Abstract
Purpose
The stability of alemtuzumab concentrations, 6.67 mcg/mL in glass bottles and, 40 mcg/mL, and 120 mcg/mL in polyolefin containers when stored at room temperature was evaluated.
Methods
Triplicate solutions of alemtuzumab in 0.9% sodium chloride were prepared in either glass bottles or polyolefin containers and stored at room temperature under normal fluorescent lightening conditions. The solutions were analyzed by a stability-indicating high-performance liquid chromatography assay at 0, 8, 14, and 24 hours after preparation. The pH of the solution was measured and the solution containers were visually inspected at these same time points. Stability was defined as retention of ≥90% of the initial alemtuzumab concentration.
Results
All solutions retained over 90% of their initial concentration with no significant change over the time period studied. The more dilute solution, 6.67 mcg/mL showed some degradation down to 91% of the initial concentration whereas the other concentrations showed greater than 99% over the 24 hour period. The pH of the solutions varied by concentration, but did not change significantly over 24 hours for each concentration studied. No particles were detected in any solution by visual inspection.
Conclusion
Alemtuzumab concentrations of 6.67 mcg/mL in glass bottles and, 40 mcg/mL, and 120 mcg/mL in polyolefin containers are stable for at least 24 hours at room temperature.
Several monoclonal antibodies have been approved by the Food and Drug Administration (FDA) to treat cancer.1 Alemtuzumab, a monoclonal antibody that targets lymphocytes, has been shown to be useful to treat certain forms of lymphoma when given in combination with standard chemotherapy.2–5 However, anticancer drugs are frequently used off-label or studied in investigational protocols at different doses and infusion times. Pharmacists often do not have the information to prepare these off-label doses in a manner that facilitates efficient drug delivery. For example, a current protocol uses alemtuzumab given at various doses over a 12-hour infusion period. The manufacturer’s preparation information is intended for a short infusion and does not accommodate the situation where the infusion may need to be interrupted due to an infusion-related reaction.
Currently there is only limited stability information for mixing alemtuzumab in solutions. Kupfer et al., using a size-exclusion chromatography (SEC) high-performance liquid chromatography (HPLC) assay, demonstrated that alemtuzumab 0.28 mg/mL solutions in 0.9% sodium chloride Injection are stable for 14 days at 6° C and at room temperature.6 Vermeulen et al. showed that alemtuzumab 0.1 to 41 ug/mL solutions in 0.9% sodium chloride injection are stable for up to 28 days at refrigerated temperatures (4° C) using a flow cytometric assay.7 Because it is not always possible to mix the alemtuzumab to these exact concentrations for the doses used in the current studies, it is necessary to divide the 12-hour infusion into two bags and infuse each over 6 hours. Also, because of the limited data, these bags are prepared right before they are needed. This puts a strain on the pharmacy and also could lead to the possibility that the infusion might be interrupted for a brief period of time due to a need to change bags in the middle of an infusion.
To provide additional data for our studies and for situations where alemtuzumab is used off-label at concentrations that might not be feasible for administration using current stability information, we examined the stability of alemtuzumab for up to 24 hours mixed in 0.9% sodium chloride injection at concentrations based on the doses used in the current studies when stored at room temperature conditions (22 – 28° C).
Methods
Sample preparation
Alemtuzumaba concentrations of 6.67 mcg/mL were prepared in 150mL glass bottles of 0.9% sodium chloride injectionb and concentrations of 40 mcg/mL and 120 mcg/mL were prepared in 250mL polyolefin bags of 0.9% sodium chloride injectionc. Each preparation was agitated 10 times to homogenize the contents of the bag. Preparations were stored under normal fluorescent lightening conditions throughout the study. Each concentration was prepared in triplicate and stored at room temperature (22–28° C).
