Abstract
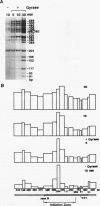
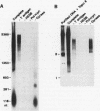
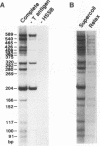
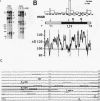
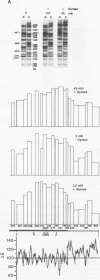
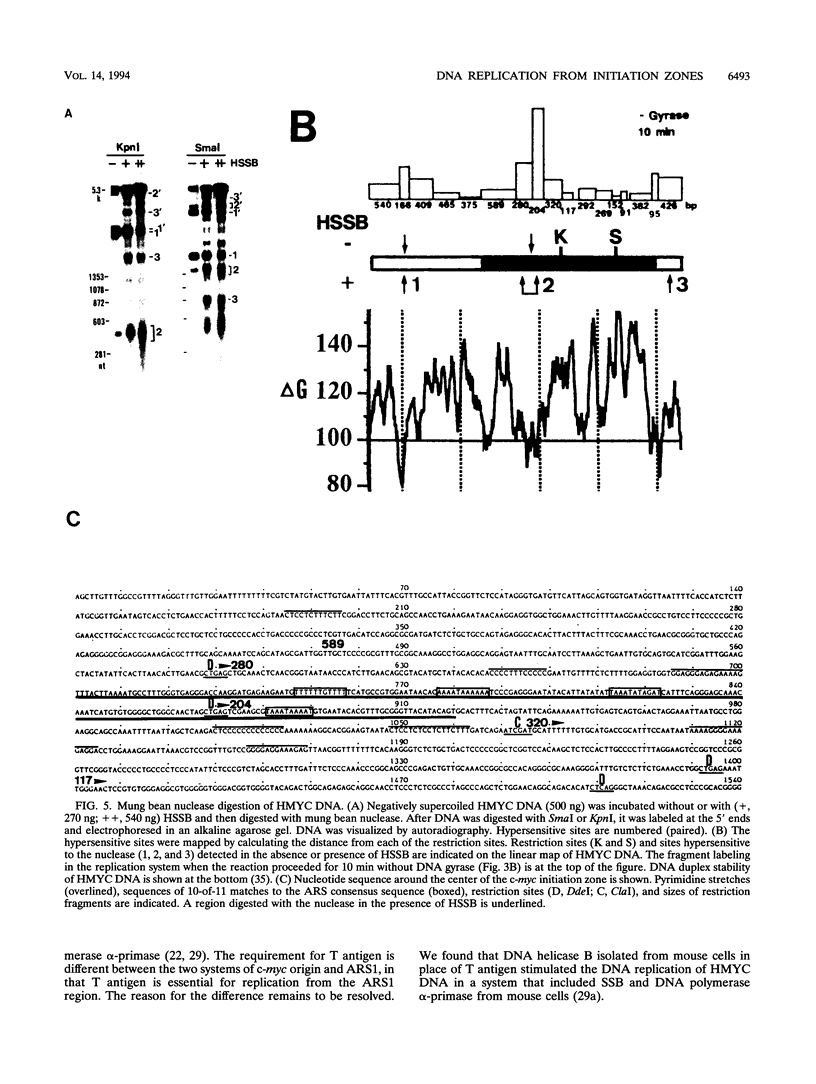
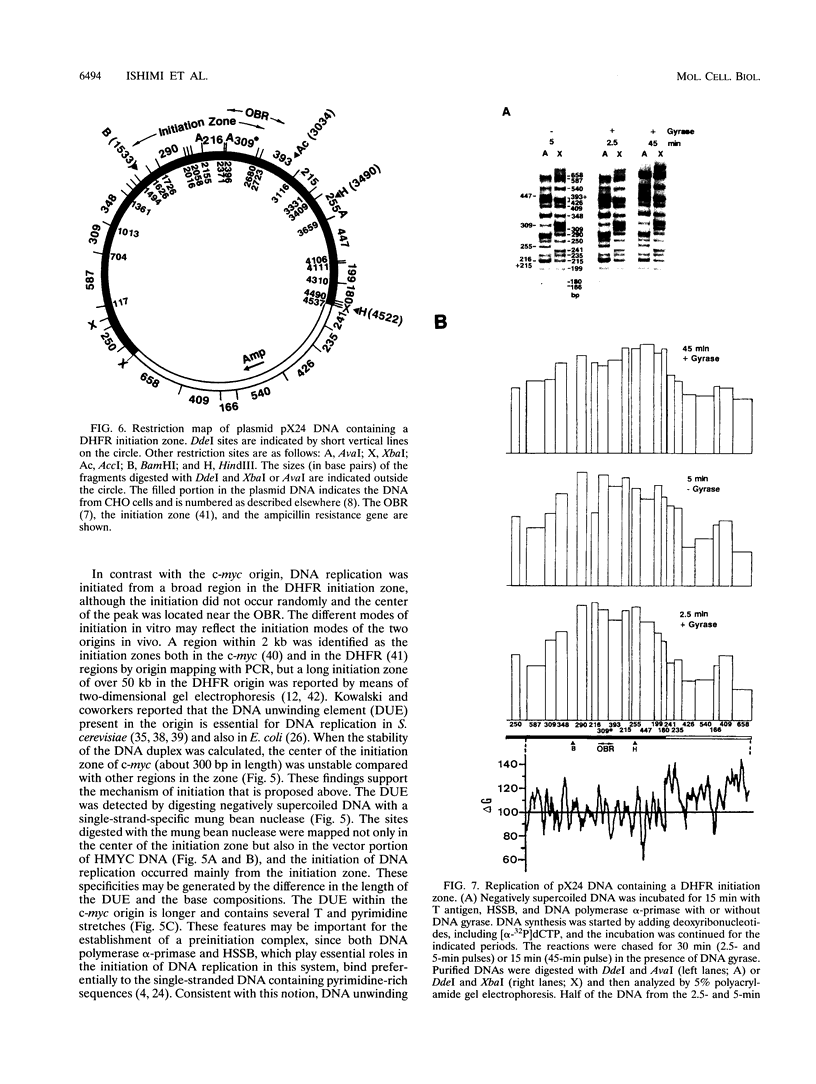
We reported that DNA replication initiates from the region containing an autonomously replicating sequence from Saccharomyces cerevisiae when negatively supercoiled plasmid DNA is incubated with the proteins required for simian virus 40 DNA replication (Y. Ishimi and K. Matsumoto, Proc. Natl. Acad. Sci. USA 90:5399-5403, 1993). In this study, the DNAs containing initiation zones from mammalian cells were replicated in this model system. When negatively supercoiled DNA containing an initiation zone (2 kb) upstream of the human c-myc gene was incubated with simian virus 40 T antigen as a DNA helicase, HSSB (also called replication protein A), and DNA polymerase alpha-primase complex isolated from HeLa cells, DNA replication was specifically initiated from the center of the initiation zone, which was elongated bidirectionally in the presence of a DNA swivelase. Without HSSB, the level of DNA synthesis was significantly reduced and the localized initiation could not be detected, indicating that HSSB plays an essential role in the initiation of DNA replication. The digestion of negatively supercoiled template DNA with a single-strand-specific nuclease revealed that HSSB stimulated DNA unwinding in the center of the initiation zone where the DNA duplex is relatively unstable. In contrast, DNA replication started from a broad region of an initiation zone downstream of the dihydrofolate reductase gene from chinese hamster ovary cells, but the center of the region was mapped near the origin of bidirectional DNA replication. These results suggested that this system mimics a fundamental process of initiation of eukaryotic DNA replication. The mechanism of initiation is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Laemmli U. K. Identification of nuclear pre-replication centers poised for DNA synthesis in Xenopus egg extracts: immunolocalization study of replication protein A. J Cell Biol. 1992 Oct;119(1):1–15. doi: 10.1083/jcb.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. P., Kobayashi R., Stillman B. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science. 1993 Dec 17;262(5141):1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992 May 14;357(6374):128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zhao J., Larson D. D. On the nature of origins of DNA replication in eukaryotes. Bioessays. 1992 Oct;14(10):661–670. doi: 10.1002/bies.950141004. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Caddle M. S., Heintz N. H., DePamphilis M. L. Identification of an origin of bidirectional DNA replication in mammalian chromosomes. Cell. 1990 Sep 7;62(5):955–965. doi: 10.1016/0092-8674(90)90270-o. [DOI] [PubMed] [Google Scholar]
