Abstract
Cue-induced cravings may hinder behavior change efforts such as smoking cessation. Correlation of cue-induced cravings across multiple stimuli would provide evidence for a cue-reactive phenotype that may have implications for behavior change therapies. The purpose of this study was to examine relationships between cue-induced cravings for cigarettes and cue-induced cravings for a highly preferred food (chocolate) in a sample of smokers not subjected to lengthy deprivation for either of these two appetitive outcomes. Adult smokers (N=164) were assessed for chocolate cravingsbefore and after exposure to chocolate cues and cigarette cravings before and after exposure to smoking cues. Consistent with previous reports, cigarette cravings increased significantly post-cue exposure and chocolate cravings increased significantly post-cue exposure (ps<.0001). Consistent with study hypotheses, the magnitude of the increase inchocolate cravings after cue-exposure was significantly related to the increase inpost-cue cigarette cravings (r=0.38; p<.0001), and was significantly related to scores on a retrospective,self-report, measure of cue-induced food cravings in daily life.These findings are consistent withthe idea of a general “cue-reactive” phenotype that varies across individuals, a conceptualization of risk that may point the way towardimproved interventions for a variety of hedonically mediated behaviors with negative health outcomes.
Keywords: Tobacco use disorder, cigarette cravings, food cravings, self-reported cravings, cue-reactivity, cue-induced craving
Introduction
Strong cravings for cigarettes are a common problem for individuals attempting to quit smoking, and high craving intensity predicts low cessation success(Ferguson & Shiffman, 2009; Fidler, Shahab, & West, 2011; Fidler & West, 2011; Waters et al., 2004).In addition to cigarette cravings associated with reductions in circulating nicotine levels, it is increasingly recognized that exposure to cues associated with cigarette smoking can trigger intense cravings, as well as generate changes in physiologic parameters, and activation ofreward pathways in the brain that mediate motivated behaviors (Balfour, 2009; Brody et al., 2002; Carter & Tiffany, 1999; Erblich, Bovbjerg, & Sloan, 2011; Miranda, Rohsenow, Monti, Tidey, & Ray, 2008). Though not undisputed, cue-induced cravings for cigarettes are thought to be a key factor in relapse to smoking even after long periods of abstinence (Ferguson & Shiffman, 2009; Perkins, 2009; Shiffman, 2009). Yet to receive research attention is the possibility that smokers with higher levels of cue-induced craving for cigarettes may also be vulnerable to other cue-induced cravings, such as for highly palatable foods.
A growing body of empirical evidence and recent theorizing supports the view that cue-induced cravings for food and drugs of abuse, including cigarettes, may share the same neurobiological pathways (Blumenthal & Gold, 2010; Kenny, 2011; Pelchat, 2009). Similar to smoking-cue exposure, food-cue exposure elicitsintense cravings, as well as generate changes in physiological responses, and activation of brain reward regions involved inmotivated behaviors, even among individuals who have recently eaten (Alsene, Li, Chaverneff, & de Wit, 2003; Cornier, 2009; Ferriday & Brunstrom, 2008, 2011). To date however, only one small study has assessed cue-induced cravings for food and cigarettes in the same individuals, and this study found correlations between the two when participants were under 18 hour selective deprivation(Mahler & de Wit, 2010). Evidence of strong correlations between cue-induced cravings for preferred foods and cigarettes would not only provide behavioral support for shared brain mechanisms, it would also point the way toward an experimental protocol with which to explore common and unique aspects of these conditioned cravings. The results of such research would have implications for optimizing treatment interventions for individuals with a propensity for multiple cue-induced behaviors(a cue-reactive phenotype) that might have negative health implications.
The purpose of this study was to examine relationships between cue-induced cravings for cigarettes and cue-induced cravings for chocolate (one of the most highly craved food items (Polivy, Coleman, & Herman, 2005)) in a sample of smokers in the absence of lengthy concurrent deprivation.
Methods
A subgroup of 164 healthy smokers (all participants who completed the food craving assessment which was added to the protocol in mid-recruitment) induced cigarette craving the parent study, participants had to report smoking at least 10 cigarettes per day for at least from a larger study of cue-induced cigarette craving (Erblich & Bovbjerg, 2004)was included in this study. To be eligible for 5 years and qualify for a current diagnosis of nicotine dependence per the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994). Exclusion criteria were: (1) current treatment for smoking cessation, (2) current or past substance abuse (other than nicotine), (3) history of hospitalization for mental illness, (4) history of smoking-related illness, (5) below the age of 23, and (6) current pregnancy. To be eligible for this sub-study, participants had to complete the parent study and be willing to complete the food craving assessment.
