Key Points
The central nervous system can be a direct target of alloreactive T cells during GVHD.
Central nervous system damage in mouse models of GVHD lead to deficits in learning and increased anxiety behavior.
Abstract
Despite significant advances in prevention and management, graft versus host disease (GVHD) is still a leading complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although skin, gut, liver, thymus, and lung are GVHD targets, neurological complications (NC) have also been reported following allo-HSCT. We demonstrate that the central nervous system (CNS) can be a direct target of alloreactive T cells following allo-HSCT in mice. We found significant infiltration of the CNS with donor T lymphocytes and cell death of neurons and neuroglia in allo-HSCT recipients with GVHD. We also found that allo-HSCT recipients with GVHD had deficits in spatial learning/memory and demonstrated increased anxious behavior. These findings highlight CNS sensitivity to damage caused by alloreactive donor T cells and represent the first characterization of target cell subsets and NC during GVHD. Therefore, these clinically relevant studies offer a novel and rational explanation for the well-described neurological symptoms observed after allo-HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is one of the only curative therapies for patients with hematological malignancies. However, its use is restricted by several major complications including graft-versus-host disease (GVHD), malignant relapse, and posttransplant immune deficiency.1,2 Although damage to gastrointestinal epithelium caused by alloreactive T cells is the primary contributor to acute GVHD-related mortality,3 there is growing experimental evidence that GVHD can be a risk factor for neurological complications (NC).4-8 NC such as central pareses, seizures, headaches, mental changes, consciousness disturbances, cerebellar signs, and cognitive deficits consistent with encephalopathies, meningoencephalitides, angiitis, atrophies, demyelination, and hemorrhages, have all been reported in patients who received an allo-HSCT.9-12 Typically, NC associated with allo-HSCT has been attributed to underlying primary disease, drug and radiation toxicity, or infection. These confounding factors have made a comprehensive assessment of the contribution of GVHD to NC in patients receiving allo-HSCT technically challenging. Thus, murine models of GVHD offer the best hope for distinguishing these competing etiological factors of NC after allo-HSCT and, in fact, lymphocytic infiltration into the brain has been reported in 2 murine models of GVHD.13,14 Given the lymphocytic infiltrate observed after allo-HSCT in both the clinical setting15,16 as well as limited animal studies, we hypothesized that the central nervous system (CNS) can in fact be a direct target of alloreactive T cells during GVHD, resulting in permanent or transient NC. Using an major histocompatibility complex–disparate murine allo-HSCT model to account for variables such as conditioning regimes, we performed immunologic, pathological, and neurobehavioral analyses to comprehensively assess the impact of GVHD on the CNS.
Methods
Mice
Female C57BL/6, B6.SJL-Ptprca Pepcb/BoyJ, BALB/c, and BALB/c-Thy1.1 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Allo-HSCT was performed as previously described17 using bone marrow that was T cell–depleted using anti–Thy-1.2 and rabbit complement (Cedarlane Laboratories, Homby, ON). Donor T cells (0.5 × 106 cells) were enriched by CD5 selection by MACS (Miltenyi, Auburn, CA). Control groups received syngeneic transplant by injecting T cell–depleted bone marrow and splenic T cells from BALB/c-Thy1.1 mice. All HSCT (syngeneic and allogeneic) recipients received total body irradiation (850 cGy split dose with each dose separated by 3 hours) as a conditioning regime. GVHD was monitored using a clinical scoring system for fur and skin pathology, motility, hunching, and weight loss. Studies were all approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
Flow cytometry
Mice were perfused with phosphate-buffered saline and their brains removed and homogenized in 1 mg/mL Collagenase D and 0.2 mg/mL DNase 1 (Roche Diagnostics, Germany). Mononuclear cells were isolated using Percoll 30% (GE Healthcare, Sweden). Cells were analyzed using an LSR II flow cytometer (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Ashland, OR).