- Caddle M. S., Lussier R. H., Heintz N. H. Intramolecular DNA triplexes, bent DNA and DNA unwinding elements in the initiation region of an amplified dihydrofolate reductase replicon. J Mol Biol. 1990 Jan 5;211(1):19–33. doi: 10.1016/0022-2836(90)90008-A. [DOI] [PubMed] [Google Scholar]
- Callan H. G. DNA replication in the chromosomes of eukaryotes. Cold Spring Harb Symp Quant Biol. 1974;38:195–203. doi: 10.1101/sqb.1974.038.01.023. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Eukaryotic DNA replication: anatomy of an origin. Annu Rev Biochem. 1993;62:29–63. doi: 10.1146/annurev.bi.62.070193.000333. [DOI] [PubMed] [Google Scholar]
- Dornreiter I., Erdile L. F., Gilbert I. U., von Winkler D., Kelly T. J., Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992 Feb;11(2):769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eki T., Matsumoto T., Murakami Y., Hurwitz J. The replication of DNA containing the simian virus 40 origin by the monopolymerase and dipolymerase systems. J Biol Chem. 1992 Apr 15;267(11):7284–7294. [PubMed] [Google Scholar]
- Eki T., Murakami Y., Enomoto T., Hanaoka F., Yamada M. Characterization of DNA replication at a restrictive temperature in a mouse DNA temperature-sensitive mutant, tsFT20 strain, containing heat-labile DNA polymerase alpha activity. J Biol Chem. 1986 Jul 5;261(19):8888–8893. [PubMed] [Google Scholar]
- Gazin C., Dupont de Dinechin S., Hampe A., Masson J. M., Martin P., Stehelin D., Galibert F. Nucleotide sequence of the human c-myc locus: provocative open reading frame within the first exon. EMBO J. 1984 Feb;3(2):383–387. doi: 10.1002/j.1460-2075.1984.tb01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Dean F. B., Kwong A. D., Lee S. H. The in vitro replication of DNA containing the SV40 origin. J Biol Chem. 1990 Oct 25;265(30):18043–18046. [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Okazaki T., Itani T., Ogata M., Sato Y., Ariga H. An initiation site of DNA replication with transcriptional enhancer activity present upstream of the c-myc gene. EMBO J. 1988 Oct;7(10):3135–3142. doi: 10.1002/j.1460-2075.1988.tb03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y., Claude A., Bullock P., Hurwitz J. Complete enzymatic synthesis of DNA containing the SV40 origin of replication. J Biol Chem. 1988 Dec 25;263(36):19723–19733. [PubMed] [Google Scholar]
