IL-10-positive CD8+ T cell responses and in all volunteers without IL-10-positive CD8+ T cell responses (Fig. 5). No significant differences in the number of PD-1+CD107a-positive PP65-specific CD8+ T cells were observed between volunteers with or without Gag- or Nef-specific IL-10-positive CD8+ T cell responses (p = 0.73 and p = 0.96 for Gag and Nef, respectively).
Blocking of PD-1 does not prevent suppression by the IL-10-positive CD8+ T cells
Blocking PD-1 engagement to its ligand (PDL-1) enhances proliferation and cytokine production by the HIV-specific CD8+ T cells (9–11). We have previously demonstrated that HIV-specific IL-10-positive CD8+ T cells suppress HIV-specific cytolysis by direct cell-cell contact (7). To determine whether PD-1/PDL-1 engagement is the mechanism by which HIV-specific IL-10-positive CD8+ T cells mediated the suppression of HIV-specific responses, we performed blocking experiments using anti-PD-1 Ab (or iso-type control) on two HIV-positive volunteers with demonstrated significant Gag- (patient no. 1) and Nef- (patient no. 2) specific IL-10-positive CD8+ T cell responses (Fig. 6). Coculture of IL-10-positive CD8+ T cells and IL-10-negative PBMC in the same well led to a strong suppression of HIV-specific CD107a-positive CD8+ T cells compared with IL-10-negative PBMC alone. These results suggest that blocking of PD-1 does not prevent suppression by the IL-10-positive CD8+ T cells.
FIGURE 6.
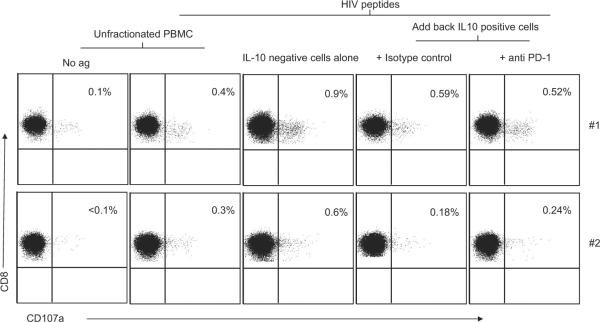
Blocking of PD-1 does not prevent suppression by the IL-10-positive CD8+ T cells. PBMC were stimulated with Gag B (patient no. 1) or Nef B (patient no. 2) peptides and then IL-10-positive cells were isolated. Eluted IL-10-negative PBMC were stimulated for an additional 6 h with Gag B (patient no. 1) or Nef B (patient no. 2) peptides and anti-CD107a FITC and then stained with anti-CD3 PerCP Cy5.5, anti-CD4 PE Cy7, and anti-CD8 Pacific blue and analyzed by flow cytometry. IL-10-positive cells were added in the presence of anti-PD-1 (or isotype control). Samples were first gated on the CD3+CD8+ lymphocyte population and then the percentages of CD107a-positive cells were determined. The results were expressed as the percentage of Gag- or Nef-specific CD8+ T cells expressing CD107a after subtraction of the background values. Plots are from two independent experiments yielding similar results.
Discussion
Regulatory T cells modulate the immune responses in chronic virus infections such as HIV (1). In this study we describe distinct populations of suppressor IL-10-positive CD8+ T cells that exhibit specificities to different HIV Ags. We have shown previously that Gag-specific, IL-10-positive CD8+ T cells impair the ability of both HIV- and non-HIV-specific CTL by suppressing CD8+ T cell degranulation (7). Our current data suggest that the concomitant presence of Gag- and Nef-specific IL-10-positive CD8+ T cells augmented the in vitro suppressive effect on effector CD8+ T cells. IL-10-positive CD8+ T cells specific to additional HIV Ags likely exist. Our findings indicate that IL-10-positive CD8+ T cells exert a suppressive effect that is additive, although the specific contributions of each population are currently unknown. The significance of Ag specificity of the suppressor IL-10-positive CD8+ T cells is another important aspect to address. It is intriguing to postulate that the level and method of Ag presentation involved in the induction and maintenance of these IL-10-positive CD8+ T cells may share similarities to those required to generate effector CD8+ T cells. We have demonstrated previously that the suppression by Gag-specific IL-10-positive CD8+ T cells appeared to be mediated by direct cell-cell contact (7). However, our current observation that multiple HIV Ag-specific IL-10-positive CD8+ T cells inhibit HIV-specific effector CD8+ T cells supports a mechanism of HIV Ag-specific induction of suppressor IL-10-positive CD8+ T cells. Additionally, our present data reintroduce the possibility of concomitant direct cell-cell contact as well as indirect inhibition. These findings further indicate that the immune regulations in HIV infection are complex and introduce new insights for immunotherapeutic approaches.