Histopathology
Coronal sections were taken from paraffin-embedded brains. Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling sections were incubated with proteinase K and terminal deoxynucleotidyl transferase buffer. Location of CD3+ cells (meninges/ependyma, vessel, and parenchyma) and the degree of infiltration with lymphocytes (0 = none/minimal, 1 = mild, 2 = moderate, and 3 = marked, and 4 = severe) were determined.
Behavioral testing
Neuromuscular function and muscle strength was tested by grip test where time to release from an inverted grid was recorded. Motor coordination was assessed in an accelerated Rotorod (Series 8, IITC Life Sciences, St. Petersburg, FL); exploratory activity and anxiety were tested in an open field test; and learning and memory were assessed in the Morris water maze test as previously described.18
Statistics
Comparisons between 2 groups were made using the nonparametric, unpaired Mann-Whitney U test. Behavioral studies between 3 or more groups were evaluated using analysis of variance. All statistics were calculated, and display graphs generated, using Graphpad Prism. Graphs show mean ± standard error of the mean unless otherwise indicated.
Results and discussion
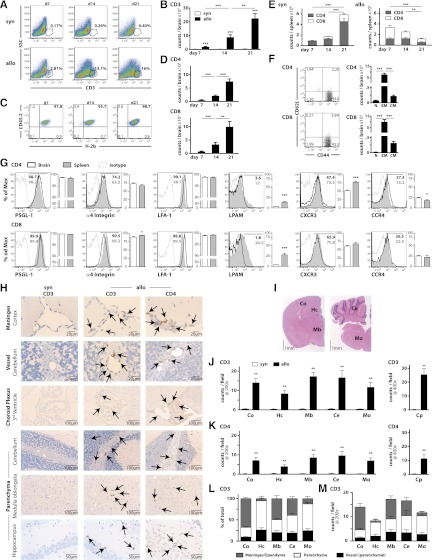
Consistent with previous studies13,14 using whole enzyme-digested brains, we found significant numbers of CD3+ T cells in the CNS on days 7, 14, and 21 in recipients of allo-HSCT compared with recipients of syngeneic HSCT (Figure 1A-B). CD3+ T cells isolated from allo-HSCT recipients were exclusively of donor origin (Figure 1C) and contained both CD4+ and CD8+ T cells (Figure 1D). Importantly, donor T cells were detectable in the periphery after both syn- and allo-HSCT (Figure 1E), demonstrating that CNS infiltration is not due to leakiness of the blood–brain barrier after irradiation. Infiltrating T cells primarily expressed an effector memory phenotype (CD44highCD62Llow) with only very few cells exhibiting a central memory phenotype (CD44lowCD62Lhigh). Naive T cells (CD44lowCD62Llow) were only detectable in CD4+ cells (Figure 1F). Compared with T cells in the periphery, CNS infiltrating T cells exhibited similar expression of PSGL-1, α4-Integrin, and LFA-1 as peripheral T cells, but lower expression of LPAM as well as a slight reduction in expression of CXCR3 (CD4 T cells) and α4-integrin (CD8 T cells) (Figure 1G). We also noted a small increase in CCR4 expression compared with splenic T cells. These findings are consistent with those of mass spectometry/experimental autoimmune encephalomyelitis in which (1) CD44 but not CD62L is important for T-cell recruitment to the CNS19; (2) expression of PSGL-1 and α4 integrin, but not LPAM, is essential for adhesion of T cells to brain vessels20-22; (3) LFA-1 is required for transendothelial migration23; and 4) CXCR3 mediates disease severity.24
Figure 1.