Basic demographic information (age, gender, education, income, ethnicity, marital status) and smoking history data (age at initiation, cigarettes per day, years having smoked) were obtained via self-administered questionnaires. The 6-item Fagerstrom Test of Nicotine Dependence (FTND) was used to measure the severity of particip ants’ dependence on nicotine(Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Finally, participants completed the Situational Appetite Measures-Urges scale (SAM-U) and Efficacy scale (SAM-E). These instruments assess “trait” level of food cue-induced craving in daily life. Each scale has 5 factors with 6 items each related to food cravings in response to specific internal and/or external stimuli: relaxation, presence of food, hunger, reward and negative feelings. Questions asked on the SAM-U and SAM-E are identical and both use a 5-point response scale; however, the SAM-U assesses urge to eat, whereas the SAM-E assesses confidence in resisting the urge to eat. Reliability coefficients for the two factors range from .74 to .95 (Stanton, Garcia, & Green, 1990).
Self-reported craving in response to the experimental cue exposures was assessed via a brief, five-item, 0–100 instrument designed specifically to make rapid assessments of craving during experimental manipulations that has been used in multiple studies (Erblich & Bovbjerg, 2004; Erblich, Boyarsky, Spring, Niaura, & Bovbjerg, 2003; Hutchison, Niaura, & Swift, 1999). Pre- and post-cue versions of the instrument separately assessed cravings for cigarettes and chocolate “right now” and “during the scene,” respectively.
Procedures
To avoid ceiling effects in cigarette craving, participants were instructed to smoke one cigarette immediately before beginning the study procedures. Participants then turned in their package of cigarettes (used for in vivo exposure; see below) and were given a carbon monoxide (CO) breath test using a MicroCO monitor (MicroDirect, Lewiston, ME). To be conservative, we exploredCO levels as a potential covariate in the analyses (see below).
Following a classic cue-reactivity paradigm, participants were exposed to neutral, food-related, and smoking-related cues. The neutral cue was a stapler, the food cue was a bar of the participant’s preferred brand of chocolate, and the smoking cue was a cigarette from the participant’s own pack. To avoid possible carryover effects (Sayette, Griffin, & Sayers, 2010), the neutral cue was always presented first, followed by the chocolate cue and then the smoking cue. Self-reported chocolate and cigarette cravings were assessed immediately before and after each cue exposure using the face-valid craving questionnaire. To reduce the possibility of carryover, participants viewed a brief nature video (Hannan, 1999) between each of the three cue exposures. During each exposure, participants held and sniffed the stimulus for 90 s.
Data Analysis
To measure reactivity, we calculated raw pre- and post-change scores on the craving scale for each of the three exposures (neutral, chocolate, cigarette). To control for initial levels, we always analyzed change scores with pre-exposure craving entered as a covariate, yielding indices of baseline-adjusted change (theoretical range = -100– 100). This approach resolves the frequently discussed problem of poor change score reliability(Pedhazur, 1997). To address the study hypotheses, we conducted partial correlation analyses between: 1) smoking-cue-induced cigarette craving and chocolate-cue-induced chocolate cravings, and 2) specific cue-induced cravings and SAM scores. These analyses controlled for demographics, time since last cigarette, CO levels, BMI, FTND, and craving responses to the neutral stimulus. It should be noted that results were comparable with or without partialling out these factors. Finally, we explored the possibility that gender and ethnicity would moderate the relationships between cigarette and food cravings by comparing subgroup correlations with a Fisher’s r to Z transformation.
Results
Participants had a mean age of 37.3 ± 10.9 years of age. The sample was 49% female, 37% African American, 29% Caucasian, and 25% Hispanic and smoked an average of 18.0 cigarettes per day (FTND=5.4). Mean BMI was 27.6 ± 7.0. Mean time since last cigarette was 32.1 ± 4.2 minutes. Annual household incomes were less than $20,000 per year for 40% of the participants, and 70% had completed at least some college.
