Abstract
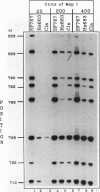
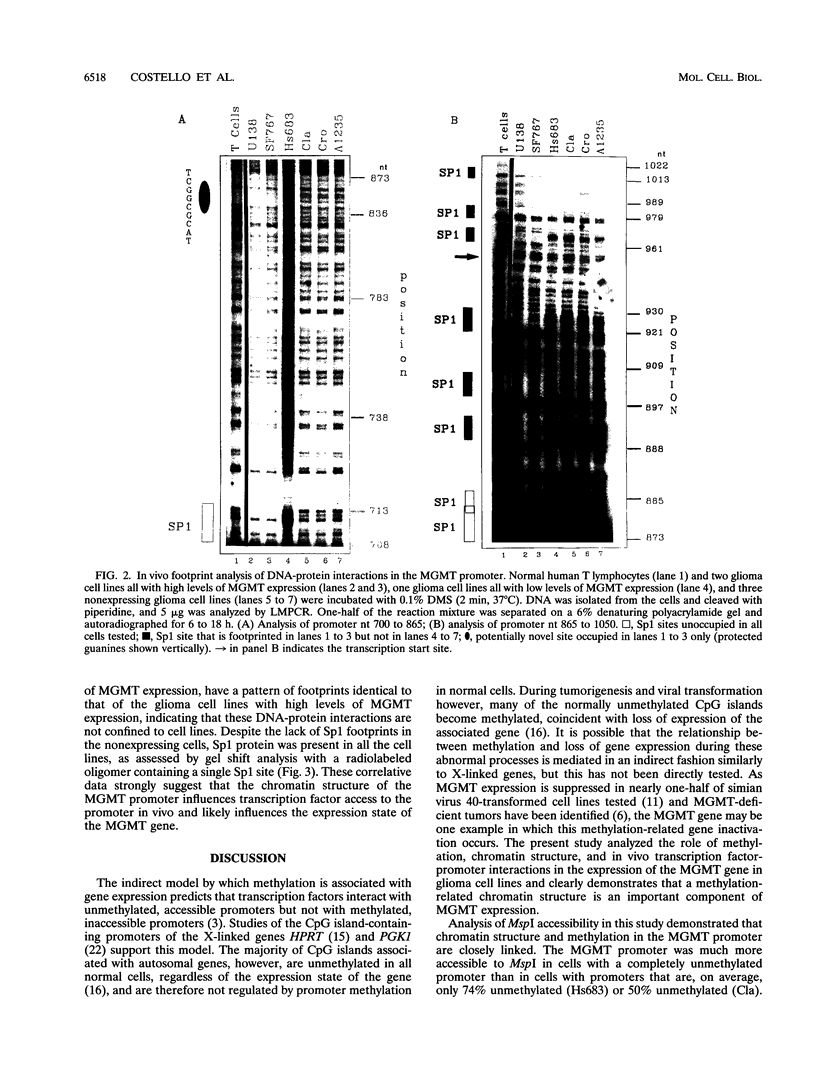
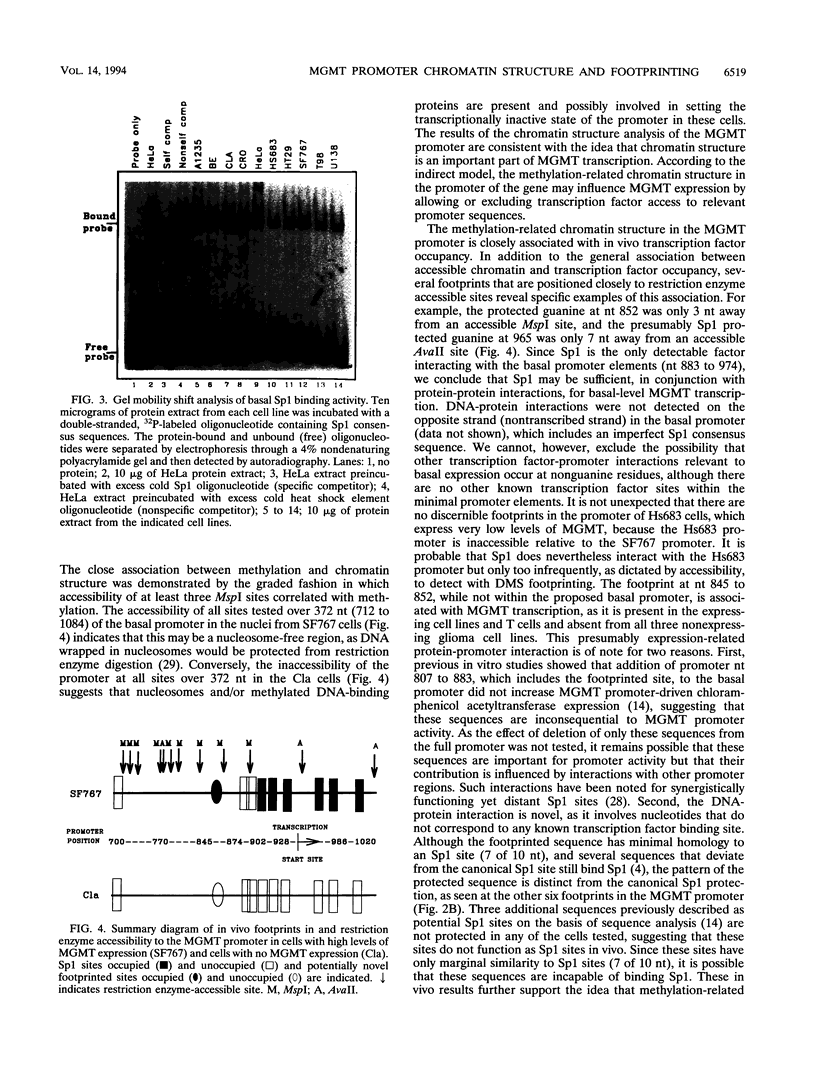
There is considerable interest in identifying factors responsible for expression of the O-6-methylguanine DNA methyltransferase (MGMT) gene, as MGMT is a major determinant in the response of glioma cells to the chemotherapeutic agent 1,3 bis(2-chloroethyl)-1-nitrosourea. Recently we have shown that MGMT expression is correlated in a direct, graded fashion with methylation in the body of the MGMT gene and in an inverse, graded fashion with promoter methylation in human glioma cell lines. To determine if promoter methylation is an important component of MGMT expression, this study addressed the complex interactions between methylation, chromatin structure, and in vivo transcription factor occupancy in the MGMT promoter of glioma cell lines with different levels of MGMT expression. Our results show that the basal promoter in MGMT-expressing glioma cell lines, which is 100% unmethylated, was very accessible to restriction enzymes at all sites tested, suggesting that this region may be nucleosome free. The basal promoter in glioma cells with minimal MGMT expression, however, which is 75% unmethylated, was much less accessible, and the basal promoter in nonexpressing cells, which is 50% unmethylated, was entirely inaccessible to restriction enzymes. Despite the presence of the relevant transcription factors in all cell lines examined, in vivo footprinting showed DNA-protein interactions at six Sp1 binding sites and one novel binding site in MGMT-expressing cell lines but no such interactions in nonexpressors. We conclude that in contrast to findings of previous in vitro studies, Sp1 is an important component of MGMT transcription. These correlations also strongly suggest that methylation and chromatin structure, by determining whether Sp1 and other transcription factors can access the MGMT promoter, set the transcriptional state of the MGMT gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abravaya K., Phillips B., Morimoto R. I. Heat shock-induced interactions of heat shock transcription factor and the human hsp70 promoter examined by in vivo footprinting. Mol Cell Biol. 1991 Jan;11(1):586–592. doi: 10.1128/mcb.11.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Antequera F., Macleod D., Bird A. P. Specific protection of methylated CpGs in mammalian nuclei. Cell. 1989 Aug 11;58(3):509–517. doi: 10.1016/0092-8674(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Baltz K. A., Edenberg H. J. Expression of the human ADH2 gene: an unusual Sp1-binding site in the promoter of a gene expressed at high levels in liver. Gene. 1992 Nov 16;121(2):313–320. doi: 10.1016/0378-1119(92)90136-d. [DOI] [PubMed] [Google Scholar]
