Abstract
Neurosteroids are potent and effective neuromodulators that are synthesized from cholesterol in the brain. These agents and their synthetic derivatives influence the function of multiple signaling pathways including receptors for γ-aminobutyric acid (GABA) and glutamate, the major inhibitory and excitatory neurotransmitters in the central nervous system (CNS). Increasing evidence indicates that dysregulation of neurosteroid production plays a role in the pathophysiology of stress and stress-related psychiatric disorders, including mood and anxiety disorders. In this paper, we review the mechanisms of neurosteroid action in brain with an emphasis on those neurosteroids that potently modulate the function of GABAA receptors. We then discuss evidence indicating a role for GABA and neurosteroids in stress and depression, and focus on potential strategies that can be used to manipulate CNS neurosteroid synthesis and function for therapeutic purposes.
Keywords: Neurosteroids, Neuroactive steroids, Allopregnanolone, Stress, Depression, Anxiety, Hippocampus
1. Introduction
Psychiatric disorders are very common with at least 30% of adults suffering from one of these illnesses at some point in their lives (Zorumski and Rubin, 2011). These mental disorders are among the leading causes of disability in western societies, and comprise approximately 40% of illnesses that result in an inability to work productively (Iglehart, 2004). Psychiatric disorders are also major contributors to mortality. While heart and lung disease, stroke, diabetes and cancer are well known causes of morbidity and mortality, it is less appreciated that these end-state illnesses often reflect longstanding consequences of alcoholism, nicotine dependence, drug abuse, and unhealthy lifestyles including obesity (Mokdad et al., 2004). Depression and schizophrenia also contribute significantly to mortality and are “risk factors” for cardiovascular disorders, diabetes and metabolic syndrome (Newcomer and Hennekens, 2007). Furthermore, the presence of co-morbid depression worsens the outcomes of primary medical illnesses and vice versa (Moussavi et al., 2007; Prince et al., 2007). Deaths from motor vehicle accidents and violence are also often associated with psychiatric disorders, particularly alcohol abuse. Suicide claims the lives of over 30,000 individuals per year in the United States, and is almost always the result of a major psychiatric illness.
Modern neuroscience and genetics are providing important insights into brain mechanisms underlying these devastating disorders. Advances are being made in understanding the fundamental biology of a spectrum of neuropsychiatric disorders including dementias such as Alzheimer’s disease, major mood disorders, schizophrenia, anxiety disorders, alcoholism, and drug dependence. This work underscores the importance of abnormalities in the activity of specific brain networks and neurocircuits that underlie the pathophysiology and clinical symptoms of these brain disorders. Functional neuroimaging in particular is helping to define defects in neural circuitry associated with altered emotion, motivation and cognition, and collectively these findings provide new opportunities for therapeutic intervention (Ressler and Mayberg, 2007; Zorumski and Rubin, 2011; Buckholtz and Meyer-Lindenberg, 2012).
At the same time, however, there has been far less progress in identifying new pharmacological treatments for psychiatric disorders. Current medications represent only marginal advances over those introduced more than 50 years ago. Despite the fact that the current psychiatric drugs are only moderately effective with the vast majority of patients only partially responding to antidepressants or antipsychotics, the pharmaceutical industry is now largely abandoning efforts to develop new psychiatric medications (Insel and Sahakian, 2012). Many factors contribute to this unfortunate state of affairs. Among these are gaps in understanding psychiatric illnesses at a molecular level and the tendency of biopharmaceutical companies to pursue well trodden paths with more immediate payoffs based on existing chemical structures and proven mechanisms of action and demonstrated efficacy. There is thus a critical need to identify viable new drug targets in order to discover more effective agents.
One consideration in neuropsychiatric drug development is whether efforts are better directed toward agents that have highly specific mechanisms of action or whether the development of agents with broader actions altering a number of systems is preferred. Roth et al. (2004) have referred to this as a “magic bullet vs. magic shotgun” approach to therapeutics. Because we have little molecular insight into the pathophysiology of common neuropsychiatric disorders, we would argue that agents that affect multiple major targets may have the highest efficacy, particularly agents that modulate key neurotransmitter and signaling systems. In this light, we propose that neurosteroids represent viable targets for drug development in neuropsychiatry. Here, we will focus on the possible role that neurosteroids play in stress-related conditions and depression, and how these agents might be developed for therapeutic purposes. Our emphasis is on steroid derivatives that alter the function of the γ-aminobutyric acid (GABA) transmitter system, but we also consider other potential targets where appropriate.
2. Neurosteroids and neuroactive steroids
The term “neurosteroid” was coined by Etienne Baulieu in the early 1980’s and refers to a class of endogenous steroids synthesized in the brain and nervous system from cholesterol that are potent and effective modulators of the two major neurotransmitter systems that govern CNS activity – glutamate, the major excitatory neurotransmitter, and GABA, the major inhibitory neurotransmitter (Baulieu, 1981, 1997). Neurosteroids are a subset of the broader class of “neuroactive steroids” that includes endogenous agents made in the body outside the nervous system as well as synthetic derivatives that have CNS actions similar to neurosteroids (Paul and Purdy, 1992).
Because they modulate transmitter systems involved in arousal, cognition, emotion and motivation, neurosteroids (or neuroactive steroids with improved drug-like properties) have the potential to become novel treatments for multiple neuropsychiatric disorders (Paul and Purdy, 1992; Zorumski et al., 2000; Frye et al., 2012). Furthermore, current evidence indicates that endogenous production of neurosteroids in the brain is altered in behavioral states of “stress” and in several neuropsychiatric disorders, including depression (Girdler and Klatzkin, 2007; Schule et al., 2011). Thus, these steroids may mediate fundamental mechanisms that underlie behavioral symptoms that cut across current diagnostic categories. Potential clinical targets for neurosteroids include mood and anxiety disorders, schizophrenia, alcoholism, sleep disorders, chronic pain, epilepsy, traumatic brain injury and neurodegenerative disorders (Zorumski et al., 2000; Gunn et al., 2011). The effects of neurosteroids in most of these disorders are thought to involve direct actions in the brain, whereas the ability of neurosteroids to modulate chronic pain likely involves the spinal cord as well as brain (Kawano et al., 2011a,b; Sasso et al., 2012). Neuroactive steroids also have potential for development as rapidly acting intravenous anesthetics (Selye, 1941; Phillipps, 1974; Morgan and Whitwam, 1985) (Fig. 1).
Fig. 1.

The list outlines potential therapeutic targets for neuroactive steroids. With the exception of Memory Dysfunction, these are targets for steroids that enhance the function of GABAA Rs. Memory Dysfunction would be a more likely target for steroids that either enhance the function of NMDARs or that inhibit GABAA Rs.
Although the GABA and glutamate neurotransmitter systems and receptors are major targets for the actions of neurosteroids, it is important to note that these steroids also alter the function of other receptors and ion channels. Alternative targets include nicotinic acetylcholine receptors (Paradiso et al., 2000), glycine receptors (Jin et al., 2009), sigma receptors (Valenzuela et al., 2008) as well as voltage-activated calcium (Todorovic et al., 1998, 2004; Pathirathna et al., 2005), voltage-activated potassium (Schlichter et al., 2006) and transient receptor potential (Majeed et al., 2011) channels (Fig. 2). Because neurosteroids are highly effective and potent modulators of GABA receptors, these will be the focus of our discussion. While some steroids interact with intracellular hormone receptors to alter gene expression, the effects we will describe involve more rapidly occurring actions on cellular signaling pathways, including ligand-gated ion channels. Nonetheless, intracellular actions, including effects on signaling pathways are also likely to be important for some agents (Frye et al., 2012).
Fig. 2.
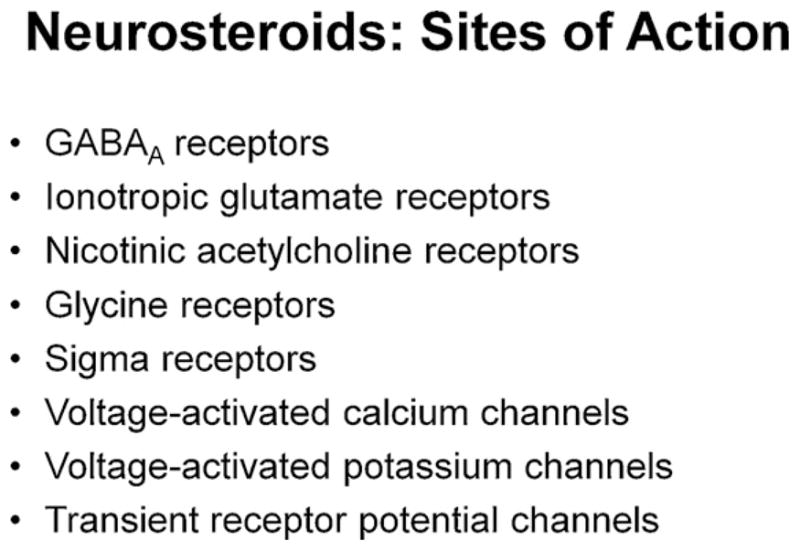
The figure lists major sites of action that have been indentified for neurosteroids. Importantly, steroids have some of their most potent and effective actions at GABAA Rs.