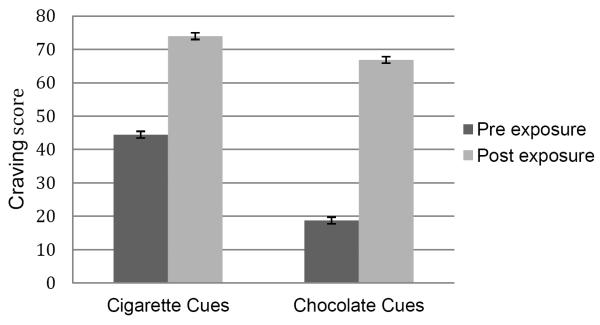
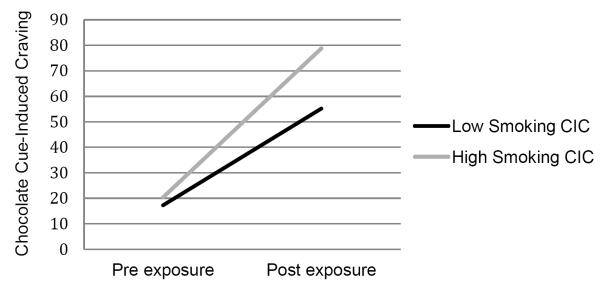
As shown in Table 1, the participants’ cigarette cravings increased significantly post-smoking cue exposure (p<.0001). Similarly, participants’ chocolate cravings increased significantly post-chocolate cue exposure (p<.0001). Consistent with the study hypothesis, there was a significant correlation between smoking-cue-induced cigarette craving and chocolate-cue-induced chocolate craving; (r=0.38; p<.0001). To better illustrate this relationship, we performed a two-group median split on cigarette craving scores and compared chocolate craving scores between the two groups. As depicted in Figure 1, smokers with above-median scores in smoking cue-induced cigarette cravings had significantly higher chocolate cue-induced chocolate cravings under laboratory conditions.
Table 1.
Mean craving scores measured pre and post cue exposure and measured with the Situational Appetite Measures instruments.
| Variable | Mean | SD |
|---|---|---|
|
| ||
| Cigarette Craving | ||
| Pre Neutral Cue | 41.5 | 35.8 |
| Post Neutral Cue | 28.5 | 35.5 |
| Pre Smoking Cue | 44.4 | 36.2 |
| Post Smoking Cue | 74.0 | 32.8 |
|
| ||
| Chocolate Craving | ||
| Pre Neutral Cue | 22.0 | 30.1 |
| Post Neutral Cue | 14.3 | 25.3 |
| Pre Chocolate Cue | 18.7 | 29.6 |
| Post Chocolate Cue | 66.9 | 35.8 |
|
| ||
| SAM - E |
79.4 | 21.6 |
|
| ||
| SAM - U |
77.7 | 17.6 |
Figure 1.
Cigarette Cue-Induced Cigarette Cravings and Chocolate Cue-Induced Chocolate Cravings
We also examined the relationship between laboratory cue-induced cravings and SAM scores (i.e., measures of “real world” cue-induced food urges). Findings revealed that laboratory cigarette craving responses were significantly correlated with both the SAM-E and SAM-U (r’s=0.22, p’s<.005). As expected, experimentally-induced cue-induced chocolate cravings were also associated with elevated SAM-E (r=0.20, p<.01) and SAM-U (r=0.22, p<.005) scores. Separate analyses by gender and ethnicity yielded statistically comparable correlations using Fisher’s r to Z transformation (p’s>.3), suggesting that effects were not moderated by gender or ethnicity. Finally, demographic factors, BMI, FTND, time since last cigarette, and CO levels were not related to any of the craving outcomes (p’s >.20).
Discussion
The present study explored the extent to which cue-induced cravings for cigarettes are related to cue-induced cravings for chocolate. Consistent with the study hypotheses, we found a significant relationship between the intensities of cravings induced by smoking cues and those induced by chocolate cues in a community sample (n=164) of nicotine-dependent smokers under controlled laboratory conditions. The intensities of these experimentally induced cravings were also found to be related to participants’ scores on a well-validated measure of cue-induced cravings for food in daily life. We found no evidence that the strength of these relationships were related to either gender or ethnicity.