- Cairns-Smith S., Karran P. Epigenetic silencing of the DNA repair enzyme O6-methylguanine-DNA methyltransferase in Mex- human cells. Cancer Res. 1992 Oct 1;52(19):5257–5263. [PubMed] [Google Scholar]
- Citron M., Decker R., Chen S., Schneider S., Graver M., Kleynerman L., Kahn L. B., White A., Schoenhaus M., Yarosh D. O6-methylguanine-DNA methyltransferase in human normal and tumor tissue from brain, lung, and ovary. Cancer Res. 1991 Aug 15;51(16):4131–4134. [PubMed] [Google Scholar]
- Costello J. F., Futscher B. W., Tano K., Graunke D. M., Pieper R. O. Graded methylation in the promoter and body of the O6-methylguanine DNA methyltransferase (MGMT) gene correlates with MGMT expression in human glioma cells. J Biol Chem. 1994 Jun 24;269(25):17228–17237. [PubMed] [Google Scholar]
- Futscher B. W., Micetich K. C., Barnes D. M., Fisher R. I., Erickson L. C. Inhibition of a specific DNA repair system and nitrosourea cytotoxicity in resistant human cancer cells. Cancer Commun. 1989;1(1):65–73. doi: 10.3727/095535489820875444. [DOI] [PubMed] [Google Scholar]
- Green M. H., Karran P., Lowe J. E., Priestley A., Arlett C. F., Mayne L. Variation in the loss of O6-methylguanine-DNA methyltransferase during immortalization of human fibroblasts. Carcinogenesis. 1990 Jan;11(1):185–187. doi: 10.1093/carcin/11.1.185. [DOI] [PubMed] [Google Scholar]
- Harrington M. A., Jones P. A., Imagawa M., Karin M. Cytosine methylation does not affect binding of transcription factor Sp1. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2066–2070. doi: 10.1073/pnas.85.7.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. C., Potter P. M., Remack J. S., Brent T. P. A comparison of human O6-methylguanine-DNA methyltransferase promoter activity in Mer+ and Mer- cells. Cancer Res. 1992 Nov 15;52(22):6404–6406. [PubMed] [Google Scholar]
- Harris L. C., Potter P. M., Tano K., Shiota S., Mitra S., Brent T. P. Characterization of the promoter region of the human O6-methylguanine-DNA methyltransferase gene. Nucleic Acids Res. 1991 Nov 25;19(22):6163–6167. doi: 10.1093/nar/19.22.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra I. K., Yang T. P. High-resolution methylation analysis of the human hypoxanthine phosphoribosyltransferase gene 5' region on the active and inactive X chromosomes: correlation with binding sites for transcription factors. Mol Cell Biol. 1994 Feb;14(2):1419–1430. doi: 10.1128/mcb.14.2.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Schaffner W. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 1989 May;3(5):612–619. doi: 10.1101/gad.3.5.612. [DOI] [PubMed] [Google Scholar]