Donor T cells with a distinct phenotype infiltrate the CNS during acute GVHD. Flow cytometric analysis and histopathological CNS findings in syngeneic and allogeneic transplanted animals. Lethal GVHD (0.5 × 106 T cells) assessed at days 7, 14, and 21. (A-G) Mononuclear cells were isolated from brains of mice given syngeneic (5 × 106 Thy1.1 BALB/c T cell–depleted bone marrow (TCD BM) cells + 5 × 105 CD5+ Thy1.1 T cells transplanted into Thy1.2 BALB/c recipients) or allogeneic (5 × 106 Ly5.1 B6 TCD BM cells + 5 × 105 CD5+ B6 T cells transplanted into Thy1.2 BALB/c recipients) HSCT after complete perfusion on days 7 (n = 10-13), 14 (n = 13-14), and 21 (n = 48). (A) Representative flow cytometry plots detailing expression of CD3. (B) Absolute number of CNS infiltrating donor CD3+ T cells. (C) Origin of allogeneic CD3+ T cells (donor = CD45.2+H-2b+). (D) Absolute number of CD4+ or CD8+ allogeneic CD3+ T cells. (E) Absolute number of CD4+ and CD8+ T cells in syngeneic and allogeneic HSCT recipients in spleen (n = 8-10). (F) Representative plots and absolute numbers of naive (N, CD62L+CD44-), effector memory (EM, CD62L+CD44+), and central memory (CM, CD62L-CD44+) T cells in allo-HSCT recipients at day 21 (n = 31-37). (G) Expression of PSGL-1, α4-Integrin, LFA-1, LPAM, CXCR3, and CCR4 on CD4+ and CD8+ T cells in CNS and spleen at day 21 (n = 10-15). (H-M) Coronal sections were taken from paraffin-embedded brains 21 days after syn- (n = 10) or allo-HSCT (n = 10). (H) CD3 or CD4 staining. (I) Hematoxylin and eosin–stained section from an untreated mice demonstrating observed areas of the brain examined; cortex (Co), hippocampus (Hc), midbrain (Mb: thalamus, basal ganglia, hypothalamus), cerebellum (Ce), medulla oblongata (Mo), and choroid plexus (CP). Quantification of total CD3+ T cells (J) and CD4+ T cell (K) numbers in indicated areas. (L) Relative and (M) absolute distribution of CD3+ T cells in meninges/ependyma, parenchyma, and parenchymal vessels. Bar graphs represent mean ± standard error of the mean. All data are representative of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
CD3+ and CD4+ T cells were consistently identified in meninges, vascular walls, and parenchyma of the cortex, hippocampus, midbrain (thalamus, hypothalamus, basal ganglia), cerebellum, medulla oblongata, and in the choroid plexus after allo-HSCT but not after syn-HSCT (Figure 1H-I). Quantification demonstrated diffuse meningeal, vascular, and parenchymal infiltration in allo-HSCT recipients, whereas in syn-HSCT controls, T cells were virtually undetectable (Figure 1J-K). In addition, CD3+ lymphocytes were also observed in the meninges and neuropil of the spinal cord as well as the sciatic nerve (data not shown). In cortex, midbrain, and cerebellum, most T cells were located in the meninges or ependyma, whereas in hippocampus and medulla oblongata, the majority of T cells were present in the parenchyma (Figure 1L). However, absolute numbers revealed equivalent parenchymal infiltration in all of these sites (Figure 1M), consistent with models of infection, ischemias, and autoinflammatory diseases.22,25
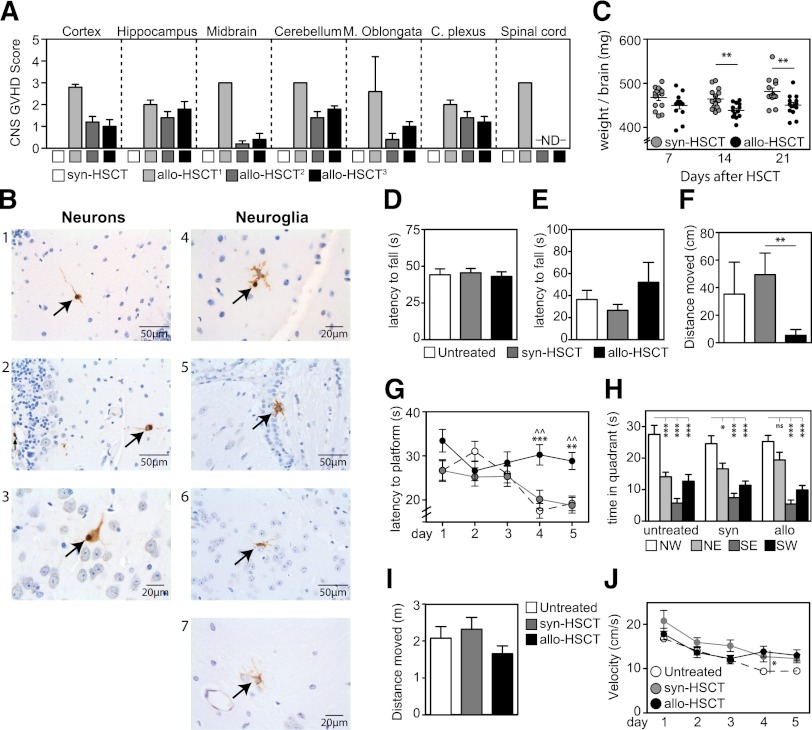