3. Neurosteroid synthesis in the brain
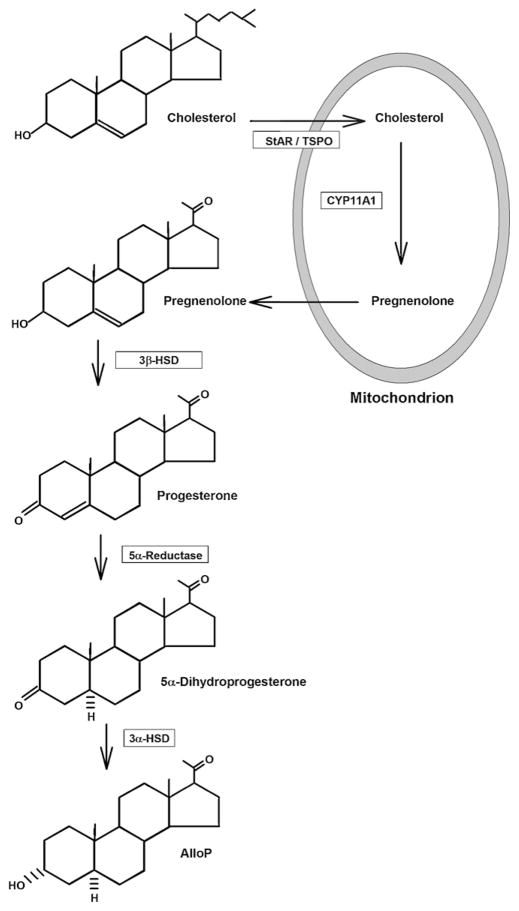
An intriguing aspect of neurosteroids is that they are synthesized locally in the brain (in addition to the periphery). Neurosteroids are generated from cholesterol by a series of steps that include the trafficking of cholesterol to the outer mitochondrial membrane by a process involving steroidogenic acute regulatory protein (StAR), shuttling of cholesterol to the inner mitochondrial membrane by translocator protein 18 kDa (TSPO) and conversion to pregnenolone by CYP11A1, a P450 side-chain cleavage enzyme (Belelli and Lambert, 2005; Rone et al., 2009; Gunn et al., 2011). Pregnenolone exits the mitochondria and is converted to neurosteroids that likely include both GABAAR potentiating and inhibiting agents. A major pathway involves the conversion of pregnenolone to alloP by the sequential actions of 3β-hydroxysteroid dehydrogenase/isomerase, 5α-reductase and 3α-hydroxysteroid dehydrogenase, with progesterone and 3α-dihydroprogesterone (3α-DHP) serving as intermediates (Belelli and Lambert, 2005; Gunn et al., 2011) (Fig. 3). The translocation of cholesterol to the inner mitochondrial membrane by TSPO is the rate limiting step (Rupprecht et al., 2010). TSPO is part of a multi-protein complex that includes voltage-dependent anion channel (VDAC) and adenine nucleotide transporter (ANT) (Papadopoulos et al., 2006; Rupprecht et al., 2010). TSPO was previously known as the peripheral (mitochondrial) BDZ receptor and some clinically used BDZs promote neurosteroidogenesis by activating TSPO (Ferrarese et al., 1993; Tokuda et al., 2010). The interaction of StAR with the TSPO complex results in what is referred to as the “transduceosome” (Midzak et al., 2011a,b). Photoaffinity labeling studies using modified neurosteroid analogues have shown labeling of VDAC, but the functional significance of this binding remains unknown (Darbandi-Tonkabon et al., 2003). Interestingly, some steroid analogues can alter TSPO’s ability to bind cholesterol and thus alter neurosteroid synthesis (Midzak et al., 2011a).
Fig. 3.
The diagram depicts key steps and enzymes involved in the synthesis of alloP from cholesterol. Conversion of cholesterol to pregnenolone occurs at the inner mitochondrial membrane.
Multiple cells in the brain synthesize neurosteroids raising the possibility that these agents are local paracrine or autocrine modulators of neural function. Early studies described a role for astrocytes and other glial cells, particularly under pathological conditions (Casellas et al., 2002; Chen and Guilarte, 2008; Rupprecht et al., 2010). In recent years, increasing evidence supports a role for principal (excitatory) neurons in the synthesis of GABAergic neurosteroids (reviewed in Chisari et al., 2010a). In the hippocampus, for example, pyramidal neurons are the primary cells that express the molecular machinery for cholesterol trafficking (Valdez et al., 2010), as well as key transporters and enzymes in steroid synthesis, including StAR (Kimoto et al., 2001; King et al., 2002; Kim et al., 2004; Lavaque et al., 2006), TSPO (Tokuda et al., 2010), the P450 side-chain cleavage enzyme (Kimoto et al., 2001; Shibuya et al., 2003) and 5α-reductase (Agís-Balboa et al., 2006). A sulfotransferase potentially involved in the synthesis of GABA inhibiting sulfated steroids is also expressed in pyramidal neurons (Kimoto et al., 2001). Based on staining with an antibody against 5α-reduced steroids including alloP, it appears that excitatory neurons are the primary cells that are immunopositive for neurosteroids under basal conditions in the brain (Saalman et al., 2007; Tokuda et al., 2010, 2011). The level of neurosteroids and neurosteroid-like immunoreactivity can be modulated by pharmacological agents including certain BDZs, other TSPO agonists, (Tokuda et al., 2010), and alcohol (Morrow et al., 1999; Sanna et al., 2004; Tokuda et al., 2011).
4. Neurosteroids and GABAA receptors
The GABA neurotransmitter system is a major target for neurosteroids. GABA acts at two classes of receptors – ligand-gated chloride (Cl−) channels called GABAA receptors (GABAARs) and metabotropic G-protein coupled receptors called GABAB receptors. Neurosteroids preferentially target GABA-gated Cl− channels (Majewska et al., 1986; Paul and Purdy, 1992; Belelli and Lambert, 2005). GABAARs are pentameric proteins structurally related to nicotinic acetylcholine receptors and are formed from multiple subunits. At least 19 distinct GABAAR subunits (termed α1-6, β1-3, γ1-3, δ, ε, π, θ and ρ1-3) have been identified to date (Hevers and Luddens, 1998; Olsen and Sieghart, 2009). Most native receptors express two α subunits, two β subunits and a single γ, δ or ε subunit. Rho (ρ) subunits can be expressed with other subunits, but usually form homomeric Cl− channels; receptors expressing ρ were previously known as GABAC receptors, but are now categorized as a subtype of GABAARs. Given the number of identified GABAAR subunits and the fact that these receptors each have five subunits, a multitude of potential receptor subtypes could be expressed. It appears, however, that a few specific subunit combinations reflect the majority of GABAARs found at synaptic and extrasynaptic sites in the brain (Sperk et al., 1997; Herd et al., 2007), with about 60% of GABAARs expressing α1β2γ2, 15–20% α2βδ2, 5% α4βδ or α4β γ and less than 5% α5β2γ2 or α6β2/3γ2 (Rudolph and Knoflach, 2011). Importantly, GABAARs expressing γ-subunits are usually targeted to synapses while receptors expressing the δ subunit form a major class of extrasynaptic receptors (Farrant and Nusser, 2005; Prenosil et al., 2006). In addition to targeting GABAARs to synapses, γ-subunits convey benzodiazepine (BDZ) sensitivity when expressed in combination with specific α-subunits (Johnston, 2005). Unlike BDZs, neurosteroids appear to modulate most, if not all GABAARs expressed in brain, although some evidence suggests that neurosteroids may have preferential effects on extrasynaptic δ-expressing receptors (Stell et al., 2003; Houston et al., 2012). The enhanced effects of neurosteroids on δ-expressing receptors does not appear to involve preferential binding to these receptors but rather reflects the fact that these GABAARs are inefficiently gated by GABA, and neurosteroids are very effective positive allosteric modulators under low efficacy conditions (Brown et al., 2002; Wohlfarth et al., 2002; Shu et al., 2012). In addition to neurosteroids and BDZs, GABAARs are positively modulated and/or directly gated by other clinically important drugs including barbiturates and a wide range of general anesthetics (Yang et al., 1992; Johnston, 2005; Korpi and Sinkkonen, 2006; Houston et al., 2012).
Neurosteroids have complex effects on GABAARs. Depending on their chemical structures, neurosteroids can enhance or inhibit GABAAR function (Majewska et al., 1986, 1988). The prototype for a GABAAR-potentiating steroid is 3α-hydroxy-5α-pregnane-20-one (also referred to as allopregnanolone (alloP or 3α5αP)). At submicromolar aqueous concentrations, alloP augments responses to sub-saturating levels of GABA, while at higher concentrations alloP directly opens (gates) GABAA channels in the absence of GABA (Majewska et al., 1986; Shu et al., 2004). The net effect is to augment synaptic and tonic inhibition in local brain regions (Yamakura et al., 2001).
Although steroids are highly lipophilic molecules and influence membrane fluidity (Makriyannis et al., 1991), neurosteroids potentiate GABAARs via specific sites on GABAAR subunits (Chisari et al., 2010a). GABA potentiating steroids show significant structural specificity. A 3α-hydrogen bond donor, a 17β-hydrogen bond acceptor and a reduced pregnane ring system all increase activity (Covey et al., 2001) (Fig. 4). Steroids with either 5α- or 5β-configurations are effective and have sedating behavioral effects (Gee et al., 1995). Neurosteroids act at sites on GABAARs that are distinct from other modulators (Turner et al., 1989). Amino acid residues in transmembrane regions 1 and 4 (TM1 and TM4) of the α1 subunit (Q241, N407 and Y410) are critical for steroid potentiation, while residues in α1 TM1 (T236) and β2 TM2 (Y284) are important for direct channel gating (Hosie et al., 2006). Corresponding residues in other α subunits govern steroid sensitivity in non-α1-containing receptors (Hosie et al., 2008). Other studies have confirmed the importance of the amino acid residues involved in GABAAR potentiation but suggest further complexity in the actions of neuroactive steroids. For instance, some studies have implicated additional residues in the TM1 region that contribute to a hydrophobic surface with which steroids interact (Akk et al., 2008), and have suggested additional sites of action on GABAARs based on complex effects on GABAAR ion channel kinetics (Li et al., 2006). Furthermore, structural modifications of neurosteroids can produce synthetic steroids that inhibit the effects of 5α-reduced (but not 5β-reduced) steroids on GABAARs (e.g. 3α5α-17-phenylandrost-16-en-3-ol (17-PA)) while having little intrinsic efficacy on their own (Mennerick et al., 2004). Other synthetic steroids potentiate GABAARs while having very weak ability to directly open GABA channels (e.g. 3α5β-18-norandrostane-17β-carbonitrile) (Evers et al., 2010).
Fig. 4.