Previous studies have shown that TGF-β-producing HIV-specific CD8+ T cells significantly reduce IFN-γ responses to HIV Ags (26), up-regulate CTLA-4 expression (28–30), and inhibit T cell proliferation and IL-2 production (31). We found no significant TGF-β production by the HIV-specific IL-10-positive CD8+ T cells, suggesting that the suppressor IL-10-positive CD8+ T cell population is likely distinct from the previously described effector Tc2 cells (reviewed in Ref. (32)). Tc2 cells were shown to be cytotoxic via the perforin and Fas pathways and produce mainly IL-4, IL-5, IL-10, and TGF-β (33). However, our results do not rule out a concomitant suppressive effect in vivo of TGF-β-positive CD8+ T cells on HIV-specific cytolysis. TGF-β is also a key regulator of the signaling pathways that maintain the suppressive function of other regulatory cells, and signaling through the TGF-β receptor may still be involved in the stimulation of the IL-10-positive CD8+ T cells (34).
We have demonstrated previously that the suppressor IL-10-positive CD8+ T cells are Foxp3 negative thus have a distinct regulatory T cell immunophenotypic profile compared with regulatory CD4+ T cells (7). In contrast to regulatory CD4+ T cells, no single surface Ag marker has been identified that is exclusively expressed by suppressor CD8+ T cells (35–38). At present, the use of functional characterization by IL-10 production is required to discriminate suppressor from effector CD8+ T cells in HIV infection. Our findings thus underscore the importance of studies specifically aimed at identifying specific phenotype and functions involved in HIV immune suppression.
PD-1 is highly expressed on HIV-specific CD8+ T cells during chronic HIV infection and indicates functional exhaustion (9–12). We found that increased expression of PD-1 on HIV-specific CD107a-positive CD8+ T cells was associated with the presence of suppressor IL-10-positive CD8+ T cells. Nevertheless, the presence of suppressor CD8+ T cells might serve as a useful marker to indicate the degree of CD8+ T cell dysfunction and disease severity. Blockade of the PD-1/PDL-1 pathway did not prevent suppression, suggesting that PD-1 engagement is not a mechanism utilized by the IL-10-positive CD8+ T cells. Other inhibitory mechanisms not yet explored include direct binding of inhibitory cell-surface molecules such as CTLA-4 to costimulatory molecules on effector T cells (39, 40). Recent studies have shown that up-regulation of CTLA-4 by HIV-specific CD4+ T cells is correlated with PD-1 expression on HIV-specific CD4+ T cells and with disease progression, while in vitro blockade of CTLA-4 augmented HIV-specific T cell function (41). Indirect inhibitory mechanisms may also be involved such as up-regulation of the inhibitory receptors Ig-like transcript (ILT)3 and ILT4 on the surface of APCs, rendering them tolerogenic (37, 42, 43). Previous studies have demonstrated up-regulation of ILT4 expression in HIV-infected individuals (44). We propose a model whereby chronic HIV infection leads to the induction of multiple populations of suppressor cells that include HIV-specific IL-10-positive CD8+ T cells that contribute to the progressive functional defect of effector CD8+ T cells. Determining the precise regulatory mechanisms by which these IL-10-positive CD8+ T cells contribute to immunosuppression may guide new preventative and therapeutic strategies.
Footnotes
This work was supported by National Institutes of Health Grants AI43885, MH54907, and AI71772.
Abbreviations used in this paper: PD-1, programmed cell death-1; ILT, Ig-like transcript; PDL-1, PD-1 ligand.
Disclosures The authors have no financial conflicts of interest.