Histopathological analysis of brain sections revealed evidence of meningoencephalitis, choroiditis, and myelitis with moderate or marked lymphocytic infiltration on day 21 after allo-HSCT (Figure 2A). Restricted to recipients of allo-HSCT, we observed significant apoptosis in neurons and neuroglia (Figure 2B). Interestingly, as an indicator of organ toxicity, recipients of allo-HSCT exhibited significantly lower brain weights compared with syn-HSCT controls (Figure 2C).26 To evaluate cognitive function in mice after allo-HSCT, we performed a series of behavioral tests before the onset of severe GVHD symptoms, determined by disease score (data not shown). There was no observable difference in motor activity, coordination, strength, or neuromuscular function between allo-HSCT, syn-HSCT, or nontransplanted groups, assessed by accelerated Rotorod and grip tests (Figure 2D-E). However, recipients of allo-HSCT demonstrated reduced exploratory activity in the open field test, suggesting increased anxiety (Figure 2F) consistent with the pathophysiology of experimental autoimmune encephalomyelitis.27 Moreover, spatial learning and memory in recipients of allo-HSCT were significantly impaired compared with both syn-HSCT and untransplanted controls in the Morris water maze: allo-HSCT recipients did not improve finding the platform (Figure 2G) and failed to recall the target quadrant as precisely as syngeneic and untreated controls (Figure 2H). This is consistent with parenchymal involvement of the hippocampus.28 Importantly, the total distance moved in the open field test (Figure 2I) as well as the total distance moved (data not shown) and velocity in the Morris water maze (Figure 2J) were unchanged.
Figure 2.
Cerebral alloreactive T-cell infiltration leads to cell damage and cognitive deficits. (A) Histopathological CNS findings in syngeneic and allogeneic transplanted animals. Lethal GVHD (0.5 × 106 T cells) assessed at day 21 (Allo1) or nonlethal GVHD (0.25 × 106 T cells) assessed at days 42 (Allo2) and 63 (Allo3). Control groups received equal number of syngeneic T cells. GVHD score (0 within normal limits, ≥ 1 pathology) and estimated infiltration with lymphocytes (0 none/rare, 1 mild, 2 moderate, 3 marked, 4 severe) using immunohistochemistry for CD3. (B) Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling staining in representative coronal brain sections 21 days after allo-HSCT in cerebellum gray matter (1, 2, 4), cortex (3), corpus callosum (5), hypothalamus (6), and hippocampus (7). (C) Wet brain weights after allo- and syn-HSCT (n = 13-14). (D) Accelerated Rotorod test measuring motor coordination by latency to fall. (E) Grip test measuring strength and neuromuscular function where latency to fall was measured. (F) Exploratory activity and anxiety was measured in an open field test where distance moved in center squares was measured. (G-H) Spatial memory and learning were assessed in the gold standard Morris water maze. (G) Time spent finding the platform over 5 consecutive days or (H) time spent in each quadrant were measured. (I) Motor function and mobility were assessed by measuring the total distance moved in the open field test. (J) Mobility was measured by examining the velocity in the Morris water maze. *Represents a comparison between syn-HSCT and allo=HSCT; ^Represents a comparison between untreated and allo-HSCT. For all behavioral tests, n = 20/treatment group. Bar graphs represent mean ± standard error of the mean of at least 2 independent experiments. *P < .05; **P < .01, ***P < .001.