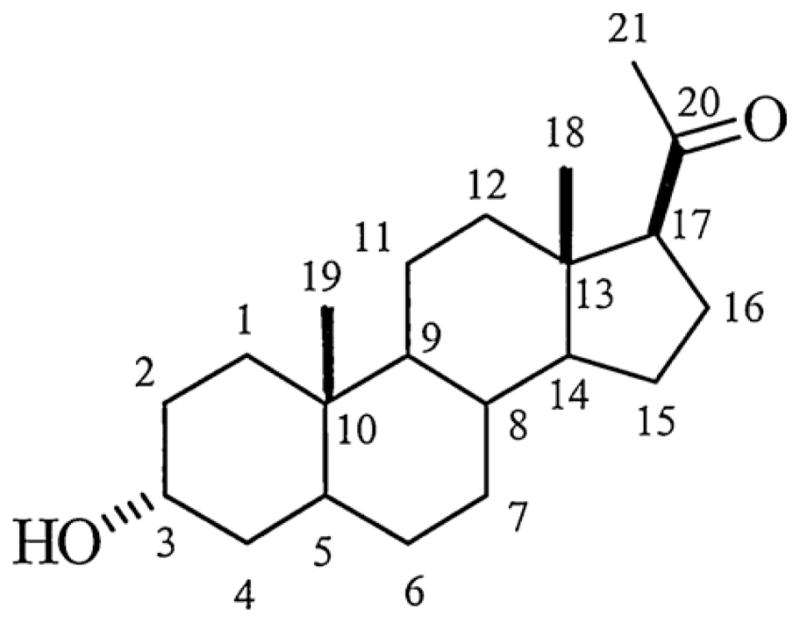
The figure shows the core structure of a 3α-hydroxy pregnane neurosteroid. In alloP, the hydrogen at C5 is in the α-conformation, while in pregnanolone, the hydrogen is in the β-configuration. Synthetic neuroactive steroids have modifications of this core structure. Key elements for enhancement of GABAA Rs include the pregnane nucleus, the presence of a 3α-hydrogen bond donor and a 17β-hydrogen bond acceptor.
Although molecular studies strongly support the notion of separate sites on GABAARs for potentiation and direct gating of GABA channels (Hosie et al., 2006), single channel recording studies suggest that as many as three distinct sites contribute to potentiation of GABA responses (Akk et al., 2004, 2007). These latter studies used cell attached patch clamp recordings to examine modulation at the single channel level. Based on analysis of clusters of channel openings gated by high concentrations of GABA, it appears that distinct sites may mediate the ability of neuroactive steroids to produce three major effects on GABAARs – an increase the frequency of long duration channel openings, a decrease in activation-dependent closed times and an increase in the duration of long channel openings, with effects on closed times making the largest contribution to enhancement of macroscopic GABA currents (Akk et al., 2004, 2007, 2010). While many neuroactive steroids produce all three effects at similar concentrations, the 18-nor-steroid described above (Evers et al., 2010) has distinct effects on these channel actions at different concentrations, with effects on the frequency of long openings occurring at an order of magnitude higher potency than effects on the duration of long channel openings (Akk et al., 2004). As noted above, the latter steroid may also distinguish sites for GABAAR potentiation from those that mediate direct channel gating (Evers et al., 2010).
Because steroids are highly lipophilic molecules and affect membrane fluidity, it was initially thought that non-specific membrane effects were responsible for actions in the brain. Neurosteroid enantiomers provided the first compelling evidence that effects in chiral (protein) environments are important in determining actions on GABAARs (Wittmer et al., 1996; Nilsson et al., 1998; Covey et al., 2000), given that enantiomers are mirror image molecules that have identical effects in lipid settings (Alakoskela et al., 2007; Covey, 2009). The identification of specific amino acids involved in neurosteroid effects on GABAARs provided unequivocal direct confirmation (Hosie et al., 2006, 2008). Subsequent studies, however, have revisited the role of cell membranes and the lipophilic properties of neurosteroids in mediating physiological effects. These studies have used a combination of single channel recordings in membrane patches and imaging of fluorescent neurosteroid analogues that are active at GABAARs to show that neuroactive steroids access intramembranous site(s) on GABAARs via partitioning in the lipid milieu of the plasma membrane (Akk et al., 2005). Furthermore, lipid solubility influences the potency and effectiveness of neuroactive steroids on GABAARs (Chisari et al., 2010a). Studies using a series of steroid analogues that varied in lipophilicity by more than two orders of magnitude (based on logP calculations describing estimated solubilities in octanol vs. water) indicate that the potency (and probably efficacy) of the steroids increases with increasing logP up to a limit. LogP also influences the onset and off-set kinetics of GABAAR modulation with more hydrophilic steroids (lower logP) having faster onset and offset of actions (Chisari et al., 2009). Effective neuroactive steroids typically have logP’s in the range of 3.0–4.5 and exert threshold effects on GABAARs at aqueous concentrations in the low nanomolar range. For an agent with a logP of 4, this means that effective concentrations in the membrane where it exerts its primary actions are in the range of at least 10–100 μM (Chisari et al., 2010a). Studies with the fluorescent neurosteroid analogues outlined above further indicate that exogenous applications readily lead to partitioning and accumulation of these steroids in plasma membrane and intracellular compartments (Akk et al., 2005), with other studies indicating that movement of steroids from the outside of a cell to intracellular compartments dampens efficacy (Li et al., 2007b).
To put the effects of neurosteroids into context, it is instructive to compare their actions to other positive allosteric modulators of GABAARs, including barbiturates and BDZs (Chisari et al., 2010a). AlloP has a threshold for potentiating GABAARs in the low nanomolar aqueous concentration range (1–10 nM). This is comparable to BDZs such as lorazepam, but is markedly more potent than pentobarbital (threshold effects in the low micromolar range). AlloP, however, is much more effective in potentiating GABA responses compared to BDZs (up to 20-fold enhancement compared to 3-fold enhancement at low GABA concentrations), but comparable to barbiturates (Fig. 5). The net effect of GABAAR-potentiating neurosteroids is to enhance the efficacy of GABA-mediated inhibition, likely contributing to sedating properties of these agents and their effects on anxiety, mood and seizures. These effects occur at both synaptic and extrasynaptic GABAARs resulting in enhanced phasic and tonic inhibition, respectively. At synapses, neurosteroids markedly prolong the duration of GABA-mediated synaptic currents while having less effect on peak synaptic responses (Harrison et al., 1987; Zorumski et al., 1998). Compared to BDZs and barbiturates, neurosteroids also have other attractive features as therapeutic targets. These include the ability to manipulate endogenous synthesis, synthetic strategies to diminish off-target actions, and perhaps more favorable abuse potential (Morrow et al., 2006). These will be discussed later in the paper.
Fig. 5.
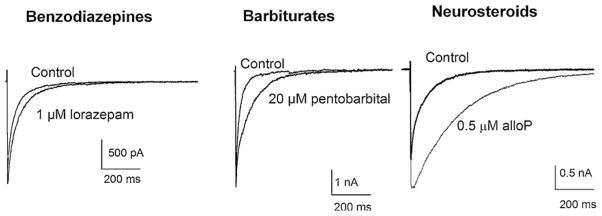
The traces depict the effects of a representative BDZ (lorazepam), barbiturate (pentobarbital) and neurosteroid (alloP) on GABAergic inhibitory postsynaptic currents (IPSCs) recorded from cultured hippocampal neurons.
In addition to GABA potentiating steroids, there are also neurosteroids that dampen responses at GABAARs (Majewska et al., 1988). These include steroids that are sulfated at the C3 position, with pregnenolone sulfate (PREGS) and dehydroepiandrosterone sulfate (DHEAS) serving as prototypes. Additionally, steroids in which a C3-hydroxyl group is in the β-configuration (as opposed to the 3α-configuration in GABA potentiating steroids) are non-competitive GABAAR antagonists, sharing many if not all properties, other than potency, with the C3-sulfated steroids (Wang et al., 2002). GABAAR inhibiting steroids exhibit GABA channel activation dependence, in that they are more potent against higher GABA concentrations than lower (sub-EC50) concentrations (Eisenman et al., 2003). GABA inhibiting steroids act at sites on GABAARs that are not yet defined but that are distinct from the site at which picrotoxin inhibits GABAARs and are distinct from the sites at which the GABA potentiating steroids act (Shen et al., 1999; Akk et al., 2001). GABA inhibiting steroids appear to act by enhancing desensitization of GABAARs (Shen et al., 2000; Eisenman et al., 2003). Interestingly, under basal conditions low concentrations of GABA inhibiting steroids have fairly subtle effects on GABA-mediated inhibitory postsynaptic currents (IPSCs), but markedly depress IPSCs that have been potentiated by other agents, including GABA potentiating steroids (Wang et al., 2002). Thus, at appropriate concentrations, these agents can serve as functional antagonists of GABAAR enhancers. GABAAR inhibiting steroids also share properties with a number of structurally diverse molecules including certain amphiphiles (Chisari et al., 2010b) and hydrophobic anions (Chisari et al., 2011), and can be effective against receptors mediating both tonic and phasic GABAergic inhibition (Shu et al., 2012).
5. GABA and depression
Before considering whether GABAergic neurosteroids play a role in stress-related disorders and depression, it is important to consider the evidence for changes in the GABA system in these conditions. This topic has been extensively reviewed recently by Luscher et al. (2011) and we will highlight only a few points here. Based on studies using biochemical measures of GABA in plasma and cerebrospinal fluid (CSF), there is evidence for decreases in GABA levels in depression (Brambilla et al., 2003; Luscher et al., 2011). This is consistent with studies measuring GABA levels in resected regions of cerebral cortex in individuals with histories of depression, and measures of GABA levels in cortex using magnetic resonance spectroscopy, with changes found in both adolescents and adults with depression (Sanacora et al., 2000; Gabbay et al., 2011; Luscher et al., 2011). Postmortem studies using brain tissue from neocortex and hippocampus also describe changes in the GABA system in individuals with mood disorders and have demonstrated a decrease in the number of GABAergic interneurons in cortex and hippocampus (Benes et al., 2008). These findings have been most clearly documented in individuals with bipolar disorder, but some evidence indicates changes in major depression and schizophrenia (Benes et al., 2008). Although effect sizes are small, there is also evidence of association of polymorphisms in several GABAAR genes in depression. These include changes in α4, α5, β1 and β3 subunits (Luscher et al., 2011). How these genetic findings translate into altered GABAergic function remains speculative at present. It is interesting to note, however, that the two alpha subunits associated with depression are largely expressed at extrasynaptic loci in the brain where they mediate tonic inhibition (Glykys et al., 2008; Brickley and Mody, 2012). For example, in the hippocampus, α4 subunits are expressed with δ-subunits at extrasynaptic sites on dentate granule cells, while α5 subunits make significant contributions to tonic GABA inhibition in pyramidal neurons (Belelli et al., 2009; Houston et al., 2012). The decreases in GABA levels noted above might also be consistent with changes in tonic inhibition, given that these measures are unlikely to reflect phasic GABA release at synapses directly. These observations suggest that tonic disinhibition may underlie risk for depression and that drugs that enhance tonic inhibition may be a therapeutic approach to treating or perhaps even preventing depression in susceptible individuals.