- Ishimi Y., Matsumoto K. Loading of a DNA helicase on the DNA unwinding element in the yeast replication origin: mechanism of DNA replication in a model system. Biochemistry. 1994 Mar 8;33(9):2733–2740. doi: 10.1021/bi00175a049. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Matsumoto K. Model system for DNA replication of a plasmid DNA containing the autonomously replicating sequence from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5399–5403. doi: 10.1073/pnas.90.12.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y., Sugasawa K., Hanaoka F., Kikuchi A. Replication of the simian virus 40 chromosome with purified proteins. J Biol Chem. 1991 Aug 25;266(24):16141–16148. [PubMed] [Google Scholar]
- Kim C., Snyder R. O., Wold M. S. Binding properties of replication protein A from human and yeast cells. Mol Cell Biol. 1992 Jul;12(7):3050–3059. doi: 10.1128/mcb.12.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsberg D., Selig S., Keshet I., Cedar H. Replication structure of the human beta-globin gene domain. Nature. 1993 Dec 9;366(6455):588–590. doi: 10.1038/366588a0. [DOI] [PubMed] [Google Scholar]
- Kowalski D., Eddy M. J. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 1989 Dec 20;8(13):4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. The two faces of higher eukaryotic DNA replication origins. Cell. 1990 Sep 7;62(5):845–847. doi: 10.1016/0092-8674(90)90258-g. [DOI] [PubMed] [Google Scholar]
- Little R. D., Platt T. H., Schildkraut C. L. Initiation and termination of DNA replication in human rRNA genes. Mol Cell Biol. 1993 Oct;13(10):6600–6613. doi: 10.1128/mcb.13.10.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Ishimi Y. Single-stranded-DNA-binding protein-dependent DNA unwinding of the yeast ARS1 region. Mol Cell Biol. 1994 Jul;14(7):4624–4632. doi: 10.1128/mcb.14.7.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Eki T., Hurwitz J. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9712–9716. doi: 10.1073/pnas.87.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney C., Leffak M. Autonomous replication of a DNA fragment containing the chromosomal replication origin of the human c-myc gene. Nucleic Acids Res. 1990 Mar 11;18(5):1233–1242. doi: 10.1093/nar/18.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendy T., Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993 Feb 15;268(5):3389–3395. [PubMed] [Google Scholar]
- Micklem G., Rowley A., Harwood J., Nasmyth K., Diffley J. F. Yeast origin recognition complex is involved in DNA replication and transcriptional silencing. Nature. 1993 Nov 4;366(6450):87–89. doi: 10.1038/366087a0. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Gasser S. M., Laemmli U. K. Relation of chromosome structure and gene expression. Philos Trans R Soc Lond B Biol Sci. 1987 Dec 15;317(1187):563–574. doi: 10.1098/rstb.1987.0081. [DOI] [PubMed] [Google Scholar]