Our findings provide additional support for the existence of a general “cue-reactive” phenotype, as proposed by Mahler and de Wit (2010). In a study of 15 college age smokers, Mahler and de Wit (2010) found a strong correlation (r=.57) between cue-induced cigarette craving and cue-induced food craving after 18 hours of abstinence from smoking and/or eating. They found no association between cue-induced cravings when participants were permitted to smoke and eat during study procedures. Although we did not allow participants to smoke or eat during study procedures, prior abstinence was not required. Indeed, participants were instructed to smoke immediately before initiating study procedures. Our results thus indicate that substantial deprivation periods are not necessary to see significant correlations in the intensity of craving responses elicited across different appetitive cues. The somewhat lower correlation between different types of cue-induced cravings in our study (r=.38) compared to that found in the Malhler and de Wit study (r=.57), may reflect the effects of deprivation status, but could also be due to a variety of other methodological differences between the two studies (e.g., their use of a set of food cues vs. a specific chocolate cue). Additional research will be required to determine the extent to which deprivation state influences the detection of a cue-reactive phenotype. Future research should also confirm correlations across additional types of cue-induced cravings (e.g., cocaine) to establish the generalizabilty of individual differences in cue-reactivity, as suggested by analogous studies of cues with incentive motivational value in animal models (Saunders & Robinson, 2011).
Recent theorizing suggests that the neurobiological pathways underlying cue-induced cravings for food, include the same mesolimbic dopamine reward circuitry involved in cue-induced craving for drugs of abuse, including nicotine, alcohol, and cocaine (Berridge, 2007; Blum, Liu, Shriner, & Gold, 2011; Blumenthal & Gold, 2010; Kenny, 2011; Pelchat, 2009). The mechanisms responsible for individual differences in the intensity of cue-induced cravings across these modalities have yet to be determined. However, accumulating evidence from animal studies and initial research with humans increasingly implicates genetic differences in dopamine pathways activated by such incentive stimuli (Blum et al., 2011; Erblich, Lerman, Self, Diaz, & Bovbjerg, 2005; Flagel, Akil, & Robinson, 2009; Flagel et al., 2011; Flagel et al., 2010).
The clinical implications of the possibility that some individuals may have a propensity for particularly intense cue-induced cravings (a high cue-reactive phenotype) have yet to be realized, but may be profound. For example, weight gain is a common side-effect of smoking cessation, but little attention has been paid to the possible role of a shared phenotype for cue reactivity. Evidence suggests that there are significant short-term benefits and no risks of combining smoking treatment and behavioral weight control (B. Spring et al., 2009); however, not all reports are supportive of dual-treatment (Parsons, Lycett, & Aveyard, 2009; Perkins et al., 2001; B. Spring, McFadden, Rademaker, & Hitsman, 2011). Individuals who are highly cue reactive may be more likely to have difficulty changing both their eating and smoking behaviors together, or they may be more particularly responsive to tailored interventions. More research to explore possible synergistic benefits would now seem to be warranted.
Key strengths of the research reported here include the large, community recruited sample (n=164) and diverse ethnic representation. Moreover, the research design included both experimental and survey based assessments to explore possible relationships between cue-induced urges for chocolate and cigarettes under controlled laboratory conditions and urges for foods in daily life. A lack of survey-based assessments of smoking cue-induced cravings in daily life is a weakness, as is the lack of data regarding time of last eating episode and, more specifically, length of abstinence from chocolate. It is also important to note that while we found increases in self-reported cravings, it is not clear whether these cravings would result in increased eating or smoking in response to the cues. Future studies should take this line of research one step further and examine to what extent specific cues elicit alterations in behavior as well as increased urges.
In summary, the present study demonstrated significant associations in the magnitude of cravings induced by smoking cuesand chocolate cues under controlled laboratory conditions, as well as in daily life. These findings contribute to a growing appreciation that a common phenotype of increased cue-reactivity, which may reflect underlying genetic variability in brain responses to incentive stimuli, could contribute to increased risk of multiple negative health outcomes in the context of addictive behaviors.
Highlights.
Self-reported cravings for chocolate increase following exposure to chocolate cues; self-reported cravings for cigarettes increase following exposure to smoking cues.
Higher levels of smoking cue-induced cigarette cravings are associated with heightened chocolate cue-induced chocolate cravings, supporting the possibility of a “cue-reactive” phenotype.
Substantial deprivation periods are not necessary to see significant correlations between these two responses to appetitive cues.
Figure 2.
Relationship Between Smoking Cue-Induced Craving and Chocolate Cue-Induced Craving
Acknowledgements
This work was funded by grants #K22CA124800, #R21CA118703, and #UL1RR029887, from the National Institutes of Health
Role of Funding Sources This work was funded by grants #K22CA124800, #R21CA118703, and #UL1RR029887, from the National Institutes of Health. The National Institutes of Health had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributors Drs. Erblich and Bovbjerg designed the study and wrote the protocol. Ms. Lipsky participated in data collection and analysis. Dr. Styn conceptualized and wrote the manuscript in collaboration with the other authors. All authors contributed and have approved the final manuscript.