Rodent studies are also consistent with a change in GABAergic function contributing to depressive symptoms. Mice that have heterozygous deletion of the γ2 GABAAR subunit (γ2+/− mice) exhibit diminished expression of synaptic GABAARs in hippocampus (35% decrease) and amygdala (6% decrease) (Earnheart et al., 2007). While there are significant limitations in interpreting mouse behavioral data in a psychiatric context (Nestler and Hyman, 2010), it appears that γ2+/− mice exhibit features that can be interpreted as “anxious” and “depressed.” These include abnormal threat bias, overactivity of the hypothalamic-pituitary-adrenal (HPA) axis with elevated plasma corticosterone, and decreases in the survival of adult-born granule cells in the dentate gyrus (neurogenesis) (Crestani et al., 1999; Earnheart et al., 2007; Shen et al., 2010). It also appears that the timing of changes in the GABA system is important with effects occurring early in development having the biggest adverse impact on adult behavioral phenotype. The “anxious-depressed” phenotype is reversed by treatment with antidepressant medications; desipramine, a tricyclic antidepressant, improves both anxious and depressed features in these mice and fluoxetine, a selective serotonin reuptake inhibitor (SSRI), primarily improves the anxiety features (Shen et al., 2010). While these studies suggest that changes in phasic GABAergic inhibition occur in the γ2+/− mice, it is also important to note that tonic inhibition could be altered because γ2 subunits can be expressed at extrasynaptic loci (Houston et al., 2012).
Based on the changes outlined above, Luscher et al. (2011) proposed a model to describe the role of GABA in depressive behaviors. In their model, stess and depression are associated with diminished GABAergic function in prefrontal cortex and hippocampus. This results in increased excitatory output from these regions and altered regulatory control over the release of corticotrophin releasing hormone (CRH) from neurons in the hypothalamic paraventricular nucleus (PVN). Increased CRH then promotes release of adrenocorticotrophic hormone (ACTH) from the pituitary and elevated cortisol (corticosterone) release from the adrenal glands. The altered hippocampal output in this model is consistent with other studies in rats exposed to chronic mild stress (Airan et al., 2007). In this latter study, a voltage-sensitive dye was used to examine the propagation of activity through slices of ventral hippocampus following chronic stress, and demonstrated an input–output mismatch with diminished activity in the dentate gyrus and enhanced outflow from area CA1 accompanying chronic stress. These physiological changes were corrected by antidepressant treatment, as were behavioral changes in the forced swim test; improvement in both hippocampal physiology and behavior were also associated with antidepressant-induced neurogenesis in the dentate gyrus (Airan et al., 2007). Given that the hippocampus generally exerts negative control over corticosterone secretion, it is important to consider how the changes outlined above result in enhanced stress responses and corticosterone secretion. We will revisit this later.
6. Neurosteroids, stress and depression
Several studies have documented decreases in plasma and CSF levels of alloP and other GABAergic neurosteroids in individuals with major depression (Uzunova et al., 1998; Romeo et al., 1998; Strohle et al., 1999; Rasmusson et al., 2006). Some evidence also suggests increases in 3β-hydroxysteroids that inhibit GABAARs (Schule et al., 2011). These changes, particularly changes in alloP, correlate with symptom severity and reverse with effective antidepressant treatment. Other studies have examined neurosteroid responses to laboratory stressors in women with mood disorders, including premenstrual dysphoric disorder (Girdler and Klatzkin, 2007). An intriguing finding is that women with no history of depression show increases in alloP levels for 30–60 min following an acute stressor, while women with mood disorders show decreases in alloP levels. Additionally, women with histories of prior depression show a similar dampening of alloP levels in response to acute stress, even when euthymic at the time of testing (Girdler and Klatzkin, 2007). This suggests that altered alloP responses to stress may be a marker for risk of depression.
Successful treatment with some antidepressants is associated with increases in alloP levels that correlate with improvement (Uzunov et al., 1996, 1998). How antidepressants alter neurosteroid levels is not entirely clear although certain psychopharmacological treatments can increase alloP levels directly and independently of effects on mood. This is seen with several, but not all antidepressant treatments including the selective serotonin reuptake inhibitors (SSRIs), fluoxetine, paroxetine, fluvoxamine and sertraline, but not mirtazepine (a non-SSRI antidepressant), electroconvulsive therapy (ECT) or repetitive transcranial magnetic stimulation (rTMS) (Barbaccia, 2004; Esser et al., 2006). It is also important to note that certain antipsychotic medications, including clozapine and olanzapine (Danovich et al., 2008; Ugale et al., 2004), also increase alloP levels, as can many clinically used BDZs (McCauley et al., 1995; Bitran et al., 2000). Among BDZs, clonazepam stands out as an exception having little apparent effect on neurosteroid production (Tokuda et al., 2010), likely because of low affinity for TSPO (Papadopoulos et al., 1992). Interestingly and conversely, it appears that agents that dampen the endogenous production of neurosteroids, such as the 5α-reductase inhibitor finasteride, may be associated with depressive symptoms and cognitive dysfunction when used to treat alopecia or prostatic hyperplasia although data supporting this idea are largely anecdoctal (Altomare and Capella, 2002; Rahimi-Ardabili et al., 2006; Irwig, 2012a,b).
Given the higher prevalence of depression in women and the relationship of neurosteroids to progesterone, there has been considerable interest in determining whether neurosteroids contribute to depression in the perimenstrual and postpartum periods (Hines et al., 2011). Progesterone, a precursor of alloP, circulates at low levels in the late luteal phase but shows 2–4-fold elevation during the follicular phase of the menstrual cycle. The low levels during the late luteal phase correlate with a period of increased risk for depressed mood (Frye et al., 2012). During pregnancy even more dramatic changes in progesterone are observed, with levels increasing 50–100-fold followed by a precipitous drop in the immediate postpartum period. In a rodent model, high levels of progesterone and alloP during pregnancy are associated with compensatory changes in GABAARs and a decrease in the expression of δ-subunits that mediate tonic inhibition. In the early postpartum period as progesterone levels fall, δ-subunits are under-expressed. This correlates with increased neural excitability and depressive-like behaviors that persist until δ-subunit expression is restored (Maguire and Mody, 2008; Brickley and Mody, 2012). Similar, but less profound changes may occur during puberty (Shen et al., 2007). Interestingly, a mouse model with diminished δ-subunit expression is also associated with depression-like behaviors and infanticide (Maguire and Mody, 2008).
Animal studies provide other support for a role of neurosteroids in stress and depression. A seminal study by Purdy et al. (1991) found that following a 10 min period of acute swim stress, levels of alloP increased in cerebral cortex, hypothalamus and plasma. Cortical alloP levels rose quickly and peaked within 10–15 min of stress onset with gradual decline to levels below baseline by 2 h after the stress. Levels in hypothalamus and plasma rose more slowly and peaked at about 1 h after stress. Importantly, alloP has also been shown to have anxiolytic activity in mice at non-sedating doses (Crawley et al., 1986). These studies provided early support for the idea that alloP and other neurosteroids are involved in stress responses and raise the possibility that these steroids may be involved in restoring homeostatic mechanisms that are disrupted by acute stress (Vallee et al., 2000).
Subsequent studies have examined how alloP levels change following more prolonged stressors. In a model of chronic social isolation stress, Dong et al. (2001) found significant decreases in two 5α-reduced neurosteroids, alloP and its precursor 5α-DHP, with no changes in pregnenolone or progesterone. Changes in the 5α-reduced steroids were accompanied by decreases in both mRNA and protein for 5α-reductase, with no change in 3α-hydroxysteroid dehydrogenase (3α-HSD), another enzyme in the alloP pathway; 3α-HSD is also referred to as hydroxysteroid-oxidoreductase when the enzyme catalyzes the degradation of alloP. Rodents exposed to chronic social isolation show enhanced contextual fear conditioning, a decrement in the ability to extinguish fear responses, but no change in cued fear responses. These changes in fear conditioning correlate inversely with the expression of 5α-reductase in hippocampus and amygdala. Changes in alloP levels in the hippocampus and enhanced fear responses are mimicked by treatment with a 5α-reductase inhibitor and are reversed by treatment with exogenous alloP or S-norfluoxetine, an antidepressant that increases alloP levels (Pinna et al., 2003; Pibiri et al., 2008). Other studies found that socially isolated mice have diminished expression of 5α-reductase mRNA and decreased alloP levels in frontal cortex and lateral and basolateral amygdala, as well as decreases in dentate granule cell and CA3 pyramidal neurons (Agis-Balboa et al., 2007). These results are consistent with a model of neurocircuit dysfunction involving frontal cortex and hippocampal inputs to the amygdala, with subsequent changes in the output of the amygdala. Importantly, decreases in the expression of 5α-reductase and alloP levels are seen as key contributors to altered stress responses, resulting in downstream changes in other stress-related modulators including corticosterone, and changes in behavior (Patchev et al., 1994, 1996; Sarkar et al., 2011; Mody and Maguire, 2012).