- Natale D. A., Schubert A. E., Kowalski D. DNA helical stability accounts for mutational defects in a yeast replication origin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2654–2658. doi: 10.1073/pnas.89.7.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Ina S. DNA replication of histone gene repeats in Drosophila melanogaster tissue culture cells: multiple initiation sites and replication pause sites. Mol Cell Biol. 1993 Jul;13(7):4098–4106. doi: 10.1128/mcb.13.7.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Kowalski D. The ease of DNA unwinding as a determinant of initiation at yeast replication origins. Cell. 1988 Feb 26;52(4):559–567. doi: 10.1016/0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Kowalski D. Thermal energy suppresses mutational defects in DNA unwinding at a yeast replication origin. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2486–2490. doi: 10.1073/pnas.87.7.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L. T., Burhans W. C., DePamphilis M. L. Mapping an origin of DNA replication at a single-copy locus in exponentially proliferating mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4685–4689. doi: 10.1128/mcb.10.9.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. An initiation zone of chromosomal DNA replication located upstream of the c-myc gene in proliferating HeLa cells. Mol Cell Biol. 1990 Sep;10(9):4899–4904. doi: 10.1128/mcb.10.9.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. P., Dijkwel P. A., Hamlin J. L. Replication initiates in a broad zone in the amplified CHO dihydrofolate reductase domain. Cell. 1990 Jun 15;61(6):1075–1087. doi: 10.1016/0092-8674(90)90071-l. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Collins K. L., Simancek P., Russo A., Wold M. S., Virshup D. M., Kelly T. J. Reconstitution of simian virus 40 DNA replication with purified proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8692–8696. doi: 10.1073/pnas.87.22.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel R., Schweizer J., Stahl H. Simian virus 40 T-antigen DNA helicase is a hexamer which forms a binary complex during bidirectional unwinding from the viral origin of DNA replication. J Virol. 1992 Feb;66(2):804–815. doi: 10.1128/jvi.66.2.804-815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig B., Wölfl S., Dorbic T., Vahrson W., Rich A. Transcription of human c-myc in permeabilized nuclei is associated with formation of Z-DNA in three discrete regions of the gene. EMBO J. 1992 Dec;11(12):4653–4663. doi: 10.1002/j.1460-2075.1992.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobbe C. R., Weissbach L., Borowiec J. A., Dean F. B., Murakami Y., Bullock P., Hurwitz J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1834–1838. doi: 10.1073/pnas.84.7.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]