Conflict of Interest All authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Alsene KM, Li Y, Chaverneff F, de Wit H. Role of abstinence and visual cues on food and smoking craving. Behavioural Pharmacology. 2003;14:145–151. doi: 10.1097/00008877-200303000-00006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4 ed Author; Washington, DC: 1994. [Google Scholar]
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handbook of Experimental Pharmacology. 2009;192:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Current Pharmaceutical Design. 2011;17(12):1158–1167. doi: 10.2174/138161211795656819. [DOI] [PubMed] [Google Scholar]
- Blumenthal DM, Gold MS. Neurobiology of food addiction. Current Opinion in Clinical Nutrition & Metabolic Care. 2010;13(4):359–365. doi: 10.1097/MCO.0b013e32833ad4d4. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG. Brain Metabolic Changes During Cigarette Craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cornier M-A. The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiology & Behavior. 2009;97(5) doi: 10.1016/j.physbeh.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH. In vivo versus imaginal smoking cue exposures: is seeing believing? Experimental & Clinical Psychopharmacology. 2004;12(3):208–215. doi: 10.1037/1064-1297.12.3.208. [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH, Sloan RP. Exposure to smoking cues: Cardiovascular and autonomic effects. Addictive Behaviors. 2011;36:737–742. doi: 10.1016/j.addbeh.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98(5):657–664. doi: 10.1046/j.1360-0443.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Molecular Psychiatry. 2005;10(4):407–414. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. How does food cue exposure lead to larger meal sizes? British Journal of Nutrition. 2008;100:1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. International Journal of Obesity. 2011;35:142–149. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Fidler JA, Shahab L, West R. Strength of urges to smoke as a measure of severity of cigarette dependence: comparison with the Fagerström Test for Nicotine Dependence and its components. Addiction. 2011;106(6):631–638. doi: 10.1111/j.1360-0443.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- Fidler JA, West R. Enjoyment of smoking and urges to smoke as predictors of attempts and success of attempts to stop smoking: a longitudinal study. Drug and Alcohol Dependence. 2011;115(1-2):30–34. doi: 10.1016/j.drugalcdep.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35(2):388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan D. Coral sea dreaming [Motion picture] Mill Reef Entertainment/DVD International; Mountain Lakes, NJ: 1999. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Experimental & Clinical Psychopharmacology. 1999;7(3):250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nature Reviews Neuroscience. 2011;12(11):638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Rohsenow DJ, Monti PM, Tidey J, Ray L. Effects of Repeated Days of Smoking Cue Exposure on Urge to Smoke and Physiological Reactivity. Addictive Behaviors. 2008;33:347–353. doi: 10.1016/j.addbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Lycett D, Aveyard P. Behavioural interventions to prevent weight gain on smoking cessation: a response. Addiction. 2009;104:2119–2120. doi: 10.1111/j.1360-0443.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- Pedhazur E. Multiple Regression in Behavioral Research. 3rd ed Harcourt Brace; Fort Worth, TX: 1997. [Google Scholar]
- Pelchat ML. Food addiction in humans. Journal of Nutrition. 2009;139(3):620–622. doi: 10.3945/jn.108.097816. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. Journal of Consulting & Clinical Psychology. 2001;69(4):604–613. [PubMed] [Google Scholar]
- Polivy J, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behaviour in restrained and unrestrained eaters. International Journal of Eating Disorders. 2005;38:301–309. doi: 10.1002/eat.20195. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Griffin KM, Sayers WM. Counterbalancing in smoking cue research: A critical analysis. Nicotine & Tobacco Research. 2010;12:1068–1079. doi: 10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Responses to smoking cues are relevant to smoking and relapse. Addiction. 2009;104:1617–1618. doi: 10.1111/j.1360-0443.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- Spring B, Howe D, Berendsen M, McFadden G, Hitchcock K, Rademaker AW. Behavioral interventions to promote smoking cessation and prevent weight gain: a systematic review and meta-analysis. Addiction. 2009;104:1472–86. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, McFadden HG, Rademaker AW, Hitsman B. Behavioral interventions to promote smoking cessation and prevent weight gain: a reply. Addiction. 2011;106(3):674–675. doi: 10.1111/j.1360-0443.2010.03169.x. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Garcia ME, Green SB. Development and validation of the situational appetite measures. Addictive Behaviors. 1990;15(5):461–472. doi: 10.1016/0306-4603(90)90033-t. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting & Clinical Psychology. 2004;72(6):1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]