Current thinking about the biology of stress and depression focuses on how these conditions alter information processing in specific neural circuits. While progress is being made in defining associated changes in intrinsic (resting state) connectivity networks indentified with functional magnetic resonance imaging (fMRI) in humans (Zorumski and Rubin, 2011; Buckholtz and Meyer-Lindenberg, 2012), less information is available at cellular and synaptic levels. Human studies highlight the importance of amygdala activation in basal stress vulnerability with changes in hippocampal activity over time being associated with stress-related behavioral symptoms (Admon et al., 2009), and changes in the function of specific cortical networks involved in attention, error detection and emotional/cognitive control (Sylvester et al., 2012). This is consistent with work in rodents examining changes in hippocampal-amygdala circuitry. Earlier we described studies using a voltage-sensitive dye to study the flow of activity in slices of ventral hippocampus prepared from rats exposed to chronic mild stress (Airan et al., 2007). In this study, several weeks of recurrent stress resulted in a “depressed” phenotype in which rats showed increased immobility in a forced swim test. Behavioral changes in the forced swim correlated with defects in activity propagation from dentate gyrus to CA1 with diminished input via the dentate and enhanced output from area CA1. The input–output defects and the altered swim test behavior were corrected by antidepressant treatment and required neurogenesis in the dentate gyrus. This study provided evidence that a depressed behavioral phenotype can be associated with specific neurocircuit changes. Correcting the input defect with enhanced dentate neurogenesis reversed both the behavioral and physiological changes. A related issue, however, concerns the factors that resulted in enhanced output of CA1 pyramidal neurons and raises questions about whether stress-related changes in GABAergic neurosteroids may contribute to altered CA1 function. It is possible that dampening CA1 output, perhaps via exogenous neuroactive steroids, may be therapeutic in stress and depression, given the decreases in hippocampal alloP levels observed in prior studies of chronic stress and the observation that pyramidal neurons are the principal cells that synthesize alloP under basal conditions (Agís-Balboa et al., 2006; Saalman et al., 2007; Tokuda et al., 2010).
7. How does stress alter neurosteroidogenesis?
The studies outlined above indicate that acute stress enhances the production of GABAergic neurosteroids but that chronic stress dampens the levels of these steroids. How acute stress promotes neurosteroid production is not certain, although several signaling pathways could contribute (Do Rego et al., 2012). These include endogenous ligands that act on key steps in neurosteroid synthesis. An example is acyl-CoA binding domain protein-1 (previously known as diazepam binding inhibitor (DBI)), a 10 kDa polypeptide, and its derivative triakontatetraneuro-peptide (DBI-17-50) (Ferrarese et al., 1991). DBI was discovered more than 20 years ago as an endogenous stress-induced agent that inhibits BDZ (diazepam) binding. DBI is an agonist for TSPO and promotes neurosteroid production (Ferrarese et al., 1993).
In peripheral tissue, ACTH and corticosterone stimulate steroid synthesis via adenylate cylase, cyclic AMP and protein kinase A (Rone et al., 2009). This is thought to enhance neuroactive steroid production by phosphorylating key molecules in the biosynthetic pathway, including StAR. Both the DBI and ACTH pathways could result in stress-induced neurosteroid synthesis, with ACTH and corticosterone being intimately involved in central and peripheral responses to stress.
Increases in intracellular calcium can also serve as a trigger for neurosteroid synthesis. In peripheral cells, both calcium influx via low-voltage-activated (T-type) calcium channels (Rossier, 2006) and calcium release from intracellular stores (Capponi et al., 1988; Charradi et al., 1996) promote cholesterol trafficking and initiate the conversion of cholesterol to pregnenolone. In the CNS, calcium influx via N-methyl-D-aspartate glutamate receptors (NMDARs) enhances pregnenolone formation and the synthesis of neurosteroids in hippocampus (Kimoto et al., 2001) and retina (Guarneri et al., 1998). Recent studies have found that low-level tonic NMDAR activation is sufficient to promote neurosteroid synthesis in hippocampal pyramidal neurons (Tokuda et al., 2011). Furthermore, neurosteroid production in the hippocampus by low-level NMDAR activation negatively modulates the induction of long-term potentiation (LTP) by initiating a form of neurosteroid-dependent metaplasticity (Tokuda et al., 2011). How NMDARs activate neurosteroid synthesis is not well understood, although it is known that the metaplastic effects of low-level NMDAR activation involve calcium, calcineurin, nitric oxide synthase and p38 mitogen activated protein kinase at the minimum (Izumi et al., 1992a, 2008; Kato and Zorumski, 1993; Zorumski and Izumi, 2012). The role of NMDARs in promoting neurosteroid synthesis in pyramidal neurons provides a potential link between acute stress and steroid production. There is evidence that acute stress increases extracellular glutamate in the CNS. These changes in glutamate levels likely reflect corticosterone-induced enhancement of glutamate release at synapses (Popoli et al., 2012) as well as changes in glutamate uptake (Sandi, 2011). Interestingly, acute stress, like low-level NMDA receptor activation, can impair LTP induction and learning via an NMDAR-dependent mechanism (Izumi et al., 1992b; Kim et al., 1996; Yang et al., 2008; Zorumski and Izumi, 2012). These findings may be relevant to clinical depression because changes in NMDAR function are thought to be associated with the depressed state (Marsden, 2011).
Because clinical depression and stress-related psychiatric disorders represent relatively persistent states lasting months to years in some cases, it is also important to consider how changes in neurosteroid levels occur as a result of repeated or chronic stress. As noted, chronic stress and depression are associated with decrements in neurosteroid levels. While the mechanisms driving these longer-term changes are not entirely clear, homeostatic changes resulting from tonic glutamate accumulation and altered glucocorticoid levels could contribute. Studies in rodents and humans also provide support for downregulation of key steps in neurosteroid synthesis, including changes in the expression of TSPO (Rupprecht et al., 2010) and 5α-reductase (Dong et al., 2001; Agis-Balboa et al., 2007) as a result of more persistent stress exposure.
8. Potential therapeutic approaches: correcting defective circuits
Neurosteroids have multiple actions that make them potentially attractive neurotherapeutic agents. We have emphasized their ability to modulate GABAARs because these receptors are a major site of action in the brain and there is increasing evidence for GABAergic dysfunction in mood disorders including depression (Luscher et al., 2011) and bipolar disorder (Benes et al., 2008). Even if defects in the GABA and endogenous neurosteroid systems play only an indirect role in the etiology of neuropsychiatric disorders (evidence above notwithstanding), neurosteroids could represent an effective way of circumventing core neurocircuit dysfunction based on their powerful modulatory actions. Additionally, some neurosteroids, particularly C3-sulfates, have effects on glutamate-mediated excitatory transmission via effects on NMDARs and these could contribute to therapeutic effects. PREGS is an example of a sulfated neurosteroid that positively modulates some NMDARs, while other sulfated steroids (e.g. pregnanolone sulfate and epipregnanolone sulfate) inhibit these receptors (Wu et al., 1991; Park-Chung et al., 1997). The effects of PREGS on NMDARs appear to be subtype selective with positive modulatory actions on NR1/NR2A and NR1/NR2B receptors, and inhibitory effects on receptors expressing NR2C or NR2D subunits in combination with NR1 (Malayev et al., 2001; Gibbs et al., 2006). Effects on NMDARs could be important for new drug development in depression given recent studies indicating the effectiveness of the NMDAR antagonist and dissociative anesthetic, ketamine, as a rapidly acting antidepressant treatment (Zarate et al., 2006; Machado-Vieira et al., 2009; aan het Rot et al., 2012). However, a current challenge in developing steroidal NMDAR antagonists for clinical use is that these agents are generally of low potency. Nonetheless, if both GABAAR enhancers and NMDAR inhibitors have potential as antidepressants, then an ideal agent might be one that combines these effects in a single molecule. Such an agent might also overcome problems with untoward psychotomimetic and pathomorphological effects of NMDAR antagonists such as ketamine (Olney et al., 1991). A proof of concept for such an agent is provided by the synthetic neuroactive steroid, 3α,5β-20-oxo-pregnane-3-carboxylic acid (3α5β-PC). This steroid has a carboxyl substitution at C3 and has dual actions, enhancing GABAARs and inhibiting NMDARs (Mennerick et al., 2001). 3α5β-PC diminishes ethanol self-administration in rodents (O’Dell et al., 2005), but has not yet been examined in models of stress and depression.
Multiple antidepressant treatments enhance neurogenesis in the dentate gyrus, and this effect is associated with improvement in some, but not all depressive symptoms in rodent models (Airan et al., 2007; Pollak et al., 2008; Santarelli et al., 2003). Certain neurosteroids such as alloP have also been reported to enhance dentate neurogenesis (Charalampopoulos et al., 2008; Wang et al., 2010; Irwin et al., 2012). Furthermore, intrahippocampal injections of alloP exert both antidepressant and anxiolytic effects in rodents, suggesting that effects on hippocampal circuitry may be germane to therapeutic actions (Nin et al., 2008). Recent studies suggest that enhanced neurogenesis in the dentate gyrus contributes to antidepressant effects because the new adult-born neurons play a significant role in dampening corticosterone release and in mediating stress-induced behaviors via regulation of the HPA axis (Snyder et al., 2011; Surget et al., 2011). AlloP and other steroids also have neuroprotective actions including effects on apoptosis and glutamate-mediated excitotoxicity (Marx et al., 2011). Thus, these steroids could be well positioned to alter hippocampal and HPA function in the face of stressors. An intriguing twist on this story is that, under conditions of recurring stress, the normally inhibitory effects of GABA and neurosteroids on hypothalamic CRH neurons may become excitatory because of depolarizing changes in the Cl− equilibrium potential resulting from altered expression of Cl− transporters. Under these conditions, GABA and neurosteroids may enhance stress responses via effects on extrasynaptic δ-containing GABAARs (Hewitt et al., 2009; Sarkar et al., 2011). How this circuitry is reset to baseline following stress and the role of changes in neurosteroid levels in modulating this circuitry remain uncertain, but these data suggest that the effects of neurosteroids are likely to be complex and dependent upon the timing of stress responses and neuroactive steroid administration (Mody and Maguire, 2012). Even with this caveat, it is clear that neurosteroids are important modulators of stress responses and the preponderance of evidence indicates that neuroactive steroids have antidepressant and anxiolytic actions in chronic stress models via their ability to enhance inhibitory effects of GABA (Dong et al., 2001).