Using murine models of allo-HSCT to account for variables such as conditioning regimes, we found CNS infiltration of donor T cells in recipients of allo-HSCT consistent with previously reported studies.13,14 Importantly, we observed significant neurocognitive deficits, which were already apparent in the early stages of GVHD. Although further studies are needed in patients to carefully separate the impacts of cytoreductive conditioning and the alloreactive T-cell response, these findings present evidence that the CNS is a potential target of alloreactive T cells and suggest that GVHD could be a contributing factor in neurological and cognitive deficits following allo-HSCT.
Acknowledgments
The authors thank the staff of the Memorial Sloan-Kettering Cancer Center Research Animal Resources Center for excellent animal care and the staff of the Comparative Pathology Laboratory for assistance with sample preparation, immunohistochemistry, and microscopy.
This work was supported by the National Institutes of Health (R01-HL069929, R01-CA107096, R01-AI080455, and R01-HL095075) (M.R.M.v.d.B.), National Institute of Mental Health (5R01-MH086883 and 1R01-MH58669) (M.T.), US Department of Defense (USAMRAA Award W81XWH-09-1-0294) (M.R.M.v.d.B.), Radiation Effects Research Foundation (RERF-NIAID) (M.R.M.v.d.B.), Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, the Lymphoma Foundation, Alex’s Lemonade Stand, the Geoffrey Beene Cancer Research Center at Memorial Sloan-Kettering Cancer Center, and the Peter Solomon Fund. This work was also supported by the German Mehr-LEBEN-für-krebskranke-Kinder - Bettina-Braeu-Stiftung and the German Bayerische Forschungsstiftung Fellowship (S.H.), fellowships from the Australian National Health and Medical Research Council (CJ Martin Biomedical Research Fellowship, 1012401) and the Leukemia and Lymphoma Society (5534-11) (J.A.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: S.H., J.A.D., and M.R.M.v.d.B. designed, analyzed, and interpreted the experiments and wrote and drafted the manuscript. L.K.J. helped design and interpret experiments. O.M.S., J.T., N.V.S., and M.L.W. provided technical assistance and helped with animal handling. A.M.H., M.H.A., B.L., and M.T. helped design experiments and provided expert interpretation. M.R.M.v.d.B. directed the research. S.H., J.A.D., L.K.J., O.M.S., J.T., N.V.S., M.L.W., A.M.H., M.H.A., B.L., M.T., and M.R.M.v.d.B. discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel van den Brink, Immunology Program and Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: vandenbm@mskcc.org.