High-performance liquid chromatographic assay
The assay published by Kupfer et al. was validated and used in our study.6 It is a stability-indicating, size-exclusive HPLC assay. An Agilent 1100 HPLC instrumentd was used which includes a degasser, a binary pump, an autosampler, and a Diode-Array detector. An Agilent Bio SEC-5 (5 μm, 300 Å, 7.8x300 mm) column maintained at 25 °C was used for separation. The elution was run isocratically with a mobile phase consisting of 0.1 M sodium phosphatee, pH 7.0 mixed with 0.3 M sodium chloridef at flow rate of 1 mL/min. An injection volume of 20 μL was used for the 120 mcg/mL preparation and 50 μL for the 40 and 6.67 mcg/mL preparations. Different injection volumes were used to improve signal strength and reduce variability for the lower concentrations. The average retention time was 7.8 minutes. Detection was set at 280 nm. ChemStation Software by Agilent Technologies was used for instrument operation, data collection and processing. USP guidelines were followed in our analysis, that is, system suitability was established by consecutively inject a reference standard six times to have variations of area under curve less than 2%; two reference standards were prepared for each preparation and were injected before, in-between, and after sample injections with variations of reference factor less than 2%.
Assay validation
We performed a limited validation of the assay because our assay was adopted from a published and validated assay procedure. A standard 7-point calibration curve was constructed by linear regression of the peak area of alemtuzumab against alemtuzumab concentrations of 6, 30, 60, 75, 120, 150 and 240 mcg/mL. The curve was linear over this range of concentrations (r2 = 0.9999). The LOD was 1.74 mcg/mL and LOQ was 5.27 mcg/mL.
Stability-indicating assay validation
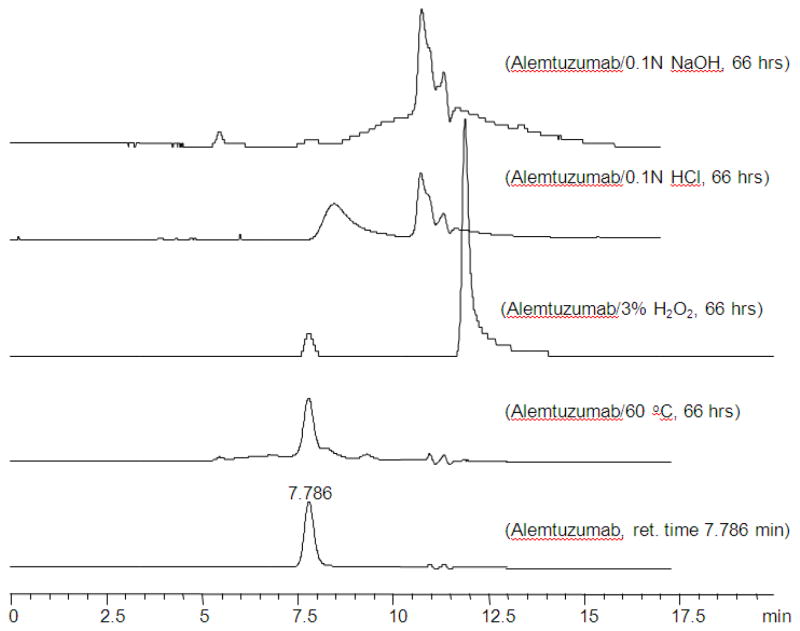
The assay was shown to be stability indicating by showing that forced degradation of the samples, using 0.1 N sodium hydroxide, 0.1 N hydrochloric acid, and 3% hydrogen peroxide, demonstrated that the peaks associated with the degradation products did not interfere with the peak from the intact alemtuzumab. Figure 1 shows representative chromatograms of alemtuzumab when subjected to forced degradation.
Figure 1.
Representative chromatograms of alemtuzumab under stress conditions
Sample analysis
Stability assessments were performed after preparation (time zero) and at 8 hours, 14 hours, and 24 hours. At each time point, 1 mL was drawn for HPLC analysis with two injections for each and 2 mL was drawn for pH measurements. The samples were also visually inspected at each time point to look for particles in the solution.