There are several potential strategies that can be used to manipulate the actions of neurosteroids in the treatment of neuropsychiatric disorders (Fig. 6). Perhaps the most straightforward approach is the use of synthetic neuroactive steroids to supplement or replenish endogenous agents. Such an approach is exemplified by the development of alphaxalone as an anesthetic drug in the 1980’s. Althesin, a combination of the 5α-reduced steroids alphaxalone and alphadolone acetate, was an effective intravenous anesthetic with relatively fast rates of onset and offset of action and favorable cardiovascular side effects (Morgan and Whitwam, 1985; Zorumski et al., 2000). Unfortunately, allergic reactions to formulation in Cremophor EL led to discontinuation of Althesin as an anesthetic in humans. The neuroactive steroid, ganaxolone, is another example of a synthetic neuroactive steroid that has been used in clinical trials for epilepsy and migraine headaches (Monaghan et al., 1997). Ganaxolone is a GABAAR-enhancing, 5α-reduced pregnane steroid with a 3β-methyl substitution that helps to protect the C3 hydrogen bond donor from enzymatic transformation in vivo. Although ganaxolone has low oral bioavailability, clinical experience with this compound, both with respect to efficacy in epilepsy and safety have been encouraging. As with many steroids, the low aqueous solubility and poor oral bioavailability of ganaxolone have been problematic although more recent efforts have been directed toward improving delivery of this agent through novel formulations (Reddy and Rogawski, 2009). Potential complicating issues with neuroactive steroids include possible endocrine effects via actions on intracellular (hormonal) steroid receptors. However, there are synthetic strategies that can circumvent these off-target actions, including the use of enantiomers that act on GABAARs but have little or no activity at conventional intracellular nuclear hormone receptors. Enantiomers of etiocholanolone and androsterone are examples in which unnatural enantiomers are effective GABAAR modulators while their natural counterparts are weak nuclear hormone receptor agonists at best (Katona et al., 2008; Krishnan et al., 2012). Interestingly, these ent-steroids may interact with sites on GABAARs that are distinct from natural steroid sites (Li et al., 2007a). An attractive feature of using exogenous neuroactive steroids as treatments is that these agents would be well positioned to circumvent defects in the synthesis of endogenous neurosteroids that have been described in depression and chronic stress.
Fig. 6.

The diagram lists potential therapeutic strategies for altering the function of neurosteroids.
An alternative approach is to use agents that alter neurosteroid biosynthesis. For example, enhanced activity of the steroidogenic enzyme, 3α-HSD, appears to underlie the ability of fluoxetine to increase brain alloP levels (Griffin and Mellon, 1999). Other strategies for altering alloP levels include the use of precursors such as pregnenolone. Indeed, Marx et al. (2009) have done human proof-of-concept studies demonstrating that oral pregnenolone can enhance the endogenous production of several neurosteroids, including alloP, and can improve negative symptoms of schizophrenia. While preliminary, these results suggest a potential avenue to treatment. The mitochondrial translocator protein, TSPO, also represents a target for enhancing endogenous neurosteroid production and there is evidence for diminished expression or function of TSPO in several psychiatric disorders including anxiety disorders and depression with anxiety (Rupprecht et al., 2010). Although there are ligands for imaging TSPO in the human brain (Owen and Matthews, 2011), the work to date on the role of TSPO in psychiatric disorders has largely focused on changes in peripheral tissues. TSPO agonists promote the movement of cholesterol from the cytoplasm to the inner mitochondrial membrane to initiate neurosteroid synthesis. Early work indicated that TSPO agonists have anxiolytic effects in rodents (Romeo et al., 1992) that are mediated at least in part via effects in the hippocampus (Bitran et al., 2000), and these agents have been shown to modulate GABAAR function (Rupprecht et al., 2009; Tokuda et al., 2010). Recent work identified a novel agent, XBD173, that increases neurosteroid production and, in preliminary human studies, has anxiolytic effects comparable to the BDZ, alprazolam, but with diminished side effects (Rupprecht et al., 2009).
A potential problem in the use of TSPO agonists or other agents that enhance neurosteroid synthesis is that these agents would be predicted to increase steroid production in both the periphery and in the CNS, possibly resulting in complex off-target systemic effects. Another significant concern with efforts to enhance neurosteroids endogenously or with exogenous synthetic agents is that these steroids are very potent and effective modulators of tonic and phasic GABAergic inhibition. Thus, sedation, incoordination, cognitive impairment and other side effects could limit therapeutic use. In both rodents and humans, however, this does not appear to be a major problem and these agents can improve anxiety and anhedonia at levels that do not cause significant adverse effects (Marx et al., 2009; Rupprecht et al., 2009). Neurosteroids have an additional benefit in that they may also have positive effects on alcoholism and substance use disorders that are often comorbid with depression and anxiety disorders (Morrow et al., 2006). Ethanol stimulates neurosteroid production acutely and it is possible that longer-term effects of repeated ethanol use on neurosteroid levels may contribute to anxiety and depressive syndromes that arise in the context of alcohol abuse (Morrow et al., 1999, 2001) as well as defects in cognition and the function of neurocircuits (VanDoren et al., 2000; Sanna et al., 2004; Izumi et al., 2007).
9. Summary and future directions
The evidence reviewed here strongly suggests that CNS and peripheral neurosteroid production is altered in depression and stress-related conditions. Furthermore, alterations in neurosteroids likely cut across current psychiatric diagnoses and include mood, anxiety, substance abuse and possibly psychotic disorders. Exogenous neuroactive steroids and approaches that enhance endogenous neurosteroid synthesis, either by precursor strategies or by targeting biosynthetic pathways, have potential for pharmaceutical development. Given their role as local endogenous modulators in the brain, neurosteroids may be uniquely positioned to modulate neuronal networks involved in several neuropsychiatric disorders, and have the potential to correct a biochemical defect contributing to multiple symptoms and dysfunction. Despite the fact that GABA-enhancing steroids are potent and highly effective modulators, their effects both in vivo and in vitro can be relatively subtle in terms of changes in behavior and neuronal circuits (Tokuda et al., 2010). It is important to note, however, that the aqueous potency of highly lipophilic neuroactive steroids such as alloP and its derivatives does not necessarily translate into high potency at membranous sites of action on receptors and ion channels where the local concentration vastly exceeds the aqueous concentration (Chisari et al., 2010a). The high lipophilicity indicates that these agents can accumulate at high concentrations in membranes, and thus, their effects can result from low affinity interactions with specific targets. Furthermore, information about the importance of membrane partitioning and intracellular pools of these neuroactive steroids in mediating their pharmacological effects is relatively sparse. Such membrane partitioning and sequestration could provide mechanisms for modulating excessive effects (Li et al., 2007b) or possibly for providing reservoirs for more prolonged activity at key sites of action (Akk et al., 2005; Chisari et al., 2009). The latter observations may underlie the fact that exogenous applications of alloP or TSPO agonists have relatively subtle effects on hippocampal network function, but can modulate and markedly potentiate other agents acting on GABAARs or other receptors (Tokuda et al., 2010, 2011).
Although we have emphasized the importance of GABA (and glutamate) receptors, neurosteroids have other synaptic and extrasynaptic “targets” that could contribute to their psychotherapeutic actions, including potent effects on other receptors and channels. Several lines of evidence also suggest a role for mitochondrial and microtubule dysfunction in psychiatric illnesses including mood and psychotic disorders (Manji et al., 2012). Effects on these systems (Midzak et al., 2011b), including the ability of some neuroactive steroids to bind to mitochondrial associated proteins such as VDAC (Darbandi-Tonkabon et al., 2003) and microtubule proteins such as MAP-2 (Bianchi and Baulieu, 2012) and tubulin (Chen et al., 2012), are also potential targets for therapeutic intervention. Indeed, recent animal studies suggest that a novel steroid, 3β-methoxy-pregnenolone, has antidepressant actions via effects on microtubules (Bianchi and Baulieu, 2012). The neuroprotective (Langmade et al., 2006) and neurorestorative effects of neurosteroids, including enhanced neurogenesis (Irwin et al., 2012), are also important to consider, in light of the repeated observation that stress-related psychiatric disorders are associated with changes in brain volume in hippocampus, neocortex and other regions (Zorumski and Rubin, 2011). Neurosteroids also have effects on pregnane xenobiotic receptors (PXRs), a class of nuclear receptors that regulates the expression of a variety of genes, including signaling pathways involved in mood, cognition and motivation (Frye et al., 2012). How their effects on alternative intracellular targets and other signaling pathways intersect with actions at plasma membrane GABA, glutamate or other ion channels remains to be determined. However, based on their interactions with multiple CNS targets, neurosteroids may represent good lead structures or starting points for further optimization into drugs that may prove useful for treating symptoms that are shared across a number of stress and mood-related neuropsychiatric disorders.
Acknowledgments
Work in the authors’ laboratories is supported by grants MH07791, GM47969, AA017413 and NS057105 from the National Institutes of Health, the Bantly Foundation and the Taylor Family Institute for Innovative Psychiatric Research. SMP and DFC are founding members and CFZ serves on the Scientific Advisory Board of Sage Therapeutics. We dedicate this paper to Robert Purdy, a good friend and pioneer in research on neuroactive steroids.