References
- 1.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol. Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 2.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 3.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, Planta M, McGlaughlin M, Jackson R, Ziegler SF, Fauci AS. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur. J. Immunol. 2005;35:2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 6.Filaci G, Fravega M, Fenoglio D, Rizzi M, Negrini S, Viggiani R, Indiveri F. Non-antigen specific CD8+ T suppressor lymphocytes. Clin. Exp. Med. 2004;4:86–92. doi: 10.1007/s10238-004-0042-3. [DOI] [PubMed] [Google Scholar]
- 7.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J. Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 10.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 11.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 12.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 14.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur. J. Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, Bangsberg DR. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin. Infect. Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 16.McEvers K, Elrefaei M, Norris P, Deeks S, Martin J, Lu Y, Cao H. Modified anthrax fusion proteins deliver HIV antigens through MHC class I and II pathways. Vaccine. 2005;23:4128–4135. doi: 10.1016/j.vaccine.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 18.Elrefaei M, El-Sheikh N, Kamal K, Cao H. HCV-specific CD27−CD28− memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology. 2003;110:513–518. doi: 10.1111/j.1365-2567.2003.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor β that can suppress HCV-specific T-cell responses. J. Virol. 2007;81:5882–5892. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElroy MD, Elrefaei M, Jones N, Ssali F, Mugyenyi P, Barugahare B, Cao H. Coinfection with Schistosoma mansoni is associated with decreased HIV-specific cytolysis and increased IL-10 production. J. Immunol. 2005;174:5119–5123. doi: 10.4049/jimmunol.174.8.5119. [DOI] [PubMed] [Google Scholar]
- 21.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Elrefaei M, Barugahare B, Ssali F, Mugyenyi P, Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J. Immunol. 2006;176:1274–1280. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 24.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 25.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 26.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-β1-producing immunoregulatory CD8+ T cells. J. Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 27.Zanussi S, Simonelli C, D'Andrea M, Caffau C, Clerici M, Tirelli U, DePaoli P. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin. Exp. Immunol. 1996;105:220–224. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HB, Paik DJ, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-β-costimulated CD4+CD25− T cells. Int. Immunol. 2004;16:1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 30.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-β-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 31.Sung JL, Lin JT, Gorham JD. CD28 co-stimulation regulates the effect of transforming growth factor-β1 on the proliferation of naive CD4+ T cells. Int. Immunopharmacol. 2003;3:233–245. doi: 10.1016/S1567-5769(02)00276-X. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin. Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 33.Vukmanovic-Stejic M, Vyas B, Gorak-Stolinska P, Noble A, Kemeny DM. Human Tc1 and Tc2/Tc0 CD8 T-cell clones display distinct cell surface and functional phenotypes. Blood. 2000;95:231–240. [PubMed] [Google Scholar]
- 34.Rich S, Seelig M, Lee HM, Lin J. Transforming growth factor beta 1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+ T cells. J. Immunol. 1995;155:609–618. [PubMed] [Google Scholar]
- 35.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 36.Scotto L, Naiyer AJ, Galluzzo S, Rossi P, Manavalan JS, Kim-Schulze S, Fang J, Favera RD, Cortesini R, Suciu-Foca N. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− T suppressor cells. Hum. Immunol. 2004;65:1297–1306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N. Alloantigen specific CD8+CD28− FOXP3+ T suppressor cells induce ILT3+ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int. Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 38.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum. Immunol. 2004;65:142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Manzotti CN, Tipping H, Perry LC, Mead KI, Blair PJ, Zheng Y, Sansom DM. Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25+ regulatory T cells. Eur. J. Immunol. 2002;32:2888–2896. doi: 10.1002/1521-4141(2002010)32:10<2888::AID-IMMU2888>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 42.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 43.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl. Immunol. 2003;11:245–258. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 44.Vlad G, Piazza F, Colovai A, Cortesini R, Della Pietra F, Suciu-Foca N, Manavalan JS. Interleukin-10 induces the upregulation of the inhibitory receptor ILT4 in monocytes from HIV positive individuals. Hum. Immunol. 2003;64:483–489. doi: 10.1016/s0198-8859(03)00040-5. [DOI] [PubMed] [Google Scholar]