References
- 1.Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 2010;10(3):213–221. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 2.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4(11):856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 3.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. [PubMed] [Google Scholar]
- 4.Antonini G, Ceschin V, Morino S, et al. Early neurologic complications following allogeneic bone marrow transplant for leukemia: a prospective study. Neurology. 1998;50(5):1441–1445. doi: 10.1212/wnl.50.5.1441. [DOI] [PubMed] [Google Scholar]
- 5.Faraci M, Lanino E, Dini G, et al. Severe neurologic complications after hematopoietic stem cell transplantation in children. Neurology. 2002;59(12):1895–1904. doi: 10.1212/01.wnl.0000036608.42104.b9. [DOI] [PubMed] [Google Scholar]
- 6.Iguchi A, Kobayashi R, Yoshida M, et al. Neurological complications after stem cell transplantation in childhood. Bone Marrow Transplant. 1999;24(6):647–652. doi: 10.1038/sj.bmt.1701969. [DOI] [PubMed] [Google Scholar]
- 7.Sostak P, Padovan CS, Yousry TA, et al. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology. 2003;60(5):842–848. doi: 10.1212/01.wnl.0000046522.38465.79. [DOI] [PubMed] [Google Scholar]
- 8.Uckan D, Cetin M, Yigitkanli I, et al. Life-threatening neurological complications after bone marrow transplantation in children. Bone Marrow Transplant. 2005;35(1):71–76. doi: 10.1038/sj.bmt.1704749. [DOI] [PubMed] [Google Scholar]
- 9.Kamble RT, Chang CC, Sanchez S, et al. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant. 2007;39(1):49–52. doi: 10.1038/sj.bmt.1705540. [DOI] [PubMed] [Google Scholar]
- 10.Kew AK, Macaulay R, Burrell S, et al. Central nervous system graft-versus-host disease presenting with granulomatous encephalitis. Bone Marrow Transplant. 2007;40(2):183–184. doi: 10.1038/sj.bmt.1705709. [DOI] [PubMed] [Google Scholar]
- 11.Saad AG, Alyea EP, III, Wen PY, et al. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation. J Clin Oncol. 2009;27(30):e147–e149. doi: 10.1200/JCO.2009.21.7919. [DOI] [PubMed] [Google Scholar]
- 12.Sostak P, Padovan CS, Eigenbrod S, et al. Cerebral angiitis in four patients with chronic GVHD. Bone Marrow Transplant. 2010;45(7):1181–1188. doi: 10.1038/bmt.2009.323. [DOI] [PubMed] [Google Scholar]
- 13.Padovan CS, Gerbitz A, Sostak P, et al. Cerebral involvement in graft-versus-host disease after murine bone marrow transplantation. Neurology. 2001;56(8):1106–1108. doi: 10.1212/wnl.56.8.1106. [DOI] [PubMed] [Google Scholar]
- 14.Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103(9):3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 15.Unger ER, Sung JH, Manivel JC, et al. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52(5):460–470. doi: 10.1097/00005072-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rouah E, Gruber R, Shearer W, et al. Graft-versus-host disease in the central nervous system. A real entity? Am J Clin Pathol. 1988;89(4):543–546. doi: 10.1093/ajcp/89.4.543. [DOI] [PubMed] [Google Scholar]
- 17.Penack O, Henke E, Suh D, et al. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. J Natl Cancer Inst. 2010;102(12):894–908. doi: 10.1093/jnci/djq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F, Dumont M, Banerjee R, et al. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J. 2010;24(12):4639–4647. doi: 10.1096/fj.10-161471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brocke S, Piercy C, Steinman L, et al. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96(12):6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battistini L, Piccio L, Rossi B, et al. CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood. 2003;101(12):4775–4782. doi: 10.1182/blood-2002-10-3309. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt B, Laschinger M, Schulz M, et al. The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin. J Clin Invest. 1998;102(12):2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169(2):1000–1006. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 23.Laschinger M, Vajkoczy P, Engelhardt B. Encephalitogenic T cells use LFA-1 for transendothelial migration but not during capture and initial adhesion strengthening in healthy spinal cord microvessels in vivo. Eur J Immunol. 2002;32(12):3598–3606. doi: 10.1002/1521-4141(200212)32:12<3598::AID-IMMU3598>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Huang D, Matsui M, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3-/- mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176(7):4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 25.Wolburg K, Gerhardt H, Schulz M, et al. Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 1999;296(2):259–269. doi: 10.1007/s004410051287. [DOI] [PubMed] [Google Scholar]
- 26.Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32(4):448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 27.Peruga I, Hartwig S, Thöne J, et al. Inflammation modulates anxiety in an animal model of multiple sclerosis. Behav Brain Res. 2011;220(1):20–29. doi: 10.1016/j.bbr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Silva AJ, Giese KP, Fedorov NB, et al. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol Learn Mem. 1998;70(1-2):44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]