Abbreviations
- 5α-DHP
5α-dihydroprogesterone
- 3α-HSD
3α-hydroxysteroid dehydrogenase
- 3α5β-PC
3α,5β-20-oxo-pregnane-3-carboxylic acid
- 17-PA
3α5α-17-phenylandrost-16-en-3-ol
- ANT
adenine nucleotide transporter
- ACTH
adrenocorticotrophic hormone
- alloP
allopregnanolone
- BDZ
benzodiazepine
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CRH
corticotrophin releasing hormone
- DHEAS
dehydroepiandrosterone sulfate
- DBI
diazepam binding inhibitor
- ECT
electroconvulsive therapy
- fMRI
functional magnetic resonance imaging
- GABA
γ-aminobutyric acid
- GABAA Rs
GABAA receptors
- HPA
hypothalamic-pituitary-adrenal
- IPSCs
inhibitory postsynaptic currents
- LTP
long-term potentiation
- NMDARs
N-methyl-D-aspartate glutamate receptors
- CYP11A1
P450 side-chain cleavage enzyme
- PVN
paraventricular nucleus
- PXRs
pregnane xenobiotic receptors
- PREGS
pregnenolone sulfate
- rTMS
repetitive transcranial magnetic stimulation
- SSRIs
selective serotonin reuptake inhibitors
- logP
solubility in octanol vs. water
- StAR
steroidogenic acute regulatory protein
- TSPO
translocator protein 18 kDa
- TM
transmembrane regions
- VDAC
voltage-dependent anion channel
References
- aan het Rot M, Zarate CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biological Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. The Journal of Physiology. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. The Journal of Physiology. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Current Neuropharmacology. 2010;8:18–25. doi: 10.2174/157015910790909458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacology & Therapeutics. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Molecular Pharmacology. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABA-A receptor. The Journal of Neuroscience. 2005;25:11606–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakoskela JM, Covey DF, Kinnunen PK. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochimica et Biophysica Acta. 2007;1768:131–145. doi: 10.1016/j.bbamem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. The Journal of Dermatology. 2002;29:665–669. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML. Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Critical Reviews in Neurobiology. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormones in the brain: several mechanisms. In: Fuxe K, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Pergamon Press; Oxford: 1981. pp. 3–14. [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Progress in Hormone Research. 1997;52:1–32. [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. The Journal of Neuroscience. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Reviews. Neuroscience. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Sugguraju S, Walsh JP. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolar. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20935–20940. doi: 10.1073/pnas.0810153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Baulieu EE. 3β-methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1713–1718. doi: 10.1073/pnas.1121485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasyanptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. British Journal of Pharmacology. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Capponi AM, Rossier MF, Davies E, Vallotton MB. Calcium stimulates steroidogenesis in permeabilized bovine adrenal cortical cells. The Journal of Biological Chemistry. 1988;263:16113–16117. [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochemistry International. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends in Endocrinology and Metabolism. 2008;19:300–307. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Charradi N, Rossier MF, Vallotton MB, Capponi AM. Calcium stimulates intramitochondrial cholesterol transfer in bovine adrenal glomerulosa cells. The Journal of Biological Chemistry. 1996;271:25971–25975. doi: 10.1074/jbc.271.42.25971. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacology & Therapeutics. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Chen LH, Akentieva N, Lichti CF, Darbandi R, Hastings R, Covey DF, Reichert DE, Townsend RR, Evers AS. A neurosteroid analogue photolabeling reagent labels the cochicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis. 2012;33:666–674. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends in Neurosciences. 2010a;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low affinity interaction. Journal of Neurophysiology. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Shu HJ, Taylor A, Steinbach JH, Zorumski CF, Mennerick S. Structurally diverse amphiphiles exhibit biphasic modulation of GABAA receptors: similarities and differences with neurosteroid actions. British Journal of Pharmacology. 2010b;160:130–141. doi: 10.1111/j.1476-5381.2010.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Wu K, Zorumski CF, Mennerick S. Hydrophobic anions potently and uncompetitively antagonize GABA-A receptor function in the absence of a conventional binding site. British Journal of Pharmacology. 2011;164:667–680. doi: 10.1111/j.1476-5381.2011.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DF, Evers AS, Mennerick S, Zorumski CF, Purdy RH. Recent developments in structure-activity relationships for steroid modulators of GABAA receptors. Brain Research Reviews. 2001;37:91–97. doi: 10.1016/s0165-0173(01)00126-6. [DOI] [PubMed] [Google Scholar]
- Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid (A) receptor modulation and anesthesia. The Journal of Pharmacology and Experimental Therapeutics. 2000;293:1009–1016. [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Research. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neuroscience. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Danovich L, Veenman L, Leschiner S, Lahav M, Shuster V, Weizman A, Gavish M. The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzoditor and steroid production. European Neuropsychopharmacology. 2008;18:24–33. doi: 10.1016/j.euroneuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Darbandi-Tonkabon R, Hastings WR, Zeng CM, Akk G, Manion BD, Bracamontes JR, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. The Journal of Biological Chemistry. 2003;278:13196–13206. doi: 10.1074/jbc.M213168200. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Vaudry D, Luu-The V, Tonon MC, Tsutsui K, Pelletier G, Vaudry H. Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Frontiers in Endocrinology. 2012;3:1–15. doi: 10.3389/fendo.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. The Journal of Neuroscience. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S. Activation dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. The Journal of Physiology. 2003;550:679–691. doi: 10.1113/jphysiol.2003.043810. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Romeo E, Baghai TC, Di Michele F, Schule C, Pasini A, Zwanzger P, Padgerg F, Rupprecht R. Neuroactive steroids as modulators of depression and anxiety. Neuroscience. 2006;138:1041–1048. doi: 10.1016/j.neuroscience.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Evers AS, Chen ZW, Manion B, Han M, Jiang X, Darbandi-Tonkabon R, Kable T, Bracamontes J, Zorumski CF, Mennerick SJ, Steinbach JH, Covey DF. A synthetic 18-norsteroid distinguishes between two neuroactive steroid binding sites on GABAA receptors. The Journal of Pharmacology and Experimental Therapeutics. 2010;333:404–413. doi: 10.1124/jpet.109.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Reviews. Neuroscience. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Appollonio I, Bianchi G, Frigo M, Marzorati C, Pecora N, Perego M, Pierpaoli C, Frattola L. Benzodiazepine receptors and diazepam binding inhibitor: a possible link between stress, anxiety and the immune system. Psychoneuroendocrinology. 1993;18:3–22. doi: 10.1016/0306-4530(93)90051-l. [DOI] [PubMed] [Google Scholar]
- Ferrarese C, Mennini T, Pecora N, Pierpaoli C, Frigo M, Marzorati C, Gobbi M, Bizzi A, Codegoni A, Garattini S. Diazepam binding inhibitor (DBI) increases after acute stress in rat. Neuropharmacology. 1991;30:1445–1452. doi: 10.1016/s0028-3908(11)80015-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and mechanisms of 3α5α-THP on emotion, motivation and reward functions involving pregnane xenobiotic receptor. Frontiers in Neuroscience. 2012;5:1–18. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC. Anterior cingluate cortex γ-aminobutyric acid in depressed adolescents. Archives of General Psychiatry. 2011;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Critical Reviews in Neurobiology. 1995;9:207–227. [PubMed] [Google Scholar]
- Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous modulators. Pharmacology, Biochemistry, and Behavior. 2006;8:555–567. doi: 10.1016/j.pbb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacology & Therapeutics. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? The Journal of Neuroscience. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri P, Russo D, Cascio C, De Leo G, Piccoli F, Guarneri R. Induction of neurosteroid synthesis by NMDA receptors in isolated rat retina: a potential early event in excitotoxicity. The European Journal of Neuroscience. 1998;10:1752–1763. doi: 10.1046/j.1460-9568.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABAA receptor interactions: a focus on stress. Frontiers in Neuroscience. 2011;5:1–20. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. The Journal of Neuroscience. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacology & Therapeutics. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Molecular Neurobiology. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kruz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nature Neuroscience. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Current Opinion in Neurobiology. 2011;22:1–7. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2008;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors via two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? The Journal of Neuroscience. 2012;32:3887–3897. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglehart JK. The mental health maze and the call for transformation. The New England Journal of Medicine. 2004;350:507–514. doi: 10.1056/NEJMhpr032740. [DOI] [PubMed] [Google Scholar]
- Insel T, Sahakian BJ. A plan for mental illness. Nature. 2012;483:269. doi: 10.1038/483269a. [DOI] [PubMed] [Google Scholar]
- Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? The Journal of Sexual Medicine. 2012a doi: 10.1111/j.1743-6109.2012.02846.x. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. The Journal of Clinical Psychiatry. 2012b;73:1220–1223. doi: 10.4088/JCP.12m07887. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Wang JM, Chen S, Brinton RD. Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Frontiers in Endocrinology. 2012;2:1–14. doi: 10.3389/fendo.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992a;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Low concentrations of N-methyl-D-aspartate inhibit the induction of long-term potentiation in rat hippocampal slices. Neuroscience Letters. 1992b;137:245–248. doi: 10.1016/0304-3940(92)90414-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Murayama K, Tokuda K, Krishnan K, Covey DF, Zorumski CF. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. The European Journal of Neuroscience. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258–265. doi: 10.1002/hipo.20383. [DOI] [PubMed] [Google Scholar]
- Jin X, Covey DF, Steinbach JH. Kinetic analysis of voltage-dependent potentiation and block of the glycine alpha 3 receptor by a neuroactive steroid analogue. The Journal of Physiology. 2009;587:981–987. doi: 10.1113/jphysiol.2008.159343. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GAR. GABAA receptor channel pharmacology. Current Pharmaceutical Design. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- Kato K, Zorumski CF. Nitric oxide inhibitors facilitate the induction of hippocampal long-term potentiation by modulating NMDA responses. Journal of Neurophysiology. 1993;70:1260–1263. doi: 10.1152/jn.1993.70.3.1260. [DOI] [PubMed] [Google Scholar]
- Katona BW, Krishnan D, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. European Journal of Medicinal Chemistry. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kawano T, Soga T, Chi H, Eguchi S, Yamazaki F, Kumagai N, Yokoyama M. Role of the neurosteroid allopregnanolone in the hyperalgesic behavior induced by painful nerve injury in rats. Journal of Anesthesia. 2011a;25:942–945. doi: 10.1007/s00540-011-1216-2. [DOI] [PubMed] [Google Scholar]
- Kawano T, Soga T, Chi H, Eguchi S, Yamazaki F, Yokoyama M. The involvement of the neurosteroid allopregnanolone n the antihyperalgesic effect of paroxetine in a rat model of neuropathic pain. Neuroreport. 2011b;22:984–988. doi: 10.1097/WNR.0b013e32834da80d. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kang SS, Cho GJ, Choi WS. Steroidogenic acute regulatory protein: its presence and function in brain neurosteroidogenesis. Archives of Histology and Cytology. 2004;67:383–392. doi: 10.1679/aohc.67.383. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizwa T, Ohta Y, Makino J, Tamura HO, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. The Journal of Neuroscience. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Sinkkonen ST. GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacology & Therapeutics. 2006;109:12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Manion BD, Taylor A, Bracamontes J, Steinbach JH, Reichert DE, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiating site on γ-aminobutyric acid type A (GABAA) receptors. Journal of Medicinal Chemistry. 2012;55:1334–1345. doi: 10.1021/jm2014925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DH, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. Steroidogenic acute regulatory protein in the brain. Neuroscience. 2006;138:741–747. doi: 10.1016/j.neuroscience.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Molecular Pharmacology. 2007a;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, Akk G. Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant alpha1beta2gamma2 GABA-A receptors. Molecular Pharmacology. 2006;69:2015–2026. doi: 10.1124/mol.106.022590. [DOI] [PubMed] [Google Scholar]