Data analysis
All experiments were performed in triplicate. Data are presented as the mean ± standard deviation percentage of the initial concentration. Stability was defined as retention of ≥90% of the initial alemtuzumab concentration.
Results and discussion
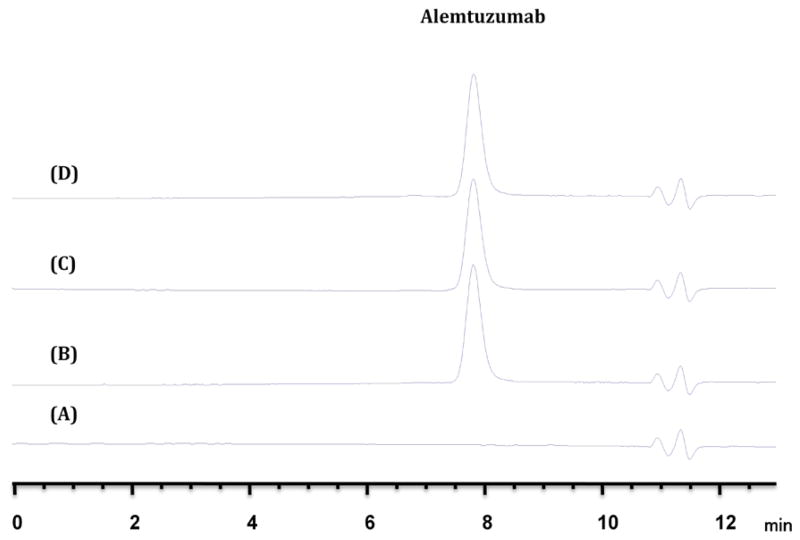
The representative chromatograms of alemtuzumab are shown in Figure 2.
Figure 2.
Representative HPLC chromatograms of alemtuzumab stability study
(A): Blank, 0.9% Sodium Chloride injection, USP.
(B): Alemtuzumab reference solution.
(C): Alemtuzumab solution, 40 mcg/ml at time zero.
(D): Alemtuzumab solution, 40 mcg/ml at time 24 hours.
All samples retained greater than or equal to 90% of the initial concentration at all time points, suggesting minimal to no loss of product due to degradation or adsorption (Table 1). There was some degradation in the most dilute solution (6.67 mcg/mL), but greater than 90% remained at the 24 hour time-point.
Table 1.
Stability of alemtuzumab in 0.9% Sodium Chloride Injection in glass bottles (6.67 mcg/mL) or polyolefin infusion bags (40 mcg/mL and 120 mcg/mL) at Room Temperature (22–28° C)
| Preparations | Mean ± S.D.* | |||
|---|---|---|---|---|
| Actual Initial Drug Concentration (mcg/mL) | % Initial Concentration Remaining | |||
| 8 hr | 14 hr | 24 hr | ||
| Alemtuzumab 120 mcg/mL | 115.15 ± 1.8 | 99.02 ± 1.2 | 99.10 ± 1.5 | 99.37 ± 0.9 |
| Alemtuzumab 40 mcg/mL | 37.71 ± 1.1 | 99.75 ± 2.0 | 100.01 ± 2.1 | 99.32 ± 2.1 |
| Alemtuzumab 6.67 mcg/mL | 8.44 ± 0.2 | 95.04 ± 1.7 | 95.21 ± 2.6 | 91.05 ± 0.9 |
n = 6
According to the manufacturer, the pH of alemtuzumab 30-mg/mL is 6.8–7.4 and the pH of 0.9% sodium chloride injection is 4.5–7.0. The pH of the solutions varied by concentration; however, there was no significant change in the solution over the 24 hour period for each of the concentrations tested (Table 2).