- Macdonald D. R., Gaspar L. E., Cairncross J. G. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Ann Neurol. 1990 May;27(5):573–574. doi: 10.1002/ana.410270519. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Mitchell R. B., Moschel R. C., Dolan M. E. Effect of O6-benzylguanine on the sensitivity of human tumor xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea and on DNA interstrand cross-link formation. Cancer Res. 1992 Mar 1;52(5):1171–1175. [PubMed] [Google Scholar]
- Nakatsu Y., Hattori K., Hayakawa H., Shimizu K., Sekiguchi M. Organization and expression of the human gene for O6-methylguanine-DNA methyltransferase. Mutat Res. 1993 Jan;293(2):119–132. doi: 10.1016/0921-8777(93)90063-m. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Mueller P. R., Wold B., Riggs A. D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989 Nov 10;246(4931):810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Tanguay R. L., Steigerwald S. D., Riggs A. D. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosomal DNA at the CpG island and promoter of human PGK-1. Genes Dev. 1990 Aug;4(8):1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- Pieper R. O., Costello J. F., Kroes R. A., Futscher B. W., Marathi U., Erickson L. C. Direct correlation between methylation status and expression of the human O-6-methylguanine DNA methyltransferase gene. Cancer Commun. 1991 Aug;3(8):241–253. doi: 10.3727/095535491820873092. [DOI] [PubMed] [Google Scholar]
- Sariban E., Kohn K. W., Zlotogorski C., Laurent G., D'Incalci M., Day R., 3rd, Smith B. H., Kornblith P. L., Erickson L. C. DNA cross-linking responses of human malignant glioma cell strains to chloroethylnitrosoureas, cisplatin, and diaziquone. Cancer Res. 1987 Aug 1;47(15):3988–3994. [PubMed] [Google Scholar]
- Schmidt M. C., Zhou Q., Berk A. J. Sp1 activates transcription without enhancing DNA-binding activity of the TATA box factor. Mol Cell Biol. 1989 Aug;9(8):3299–3307. doi: 10.1128/mcb.9.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Tazi J., Bird A. Alternative chromatin structure at CpG islands. Cell. 1990 Mar 23;60(6):909–920. doi: 10.1016/0092-8674(90)90339-g. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kato T., Ayaki H., Ishizaki K., Tano K., Mitra S., Ikenaga M. Correlation between DNA methylation and expression of O6-methylguanine-DNA methyltransferase gene in cultured human tumor cells. Mutat Res. 1992 Mar;273(2):221–230. doi: 10.1016/0921-8777(92)90083-f. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Hurst-Calderone S., Kohn K. W. Measurement of O6-alkylguanine-DNA alkyltransferase activity in human cells and tumor tissues by restriction endonuclease inhibition. Cancer Res. 1987 Dec 1;47(23):6229–6235. [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wronski M. A., Harris L. C., Tano K., Mitra S., Bigner D. D., Brent T. P. Cytosine methylation and suppression of O6-methylguanine-DNA methyltransferase expression in human rhabdomyosarcoma cell lines and xenografts. Oncol Res. 1992;4(4-5):167–174. [PubMed] [Google Scholar]