- Li P, Shu H-J, Wang C, Mennerick S, Zorumski CF, Covey DF, Steinbach JH, Akk G. Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation. The Journal of Physiology. 2007b;584:789–800. doi: 10.1113/jphysiol.2007.142794. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacology & Therapeutics. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to post-partum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Amer MS, Agarwal AK, McKeown L, Porter KE, O’Regan DJ, Naylor J, Fishwick CW, Muraki K, Beech DJ. Stereo-selecive inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. British Journal of Pharmacology. 2011;162:1509–1520. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neuroscience Letters. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Makriyannis A, DiMeglio CM, Fesik SW. Anesthetic steroid mobility in model membrane preparations as examined by high-resolution 1H and 2H NMR spectroscopy. Journal of Medicinal Chemistry. 1991;34:1700–1703. doi: 10.1021/jm00109a024. [DOI] [PubMed] [Google Scholar]
- Malayev AA, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulfate reveals subtype selective modulation of NMDA receptors by sulfated steroids. British Journal of Pharmacology. 2001;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nature Reviews. Neuroscience. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Marsden WN. Stressor-induced NMDAR dysfunction as a unifying hypothesis for the aetiology, pathogenesis and comorbidity of clinical depression. Medical Hypotheses. 2011;77:508–528. doi: 10.1016/j.mehy.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Marx CE, Bradford DW, Hamer RM, Naylor JC, Allen TB, Lieberman JA, Strauss JL, Kilts JD. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78–90. doi: 10.1016/j.neuroscience.2011.06.076. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley LD, Park CH, Lan NC, Tomich JM, Shively JE, Gee KW. Benzodiazepines and peptides stimulate pregnenolone synthesis in brain mitochondria. European Journal of Pharmacology. 1995;276:145–153. doi: 10.1016/0014-2999(95)00036-k. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Molecular Pharmacology. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zeng CM, Benz A, Shen W, Izumi Y, Evers AS, Covey DF, Zorumski CF. Effects on GABAA receptors of a neuroactive steroid that negatively modulates glutamate neurotransmission and augments GABA neurotransmission. Molecular Pharmacology. 2001;60:732–741. [PubMed] [Google Scholar]
- Midzak A, Akula N, Lecanu L, Papadopoulos V. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. The Journal of Biological Chemistry. 2011a;286:9875–9887. doi: 10.1074/jbc.M110.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Molecular and Cellular Endocrinology. 2011b;336:70–79. doi: 10.1016/j.mce.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABAA receptors. Frontiers in Cellular Neuroscience. 2012;6:1–6. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JE. Actual causes of death in the United States, 2000. JAMA: the Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA. Initial human experience with ganaxalone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1997;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Morgan M, Whitwam JG. Althesin. Anaesthesia. 1985;40:121–123. doi: 10.1111/j.1365-2044.1985.tb10700.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcoholism, Clinical, and Experimental Research. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues in Clinical Neuroscience. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Research Reviews. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature Neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA: the Journal of the American Medical Association. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- Nilsson KR, Zorumski CF, Covey DF. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dephydroepiandrosterone sulfate, pregnenolone sulfate, and (3 alpha, 5 beta)-3-hydroxypregnan-20-one sulfate. Journal of Medicinal Chemistry. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- Nin MS, Salles FB, Azeredo LA, Frazon AP, Gomez R, Barros HM. Antidepressant effect and changes of GABA(A) receptor gamma2 subunit after hippocampal administration of allopregnanolone in rats. Journal of Psychopharmacology. 2008;22:477–485. doi: 10.1177/0269881107081525. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacology, Biochemistry, and Behavior. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Matthews PM. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. International Review of Neurobiology. 2011;101:19–39. doi: 10.1016/B978-0-12-387718-5.00002-X. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends in Pharmacological Sciences. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Guarneri P, Krueger KE, Guidotti A, Costa E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5113–5117. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH. Steroid inhibition of rat neuronal nicotinic α4β2 receptors expressed in HEK 293 cells. Molecular Pharmacology. 2000;58:341–351. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Molecular Pharmacology. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotrophin-releasing hormone-induced anxiety and alters the release and gene expression of corticotrophin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Brimelow BC, Jagodic MM, Krishnanm K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. The FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Phillipps GH. Structure-activity relationships in steroidal anaesthetics. In: Halsey MJ, Millar RA, Sutton JA, editors. Molecular Mechanisms in General Anaesthesia. Churchill Livingstone; Edinburgh: 1974. pp. 32–46. [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews. Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil GA, Schneider-Gasser EM, Rudolph U, Kreist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. Journal of Neurophysiology. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clinical Pharmacology. 2006;6:7. doi: 10.1186/1472-6904-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biological Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Auta J, Kozikowski AP, Ma D, Papadopoulos V, Puia G, Costa E, Guidotti A. 2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR) The Journal of Pharmacology and Experimental Therapeutics. 1992;262:971–978. [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. The American Journal of Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochimica et Biophysica Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier MF. T channels and steroid biosynthesis: in search of a link with mitochondria. Cell Calcium. 2006;40:155–164. doi: 10.1016/j.ceca.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature Reviews. Drug Discovery. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature Reviews. Drug Discovery. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature Reviews. Drug Discovery. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Saalman YB, Kirkcaldie MTK, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. Journal of Neuroendocrinology. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Critical Reviews in Neurobiology. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends in Neurosciences. 2011;34:165–176. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABA-A receptor activity in rat hippocampus. The Journal of Neuroscience. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstraub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. The Journal of Neuroscience. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso O, Russo R, Vitiello S, Raso GM, D’Agostino G, Iacono A, Rana GL, Vallee M, Cuzzocrea S, Piazza PV, Meli R, Calignano A. Implication of allo-pregnanolone in the antinociceptive effect of N-palmitoylethanolamine in acute or persistent pain. Pain. 2012;153:33–41. doi: 10.1016/j.pain.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Schlichter R, Keller AF, Do Roo M, Breton JD, Inquimbert P, Poisbeau P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. Journal of Molecular Neuroscience. 2006;28:33–51. doi: 10.1385/jmn:28:1:33. [DOI] [PubMed] [Google Scholar]
- Schule C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetic effect of steroid hormones. Proceedings of the Society for Experimental Biology and Medicine. 1941;46:116–121. [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reveral of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nature Neuroscience. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. Gamma-aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biological Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. The Journal of Neuroscience. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Zorumski EC, Covey DF, Zorumski CF. Pregnenolone sulfate and dehydroepiandrosterone sulfate inhibit GABA-gated chloride currents in Xenopus oocytes expressing picrotoxin-insensitive GABAA receptors. Neuropharmacology. 1999;38:267–271. doi: 10.1016/s0028-3908(98)00172-5. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori T, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochimica et Biophysica Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, McCollum M, Akk G, Manion B, Evers AS, Krishnan K, Covey DF, Zorumski CF, Steinbach JH, Mennerick S. Characteristics of α4/δ-containing GABAA receptors expressed in Xenopus oocytes. British Journal of Pharmacology. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABA-A receptors. The Journal of Neuroscience. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviors. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biological Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Molecular Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh T, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5β-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Molecular Pharmacology. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, Covey DF, Lingle CJ. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid lacking GABA-modulatory activity. Molecular Pharmacology. 1998;54:918–927. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. The Journal of Neuroscience. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K, O’Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. The Journal of Neuroscience. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DM, Ransom RW, Yang JSJ. Olsen RW: steroid anesthetics and naturally occurring analogs modulate the (-aminobutyric acid receptor complex at a site distinct from barbiturates. The Journal of Pharmacology and Experimental Therapeutics. 1989;248:960–966. [PubMed] [Google Scholar]
- Ugale RR, Hirani K, Morelli M, Chopde CT. Role of neuroactive steroid allopregnanolone in antipsychotic-like action of olanzepine in rodents. Neuropsychopharmacology. 2004;29:1597–15609. doi: 10.1038/sj.npp.1300460. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez CM, Smith MA, Perry G, Phelix CF, Santamaria F. Cholesterol homeostasis markers are localized to mouse hippocampal pyramidal and granule layers. Hippocampus. 2010;20:902–905. doi: 10.1002/hipo.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Partridge LD, Mameli M, Meyer DA. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Research Reviews. 2008;57:506–519. doi: 10.1016/j.brainresrev.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Analytical Biochemistry. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RE, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3β-Hydroxypregnane steroids are pregnenolone sulfate-like GABAA-receptor antagonists. The Journal of Neuroscience. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Molecular Pharmacology. 1996;50:1581–1586. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA (A) receptors containing the delta subunit. The Journal of Neuroscience. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Molecular Pharmacology. 1991;40:333–336. [PubMed] [Google Scholar]
- Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annual Review of Pharmacology and Toxicology. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- Yang J, Isenberg KE, Zorumski CF. Volatile anesthetics gate a chloride current in postnatal rat hippocampal neurons. The FASEB Journal. 1992;6:914–918. doi: 10.1096/fasebj.6.3.1740240. [DOI] [PubMed] [Google Scholar]
- Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. The Journal of Biological Chemistry. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neuroscience and Biobehavioral Reviews. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Covey DF. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–171. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Isenberg KE, Covey DF. Potential clinical uses of neuroactive steroids. Current Opinion in Investigational Drugs. 2000;1:360–369. [PubMed] [Google Scholar]
- Zorumski CF, Rubin EH. Psychiatry and Clinical Neuroscience: A Primer. Oxford University Press; New York: 2011. [Google Scholar]