Table 2.
pH of alemtuzumab in 0.9% Sodium Chloride Injection in glass bottles (6.67 mcg/mL) or polyolefin infusion bags (40 mcg/mL and 120 mcg/mL) at room temperature (22–28° C)
| Preparations | Mean ± S.D.* | |||
|---|---|---|---|---|
| Initial | 8 hr | 14 hr | 24 hr | |
| Alemtuzumab 120 mcg/mL | 7.02 ± 0.03 | 6.37 ± 0.05 | 6.36 ± 0.02 | 6.35 ± 0.02 |
| Alemtuzumab 40 mcg/mL | 5.95 ± 0.18 | 5.08 ± 0.27 | 5.15 ± 0.26 | 5.13 ± 0.23 |
| Alemtuzumab 6.67 mcg/mL | 4.89 ± 0.21 | 4.93 ± 0.26 | 5.00 ± 0.27 | 4.96 ± 0.20 |
n = 3
Visual inspection did not demonstrate any particles in the solution containers at any time during the study period.
Our results are consistent with other studies in that it appears that alemtuzumab is stable for extended time periods over a wide variety of concentrations.6,7 The knowledge that the concentrations used in this study (6.67, 40, and 120 mcg/mL) are stable at room temperature will allow the solutions to be prepared in a single container for 12-hour infusions. This information will also allow pharmacy to prepare the solutions slightly in advance of when they are needed and obviate the need to prepare a new solution if the infusion needs to be prolonged.
Conclusion
Alemtuzumab concentrations of 6.67 mcg/mL in glass bottles and, 40 mcg/mL, and 120 mcg/mL in polyolefin containers are stable for at least 24 hours at room temperature.
Footnotes
Alemtuzumab injection 30 mg/mL, Genzyme Corporation, Cambridge, MA 02142, Lot F0001C02
150 mL 0.9% sodium chloride injection in glass bottles USP, B. Braun Medical Inc., Irvine, CA USA 92614-5895 Lot # J1E003
250 mL 0.9% sodium chloride injection in polyolefin bags USP, B. Braun Medical Inc., Irvine, CA USA 92614-5895 Lot # J1D515
Agilent Technologies, Santa Clara, CA
Mallinkrodt Baker, Inc., Paris, Kentucky 40361 LOT 7914 V01602
Fisher Chemicals, Fair Lawn, NJ 07410, Lot 015935
All authors have no conflicts of interest to disclose.
References
- 1.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterborg A, Foà R, Bezares RF, et al. Management guidelines for the use of alemtuzumab in chronic lymphocytic leukemia. Leukemia. 2009;23:1980–8. doi: 10.1038/leu.2009.146. [DOI] [PubMed] [Google Scholar]
- 3.Wilson WH, Hernandez-Ilizaliturri FJ, Dunleavy K, Little RF, O’Connor OA. Novel disease targets and management approaches for diffuse large B-cell lymphoma. Leuk Lymphoma. 2010;51 (Suppl 1):1–10. doi: 10.3109/10428194.2010.500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi JH, Kim SJ, Ko YH, Kim WS. Treatment of patients with refractory diffuse large B-cell lymphoma or mantle cell lymphoma with alemtuzumab, alone or in combination with cytotoxic chemotherapy. Leuk Lymphoma. 2011;52:317–20. doi: 10.3109/10428194.2010.529203. [DOI] [PubMed] [Google Scholar]
- 5.Dunleavy K, Wilson WH. Targeting CD52 as a novel therapeutic strategy in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2010;51:1583–4. doi: 10.3109/10428194.2010.508190. [DOI] [PubMed] [Google Scholar]
- 6.Kupfer M, Scriba G, Hartmann M. Stability of Alemtuzumab in Infusion-Bags. Pharmazie. 2009;64:622–623. [PubMed] [Google Scholar]
- 7.Vermeulen LC, Puffer EB, Hallam MB, et al. Stability of Aleztuzumab for Low-Dose Induction and Test Doses. American Journal of Transplantation. 2009;9:651–652. doi: 10.1111/j.1600-6143.2008.02506.x. Letter. [DOI] [PubMed] [Google Scholar]